Introduction
The family of Trefoil factor (TFF)-peptides,
previously known as P-domain peptides, consists of 3 members (TFF1,
2 and 3) (1). TFF-peptides are small
proteins secreted by a number of mucosal epithelial cells (2). TFFs possess a common trefoil domain
structural motif, forming characteristic disulfide bonds through
six conserved cysteine residues, and are highly gastric acid and
protease-resistant (2). TFFs help
maintain mucosal barrier integrity by protecting the
gastrointestinal (GI) mucosa against injury and improving repair
following injury (3–5). The pit cells in gastric mucosa
primarily secret TFF1, whereas TFF2 is synthesized by the neck
cells of the gastric mucosa and the Brunner's glands of the
duodenal submucosa (6). The
distribution of TFF1 and TFF2 is confined to the proximal GI tract,
whereas TFF3 is produced by the goblet cells and secreted
abundantly throughout the intestinal tract (3). TFF3 protects and repairs the
gastrointestinal mucosa, maintains the tight junction barrier
integrity and restores normal intestinal permeability during
inflammatory bowel disease pathogenesis (7). TFF3 is also present in the neural lobe
of the porcine pituitary gland (8,9).
The GI tract acts as a barrier against bacteria and
toxins preventing digestive disorders. Weaning is an important
process during development of the pig (10). The structure and function of the
digestive system of a growing pig begins immediately to change when
weaning begins (10). Weaning
involves extensive exposure to novel antigens, improving the
redistribution of the microbial community in the small intestine
(10). Piglets are susceptible to
disease after weaning. When the GI mucosal barrier function is
impaired, the ecological balance among gut microbes is destroyed
leading to enterogenous infection, resulting in diarrhea (11). Diarrheal piglets are of significant
concern to the pig industry (10).
Previous studies have revealed that TFFs can maintain the integrity
of the intestinal epithelia in mice, heal wounds in humans and
participate in cell adhesion, motility and apoptosis in
vitro (12–17). TFFs are involved in mucosal immune
modulation by regulating leukocyte recruitment and repairing tissue
to ensure healthy mucosal surfaces (18). In addition, the level of TFF3
expression is increased in the intestine of weaning rats (19). Thus, the present study hypothesized
that TFF3 may serve an important role in preventing diarrhea in
weaning piglets.
In previous studies, TFF3 has been expressed in
Escherichia coli in an intracellular and soluble form
(20–22). However, this method of production is
not ideal because of the low yield and bioactivity of the produced
TFF3 (20–22). The present study developed a
recombinant expression system for sus scrofa TFF3 fused with
a 6×his-tag in a strain of B. choshinensis. B.
choshinensis is a Gram-positive bacterium, which has the
excellent advantage of secreting various extracellular proteins
with high efficiency (23). The
spore forming ability of B. choshinensis has been removed by
genetic engineering. In addition, this expression system is very
powerful for producing proteins with native structures, even when
they contain disulfide bonds (23).
pNCMO2 is a B. choshinensis-E. coli shuttle vector (24). The pNCMO2 vector includes the P2
promoter, which drives the transcription of cell wall protein but
has no role in E. coli (24).
The objective of the present study was to explore the production of
sus scrofa TFF3 by B. choshinensis in vitro.
Materials and methods
Bacteria, plasmids and media
B. choshinensis strain HB116 (Takara Bio,
Inc.) was used in this study. E. coli DH5α competent cells
(Sangon Biotech Co, Ltd.) were used for DNA manipulation. pNCMO2
(Takara Bio, Inc.) and pMD19-T (Takara Bio, Inc.) were used as the
vector and subcloning plasmid, respectively. Milk-Tween (MT) medium
containing 2% yeast extract, 10% glucose, 10% polypeptone, 5% meat
extract, 0.01 % FeSO4·7H2O, 0.001%
ZnSO4·6H2O and 0.01%
MnSO4·4H2O was used to culture strain HB116.
E. coli DH5α cells were cultured in Luria Broth medium
(Oxoid; Thermo Fisher Scientific, Inc.). NaOH was used to adjust
the pH of all media to 7.0. Neomycin (20 µg/ml; Beijing Solarbio
Science & Technology Co, Ltd.) was added to the media used to
culture bacteria containing pNCMO2 and derivatives.
RNA extraction and PCR
Total RNA from sus scrofa spleen tissue
(preserved in our laboratory) was isolated using a
TRIzol® reagent kit (Takara Bio, Inc.) according to the
manufacturer's instructions. cDNA synthesis was performed using a
PrimeScript Reverse Transcriptase kit (Takara Bio, Inc.). Primers
for the sus scrofa TFF3 gene were designed using Primer
v.5.0 software (Sangon Biotech. Co. Ltd.), according to the gene
sequence in the GenBank database (accession no. NM_001243483). mRNA
specific primers (Sangon Biotech Co, Ltd.) were: TFF3 forward,
5′-GCATGGAGGCCAGGATGT-3′ and reverse, 5′-CGGTTAGAAGGTGCATTCT-3′.
