Introduction
Osteoarthritis is a common joint disease mainly
characterized by the degeneration and breakdown of articular
cartilage, the formation of osteophytes and subchondral cysts, and
the thickening of subchondral bone (1). Articular cartilage is a resilient and
smooth tissue that covers and protects the ends of bones in joints,
and primarily consists of a dense extracellular matrix with no
blood vessels, lymphatic vessels or nerves, with a sparse
chondrocyte population (2,3).
The extracellular matrix is a multifaceted meshwork
of proteoglycans, polysaccharides and proteins secreted by resident
supporting cells. The extracellular matrix not only represents a
critical structural component of articular cartilage, but is also
involved in the pathological degradation of articular cartilage
(4-6).
Physical and biochemical alterations in the extracellular matrix
may elicit numerous signaling events by communicating with the
intracellular cytoskeleton, stimulating chondrocyte responses and
thus inducing osteoarthritis (7-9).
The dynamic remodeling and homeostasis of the extracellular matrix
is regulated by a variety of proteinases, especially proteinases in
the matrix metalloproteinase (MMP) family (10,11).
Facet joint osteoarthritis (FJOA) is a common form
of osteoarthritis that occurs in the facet joints located in the
posterior aspect of the vertebral column (12). However, to the best of our knowledge,
FJOA is not as well studied as other forms of osteoarthritis, such
as hip osteoarthritis and knee osteoarthritis. Efforts have been
made to examine the anatomy, biomechanics, epidemiology and
clinical manifestations of FJOA (13). The expression of key molecules in the
degenerative process of facet joints such as MMP13, tumor necrosis
factor-a and interleukin-6 have also been identified (14). A previous study determined the
molecular changes in FJOA by collecting human facet joint tissues
from healthy controls and patients with FJOA and conducting
transcriptome sequencing (15).
The current study investigated the expression
profiles of genes in healthy controls and patients with FJOA by
filtering genes with the most significant fold-changes and
screening the most enriched Gene Ontology (GO) categories. Analysis
results showed that MMP12, a member of the MMP family, was the most
downregulated gene in FJOA. In addition, many enriched GO
categories were related to the extracellular matrix. Therefore, the
canonical signaling pathway ‘inhibition of matrix
metalloproteinases’ was investigated through the joint use of
Ingenuity Pathway Analysis (IPA) software, heatmap and hierarchical
cluster analysis, and reverse transcription-quantitative PCR
(RT-qPCR) validation to study the involvement of MMPs in FJOA.
Materials and methods
Facet joint tissue collection and
transcriptome sequencing
Human facet joint tissues were collected from 48
patients with FJOA (FJOA group) and 10 healthy patients with
vertebral fractures (control group) as previously described
(15). Magnetic resonance imaging
was applied to determine the degree of cartilage degeneration. The
facet joint tissues of patients in the FJOA group were of Grade 2
(moderate degeneration) and Grade 3 (severe degeneration), while
the facet joint tissue of patients in the control group was of
Grade 0 (normal) and Grade 1 (mild degeneration) (16). Patients with infection, inflammatory
diseases or autoimmune diseases were excluded from the current
study. The present study was ethically approved by the Human Ethics
Committee of the Second Affiliated Hospital of Nantong University.
All participants provided written informed consent.
Facet joint tissues from the FJOA and control groups
were divided into three parts for technical repetitions for
subsequent RNA isolation and sequencing experiments. Total RNA was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Extracted total RNA was used to fragment RNA and
synthesize cDNA. A cDNA library was amplified and sequenced using a
Hiseq X Ten sequencing platform (Illumina, Inc.) to produce raw
reads. Dirty reads were filtered to obtain clean reads. Clean reads
were then mapped to the reference genome using TopHat v2.1.1
(https://ccb.jhu.edu/software/tophat/index.shtml)
(17,18).
Bioinformatics analysis
Transcriptome sequencing data were used to identify
the fragments per kilobase of transcript per million mapped reads
(FPKM) values of each gene. The FPKM values of genes in the FJOA
group were compared with the FPKM values in the control group to
screen differentially expressed genes with a fold-change >2 or
<-2 (log2 ratio >1 or <-1) and a q-value
(adjusted P-value) <0.05. The Database for Annotation,
Visualization, and Integrated Discovery (DAVID) 6.7 (https://david.ncifcrf.gov/) was used to enrich
differentially expressed genes to GO categories (19-21).
IPA software (2018 release Qiagen, Inc.) was used to analyze the
canonical signaling pathway ‘inhibition of matrix
metalloproteinases’. Heatmap and hierarchical cluster analyses were
performed using R v2.13.0 (https://www.r-project.org/) to display the expression
patterns of differentially expressed genes.
