Introduction
According to the World Health Organization,
approximately 200,000-400,000 new cases of visceral leishmaniasis
(VL) occur every year in the world, 90% of which are noted in
Southeast Asia, Latin America and East Africa (1). Based on the continuous emergence of new
VL cases, the Indian subcontinent, Nepal, and Bangladesh were
affected by VL and had to postpone the plan to eliminate VL from
2015 until 2020(2). Western cities
of China such as Xinjiang, Gansu, Sichuan and other provinces as
well as autonomous regions were endemic areas of VL (3). The abundant epidemiological data and
the high incidence rate provided the clinicians with sufficient
knowledge regarding the incidence of the disease in endemic areas,
leading to a high detection rate. However, it is difficult to
diagnose imported cases in non-epidemic areas due to factors, such
as the population mobility and low attention to the disease
(4). Moreover, clinical
manifestations and disease symptoms or signs may produce additional
confusion. The absence of the medical history of patients resident
in epidemic areas may lead to misdiagnosis of the disease (5). Based on research statistics, the
misdiagnosis rate of clinical VL can reach 23.4% (6). VL is characterized by intermittent
fever, hepatosplenomegaly, pancytopenia and polyclonal hyperimmune
globulinemia. These symptoms are easily confused with AIDS,
aplastic anemia, and plasma cell systemic diseases (7,8). The
diagnosis of VL is based on the detection of Leishmania
amastigotes in the human body (3,9). This
should be differentiated from Penicillium marneffei,
Histoplasma, and Plasmodium (10). The prognosis of VL is very poor and
it mainly depends on early diagnosis and targeted therapy. Sodium
antimony gluconate treatment was recommended for the treatment of
Leishmaniasis in China. However, certain studies have
applied sodium antimony gluconate and amphotericin B in the
treatment of relapse and refractory cases due to the development of
clinical resistance (11,12). In order to improve the diagnostic
rate and curative effect of VL, the present study combined the
latest domestic and overseas research progress with the clinical
data derived from VL-suspected cases, including laboratory
diagnosis, diagnostic methods, relapse and other refractory
cases.
Case report
In November 2010, a 25-year-old man from the Jiangxi
Province of China was admitted to a local hospital due to left
oblique hernia. He had hepatosplenomegaly during physical
examination and his abdominal magnetic resonance imaging scan
indicated portal vein dilatation (14 mm). The blood examinations
revealed severe pancytopenia and chronic HBV infection. Significant
plasma cell infiltration (12.5%) was noted following bone marrow
aspiration. Therefore, multiple myeloma was suspected. On the 29th
of December 2010, the patient was transferred to our clinic. The
patient worked as an excavator driver in a gold mine in the Sichuan
Province of China in the past two years. A year ago, he began to
suffer from low fever and night sweats, accompanied with fatigue.
Administration of a Chinese herbal medicine resulted in the
elimination of the fever, whereas fatigue and intermittent
nighttime sweat continued until his hospitalization. He did not
receive any further treatment.
The patient exhibited no past medical history, and
did not receive any supplements, illicit drugs, raw meat or
unpasteurized milk. His family and personal history did not include
associated diseases or disorders. However, it was reported that he
had frequent sexual intercourse with different female partners. In
addition, he was indirectly exposed to farm animals and pets
without having close contact with them, whereas his family was not
engaged in agricultural works. He denied tick or flea infections,
blood transfusion and the presence of osteodynia or arthralgia. He
never smoked and was not an alcoholic. On physical examination the
patient did not exhibit fever and appeared pale and sweaty. The
cardiorespiratory examination was unremarkable. A markedly tender
and enlarged liver and spleen were noted that were located 5 and 11
cm below the costal margins, respectively. No rashes, dryness of
eyes, mouth ulcers, or mucocutaneous bleeding were noted. Several
palpable lymph nodes with an approximate diameter of 1 cm were
noted in the supraclavicular fossa and groin. No skin lesion or
sedimentation was noted. Periorbital or peripheral extremity edema
was not present. The patient exhibited a left oblique hernia, which
appeared two months ago.