The PCR program to amplify the TFF3 gene from cDNA was 95°C for 5
min, followed by 35 cycles of 95°C for 30 sec, 55°C for 20 sec and
72°C for 30 sec with a final extension step at 72°C for 10 min. PCR
products were separated by 2% agarose gel electrophoresis. Purified
PCR fragments were retrieved using a PCR gel recovery kit (Takara
Bio Inc.). Bands were visualized using the GelDoc XR+
(Bio-Rad Laboratories, Inc.) gel imaging system through nucleic
acid staining. Densitometric analysis was performed using Gel-Pro
Analyzer 4.0 software (Media Cybernetics, Inc.).
Construction of pNCMO2-TFF3-6×his
According to the manufacturer's instructions of the
pMD19-T vector kit (Takara Bio, Inc.), the TFF3-encoding gene and
6×his tag were linked to the T vector to generate
pMD19-T-TFF3-6×his and transformed into E. coli DH5α.
Positive clones were identified by colony PCR, where a single
bacterium is used as a template, and can quickly identify whether
the colony is a positive colony with the target gene. Plasmids
extracted from the positive clones were sequenced by Takara Bio,
Inc. The primers were forward, 5′-GCgtcgacATGGAGGCCAGGATGT-3′ and
reverse, 5′-CGGggtaccTTAGTGATGTGATGGTGATGGAAGGTGCATTCT-3′;
lower-case letters denote the enzyme restriction sites for
SalI and KpnI and the underlined bases denote the
nucleotide sequence encoding the 6×his tag. To construct the sus
scrofa TFF3 expression vector pNCMO2-TFF3-6×his, the fused
TFF3-6×his combined fragment from pMD19-T-TFF3-6×his was amplified
by PCR. The following thermocycling conditions were used for the
PCR: initial denaturation at 95°C for 10 min; followed by 35 cycles
of 95°C for 30 sec, 56°C for 30 sec and 72°C for 30 sec; and a
final extension 72°C for 10 min. Digestion with SalI and
KpnI followed by ligation at 16°C overnight was used to
clone the amplified fragment into the pNCMO2 vector to generate
pNCMO2-TFF3-6×his. DNA sequencing was performed to confirm the
cloned DNA sequence (Takara Bio, Inc.).
B. choshinensis transfection
Competent cells of B. choshinensis HB116 were
prepared by inoculating 1 ml bacterial solution in 100 ml MT medium
(Shanghai Kemin Biotechnology Ltd.), which were then cultured at
37°C for 18 h. Bacteria were then collected by centrifugation at
4,000 × g for 5 min at room temperature, and suspended in 50 mmol/l
Tris-HCl (pH=8.5; Beijing Dingguo Changsheng Biotechnology Co,
Ltd.). Bacteria were then transfected with vector DNA using
electrophoretic transfer according to the manufacturer's
instructions (Takara Bio, Inc.). The empty pNCMO2 vector was
transfected as a negative control. A MicroPulser (Bio-Rad
Laboratories, Inc.) was used with the Ec2 program.
Protein expression of recombinant sus
scrofa TFF3
B. choshinensis containing pNCMO2-TFF3-6×his
or pNCMO2 was cultured in liquid MT medium containing 20 mg/l
neomycin at 30°C for 60 h. Once the bacteria reached the
logarithmic growth period at 37°C, isopropyl
β-D-1-thiogalactopyranoside (final concentration 1 mmol/l) was
added to the bacteria. Following induction, the collected bacteria
were disintegrated by ultrasound (JY88-II Ultrasonic Cell
Disruptor; Bio-Equip) and lysed in PBS (pH=7.4). The bacterial
precipitate and supernatant were subsequently used in SDS-PAGE.
Supernatants and cells were separated by centrifugation at 12,000 ×
g for 20 min at 4°C. Proteins were detected by reducing 14%
SDS-PAGE stained with Coomassie Brilliant Blue, and the amount of
secreted proteins was evaluated. Gel-Pro Analyzer 4.0 software
(Media Cybernetics, Inc.) was used to scan and measure the density
of bands on gels to evaluate the expression level of the target
protein. For western blotting, protein samples were boiled in
SDS/b-mercaptoethanol loading buffer (192 mM glycine and 25 mM
Tris; pH 8.3; Beijing Dingguo Changsheng Biotechnology Co, Ltd.)
and subsequently electrophoresed. A total of 10 µg protein/lane was
separated via SDS-PAGE on a 14% gel. Proteins in the gel were
transferred onto PVDF membranes (Merck KGaA) by electrophoretic
transfer. Next, the membrane was blocked for 1 h in 5% skim milk at
room temperature. TBST [20 mM Tris (pH 8.0), 200 mM NaCl and 0.1%
Tween 20] was used to dilute the mouse anti-6×his-tag monoclonal
primary antibody (Abcam; cat. no. ab18184; 1:2,000). The membrane
was incubated overnight with the primary antibody at 4°C and washed
with TBST three times for 15 min. Horseradish peroxide-conjugated
secondary antibody (Abcam; goat-mouse IgG; cat. no. ab150113) was
diluted 1:3,000 with TBST and incubated with the membrane for 1 h
at room temperature. Washing with TBST was subsequently performed
three times for 15 min. ECL development methods and X-ray film
exposure were used to visualize the bands (Merck KGaA).