RT-qPCR
RT-qPCR was conducted to validate transcriptome
sequencing outcomes. RNA samples were first reverse transcribed to
cDNA using the PrimeScript RT Reagent kit (Takara Biotechnology
Co., Ltd.) according to the manufacturer's protocol. qPCR was
performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd)
on a StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The following thermocycling conditions
were used for the PCR: Initial denaturation at 95˚C for 5 min; 40
cycles of 95˚C at 30 sec, 45 sec at 56.5˚C and 72˚C for 30 sec; and
a final extension at 72˚C for 5 min. The relative expression of
target genes MMP7, disintegrin and metalloproteinase
domain-containing protein 12 (ADAM12), tissue inhibitor of
metalloproteinases 3 (TIMP3) and TIMP4 were calculated using the
2-ΔΔCq method with GAPDH as the internal reference gene
(22). The sequences of primers
pairs used in the qPCR are listed in Table I.
 | Table IPrimer pairs for reverse
transcription-quantitative PCR. |
Table I
Primer pairs for reverse
transcription-quantitative PCR.
| Gene | Primer | Sequence
(5'-3') |
|---|
| MMP7 | Forward |
AGCAGCTATGCAGCTGGCCGT |
| | Reverse |
GCCCTGAGCCTGTTCCCACTGC |
| ADAM12 | Forward |
TCTTGCTGCCGGATTTGTGGTT |
| | Reverse |
AAGGGCGCACACACCTTAGTTT |
| TIMP3 | Forward |
AGGACGCCTTCTGCAAC |
| | Reverse |
CTCCTTTACCAGCTTCTTCC |
| TIMP4 | Forward |
ACCTGTCCTTGGTGCAGA |
| | Reverse |
TGTAGCAGGTGGTGATTTGG |
| GAPDH | Forward |
CCAAGGTCATCCATGACAAC |
| | Reverse |
TGTCATACCAGGAAATGAGC |
Statistical analysis
Data are presented as the mean ± SEM and were
analyzed using GraphPad Prism 5.0 (GraphPad Software, Inc.).
Student's t-test was used to compare differences between the FJOA
and control groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of the most
differentially expressed genes in FJOA
Previous transcriptome sequencing determined the
expression levels of >19,000 genes in human facet joint tissues
in both FJOA and control groups (15). To obtain a global view of the
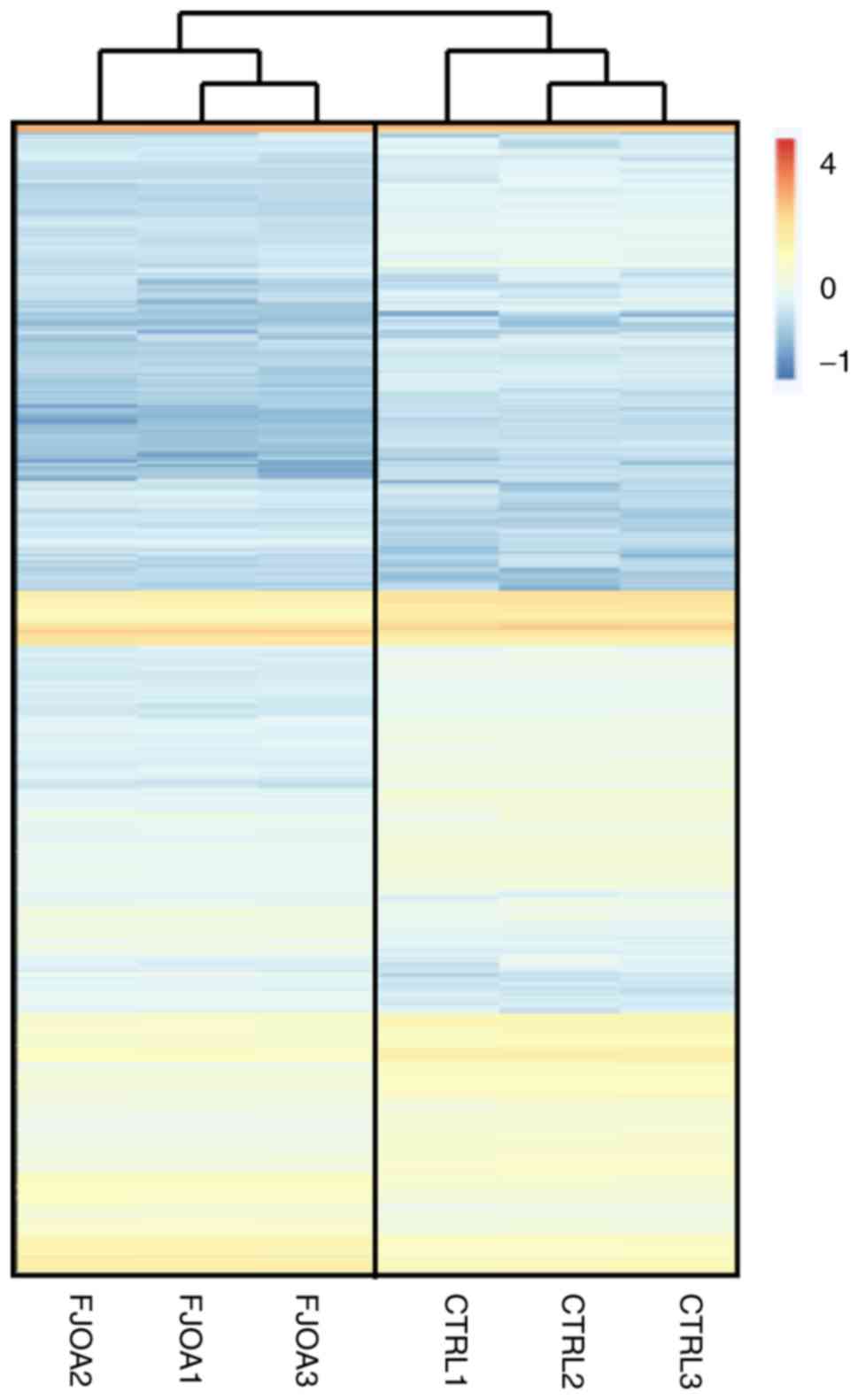
expression patterns of identified genes, the Heatmap function in
the software platform R (v.2.13.0) was used to draw a heatmap of
gene expression (Fig. 1). The
