Introduction
Diabetes is one of the most common chronic metabolic
disease in the world caused by multiple factors and has become one
of the diseases with a high mortality rate globally. Patients with
diabetes take drugs throughout their lives, which poses long-term
economic pressure (1). Besides,
diabetes has many complications threatening the vision. Cataract
occurs earlier in diabetic patients. In many developed countries,
diabetes is the major cause of non-invasive amputation, blindness
and visual impairment of adults, and diabetic patients are more
prone to suffering from glaucoma (2). Diabetes retinopathy (DR), a kind of
retinal disease, is the main cause of blindness in developed
countries. Its pathology is complex and affects the nerves and
vascular components of the retina (3,4). DR is
featured with neurological dysfunction and the destruction of the
retinal vascular system. Vascular complications are crucial factors
for the progression of the disease. Retinal neuronal dysfunction
arises in DR in the early stage and may even occur before vascular
rupture (5,6). Early neurological dysfunction is proved
to be an abnormal electroretinogram response in diabetic rats and
humans prior to any visible vascular injury (7). Additionally, decreased functional
hyperemia is one of the earliest retinal changes in diabetic
patients that are observed (8). The
hyperglycemia activation of metabolic and biochemical pathways
leads to irreversible changes, which are the adverse results of DR.
DR is a continuously dependent disease that develops in stages. The
incidence rate of DR is relatively low in the first few years of
diabetes onset, but it will increase significantly after the onset
of diabetes for many years. DR is the leading cause of acquired
blindness in adults. If microvascular damage in the retina and
vascular swelling and exudation are not prevented, new blood
vessels will begin to grow, ultimately causing retinal detachment
(9,10). Inflammation is regarded as the key
driving factor of DR pathophysiology. Raising the expression of
pro-inflammatory cytokines, such as tumor necrosis factor-α
(TNF-α), will further promote the occurrence of retinopathy in
diabetic rats (11). Extensive
attention has been paid to the function of oxidative stress in DR.
Edaravone treatment for DR reduces the concentration of oxygen free
radicals and suppresses delayed neuronal death (12).
Aminoguanidine (AG), a specific nitric oxide
synthase (NOS) inhibitor, has been proven to slow down the
progression of DR in human and animal diabetes models (13). These beneficial roles of AG are
largely attributed to the repression on the formation of advanced
glycation end products (AGEs). However, the results of multiple
studies showed that AG may also function through inhibiting iNOS,
especially in the early stage of the disease (14). Intravitreal triamcinolone acetonide
(TA) can effectively eliminate macular edema and improve vision,
especially for DR that cannot b(160e effectively treated by laser
therapy, and has been widely applied in recent years. A study has
revealed that TA can alleviate macular edema, prevent the
aggravation of macular edema secondary to PRP, and facilitate the
improvement of retinopathy (15).
In this study, the effects of TA combined with AG on
vascular endothelial function, vascular endothelial growth factor
(VEGF) expression and retinal function in DR patients were
observed. DR patients were admitted and treated. The therapeutic
effect and adverse reactions were observed, the changes in serum
inflammatory cytokines and oxidative stress factors before
treatment were detected, the level of serum VEGF was measured, and
central macular thickness (CMT), retinal neovascularization (RNV),
best corrected visual acuity (BCVA), nitric oxide (NO),
endothelin-1 (ET-1), intercellular adhesion molecule-1 (ICAM-1) and
other vascular endothelial function indexes were observed, to
confirm that TA combined with AG could inhibit inflammation and
oxidative stress and improve vascular endothelial function and
retinal function in DR patients.
Patients and methods
Clinical data
The clinical research protocol was approved by the
Ethics Committee of the Taizhou Second People's Hospital Affiliated
to Yangzhou University (Taizhou, China). Signed informed consents
were obtained from the patients and/or guardians. One hundred
patients with DR admitted to the hospital from January 2016 to
December 2018 were selected as research subjects. The patients were
enrolled after signing an informed consent. They were divided into
the observation group (n=50) and the control group (n=50) using
random control method and informed of the condition of this study.