Results
Cloning of the TFF3 gene
The coding DNA sequence (CDS) region of the sus
scrofa TFF3 gene (NM_001243483) was searched for in the NCBI
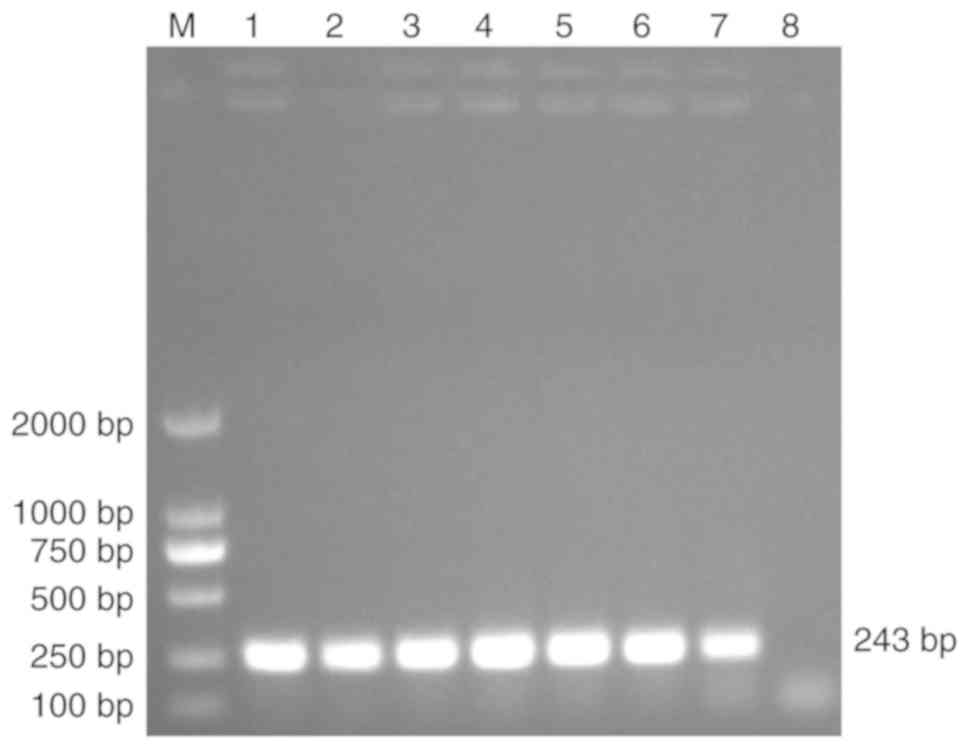
database. The length of the TFF3 CDS is 243 bp (25). In the present study, the TFF3 CDS was
cloned and a ~250 bp PCR product was obtained (Fig. 1). Sequencing confirmed the nucleotide
sequence and length of the target band (data not shown).
Verification of pNCMO2-TFF3-6×his
protein fragment
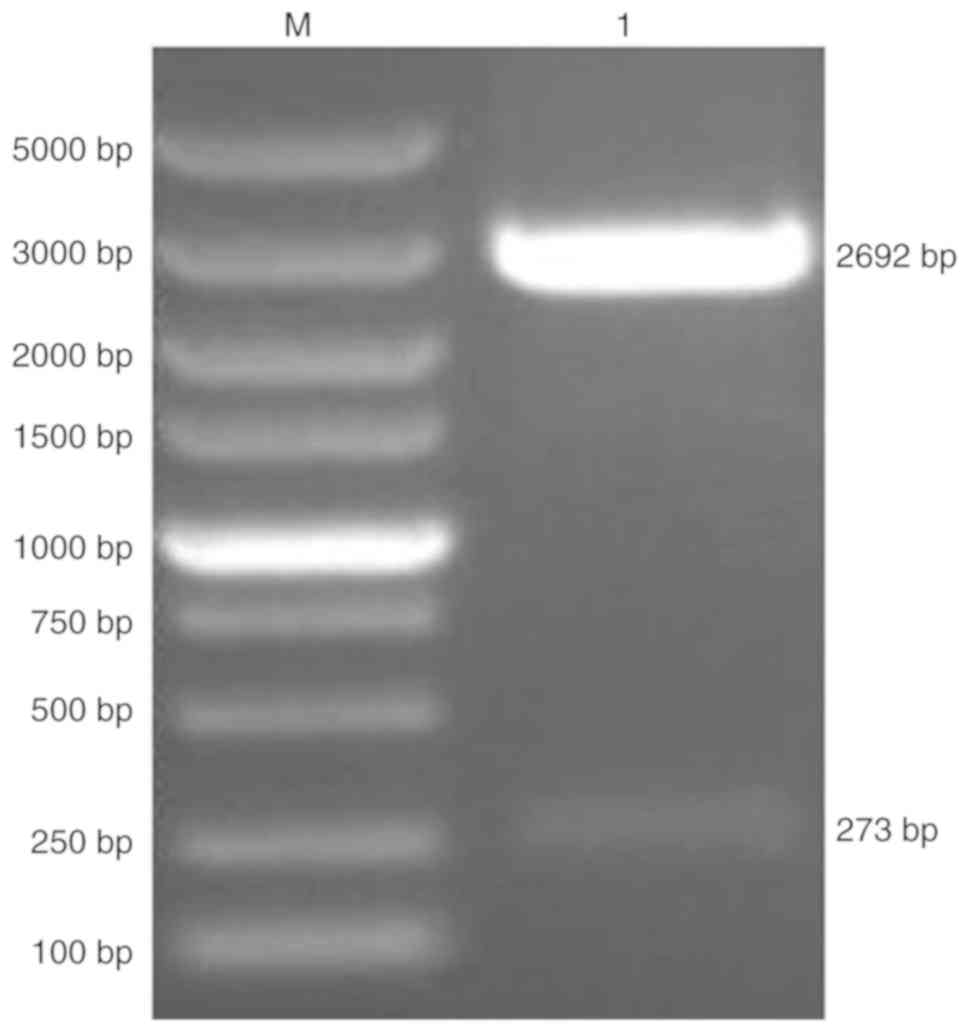
pNCMO2-TFF3-6×his was generated by cloning the TFF3
CDS into pNCMO2. pMD19-T-TFF3-6×his was verified by a restriction
enzyme digest, which produced two fragments of the expected sizes
273 and 2,692 bp (Fig. 2).