Euclidean distance was calculated using the Hierarchical Clustering
module from GenePattern (https://software.broadinstitute.org/cancer/software/genepattern/)
to cluster the identified genes (Fig.
1) (23). Parallelizing
hierarchical clustering methods were used to study the gene
expression profiles in the three control group replicates (CTRL1,
CTRL2 and CTRL3) and three FJOA group replicates (FJOA1, FJOA2 and
FJOA3) (Fig. 1). Compared with genes
in the control group, a smaller number of genes in the FJOA group
were upregulated while a relatively large number of genes were
downregulated.
A detailed investigation of these differentially
expressed genes showed that the expression level of the most
upregulated gene in FJOA, corticotropin releasing hormone receptor
1 (CRHR1), was elevated >28.8 fold (log2 ratio=4.85)
compared with the control group. The expression level of the most
downregulated gene, MMP12, was reduced to ~1/14 of its expression
level compared with the control group (log2
ratio=-3.86). The 10 most upregulated and downregulated genes in
FJOA are listed in Table II.
 | Table IITop 10 upregulated and downregulated
genes in FJOA. |
Table II
Top 10 upregulated and downregulated
genes in FJOA.
| A, Upregulated |
|---|
| GeneID | CTRL_FPKM | FJOA_FPKM | log2
(FJOA/CTRL) | q-value | Description |
|---|
| CRHR1 | 0.022303 | 0.643232 | 4.85003 | 0.00463 | Corticotropin
releasing hormone receptor 1 |
| CSF3 | 0.230299 | 3.21825 | 3.80469 | 0.000259 | Colony stimulating
factor 3 |
| CRLF1 | 6.24624 | 81.3432 | 3.70296 | 0.000259 | Cytokine receptor
like factor 1 |
| DAW1 | 0.090234 | 1.12819 | 3.6442 | 0.022496 | Dynein assembly
factor with WD repeats 1 |
| HAS1 | 2.90011 | 35.0671 | 3.59594 | 0.000259 | Hyaluronan synthase
1 |
| POSTN | 23.7872 | 265.481 | 3.48035 | 0.000259 | Periostin |
| TMEM59L | 0.181724 | 1.78416 | 3.29542 | 0.005947 | Transmembrane
protein 59 like |
| PVRL4 | 0.170113 | 1.61362 | 3.24574 | 0.000259 | Poliovirus
receptor-related protein 4 |
| LINC00702 | 0.924058 | 8.50754 | 3.20269 | 0.002128 | Long intergenic
non-protein coding RNA 702 |
| HMCN2 | 0.158359 | 1.36891 | 3.11176 | 0.000259 | Hemicentin 2 |
| B,
Downregulated |