Inclusion criteria: Patients with a history of diabetes, reduced
vision in different degrees and different degrees of retinopathy
shown in retinal fundus examination. Exclusion criteria: patients
taking glucocorticoid drugs, those with severe organ dysfunction,
those who could not receive intravenous injection, or those with
gestational diabetes, hepatic and renal insufficiency,
ketoacidosis, acute or chronic infection or immune system diseases.
All clinical specimens in this experiment were collected with the
consent of the patients and their family members according to the
Helsinki Declaration. The specific clinical data collected on
admission included age, sex, weight, course of disease and DR stage
(Table I). No significant
differences in the general data were found between the two groups
of patients.
 | Table IClinical data. |
Table I
Clinical data.
| Indicator | Control group | Observation
group |
|---|
| Sample (n) | 50 | 50 |
| Male patient (n) | 30 | 29 |
| Average age
(years) | 48±15 | 49±16 |
| Average body weight
(kg) | 52±10.5 | 54±11.5 |
| Course of disease
(years) | 3.5±1.5 | 4.0±0.9 |
| DR in stage III | 22 | 23 |
| DR in stage IV | 20 | 21 |
| DR in stage V | 8 | 6 |
Treatment methods
All patients were treated with levofloxacin eye
water in drops (1-2 drops/time and 4 drops/d) on the affected eye
(until the water covered the whole eyeball) for 3 days before
treatment, followed by conventional treatment for 3 consecutive
days. After that, the patients in the control group underwent
treatment with TA (4 mg), while those in the observation group were
treated with AG (50 mg/kg) in addition. The specific injection
method is as follows: Local anesthesia was carried out on the
affected eye. After disinfection, the eyelid opener was applied to
open the eyelid of the patients. Then the needle was inserted into
the flat part of the corneal limbus of the patients' affected eye
at ~4 mm. The direction of needle insertion was toward the center
of the eye, with the depth of ~4 mm. The puncture site was ensured
in the vitreous body. After withdrawing the needle, the needle site
was pressed using cotton swabs. According to medical advice, the
patients lied in the supine position for 1-2 h after the injection
to ensure that the drug completely reached the posterior polar
retina. They were treated once a day for 12 weeks.
Observation of therapeutic effect
Evaluation results of the therapeutic effect via
optical coherence tomography (OCT) after treatment was divided into
three types according to whether the morphology and edematous
height were reduced, i.e. returning to normal, obvious improvement
(the edematous height was reduced by more than 100 µm compared with
that before treatment) and no improvement (the edematous height was
unchanged or increased compared with that before treatment).
Observation of adverse reactions
The complications of the two groups of patients
after treatment were observed and recorded. The specially-assigned
medical staff recorded the complications of anterior chamber
inflammation, corneal edema, ocular hypertension and macular for
the two groups of patients, respectively, counted the specific
types of complications and made detailed records. Finally, the
postoperative complications of the two groups of patients were
summarized.
Detection of blood glucose (GLU),
triglyceride (TG) and total cholesterol (TC)
The biochemical indexes such as blood GLU and lipid
will change in patients with diabetes. The occurrence and
development of the disease can be indicated through the detection
of the changes in the indexes. In the morning, 5 ml of fasting
peripheral venous blood of the patients was extracted and placed in
an Eppendorf (EP) tube containing anticoagulant
ethylenediaminetetraacetic acid (EDTA). The blood was centrifuged
at 3,500 x g at room temperature for 10 min, and the supernatant
was collected to detect the changes in GLU, TG, TC and other
indexes, so as to provide an important theoretical reference for
early treatment.
Detection of serum inflammatory
factors via enzyme-linked immunosorbent assay (ELISA)
A total of 5 ml of venous blood was extracted from
the arm, placed in the EP tube containing anticoagulant and
centrifuged at 2,500 x g at room temperature for 15 min. Next, the
supernatant was collected for the detection of serum inflammatory
factors [interleukin-6 (IL-6), myeloperoxidase (MPO) and TNF-α].
The specific operation was in accordance with the instructions of
ELISA kit (Nanjing Jiancheng Bioengineering Institute). Then, the
absorbance in each group was examined under a microplate
reader.
Examination of serum oxidative stress
indicators via ELISA
A total of 5 ml of venous blood was extracted from
the arm, placed in the EP tube containing anticoagulant and
centrifuged at 2,500 x g at room temperature for 15 min.