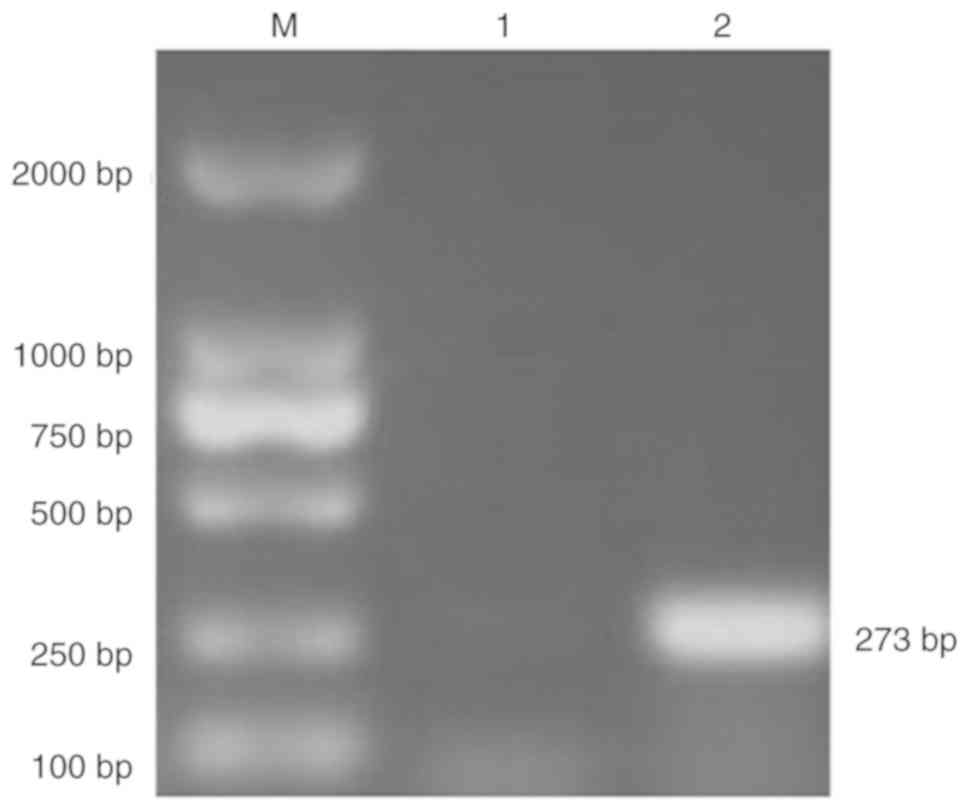
pNCMO2-TFF3-6×his was verified by PCR using the total extracted DNA
as the template (Fig. 3).
Verification of recombinant B.