| GeneID | CTRL_FPKM | FJOA_FPKM | log2
(FJOA/CTRL) | q-value | Description |
| MMP12 | 1.5777 | 0.10857 | -3.86112 | 0.013616 | Matrix
metallopeptidase 12 |
| HBG1 | 57.9551 | 4.16107 | -3.79991 | 0.000259 | Hemoglobin subunit
γ1 |
| SPIC | 2.63717 | 0.231806 | -3.508 | 0.00394 | Spi-c transcription
factor |
| TPSB2 | 8.40967 | 0.772965 | -3.44357 | 0.000259 | Tryptase β2
(gene/pseudogene) |
| SEC14L3 | 0.596955 | 0.059692 | -3.32201 | 0.007555 | Sec14 like lipid
binding 3 |
| PYHIN1 | 2.77976 | 0.280398 | -3.30942 | 0.000259 | Pyrin and hin
domain family member 1 |
| CHI3L1 | 1078.35 | 114.902 | -3.23036 | 0.000259 | Chitinase 3 like
1 |
| CD19 | 1.2214 | 0.131175 | -3.21897 | 0.004793 | Cd19 molecule |
| GP5 | 3.14407 | 0.360364 | -3.12511 | 0.000259 | Glycoprotein v
platelet |
| GPR15 | 1.43198 | 0.166889 | -3.10106 | 0.033956 | G protein-coupled
receptor 15 |
Identification of most enriched GO
categories in FJOA
To have a better understanding of the molecular
mechanisms underlying FJOA, the DAVID database was used to group
differentially expressed genes into GO biological processes,
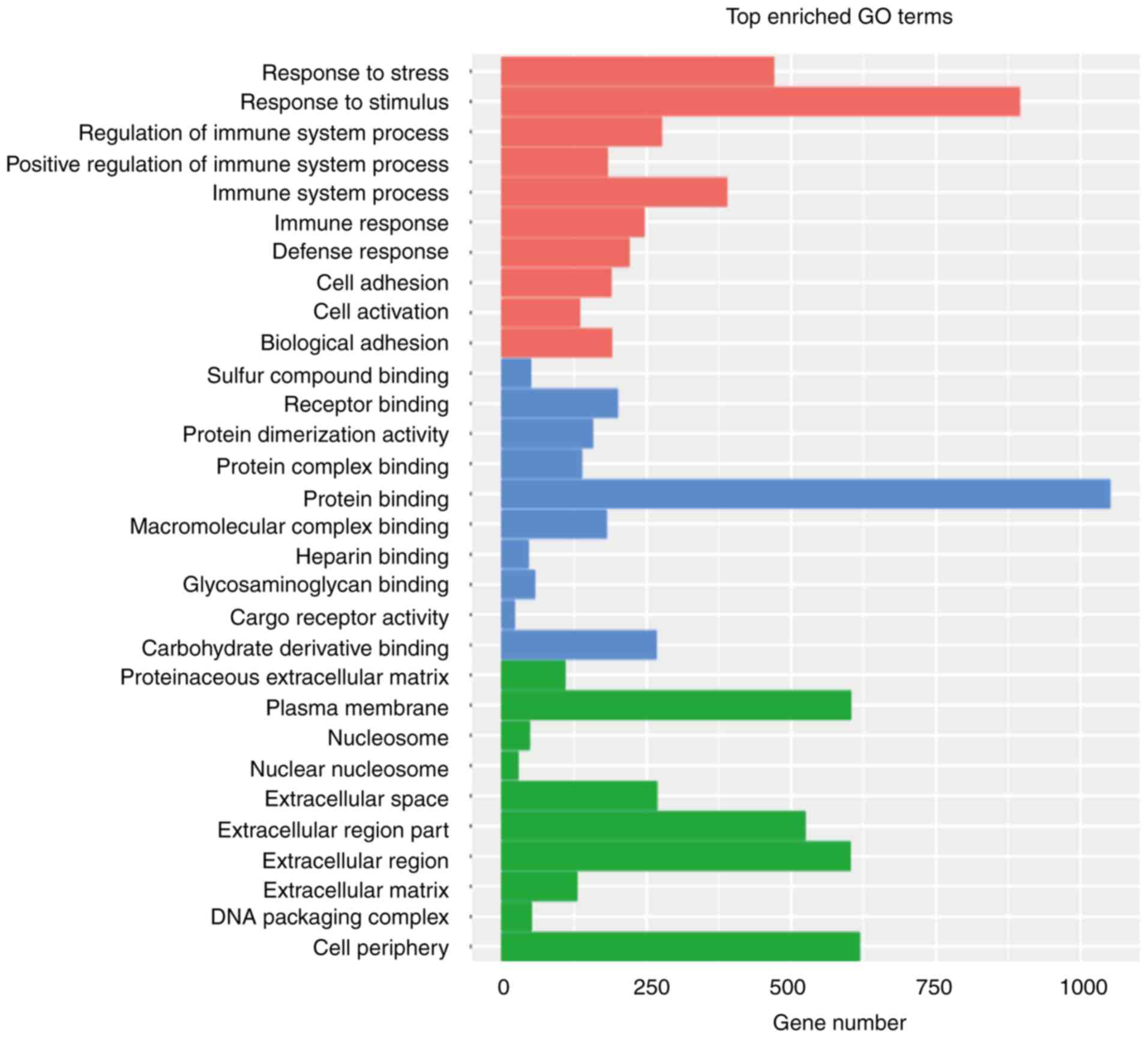
molecular functions and cellular components (Fig. 2). The ten most enriched GO biological
processes were associated with the response of the organism
(‘response to stress’ and ‘response to stimulus’), immune response
(‘regulation of immune system process’, ‘positive regulation of
immune system process’, ‘immune system process’, ‘immune response’
and ‘defense response’) and cellular behaviors (‘cell adhesion’,
‘cell activation’ and ‘biological adhesion’). The significant
involvement of the immune response was consistent with the high
expression of CRHR1 observed in FJOA tissue, as CRHR1 is essential
for the activation of various signaling pathways that regulate the
immune response. The ten most enriched GO molecular functions were
mainly related to molecular binding, while the ten most enriched GO
cellular components included various components, especially those
involving the extracellular matrix such as ‘proteinaceous
extracellular matrix’, ‘extracellular space’, ‘extracellular region
part’, ‘extracellular region’ and ‘extracellular matrix’.