Thereafter, the supernatant was collected for the detection of
serum oxidative stress indicators [malondialdehyde (MDA),
superoxide dismutase (SOD) and catalase (CAT)]. The specific
operation was in line with the instructions of ELISA kit. Then, the
absorbance in each group was measured by a microplate reader.
Detection of VEGF, NO, ET-1 and ICAM-1
in serum
A total of 5 ml of venous blood was taken from
patients and placed in the EP tube containing anticoagulant,
followed by centrifugation at 2,500 x g at room temperature for 15
min. The supernatant was collected to examine specific changes in
the serum levels of VEGF, ET-1 and ICAM-1 using the kit. NO was
determined by nitrate reductase colorimetry using a kit (Shanghai
Hufeng Biotechnology Co., Ltd.). The specific operation followed
the instructions of the kit. The absorbance in each group was
examined under the microplate reader.
Observation of CMT, RNV and BCVA
OCT system (Zeiss, type: OCT3000OI-STD) was used to
observe the CMT and RNV before and after treatment. The specific
operation was performed by specially-assigned personnel according
to the instrument instructions. The BCVA before and after treatment
was observed on the basis of the international Visual Acuity Chart
(version 2.0).
Statistical analysis
All the data originally recorded in the study were
processed SPSS 20.0 (IBM Corp.) analysis software and compared in
multiple ways. The percentage was tested using χ2 test.
The experimental results were expressed as mean ± standard
deviation (mean ± SD). P<0.05 indicates that the difference is
statistically significant. The histograms were plotted using
GraphPad Prism 6.0 (GraphPad Software).
Results
Observation results of the therapeutic
effect in the two groups of patients
As shown in Table
II, the total effective rate was 96% in the observation group
and 74% in the control group, with a statistically significant
difference (P<0.05).
 | Table IIObservation results of the therapeutic
effect. |
Table II
Observation results of the therapeutic
effect.
| Groups | Returning to
normal | Obvious
improvement | No improvement | Total effective rate
(%) |
|---|
| Control | 17 | 25 | 8 | 74 |
| Observation | 25a | 23a | 2a | 96a |
Observation results of adverse
reactions
The control group had 17 cases of complications,
mainly including anterior chamber inflammation, corneal edema,
ocular hypertension and macula, with the total adverse reaction
rate of 34%, while the observation group had only 4 cases of
complications, with the total adverse reaction rate of 8%,
displaying a statistically significant difference (P<0.05)
(Table III). Thus, TA combined
with AG exerts an obvious effect on DR patients, with fewer adverse
reactions and complications.
 | Table IIIAdverse reactions. |
Table III
Adverse reactions.
| Groups | Inflammation of
anterior chamber | Corneal edema | Intraocular
hypertension | Macula | Total adverse
reaction rate (%) |
|---|
| Control | 3 | 2 | 5 | 7 | 34 |
| Observation | 1a | 1a | 1a | 1a | 8a |
Detection results of GLU, TG and total
TC
As shown in Table
IV, GLU, TG and TC in the two groups were decreased after
treatment, but the serum levels of TG, TC and GLU in the
observation group were notably decreased compared with those in the
control group (P<0.05), and the variation amplitude at 3 months
after treatment was more evident than that at 1 month after
treatment, indicating that the hyperlipemia indexes in DR patients
will be clearly improved after treatment with TA combined with
AG.
 | Table IVDetection results of blood GLU, TG and
TC. |
Table IV
Detection results of blood GLU, TG and
TC.