choshinensis
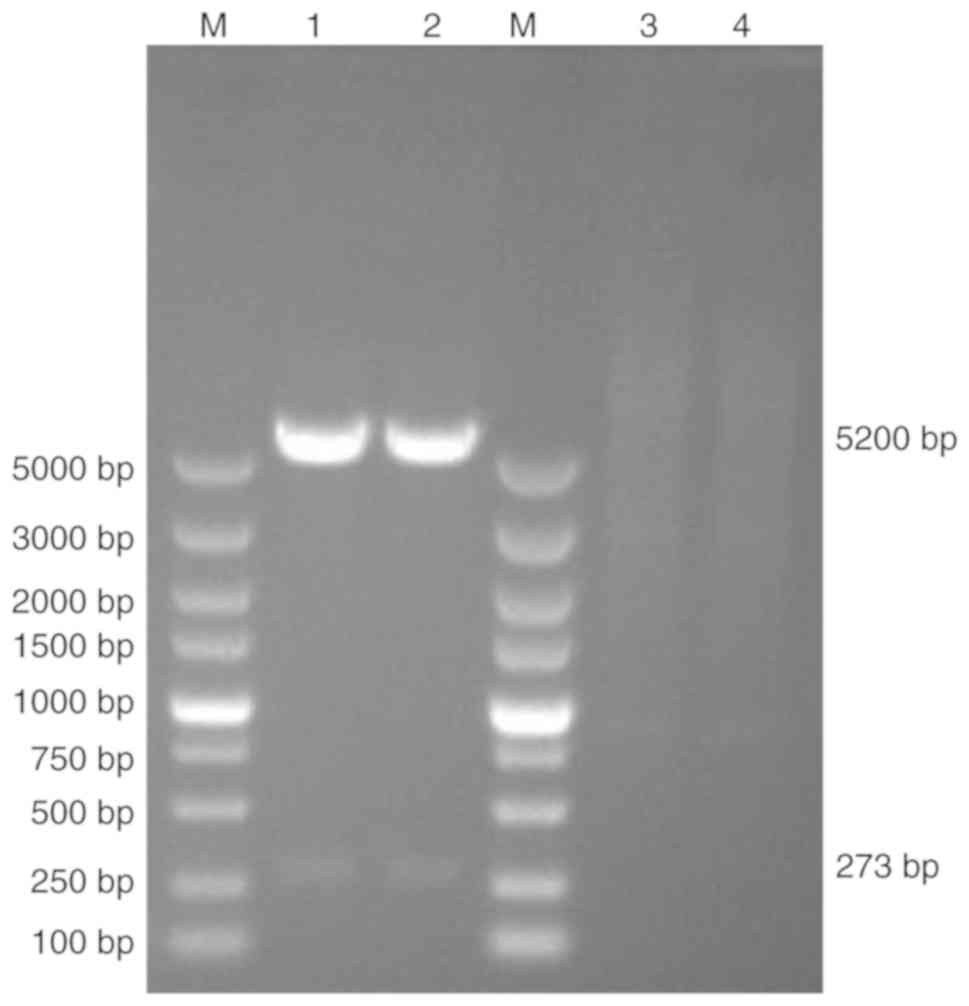
pNCMO2-TFF-6× his was electroporated into the B.
choshinensis strain HB116. The resulting strain
HB116-pNCMO2-TFF3-6×his was verified by double enzyme digestion
using extracted plasmid DNA as the template, SalI and
KpnI were used as restriction enzymes to digest plasmid. The
size of target band and plasmid were 273 and 5,200 bp, respectively
(Fig. 4).
Analysis of the recombinant fusion
protein
Protein samples from the culture medium of HB116
cells, which were transformed with the pNCMO2-TFF3-6×his plasmid,
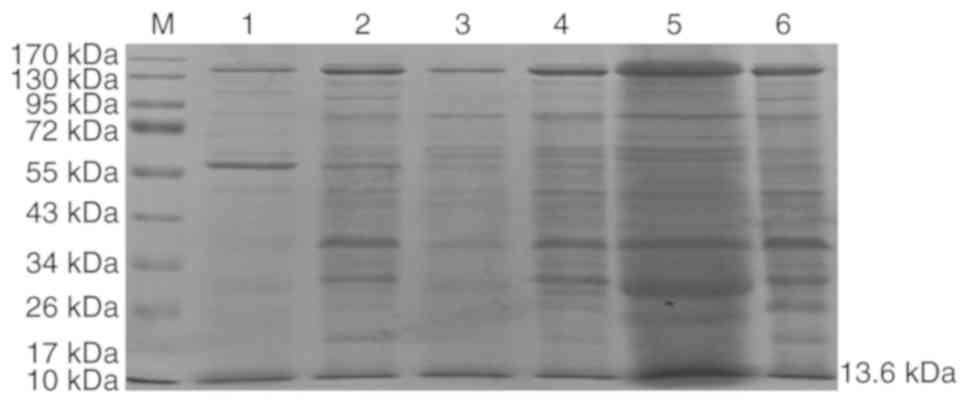
were detected using SDS-PAGE. A band with a molecular weight of ~55
kDa in the supernatant (lane 1; Fig.
5) and precipitate (lane 2; Fig.
5) of the lysate of positive control vector-amylase, while a
recombinant protein with a molecular weight of ~13 kDa was secreted
by the supernatant and precipitate of HB116-pNCMO2-TFF3-6×his (lane
5 and 6, respectively; Fig. 5). In
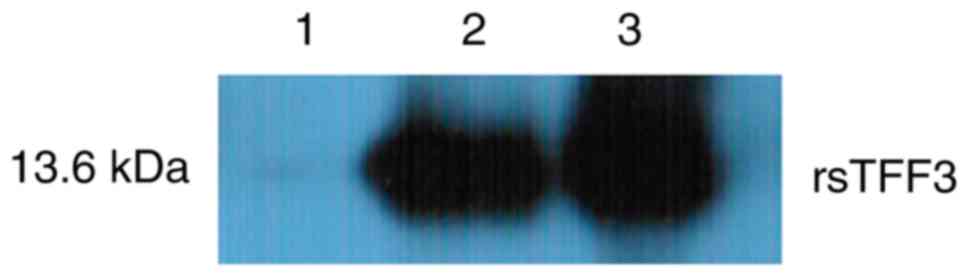
western blotting, no immunoreactive band was present in the
negative control lane, in which vector-free bacterium were induced.
However, bacteria transfected with pNCMO2-TFF3-6×his plasmid after
induction displayed high immunoreactivity for the his-tag antibody
(Fig. 6).