Analysis of the canonical signaling
pathway ‘inhibition of matrix metalloproteinases’
GO cellular component analysis showed that the
extracellular matrix was highly associated with FJOA. In addition,
MMP12, a gene that encodes for an enzyme involved in the breakdown
of the extracellular matrix, was most significantly downregulated
in FJOA. The results of the bioinformatics analysis suggested that
dysregulated MMPs and the resulting modulated extracellular matrix
might be critical in the pathological process of FJOA. Therefore,
IPA bioinformatics analysis was performed to identify the
involvement of the canonical signaling pathway ‘inhibition of
matrix metalloproteinases’ in FJOA.
The schematic diagram of the canonical signaling
pathway ‘inhibition of matrix metalloproteinases’ is shown in
Fig. 3. MMPs are calcium-dependent
zinc-containing endopeptidases that are critical for the modulation
of both cell-cell and cell-extracellular matrix interactions
(24). These MMPs are tightly
regulated at multiple levels, including gene transcription, enzyme
activation and inhibitor inactivation (25). TIMPs are a family of protease
inhibitors comprising TIMP1, TIMP2, TIMP3 and TIMP4. TIMPs form
non-covalent bonds with the latent and active forms of MMPs with a
1:1 stoichiometry and thus inhibit the activity of MMPs (26). Members of the ADAM family, such as
ADAM10, ADAM12 and ADAM13 also regulate cell adhesion and mediate
the extracellular matrix (27)
(Fig. 3).
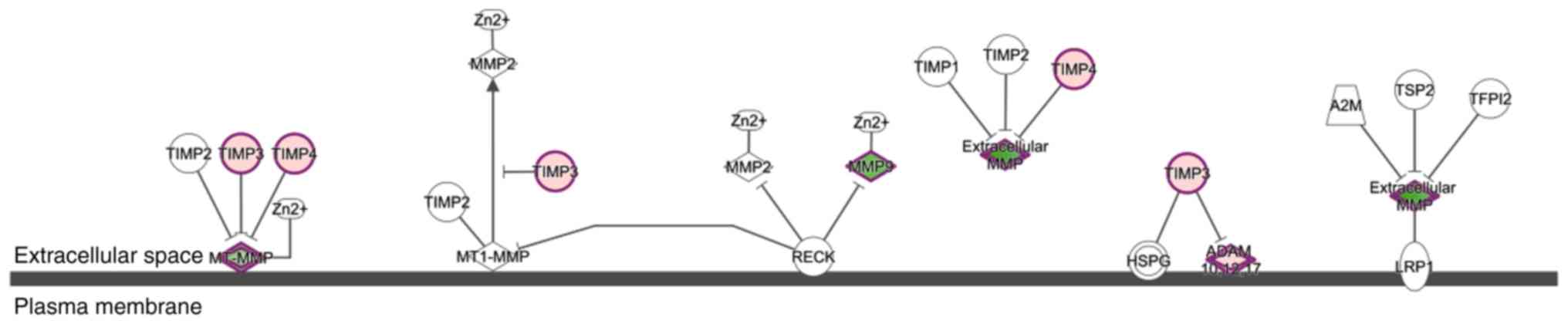 | Figure 3.Schematic diagram of the canonical
signaling pathway ‘inhibition of matrix metalloproteinases’.