| Group | TC (mmol/l) | TG (mmol/l) | GLU (mmol/l) |
|---|
| Control group |
|
Before
treatment | 5.5±0.6 | 2.5±0.06 | 14.4±1.7 |
|
1 month
after treatment | 3.9±0.8a | 1.9±0.04a | 9.9±1.2a |
|
3 months
after treatment | 2.8±0.1a | 1.2±0.09a | 5.5±1.5a |
| Observation
group |
|
Before
treatment | 5.7±0.5 | 2.7±0.03 | 15.0±4.7 |
|
1 month
after treatment |
2.3±0.4a,b |
1.3±0.04a,b |
6.7±2.6a,b |
|
3 months
after treatment |
1.2±0.3a,b |
0.8±0.02a,b |
3.1±1.3a,b |
Detection results of serum
inflammatory factors
Before treatment, there were no statistically
significant differences in the levels of IL-6, MPO and TNF-α
between the two groups of patients (P>0.05). After treatment,
the levels of IL-6, MPO and TNF-α in the two groups of patients
were remarkably lower than those before treatment, and the
variation degree at 3 months after treatment was more obvious than
that at 1 month after treatment. The levels in the observation
group were significantly lower than those in the control group
(P<0.05) (Table V).
 | Table VSerum levels of IL-6, MPO and
TNF-α. |
Table V
Serum levels of IL-6, MPO and
TNF-α.
| Groups | IL-6 (pg/ml) | TNF-α
(fmol/ml) | MPO (ng/ml) |
|---|
| Control |
|
Before
treatment | 69.5±2.6 | 49.4±2.8 | 218.5±1.8 |
|
1 month
after treatment |
46.7±2.4a |
34.1±2.1a |
155.9±1.7a |
|
3 months
after treatment |
35.5±2.1a |
28.6±2.5a |
103.4±1.1a |
| Observation |
|
Before
treatment | 69.1±1.3 | 51.4±1.9 | 217.9±4.7 |
|
1 month
after treatment |
34.6±2.4a,b |
26.7±1.1a,b |
119.8±2.6a,b |
|
3 months
after treatment |
23.1±2.5a,b |
19.5±1.6a,b |
89.6±2.4a,b |
ELISA results of serum oxidative
stress indicators
The examination results of oxidative stress indexes
(SOD, MDA and CAT) are shown in Table
VI. It was found that after treatment, the levels of MDA and
CAT in the observation group declined markedly (P<0.05), the SOD
level was evidently raised (P<0.05). The amplitude of variation
at 3 months after treatment was larger than that at 1 month after
treatment, and it was also larger in the observation group than
that in the control group.
 | Table VISerum oxidative stress
indicators. |
Table VI
Serum oxidative stress
indicators.
| Groups | CAT (IU/ml) | MDA (mmol/g) | SOD (µ/mg) |
|---|
| Control |
|
Before
treatment | 30.5±2.1 | 12.4±1.0 | 15.5±1.4 |
|
1 month
after treatment |
20.1±2.4a |