The laboratory values are presented in Table I. Urine and stool examinations were
normal. The erythrocyte sedimentation rate was 120 mm/h and the
C-reactive protein was 12 mg/dl. Severe pancytopenia was present
with neutropenia, mild thrombopenia and normocytic normochromic
anemia. In addition, the patient experienced hypoalbulinemia and
hyperglobulinemia and immunoelectrophoresis demonstrated an
elevation of IgG and IgA (Table I).
X-ray imaging indicated no evidence of bone damage. Subsequently, a
new bone marrow aspiration and biopsy were performed to confirm the
previous diagnosis on the 27th of December 2010. Bone marrow smears
were rich in mononuclear cells. The percentage of erythrocyte
series was 44% and the cells were uncoiled, with erythroblastic
anisocytosis. The percentage of granulocyte series was 36.5% and it
exhibited a light deviation to the left side. The percentage of
lymphoplasmocyte series was 17.5%, including heteromorphic
plasmocytes (uni- and binuclear), lympho-plasmocytes and
lymphocytes. The percentage of the megakaryocyte series was
estimated to 2%, with frequent thrombocytogenic megakaryocytes.
Bone marrow biopsy indicated hypercellular bone marrow with
infiltration of plasmocytes. At this stage, reactive plasmacytosis
was highly suspected, although it was previously shown that certain
viruses, such as hepatitis viruses, rarely cause this degree of
hepatosplenomegaly. The potential interactions caused by