Discussion
TFF3 is a small secreted peptide and potential
cytokine involved in protecting the GI mucosa (3). TFF3 can alleviate injuries in the GI
mucosa caused by numerous types of stimulus and quicken repair of
damaged mucosa (2,3,7). TFF3
enhances the integrity of the mucosal epithelial surface and
promotes the process of reconstitution through cell migration
(7). The interaction between TFF3
and mucins has also been reported to protect intestinal epithelia
from injuries, maintaining mucosal barrier function (26). Furthermore, mitogenic effects of TFF3
have been identified in vitro in primary cell culture, and
TFF3 has been demonstrated to inhibit apoptosis, induce cellular
invasion, act as an inflammatory modulator and exhibit
pro-angiogenic activity (27–32). A
protective or healing effect of TFF3 has also been reported in a
series of experiments, such as cells in vitro, mice, rats
and human (12–17). In addition, a previous study in
TFF3-deficient mice demonstrated that the intestinal mucosa was
damaged and apoptosis increased in the colon of the model mice
compared with TFF3-competent controls (6).
TFF3 is mainly secreted by the small intestine and
colon goblet cells. However, it is very difficult to directly
isolate natural TFF3 from these tissues (20–22).
Thus, it is important to use genetic engineering strategies to
produce large amounts of recombinant TFF3 for biochemical and
biomedical applications. B. choshinensis has an exceptional
capacity to produce heterologous proteins (23,24,33–35).
Target proteins can be produced and secreted efficiently with a
high yield by Brevibacillus expression systems (33). The Brevibacillus expression
system is suitable for producing eukaryotic proteins (23,24,33,34).
Compared with traditional E. coli expression systems, the
genus Brevibacillus, including thermophilic, alkalophilic,
psychrophilic, acidophilic and halophilic strains, use more carbon
for heterotrophic or autotrophic growth (34). Brevibacillus expression
systems can produce certain secretory or cytoplasmic proteins that
E. coli expression systems fail to produce (35). Brevibacillus expression
systems possess a good protein folding environment, lack proteases
and have a convenient downstream processing model, e.g. cell
separation and purification of secreted proteins from the culture
medium (36). Thus a number of
genetically engineered enzymes and heterologous proteins including
cytokines, antigens and antibody fragments are expressed using
Brevibacillus systems (33).
Extracellular proteins, including several bacterial and mammalian
proteins (with yields ranging between 10–1,250 mg/l) have been
produced using Brevibacillus systems (37–41).
Recombinant TFF1 was secreted extracellularly with a high yield by
the Brevibacillus system and had better wound healing
capability compared with TFF1 produced by E. coli (37).
In the present study, the sus scrofa TFF3
gene was cloned into the pNCMO2 shuttle vector. B.
choshinensis was used as a host bacterium to express sus
scrofa TFF3 and produced 30 mg/l protein fused with a
6×his-tag. Protein production was confirmed by SDS-PAGE and western
blot analyses. The obtained fusion protein exhibited good
antigenicity and specificity. Thus, Brevibacillus may be
used to produce useful mucosal factors, which can be analyzed in
terms of protein structure, bioactivity and kinetics as well as for
their mucosal-protection activities. This expression system may be
used in industrial applications to produce novel inhibitors of
diarrhea.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Key Research and Development Program of China (grant no.
2017YFD0501100) and the Colleges and Universities Key Research
Plans in Henan (grant nos. 18A230009 and 19B230013).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YYW, HPL, CMX, BYW and KZ designed the study. YYW,
HPL, CMX, BYW, KZ, YZZ, LPF and YDC performed the experiments. GMZ,
XYJ, GYY and AQL analyzed the data. HPL, KZ and YYW wrote the
manuscript. AQL drafted and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kjellev S: The trefoil factor family-small
peptides with multiple functionalities. Cell Mol Life Sci.
66:1350–1369. 2009.PubMed/NCBI
|
|
2
|
Aihara E, Engevik KA and Montrose MH:
Trefoil factor peptides and gastrointestinal function. Annu Rev
Physiol. 79:357–380. 2017.PubMed/NCBI
|
|
3
|
Sun Z, Liu H, Yang Z, Shao D, Zhang W, Ren
Y, Sun B, Lin J, Xu M and Nie S: Intestinal trefoil factor
activates the PI3K/Akt signaling pathway to protect gastric mucosal
epithelium from damage. Int J Oncol. 45:1123–1132. 2014.PubMed/NCBI
|
|
4
|
Huynh E and Li J: Generation of
lactococcus lactis capable of coexpressing epidermal growth factor
and trefoil factor to enhance in vitro wound healing. Appl
Microbiol Biotechnol. 99:4667–4677. 2015.PubMed/NCBI
|
|
5
|
Fabisiak A, Bartoszek A, Kardas G,
Fabisiak N and Fichna J: Possible application of trefoil factor
family peptides in gastroesophageal reflux and Barrett's esophagus.
Peptides. 115:27–31. 2019.PubMed/NCBI
|
|
6
|
Jeffrey GP, Oates PS, Wang TC, Babyatsky
MW and Brand SJ: Spasmolytic polypeptide: A trefoil peptide
secreted by rat gastric mucous cells. Gastroenterology.