7.1±1.1a |
22.9±1.8a |
|
3 months
after treatment |
15.6±2.0a |
5.0±1.4a |
33.5±0.8a |
| Observation |
|
Before
treatment | 31.1±1.2 | 11.9±0.9 | 16.1±1.3 |
|
1 month
after treatment |
16.4±2.0a,b |
5.5±0.8a,b |
30.4±1.6a,b |
|
3 months
after treatment |
9.8±2.1a,b |
2.3±0.1a,b |
43.1±1.7a,b |
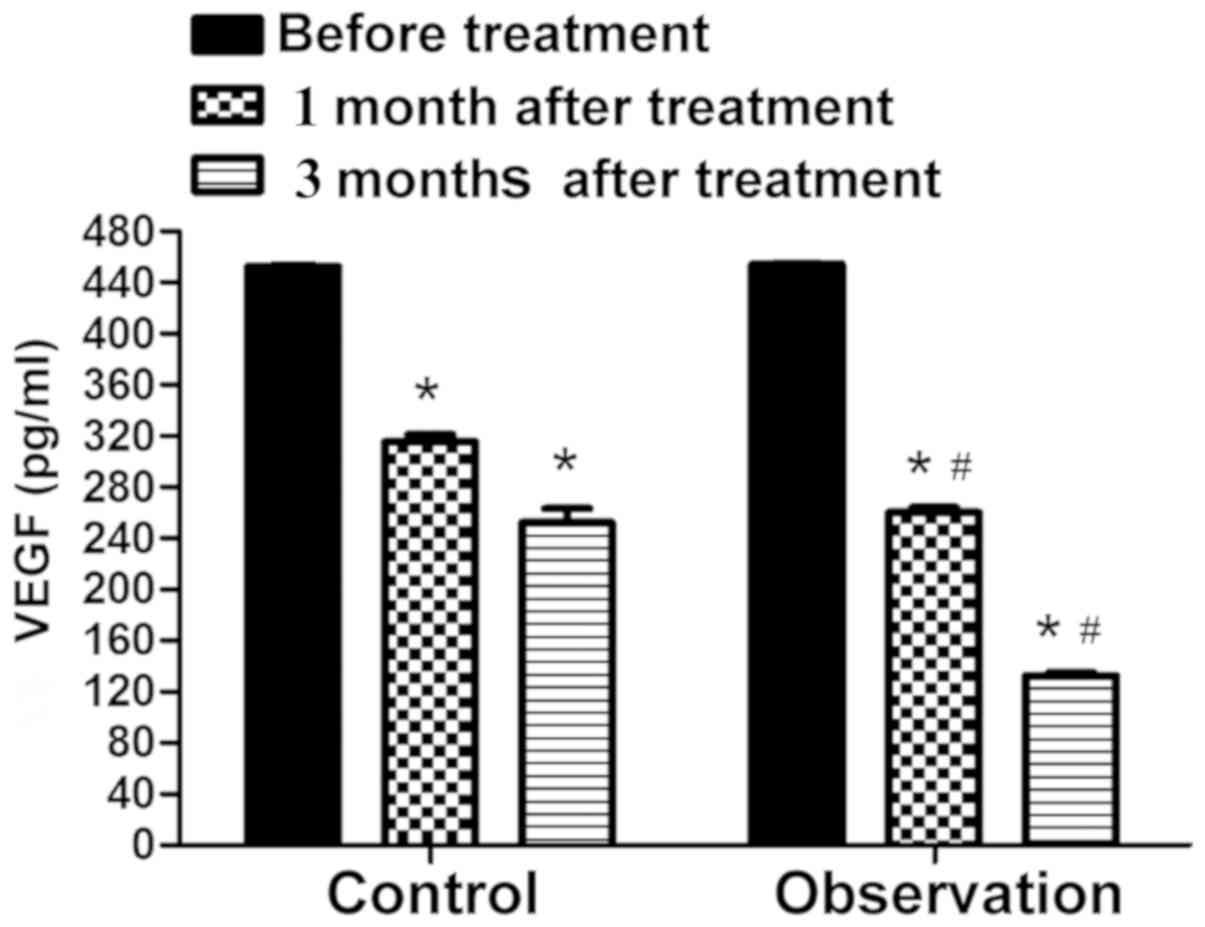
Serum VEGF expression
Fig. 1 shows that
before treatment, there was no significant difference in the VEGF
level between the two groups of patients (P>0.05). After
treatment, the VEGF level in the two groups of patients was notably
lower than that before treatment, and it was markedly lower in the
observation than that in the control group (P<0.05).
Examination results of vascular
endothelial function indexes
Before treatment, the differences in the levels of
NO, ET-1 and ICAM-1 were not statistically significant between the
two groups of patients (P>0.05). After treatment, the levels of
NO, ET-1 and ICAM-1 in the two groups of patients were remarkably
lower than those before treatment, and the decrease at 3 months
after treatment were more obvious than those at 1 month after
treatment. The levels in the observation group were evidently lower
than those in the control group (P<0.05) (Table VII).
 | Table VIILevels of NO, ET-1 and ICAM-1. |
Table VII
Levels of NO, ET-1 and ICAM-1.
| Groups | NO (µmol/l) | ET-1 (ng/l) | ICAM-1 (µg/l) |
|---|
| Control |
|
Before
treatment | 82.4±4.7 | 25.4±1.7 | 325.5±8.4 |
|
1 month
after treatment |
55.1±5.1a |
20.1±1.2a |
271.9±9.8a |
|
3 months
after treatment |
44.8±5.5a |
15.4±1.5a |
230.7±7.8a |
| Observation |
|
Before
treatment | 82.9±4.9 | 26.9±1.3 | 326.1±10.3 |
|
1 month
after treatment |
46.5±3.8a,b |
14.6±1.1a,b |
209.4±7.6a,b |
|
3 months
after treatment |
36.7±3.4a,b |
5.6±1.8a,b |
150.5±7.3a,b |
Examination results of CMT, RNV and
BCVA
As shown in Table
VIII, statistically significant differences in the levels of
CMT, RNV and BCVA were detected prior to treatment between the two
groups of patients. After treatment, the levels of CMT and RNV were
remarkably decreased while BCVA level was markedly increased. The
amplitude of variation at 3 months after treatment was more
significant than that at 1 month after treatment, and it was also
more obvious in the observation group than that in control group
(P<0.05).