inflammatory conditions, chronic infections, autoimmune diseases,
hypersensitivity states and malignancy, were also taken into
account.
 | Table ILaboratory values. |
Table I
Laboratory values.
| Examination | Value |
|---|
| Urinalysis |
|
Glucose | (-) |
|
Protein | (-) |
|
Erythrocytes
(µl) | 2 |
|
Leucocytes
(µl) | 3 |
|
Cast
(/HP) | 0 |
|
Bacteria
(/HP) | 0 |
| Stool |
|
Occult
blood | (-) |
|
Ova | (-) |
| Hematological
values |
|
ESR
(mm/h) | 120 |
|
Leucocyte
(/µl) | 1,300 |
| Differential count
(/µl) |
|
Neutrophils
(/µl) | 600 |
|
Lymphocytes
(/µl) | 500 |
|
Monocytes
(/µl) | 200 |
|
Eosinophils
(/µl) | 0 |
|
Erythrocyte
(104/µl) | 272 |
|
Hemoglobin
(g/ml) | 7.4 |
|
Hematocrit
(%) | 23.4 |
|
Platelets
(104/µl) | 8.5 |
|
Anti-platelet
IgG (ng/107 PA) | 352.1 |
|
Anti-platelet
IgM | 50.3 |
|
Anti-platelet
IgA | 1.8 |
|
CRP
(mg/l) | 12 |
| Blood chemical
values |
|
Total
protein (g/dl) | 84.3 |
|
Albumin
(g/dl) | 22.7 |
|
Globulin
(g/dl) | 61.6 |
|
IgG
(g/l) | 39.20 |
|
IgA
(g/l) | 0.52 |
|
IgM
(g/l) | 1.97 |
|
κ (g/l) | 10.4 |
|
λ (g/l) | 4.47 |
|
BUN
(mmol/l) | 4.2 |
|
Creatinine
(mmol/l) | 51.6 |
|
Na
(mmol/l) | 130.3 |
|
K
(mmol/l) | 4.0 |
|
Cl
(mmol/l) | 107.1 |
|
β2-MG
(ng/ml) | 6,246 |
|
Urinoβ2-MG
(µg/l) | 248 |
|
CD3% | 23.9 |
|
CD4% | 6.7 |
|
CD8% | 6.9 |
|
CD16+56
(NK,%) | 1.0 |
|
LDH
(U/l) | 187 |
|
AST
(U/l) | 11 |
|
ALT
(U/l) | 16 |
|
γ-GT
(U/l) | 32 |
|
Alkaline