Differentially expressed genes in the canonical signaling pathway
‘inhibition of matrix metalloproteinases’ are represented in red
and green. Red indicates upregulated genes while green indicates
downregulated genes. TIMP, tissue inhibitor of metalloproteinases;
MMP, matrix metalloproteinase; RECK, reversion-inducing
cysteine-rich protein with Kazal motifs; HSPG, basement
membrane-specific heparan sulfate proteoglycan core protein; ADAM,
disintegrin and metalloproteinase domain-containing protein; TSP2,
thrombospondin-2; A2M, α-2-macroglobulin; TFPI2, tissue factor
pathway inhibitor 2; LRP1, prolow-density lipoprotein
receptor-related protein 1; MT1-MMP, matrix metallopeptidase 14
(membrane-inserted). |
Upregulated and downregulated genes in the canonical
signaling pathway ‘inhibition of matrix metalloproteinases’ in FJOA
are labeled in red and green, respectively (Fig. 3). The expression levels of genes in
the pathway are shown in Table
III. Heatmap and relevant hierarchical clustering analyses were
performed to illustrate the expression levels of genes in the FJOA
and control groups (Fig. 4). Most
members of the MMP family (including MMP12, MMP9, MMP3, MMP16,
MMP7, MMP8 and MMP25) were significantly downregulated in FJOA
compared with controls. Meanwhile, MMP23B was upregulated and the
expression levels of other members of the MMP family were not
significantly altered compared with controls. ADAM12 and MMP
inhibitors TIMP3 and TIMP4 were upregulated in FJOA compared with
controls.
 | Figure 4.Heatmap of differentially expressed
genes in the canonical signaling pathway ‘inhibition of matrix
metalloproteinases’. Red indicates upregulated genes while green
indicates downregulated genes. CTRL, control group; FJOA, facet
joint osteoarthritis; TIMP, tissue inhibitor of metalloproteinases;
MMP, matrix metalloproteinase; RECK, reversion-inducing
cysteine-rich protein with Kazal motifs; HSPG, basement
membrane-specific heparan sulfate proteoglycan core protein; ADAM,
disintegrin and metalloproteinase domain-containing protein; TSP2,
thrombospondin-2; A2M, α-2-macroglobulin; TFPI2, tissue factor
pathway inhibitor 2; LRP1, prolow-density lipoprotein
receptor-related protein 1. |
 | Table IIIExpression levels of differentially
expressed genes in the canonical signaling pathway ‘inhibition of
matrix metalloproteinases’. |
Table III
Expression levels of differentially
expressed genes in the canonical signaling pathway ‘inhibition of
matrix metalloproteinases’.
| GeneID | CTRL_FPKM | FJOA_FPKM | log2
(FJOA/CTRL) | q-value | Description |
|---|
| A2M | 152.6 | 144.685 | -0.07684 | 0.580386 |
α-2-macroglobulin |
| ADAM10 | 23.1911 | 18.2402 | -0.34645 | 0.00269 | Disintegrin and
metalloproteinase domain-containing protein 10 |
| ADAM12 | 5.90052 | 19.1302 | 1.69694 | 0.000259 | Disintegrin and
metalloproteinase domain-containing protein 12 |