 | Table VIIIExamination results of CMT, RNV and
BCVA. |
Table VIII
Examination results of CMT, RNV and
BCVA.
| Groups | CMT (µm) | RNV
(mm2) | BCVA |
|---|
| Control |
|
Before
treatment | 442.4±4.7 | 26.7±1.2 | 0.42±0.04 |
|
1 month
after treatment |
355.1±5.8a |
20.1±1.6a |
0.55±0.08a |
|
3 months
after treatment |
267.8±5.1a |
12.5±1.7a |
0.65±0.07a |
| Observation |
|
Before
treatment | 445.9±4.3 | 26.9±1.4 | 0.41±0.06 |
|
1 month
after treatment |
320.5±3.8a,b |
13.1±1.5a,b |
0.70±0.03a,b |
|
3 months
after treatment |
220.1±2.8a,b |
5.2±1.1a,b |
0.81±0.06a,b |
Discussion
DR is a serious complication related to diabetes
(16). During diabetes, the age of
retinal pericytes gradually increases, producing adverse effects on
cell function and survival. Early DR is featured with loss of
retinal pericytes that may result in an increase in the ratio of
endothelial cells to pericytes (17). In vivo, hyperglycemia-induced
AGEs are deposited in retinal vessels, which play crucial roles in
the occurrence and development of DR. Age also causes the loss of
retinal pericytes in healthy rats. Inhibition of cytotoxicity
mediated by age has been adopted as a treatment regimen to prevent
diabetic complications (18). In
this study, DR patients were enrolled and treated, the therapeutic
effect, adverse reactions, CMT, RNV and BCVA were observed, and NO,
ET-1, ICAM-1 and other vascular endothelial function indexes were
detected. The total effective rate was 96% in the observation group
and 74% in the control group. The control group had 17 cases of
complications, mainly including anterior chamber inflammation,
corneal edema, ocular hypertension and macula, with the total
adverse reaction rate of 34%, while observation group had 4 cases
of complications, with the total adverse reaction rate of 8%,
displaying a statistically significant difference. Thus, TA
combined with AG exerts an obvious effect on DR patients, with
fewer adverse reactions and complications. In addition, it was
discovered that GLU, TG and TC in the two groups were decreased
after treatment, but the serum levels of TG, TC and GLU in the
observation group were notably decreased compared with those in the
control group, indicating that the hyperlipemia indexes in DR
patients will be evidently improved after treatment with TA
combined with AG. Research has shown that inflammation exerts
effects in the occurrence and development of DR. As the
inflammatory cells increase, the clinopathological changes of DR
include increased inflammatory cytokines and oxidative stress
damage (19). This study revealed
that after treatment, the levels of IL-6, MPO and TNF-α in the two
groups of patients were remarkably lower than those before
treatment, and those in the observation group were significantly
lower than those in the control group. After treatment, the levels
of MDA and CAT in the observation group were markedly reduced,
while the SOD level was obviously increased, and the variation
amplitude in the observation group was more significant than that
in the control group. The combination of TA and AG in the treatment
of DR can prevent excessive production of inflammatory cytokines to
cause irreversible oxidative stress damage to cells, similarly to
previously reported (20).