phosphatase (U/l) | 99 |
|
Total
bilirubin (mg/dl) | 20.1 |
Parasite infectious etiology was suspected from the
epidemic of schistosomiasis in the patient's hometown at the
Jiangxi Province. However, serological analyses did not reveal
bacterial infections with the exception of a past infection of
respiratory syncytial viruses and HBV (Table II). The presence of antibodies
against viruses, bacteria or parasites was not detected and
multiple cultures of blood, urine and stool revealed negative
results. The presence of anti-nuclear antibodies was negative and
the concentration levels of the complement proteins were within the
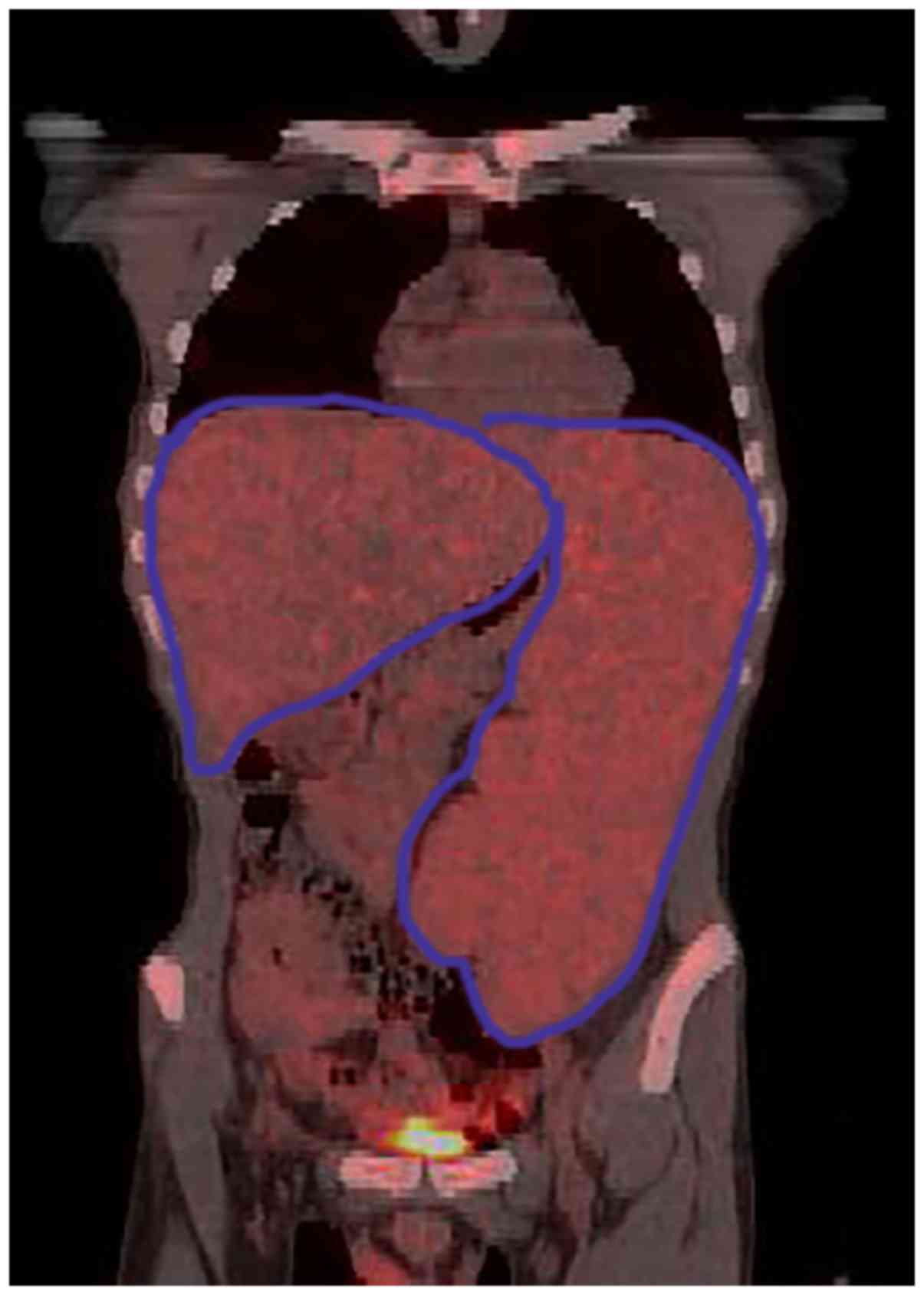
normal range. Positron Emission Tomography/Computed Tomography
(PET/CT) revealed no evidence of malignancy, although substantial
hepatosplenomegaly was evident in combination with dilatation of
the portal vein (14 mm) and small levels of ascites (Fig. 1). Magnetic resonance portal
venography (MRPVG) indicated dilatation of the spleen and the
portal veins at a diameter of 1.6 and 1.5 cm, respectively, whereas
no evidence of canalization of collateral circulation, stenosis or
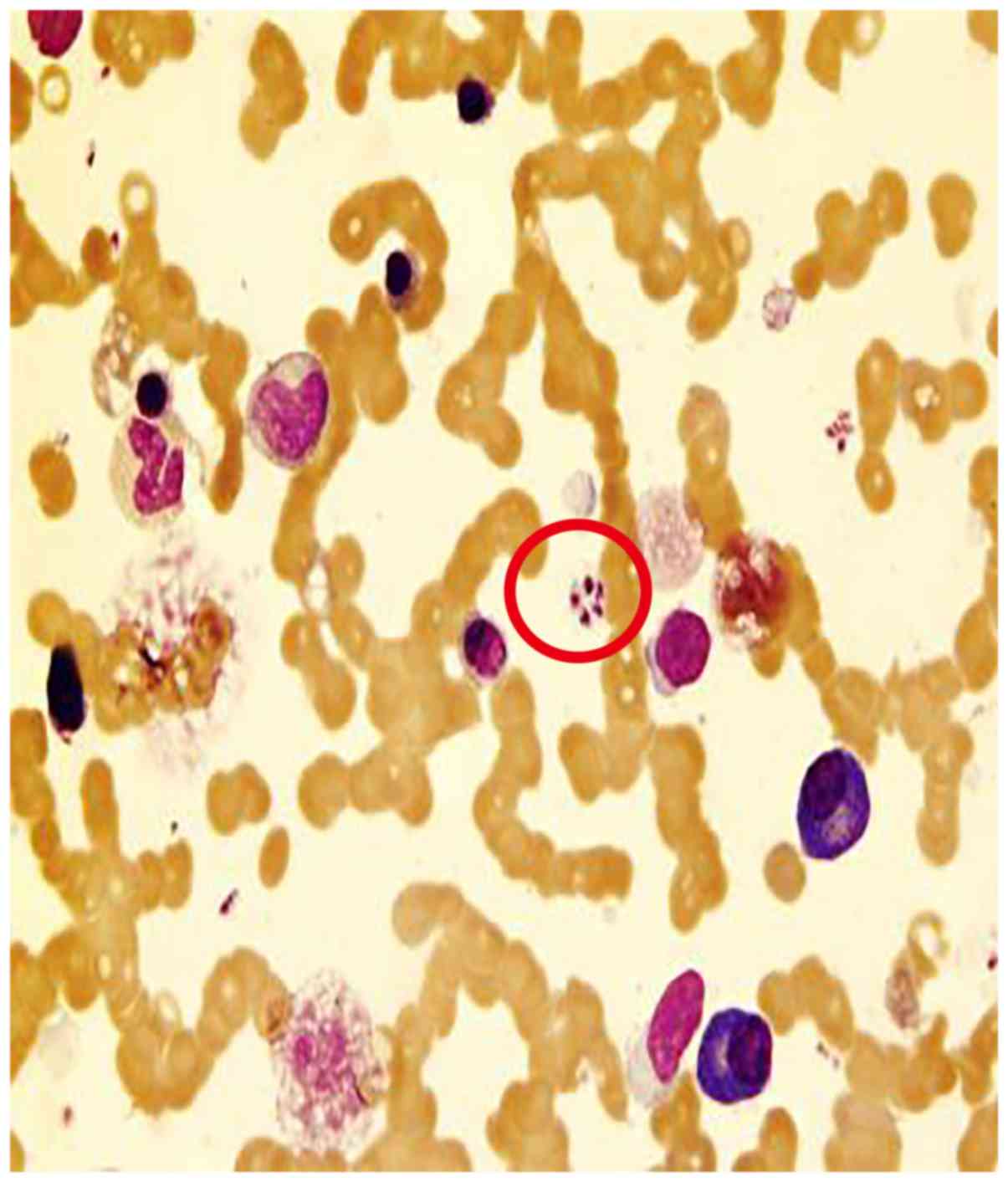
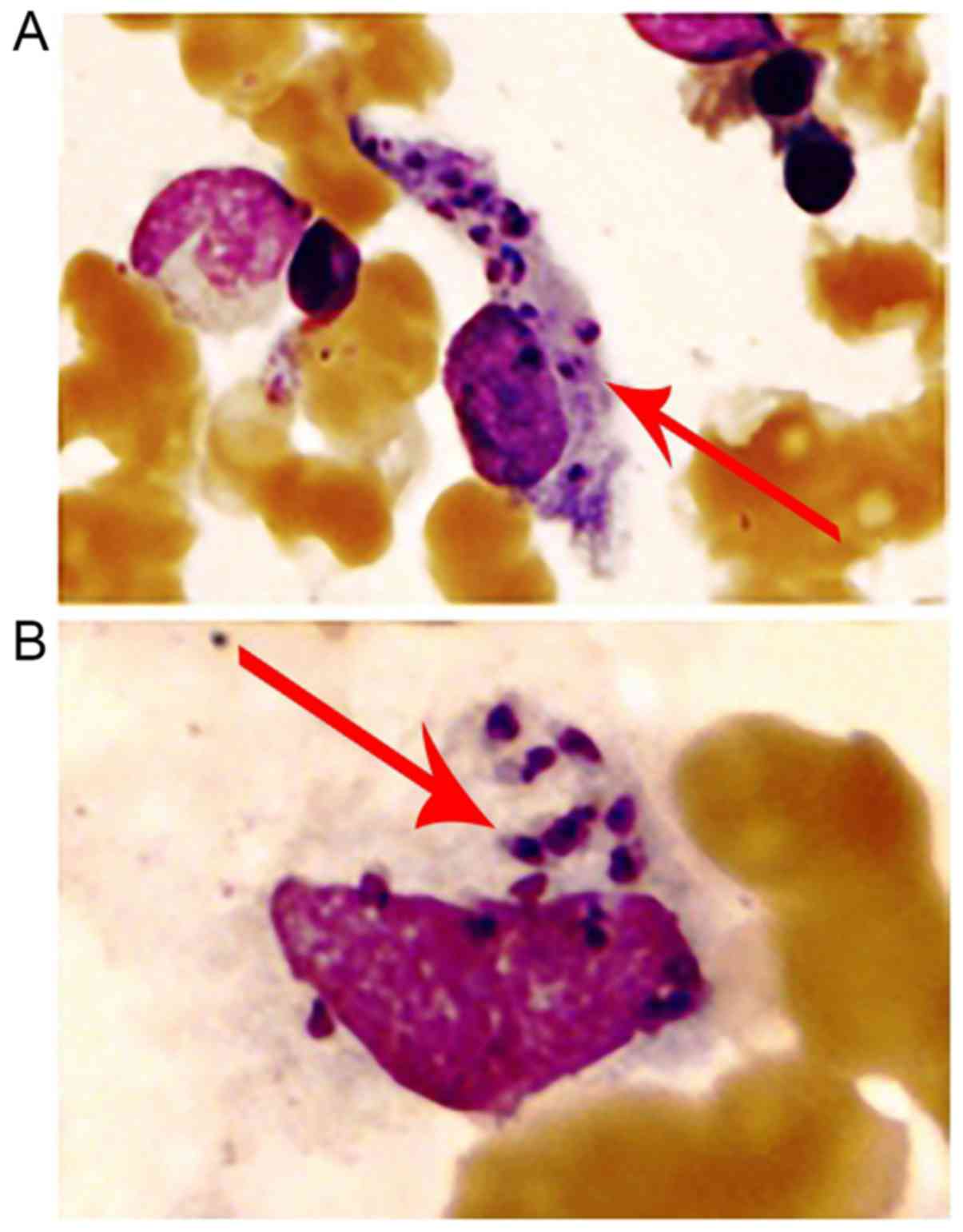
arteriovenous shunt of major vessels was noted. On the 10th of
January 2011, bone marrow biopsy was performed again and the smear
sample indicated the presence of extracellular (Fig. 2) and intra-macrophage amastigotes