| ADAM17 | 12.845 | 12.1117 | -0.08481 | 0.581591 | Disintegrin and
metalloproteinase domain-containing protein 17 |
| HSPG2 | 79.3027 | 132.242 | 0.737738 | 0.000259 | Basement
membrane-specific heparan sulfate proteoglycan core protein |
| LRP1 | 251.144 | 326.912 | 0.38039 | 0.012286 | Prolow-density
lipoprotein receptor-related protein 1 |
| MMP2 | 108.741 | 175.485 | 0.690452 | 0.000259 | Matrix
metalloproteinase 2 |
| MMP9 | 314.457 | 91.4777 | -1.78137 | 0.000259 | Matrix
metalloproteinase 9 |
| MMP17 | 1.17006 | 1.48701 | 0.345823 | 0.353116 | Matrix
metalloproteinase 17 |
| MMP11 | 5.50944 | 8.16829 | 0.568128 | 0.001552 | Matrix
metalloproteinase 11 |
| MMP3 | 373.253 | 167.156 | -1.15896 | 0.000259 | Matrix
metalloproteinase 3 |
| MMP19 | 6.38089 | 10.853 | 0.766262 | 0.000497 | Matrix
metalloproteinase 19 |
| MMP13 | 125.361 | 75.5355 | -0.73086 | 0.000259 | Matrix
metalloproteinase 13 |
| MMP28 | 2.48386 | 4.40147 | 0.825399 | 0.026506 | Matrix
metalloproteinase 28 |
| MMP23B | 0.04404 | 0.828743 | 4.23405 | 0.247905 | Matrix
metalloproteinase 23B |
| MMP14 | 74.0875 | 118.202 | 0.673953 | 0.000259 | Matrix
metalloproteinase 14 |
| MMP24 | 1.21904 | 1.34362 | 0.14038 | 0.778023 | Matrix
metalloproteinase 24 |
| MMP8 | 37.9945 | 14.832 | -1.35708 | 0.000259 | Matrix
metalloproteinase 8 |
| MMP1 | 6.78886 | 4.20773 | -0.69013 | 0.493474 | Matrix
metalloproteinase 1 |
| MMP12 | 1.5777 | 0.10857 | -3.86112 | 0.013616 | Matrix
metalloproteinase 12 |
| MMP15 | 1.65603 | 2.41486 | 0.54421 | 0.016667 | Matrix
metalloproteinase 15 |
| MMP16 | 9.42333 | 4.70063 | -1.00338 | 0.000259 | Matrix
metalloproteinase 16 |
| MMP7 | 2.13341 | 1.03658 | -1.04133 | 0.028971 | Matrix
metalloproteinase 7 |
| MMP25 | 6.06705 | 2.5246 | -1.26494 | 0.000259 | Matrix
metalloproteinase 25 |
| RECK | 9.33448 | 14.4833 | 0.633749 | 0.000259 | Reversion-inducing
cysteine-rich protein with Kazal motifs |
| TFPI2 | 0.973482 | 0.789919 | -0.30145 | 0.53558 | Tissue factor
pathway inhibitor 2 |
| TIMP1 | 322.419 | 227.107 | -0.50557 | 0.000259 | Tissue inhibitor of
metalloproteinases 1 |
| TIMP2 | 216.411 | 377.86 | 0.804078 | 0.000259 | Tissue inhibitor of
metalloproteinases 2 |
| TIMP3 | 91.4082 | 261.077 | 1.51408 | 0.000259 | Tissue inhibitor of
metalloproteinases 3 |
| TIMP4 | 27.8848 | 57.9526 | 1.05539 | 0.000259 | Tissue inhibitor of
metalloproteinases 4 |
| TSP2 | 37.7629 | 73.7939 | 0.966534 | 0.000259 |
Thrombospondin-2 |
Validation using RT-qPCR
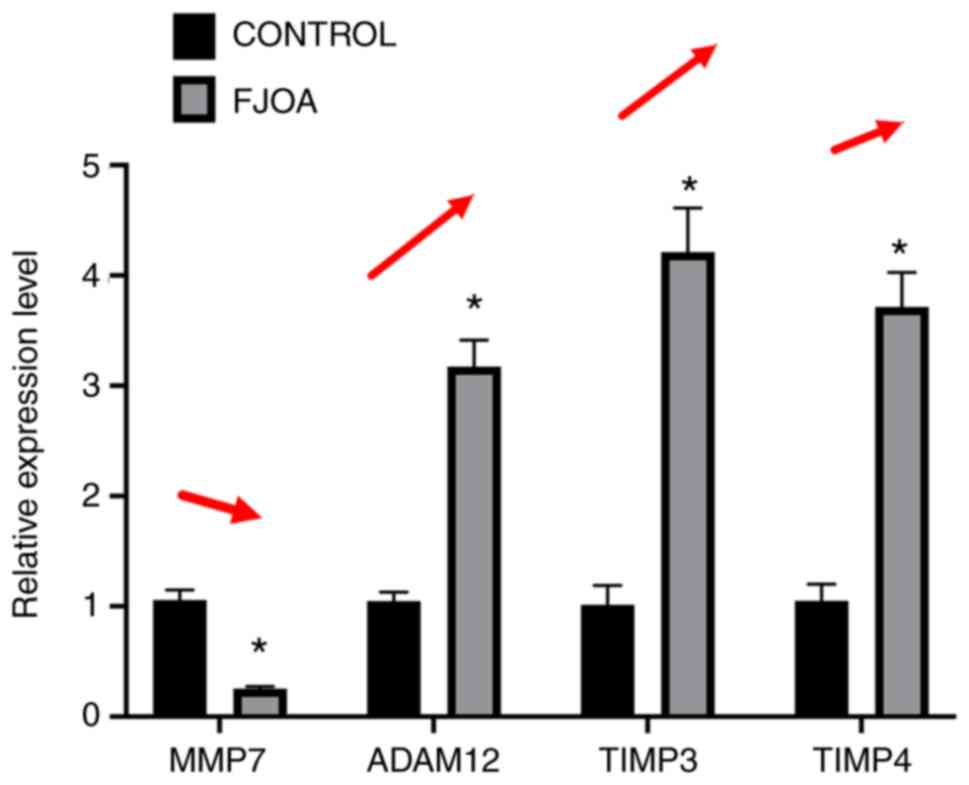
RT-qPCR experiments were further conducted to
measure the expression of genes in FJOA and control groups in order
to validate transcriptome sequencing results. Representative genes
in the canonical signaling pathway ‘inhibition of matrix
metalloproteinases’, MMP7, ADAM12, TIMP3 and TIMP4 were selected
for RT-qPCR experiments (Fig. 5).