106:336–345. 1994.PubMed/NCBI
|
|
7
|
Lin N, Xu LF and Sun M: The protective
effect of trefoil factor 3 on the intestinal tight junction barrier
is mediated by toll-like receptor 2 via a PI3K/Akt dependent
mechanism. Biochem Bioph Res Commun. 440:143–149. 2013.
|
|
8
|
Scholven J, Taras D, Sharbati S, Schon J,
Gabler C, Huber O, Meyer zum Büschenfelde D, Blin N and Einspanier
R: Intestinal expression of TFF and related genes during postnatal
development in a piglet probiotic trial. Cell Physiol Biochem.
23:143–156. 2009.PubMed/NCBI
|
|
9
|
Graziani F, Pinton P, Olleik H, Pujol A,
Nicoletti C, Sicre M, Quinson N, Ajandouz EH, Perrier J, Pasquale
ED, et al: Deoxynivalenol inhibits the expression of trefoil
factors (TFF) by intestinal human and porcine goblet cells. Arch
Toxicol. 93:1039–1049. 2019.PubMed/NCBI
|
|
10
|
Heo JM, Opapeju FO, Pluske JR, Kim JC,
Hampson DJ and Nyachoti CM: Gastrointestinal health and function in
weaned pigs: A review of feeding strategies to control post-weaning
diarrhoea without using in-feed antimicrobial compounds. J Anim
Physiol an N. 97:207–237. 2013.
|
|
11
|
Pluske JR, Turpin DL and Kim JC:
Gastrointestinal tract (gut) health in the young pig. Anim Nutr.
4:187–196. 2018.PubMed/NCBI
|
|
12
|
Meyer zum Buschenfelde D, Hoschutzky H,
Tauber R and Huber O: Molecular mechanisms involved in TFF3
peptide-mediated modulation of the E-cadherin/catenin cell adhesion
complex. Peptides. 25:873–883. 2004.PubMed/NCBI
|
|
13
|
Durer U, Hartig R, Bang S, Thim L and
Hoffmann W: TFF3 and EGF induce different migration patterns of
intestinal epithelial cells in vitro and trigger increased
internalization of E-cadherin. Cell Physiol Biochem. 20:329–346.
2007.PubMed/NCBI
|
|
14
|
Paulsen FP, Woon CW, Varoga D, Jansen A,
Garreis F, Jager K, Amm M, Podolsky DK, Steven P, Barker NP and Sel
S: Intestinal trefoil factor/TFF3 promotes re-epithelialization of
corneal wounds. J Biol Chem. 283:13418–13427. 2008.PubMed/NCBI
|
|
15
|
Jiang GX, Zhong XY, Cui YF, Liu W, Tai S,
Wang ZD, Shi YG, Zhao SY and Li CL: IL-6/STAT3/TFF3 signaling
regulates human biliary epithelial cell migration and wound healing
in vitro. Mol Biol Rep. 37:3813–3818. 2010.PubMed/NCBI
|
|
16
|
Manko A, Motta JP, Cotton JA, Feener T,
Oyeyemi A, Vallance BA, Wallace JL and Buret AG: Giardia
co-infection promotes the secretion of antimicrobial peptides
beta-defensin 2 and trefoil factor 3 and attenuates attaching and
effacing bacteria-induced intestinal disease. PLoS One.
12:e01786472017.PubMed/NCBI
|
|
17
|
Nakov R, Velikova T, Nakov V, Ianiro G,
Gerova V and Tankova L: Serum trefoil factor 3 predicts disease
activity in patients with ulcerative colitis. Eur Rev Med Pharmacol
Sci. 23:788–794. 2019.PubMed/NCBI
|
|
18
|
Soriano-Izquierdo A, Gironella M,
Massaguer A, May FE, Salas A, Sans M, Poulsom R, Thim L, Pique JM
and Panes J: Trefoil peptide TFF2 treatment reduces VCAM-1
expression and leukocyte recruitment in experimental intestinal
inflammation. J Leukoc Biol. 75:214–223. 2004.PubMed/NCBI
|
|
19
|
Lin J, Holzman IR, Jiang P and Babyatsky
MW: Expression of intestinal trefoil factor in developing rat
intestine. Biol Neonate. 76:92–97. 1999.PubMed/NCBI
|
|
20
|
Fang M, Wang W, Wang Y and Ru B: Bacterial
expression and purification of biologically active human TFF3.
Peptides. 25:785–792. 2004.PubMed/NCBI
|
|
21
|
Lu R, Wang X, Chen JP, Chen X and Xu JN:
Expression of human TFF3 in Escherichia coli. Sichuan Da Xue Xue
Bao Yi Xue Ban. 41:114–117. 2010.(In Chinese). PubMed/NCBI
|
|
22
|
Wang H, Tong Y, Fang M and Ru B:
High-level expression of human TFF3 in Escherichia coli. Peptides.
26:1213–1218. 2005.PubMed/NCBI
|
|
23
|
Mizukami M, Hanagata H and Miyauchi A:
Brevibacillus expression system: Host-vector system for efficient
production of secretory proteins. Curr Pharm Biotechnol.
11:251–258. 2010.PubMed/NCBI
|
|
24
|
Tokunaga M, Mizukami M, Yamasaki K,
Tokunaga H, Onishi H, Hanagata H, Ishibashi M, Miyauchi A, Tsumoto
K and Arakawa T: Secretory production of single-chain antibody
(scFv) in Brevibacillus choshinensis using novel fusion partner.