(Fig. 3), which lacked an
exteriorized flagellum. Detection method of worms in bone marrow
smear: Bone marrow slides containing particles were prepared
according to ICSH guidelines, using a Wright-Giemsa Stain kit (Baso
Diagnostic Inc.), and then examined under a microscope. The marrow
cellularity was observed under low power magnification (x100) to
find suspicious cell or extracellular protozoa, and then confirm
under the oil mirror (x1,000) whether it is a Leishmania
(Microscope model: Olympus CX23). The cells were morphologically
assessed and the data revealed that they were possibly amastigotes
of Leishmania (Leishmania) donovani. To
confirm this diagnosis, blood samples were sent to the Institute of
parasitic disease, at the Chinese Center for Disease Control and
Prevention. The plasma Leishmania-donovani antibody was
positive. Therefore, the present case was diagnosed as VL. The
patient was immediately treated on the 12th of January with
anti-leishmanial therapy intramuscularly, which consisted of 600 mg
sodium stibogluconate. The patient tolerated the 10-day-treatment
and exhibited only a minimal arthralgia. Moreover, leukopenia and
monocytosis disappeared at the beginning of February. Bone marrow
biopsy was performed on the 21st of March in 2011 and was negative
for amastigotes. The patient was discharged on the 25th of March
2011 with no medication. No relapse has been reported to date.