VEGF is a highly conserved glycoprotein, a powerful
vascular permeability factor, an endothelial cell-specific mitogen
and an angiogenic factor, which plays a pivotal role in
physiological and pathological angiogenesis as well as the etiology
of DR. VEGF stimulates and facilitates vascular permeability,
vasodilation dependent on endothelium, monocyte chemotaxis and
tissue factor production, and they can lead to microvascular
complications (21,22). It was discovered in this study that
before treatment, there was no significant difference in the VEGF
level between the two groups. After treatment, the VEGF level in
the two groups of patients was notably lower than that before
treatment, and it was markedly lower in the observation group than
that in the control group. Some studies have indicated that
inhibition of CMT and RNV after laser treatment can effectively
improve retinal thickness and neovascular leakage. Other studies
have reported that TA therapy notably improves retinopathy and
vision (23,24). In this study, no significant
differences in the levels of CMT, RNV and BCVA were found between
the two groups of patients before treatment. After treatment, CMT
and RNV levels in the observation group declined prominently, while
the BCVA level was raised significantly, and the variation
amplitude in the observation group was larger than that in the
control group. Some scholars consider that ET-1 has abnormal
changes in DR in the early stage. Non-specific NOS inhibitors can
protect the retina from ischemic damage, denoting that NO exerts a
pivotal effect in the pathogenesis of DR. ICAM-1 also has certain
clinical reference value in DR (25,26).
This study revealed that before treatment, there were no
significant differences in the levels of NO, ET-1 and ICAM-1
between the two groups of patients. After treatment, the levels of
NO, ET-1 and ICAM-1 in the two groups of patients were obviously
lower than those before treatment, and those in the observation
group were evidently lower than those in the control group, which
are similar to the above study results.
In conclusion, DR patients were enrolled and
treated. The therapeutic effect and adverse reactions were
observed, the changes in serum inflammatory factors and oxidative
stress factors before treatment were detected, the level of serum
VEGF was measured, CMT, RNV and BCVA were observed, the effects of
TA combined with AG on vascular endothelial function and retinal
function of DR patients were examined, to confirm that TA combined
with AG inhibited inflammation and oxidative stress and improved
vascular endothelial function and retinal function in DR patients.
The overall effect of the combined therapy is good, and its
application is worthy of popularizing.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data generated or analyzed during this study are
included in this published article.
Authors' contributions
KX and MZ designed the study and performed the
experiments, KX and HQ collected the data, MZ and HQ analyzed the
data, KX and MZ prepared the manuscript. All the authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Taizhou Second People's Hospital Affiliated to Yangzhou
University (Taizhou, China). Signed informed consents were obtained
from the patients and/or guardians.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang HY, Ruan LB, Li Y, Yang TR, Liu WJ,
Jiang YX, Li TR, Quan J and Xuan W: ICOS/ICOSL upregulation
mediates inflammatory response and endothelial dysfunction in type
2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 22:8898–8908.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sharma S, Oliver-Fernandez A, Liu W,
Buchholz P and Walt J: The impact of diabetic retinopathy on
health-related quality of life. Curr Opin Ophthalmol. 16:155–159.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zheng Y, He M and Congdon N: The worldwide
epidemic of diabetic retinopathy. Indian J Ophthalmol. 60:428–431.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fletcher EL, Phipps JA, Ward MM,
Puthussery T and Wilkinson-Berka JL: Neuronal and glial cell
abnormality as predictors of progression of diabetic retinopathy.
Curr Pharm Des. 13:2699–2712. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Barber AJ: A new view of diabetic
retinopathy: A neurodegenerative disease of the eye. Prog
Neuropsychopharmacol Biol Psychiatry. 27:283–290. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Antonetti DA, Barber AJ, Bronson SK,
Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady
JK, LaNoue KF, et al: JDRF Diabetic Retinopathy Center Group:
Diabetic retinopathy: Seeing beyond glucose-induced microvascular
disease. Diabetes. 55:2401–2411. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lieth E, Gardner TW, Barber AJ and
Antonetti DA: Penn State Retina Research Group: Retinal
neurodegeneration: Early pathology in diabetes. Clin Exp
Ophthalmol. 28:3–8. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pemp B, Garhofer G, Weigert G, Karl K,
Resch H, Wolzt M and Schmetterer L: Reduced retinal vessel response
to flicker stimulation but not to exogenous nitric oxide in type 1
diabetes. Invest Ophthalmol Vis Sci. 50:4029–4032. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luo D, Fan Y and Xu X: The effects of
aminoguanidine on retinopathy in STZ-induced diabetic rats. Bioorg
Med Chem Lett. 22:4386–4390. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mahmood D, Singh BK and Akhtar M: Diabetic
neuropathy: Therapies on the horizon. J Pharm Pharmacol.