Appl Microbiol Biot. 97:8569–8580. 2013.
|
|
25
|
Uenishi H, Eguchi T, Suzuki K, Sawazaki T,
Toki D, Shinkai H, Okumura N, Hamasima N and Awata T: PEDE (Pig EST
Data Explorer): Construction of a database for ESTs derived from
porcine full-length cDNA libraries. Nucleic Acids Res. 1:D484–D488.
2004.
|
|
26
|
Kondo S, Araki T, Toiyama Y, Tanaka K,
Kawamura M, Okugawa Y, Okita Y, Saigusa S, Inoue Y, Uchida K, et
al: Downregulation of trefoil factor-3 expression in the rectum is
associated with the development of ulcerative colitis-associated
cancer. Oncol Lett. 16:3658–3664. 2018.PubMed/NCBI
|
|
27
|
Gao F, Pan SX, Liu B and Zhang HZ: TFF3
knockout in human pituitary adenoma cell HP75 facilitates cell
apoptosis via mitochondrial pathway. Int J Clin Exp Patho.
8:14568–14573. 2015.
|
|
28
|
Arnold P, Rickert U, Helmers AK, Spreu J,
Schneppenheim J and Lucius R: Trefoil factor 3 shows
anti-inflammatory effects on activated microglia. Cell Tissue Res.
365:3–11. 2016.PubMed/NCBI
|
|
29
|
Große-Kreul J, Busch M, Winter C, Pikos S,
Stephan H and Dunker N: Forced trefoil factor family peptide 3
(TFF3) expression reduces growth, viability, and tumorigenicity of
human retinoblastoma cell lines. PLoS One.
11:e01630252016.PubMed/NCBI
|
|
30
|
Bastholm SK, Samson MH, Becher N, Hansen
LK, Stubbe PR, Chronakis IS, Nexo E and Uldbjerg N: Trefoil factor
peptide 3 is positively correlated with the viscoelastic properties
of the cervical mucus plug. Acta Obstet Gyn Scan. 96:47–52.
2017.
|
|
31
|
Li Q, Wang KK, Su C and Fang JY: Serum
trefoil factor 3 as a protein biomarker for the diagnosis of
colorectal cancer. Technol Cancer Res T. 16:440–445. 2017.
|
|
32
|
Al-Salam S, Sudhadevi M, Awwad A and Al
Bashir M: Trefoil factors peptide-3 is associated with residual
invasive breast carcinoma following neoadjuvant chemotherapy. Bmc
Cancer. 19:1352019.PubMed/NCBI
|
|
33
|
Mizukami M, Onishi H, Hanagata H, Miyauchi
A, Ito Y, Tokunaga H, Ishibashi M, Arakawa T and Tokunaga M:
Efficient production of Trastuzumab Fab antibody fragments in
Brevibacillus choshinensis expression system. Protein Expres Purif.
150:109–118. 2018.
|
|
34
|
Mizukami M, Tokunaga H, Onishi H, Ueno Y,
Hanagata H, Miyazaki N, Kiyose N, Ito Y, Ishibashi M, Hagihara Y,
et al: Highly efficient production of VHH antibody fragments in
Brevibacillus choshinensis expression system. Protein Expres Purif.
105:23–32. 2015.
|
|
35
|
Hu W, Xiang JY, Kong P, Liu L, Xie QH and
Xiang HY: Expression and characterization of a single-chain
variable fragment against human LOX-1 in Escherichia coli and
Brevibacillus choshinensis. J Microbiol Biotechn. 27:965–974.
2017.
|
|
36
|
Zou C, Duan XG and Wu J: Efficient
extracellular expression of Bacillus deramificans pullulanase in
Brevibacillus choshinensis. J Ind Microbiol Biot. 43:495–504.
2016.
|
|
37
|
Cheng YM, Lu MT and Yeh CM: Functional
expression of recombinant human trefoil factor 1 by Escherichia
coli and Brevibacillus choshinensis. Bmc Biotechnol.
15:322015.PubMed/NCBI
|
|
38
|
Hanagata H and Mizukami M: Production of
recombinant CCN proteins by Brevibacillus choshinensis. Methods Mol
Biol. 1489:85–93. 2017.PubMed/NCBI
|
|
39
|
Li Z, Su L, Duan X, Wu D and Wu J:
Efficient expression of maltohexaose-forming α-amylase from
Bacillus stearothermophilus in Brevibacillus
choshinensis SP3 and its use in maltose production. Biomed Res Int.
2017:54797622017.PubMed/NCBI
|
|
40
|
Panfertsev EA, Baranova EV, Mochalov VV,
Soloviev PV, Gorbatov AA and Biketov SF: Construction of
recombinant strain Brevibacillus choshinensis for chimeric borrelia
Dbpa antigen production. Klin Lab Diagn. 63:450–454. 2018.(In
Russian). PubMed/NCBI
|
|
41
|
Duan X, Shen Z, Zhang X, Wang Y and Huang
Y: Production of recombinant beta-amylase of Bacillus aryabhattai.
Prep Biochem Biotech. 49:88–94. 2019.
|