 | Table IISerological studies. |
Table II
Serological studies.
| Tests | Result |
|---|
| HBV-sAg, cAb,
eAb | (+) |
| Anti-HCV Ab | (-) |
| Anti-CMV IgM,
IgG | (-) |
| Anti-EBV IgM,
IgG | (-) |
| Anti-RSV IgG | (+) |
| Anti-HIV Ab | (-) |
| Anti-HSV-1 IgG | (-) |
| Anti-toxoplasma
IgG | (-) |
| Brucellosis | (-) |
| Anti-mycoplasma
Ab | (-) |
| Candida Ag | (-) |
| Vidal's reaction | (-) |
| Rheumatoid factor
(IU/ml) | 15.6 |
| Antistreptolysin
O | (-) |
| Anti-dsDNA Ab | (+) |
| Candida Ag | (-) |
| Anti-nuclear
Ab | (-) |
| CH50 (U/ml) | 45.18 |
| C3 (g/l) | 0.86 |
| C4 (g/l) | 0.09 |
Discussion
VL, which is caused by the Leishmania
donovani group of protozoa, is highly prevalent in mainland
China. However, since the end of the 1950s, a national campaign was
conducted, which resulted in the significant reduction of the
disease epidemic (13). Sporadic
cases have occurred in the newly reclaimed desert areas, such as
the Xinjiang, Gansu, Sichuan, Shaanxi and Shanxi provinces
(14). Sporadic cases have also
occurred in Inner Mongolia and the Xinjiang Uygur Autonomous Region
(14). However, the disease was
still prevalent or sporadic in some cities in western China after
the 1990s (4). In the present case
report, a patient was included who lived during the recent two
years in the Sichuan province. This region is located in the
southwest of China. The north area of the Sichuan province is known
as an endemic area of VL. It was speculated that he was infected
there.
The immune response during the chronic infection of
Leishmania is complicated. In the present case, the
polyclonal hyperimmunoglobulinemia, which led to the diagnosis of
reactive plasmocytosis, reflected the overstimulated state of
humoral immunity. However, the immunoglobins stimulated by the
parasite antigen were not primarily important for the protection of
the patient and were involved in the pathogenesis of tissue lesions
(15). The assessment of
cytoimmunity was assessed by lymph node biopsy, which demonstrated
a diffuse reactive proliferation of T cells. However, the
peripheral blood examination demonstrated that the patient
experienced severe leukopenia and low levels of CD4,
CD8 T cells and NK cells. This maybe the result of a
chronic stimulation of the Leishmania antigen and of a
variety of general suppressive mechanisms, including soluble
factors (16), sensitivity of
different cell types (17) and
interactions of cytokines (18).