61:1137–1145. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Joussen AM, Doehmen S, Le ML Koizumi K,
Radetzky S, Krohne TU, Poulaki V, Semkova I and Kociok N: TNF-alpha
mediated apoptosis plays an important role in the development of
early diabetic retinopathy and long-term histopathological
alterations. Mol Vis. 15:1418–1428. 2009.PubMed/NCBI
|
|
12
|
Noor JI, Ikeda T, Mishima K, Aoo N, Ohta
S, Egashira N, Iwasaki K, Fujiwara M and Ikenoue T: Short-term
administration of a new free radical scavenger, edaravone, is more
effective than its long-term administration for the treatment of
neonatal hypoxic-ischemic encephalopathy. Stroke. 36:2468–2474.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bolton WK, Cattran DC, Williams ME, Adler
SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P,
Ratner RE, et al: ACTION I Investigator Group: Randomized trial of
an inhibitor of formation of advanced glycation end products in
diabetic nephropathy. Am J Nephrol. 24:32–40. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eldred WD and Blute TA: Imaging of nitric
oxide in the retina. Vision Res. 45:3469–3486. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jonas JB, Kreissig I, Söfker A and
Degenring RF: Intravitreal injection of triamcinolone for diffuse
diabetic macular edema. Arch Ophthalmol. 121:57–61. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jakus V and Rietbrock N: Advanced
glycation end-products and the progress of diabetic vascular
complications. Physiol Res. 53:131–142. 2004.PubMed/NCBI
|
|
17
|
Qazi Y, Maddula S and Ambati BK: Mediators
of ocular angiogenesis. J Genet. 88:495–515. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li ZP, Xu X, Huang YF, Zhu JF, Wang XJ, Hu
HH and He ZP: Exogenous advanced glycosylation end products induce
diabetes-like vascular dysfunction in normal rats: A factor for
occurrence of diabetic retinopathy. Zhonghua Yan Ke Za Zhi.
39:352–356. 2003.(In Chinese). PubMed/NCBI
|
|
19
|
Yin Y, Chen F, Wang W, Wang H and Zhang X:
Resolvin D1 inhibits inflammatory response in STZ-induced diabetic
retinopathy rats: Possible involvement of NLRP3 inflammasome and
NF-κB signaling pathway. Mol Vis. 23:242–250. 2017.PubMed/NCBI
|
|
20
|
Mishra A and Newman EA: Aminoguanidine
reverses the loss of functional hyperemia in a rat model of
diabetic retinopathy. Front Neuroenergetics. 3(10)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun W, Yu WY, Yu DJ, Zhao TL, Wu LJ and
Han WY: The effects of recombinant human growth hormone (rHGH) on
survival of slender narrow pedicle flap and expressions of vascular
endothelial growth factor (VEGF) and classification determinant 34
(CD34). Eur Rev Med Pharmacol Sci. 22:771–777. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lenz T, Haak T, Malek J, Gröne HJ, Geiger
H and Gossmann J: Vascular endothelial growth factor in diabetic
nephropathy. Kidney Blood Press Res. 26:338–343. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mehta H, Gillies MC and Fraser-Bell S:
Combination of vascular endothelial growth factor inhibitors and
laser therapy for diabetic macular oedema: A review. Clin Exp
Ophthalmol. 44:335–339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Riches K, Franklin L, Maqbool A, Peckham
M, Adams M, Bond J, Warburton P, Feric NT, Koschinsky ML, O'Regan
DJ, et al: Apolipoprotein(a) acts as a chemorepellent to human
vascular smooth muscle cells via integrin αVβ3 and RhoA/ROCK-
mediated mechanisms. Int J Biochem Cell Biol. 45:1776–1783.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Arden GB and Sivaprasad S: The
pathogenesis of early retinal changes of diabetic retinopathy. Doc
Ophthalmol. 124:15–26. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Giove TJ, Deshpande MM, Gagen CS and
Eldred WD: Increased neuronal nitric oxide synthase activity in
retinal neurons in early diabetic retinopathy. Mol Vis.
15:2249–2258. 2009.PubMed/NCBI
|