Human infections can be asymptomatic or can manifest
as oligosymptomatic and progressive diseases. The diagnosis of VL
in an asymptomatic patient is challenging. The symptoms of VL
consist of malaise, weakness, weight loss and high-grade
intermittent fever. The liver and spleen progressively increase in
size. Hyperpigmentation is also common (black fever). Peripheral
blood exhibits progressive leukopenia with relative and absolute
monopenia, normocytic anemia and thrombocytopenia that are
encountered in the initial and late stages, respectively (19). These symptoms and the incidence of
AIDS and aplastic anemia are similar with the exception that the
latter two diseases are generally not associated with progressive
hepatosplenomegaly. In the present case report, the patient did not
exhibit fever during his admission in our clinic and following the
administration of Chinese herbal drugs. It remains unclear whether
the patient's left oblique hernia was caused by a prolonged
infection with Leishmania that enlarged the liver and spleen
and increased abdominal pressure, or whether other factors were
present that caused abnormal changes in the peritoneum. The bone
marrow biopsy presented as reactive plasmacytosis and this might
have led the physician to misdiagnosis. To avoid misdiagnosis of
leishmaniasis with malignant B cell diseases, it is important to be
aware of the differences between reactive plasmacytosis and
multiple myeloma that are both present in the clinical features and
pathological characteristics of these two conditions. The
immunohistochemical study revealed a polytypic nature of the plasma
cells. Moreover, electrophoresis was mainly indicative of a
broad-based polyclonal hyperimmunoglobulinemia in reactive
plasmacytosis.
The diagnosis of VL is mainly focused on the
detection of Leishmania malformans in human tissues.
The sensitivity of detection depends on the type of tissue.
Although, the spleen aspirate is higher than 90%, the risk
retrieving the sample from the spleen is considerably high. The
positive rate of detection is estimated from 50 to 80% in the lymph
node and in blood samples (20).
Leishmania no-flagellate should be readily distinguished
from Penicillium marneffei, histoplasma and
Plasmodium. Penicillium marneffei is a fungus and its cells
are sausage-shaped, with a transverse septum. In the present study,
the cell wall was detected by periodic acid-Schiff staining and the
cytoplasm was not colored. The histoplasma was a fungus, which
exhibited a round or oval shape. It resembled a capsule and the
periodic acid-Schiff stained cell wall was red and clear. The
cytoplasm was not stained. The Plasmodium was stained red-nuclear
following Wright's staining and the cytoplasm was azure to dark
blue. The malaria pigment has been shown to be brownish yellow and
brown or dark brown (4).
The detection of protozoa may yield certain false
negative results and it is complemented by clinical detection with
serological antibody detection technology (rk39 kit). Although this
method is fast and sensitive, it is used in patients with
immunodeficiency with very low detection rate. Moreover, this
method lacks the ability to distinguish between active or previous
infections (21). Recent advances in
molecular biology techniques and second-generation sequencing can
improve the current method and increase the diagnostic value and
dynamic monitoring of VL diseases (1).
Currently, the first-line drug used for VL in China
is mainly sodium antimony gluconate. However, in other countries
this treatment is combined with amphotericin B (3).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GZ was mainly responsible for writing the article
and data collation. JZ provided valuable assistance and information
regarding the identification of cell morphology. TW was mainly
responsible for the literature search work and the diagnosis of
this case and the collection of differential diagnostic data. LZ
directed clinical treatment and efficacy evaluation. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The case has been approved by the Ethics Committee
of Renji Hospital Affiliated to Shanghai Jiaotong University School
of Medicine [approval no. (2019) 023]. The patient also provided
written informed consent for this article.
Patient consent for publication
The patient included in the current study consented
to the publication of this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sundar S and Om PS: Molecular diagnosis of
visceral leishmaniasis. Mol Diagn Ther. 22:443–457. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Medley GF, Hollingsworth TD, Olliaro PL
and Adams ER: Health-seeking behaviour, diagnostics and
transmission dynamics in the control of visceral leishmaniasis in
the Indian subcontinent. Nature. 528:S102–S108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zheng CJ, Wang LY, Xu X, Zhu XH and Wu WP:
Visceral leishmaniasis in China during 2004-2007. Zhongguo Ji Sheng
Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 27:344–347. 2009.In
Chinese. PubMed/NCBI
|
|
4
|
Wang JY, Cui G, Chen HT, Zhou XN, Gao CH
and Yang YT: Current epidemiological profile and features of
visceral leishmaniasis in people's republic of China. Parasit
Vectors. 5(31)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang WW, Ghosh AK, Mohamath R, Whittle J,
Picone A, Lypaczewski P, Ndao M, Howard RF, Das P, Reed SG and
Matlashewski G: Development of a sandwich ELISA to detect
Leishmania 40S ribosomal protein S12 antigen from blood samples of
visceral leishmaniasis patients. BMC Infect Dis.
18(500)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao Q, Liu YB, Zhong CJ and Lv XJ:
Clinical analysis of 137 patients with visceral leishmaniasis.
Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi.
31:135–157. 2013.In Chinese. PubMed/NCBI
|
|
7
|
Sayyahfar S, Ansari S, Mohebali M and
Behnam B: Visceral leishmaniasis without fever in an 11-month-old
infant: A rare clinical feature of Kala-azar. Korean J Parasitol.
52:189–191. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tatarelli P, Fornaro G, Del Bono V,
Nicolini LA, Raiola AM, Gualandi F, Varaldo R, Di Muccio T,
Gramiccia M, Gradoni L, et al: Visceral leishmaniasis in
hematopoietic cell transplantation: Case report and review of the
literature. J Infect Chemother. 24:990–994. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kiros YK and Regassa BF: The role of rk39
serologic test in the diagnosis of visceral leishmaniasis in a
Tertiary Hospital, Northern Ethiopia. BMC Res Notes.
10(169)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gui XE and Guan LR: Differential diagnosis
of visceral leishmaniasis, progressive disseminated histoplasmosis
and penicilliosis marneffei. Zhongguo Ji Sheng Chong Xue Yu Ji
Sheng Chong Bing Za Zhi. 25:69–72. 2007.(In Chinese). PubMed/NCBI
|
|
11
|
Editorial Board of Chinese Journal of
Infectious Diseases. China expert consensus on the diagnosis and
treatment of Leishmania donovani isolates from infection. Chinese
Journal of Infectious Diseases 35: 513-518, 2017.
|
|
12
|
Hasnain MG, Nath P, Maruf S, Nabi SG,
Hossain AF, Ahmed BN, Mondal D and Basher A: Amphotericin B
deoxycholate for relapse visceral leishmaniasis in Bangladesh: A
cross-sectional study. BMC Res Notes. 11(918)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fu Q, Li SZ, Wu WP, Hou YY, Zhang S, Feng
Y, Zhang LP and Tang LH: Endemic characteristics of infantile
visceral leishmaniasis in the People's Republic of China. Parasit
Vectors. 6(143)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guan LR: Present situation of visceral
leishmaniasis and prospect for its control in China. Zhongguo Ji
Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 27:394–397. 2009.(In
Chinese). PubMed/NCBI
|
|
15
|
Miles SA, Conrad SM, Alves RG, Jeronimo SM
and Mosser DM: A role for IgG immune complexes during infection
with the intracellular pathogen Leishmania. J Exp Med. 201:747–754.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Barral A, Carvalho EM, Badaró R and
Barral-Netto M: Suppression of lymphocyte proliferative responses
by sera from patients with American visceral leishmaniasis. Am J
Trop Med Hyg. 35:735–742. 1986.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Carvalho EM, Bacellar O, Barral A, Badaro
R and Johnson WD Jr: Antigen-specific immunosuppression in visceral
leishmaniasis is cell mediated. J Clin Invest. 83:860–864.
1989.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bacellar O, Brodskyn C, Guerreiro J,
Barral-Netto M, Costa CH, Coffman RL, Johnson WD and Carvalho EM:
Interleukin-12 restores interferon-gamma production and cytotoxic
responses in visceral leishmaniasis. J Infect Dis. 173:1515–1518.
1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Carranza-Tamayo CO, Carvalho Mdo S, Bredt
A, Bofil MI, Rodrigues RM, Silva AD, Cortez SM and Romero GA:
Autochthonous visceral leishmaniasis in Brasília, Federal District,
Brazil. Rev Soc Bras Med Trop. 43:396–399. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Burza S, Croft SL and Boelaert M:
Leishmaniasis. Lancet. 392:951–970. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Antinori S, Calattini S, Longhi E,
Bestetti G, Piolini R, Magni C, Orlando G, Gramiccia M, Acquaviva
V, Foschi A, et al: Clinical use of polymerase chain reaction
performed on peripheral blood and bone marrow samples for the
diagnosis and monitoring of visceral leishmaniasis in HIV-infected
and HIV-uninfected patients: A single-center, 8-year experience in
Italy and review of the literature. Clin Infect Dis. 44:1602–1610.
2007.PubMed/NCBI View
Article : Google Scholar
|