Consistent with the transcriptome sequencing results, the
expression levels of MMP7 were significantly downregulated in the
FJOA group compared with the control group. By contrast, the
expression levels of ADAM12, TIMP3 and TIMP4 were significantly
higher in the FJOA group as compared with the control group
(Fig. 5).
Discussion
In the current study, transcriptome sequencing
outcomes from human facet joint tissues in FJOA patients and
healthy controls were analyzed. The ten most significantly
upregulated and downregulated genes were identified and the ten
most enriched GO biological processes, molecular functions and
cellular components were categorized. The analytical results showed
that the extracellular matrix was robustly involved in the genetic
changes involved in FJOA. MMP12, a calcium-dependent
zinc-containing endopeptidase capable of degrading the
extracellular matrix, was the most downregulated gene in FJOA.
CRHR1, which encodes a G-protein coupled receptor that binds
neuropeptides of the corticotropin releasing hormone family that
are major regulators of the hypothalamic-pituitary-adrenal pathway,
was the most differentially expressed gene in FJOA. From a gene
expression aspect, the results suggested that modulation of the
extracellular matrix is a critical biological process in FJOA, and
the CRHR1-regulated immune response might be critical in the
development of FJOA. Treatments targeting CRHR1 and MMP might be
potential therapeutic methods. Therefore, gene changes in the
canonical signaling pathway ‘inhibition of matrix
metalloproteinases’ were examined. Heatmap analysis and RT-qPCR
experiments illustrated that several genes in ‘inhibition of matrix
metalloproteinases’, especially MMPs and TIMPs, were dysregulated
in FJOA.
The extracellular matrix is a non-cellular meshwork
of extracellular molecules that provides a three-dimensional
physical and mechanical support for surrounding cells (28). The extracellular matrix is highly
dynamic and is continuously remodeled by matrix-degrading enzymes
under various physiological and pathological conditions, including
cell migration, cell differentiation, cell growth, wound healing
and fibrosis (28-31).
The remodeling of the extracellular matrix is principally mediated
by MMPs (32,33). Changes in the composition and
structure of the extracellular matrix, such as loss of
proteoglycans, mineralization of the extracellular matrix and
accumulation of malformed extracellular matrix are hallmarks of
osteoarthritis (8,34). The present study showed that genes
coding for many MMPs were downregulated in FJOA. Consistent with
sequencing data, MMP7 was significantly downregulated in FJOA
compared with controls. Since MMP12 was the most downregulated gene
in FJOA, the biological functions of MMP12 can be investigated in
future studies. In addition, TIMP3 and TIMP4 were found to be
upregulated in FJOA. Elevated TIMPs further inactivate MMPs and
influence the dynamics of the extracellular matrix (35).
In contrast with the present observations, many
members of the MMP family were upregulated and/or activated in many
other forms of osteoarthritis (36-38).
The contradictory results implied that the molecular changes in
joint tissues in osteoarthritis might be tissue-specific. Kim et
al (39) reported that MMP1,
MMP3 and MMP13 were upregulated in human degenerative facet joint
tissues, however, MMP3 expression was downregulated in the present
study. Aside from the differences in the collection of human facet
joint tissues and the classification of FJOA, another difference
was that the current study focused on the Chinese population while
the study performed by Kim et al performed studies on
tissues collected from an American population (39). Meta analyses could be conducted to
investigate the association between genetic changes in FJOA and
different populations. Further studies will be conducted to examine
the protein expression and localization of these MMPs in FJOA.
Meanwhile, the expression levels of another group of
metalloproteinases, ADAM proteins, were upregulated in FJOA.
Similar to MMPs, ADAM proteins are also key modulators of the
extracellular matrix (40,41). Upregulated ADAM expression might
execute opposite functions to downregulated MMPs in the breakdown
and remodeling of the extracellular matrix by shedding
transmembrane ligands of the epidermal growth factor (EGF) receptor
and thus stimulate EGF receptor signaling. ADAM12 exhibits elevated
expression levels in synovitis and post-inflammatory fibrosis of
the synovial membrane in patients with early radiographic
osteoarthritis (42). Polymorphisms
of ADAM12, especially ADAM12 polymorphism rs3740199, are highly
associated with knee osteoarthritis (43,44).
These previous studies indicated the involvement of ADAM12 in
osteoarthritis and proposed the potential roles of ADAM in FJOA.
Therefore, changes in ADAM expression and corresponding alterations
in the extracellular matrix in FJOA also need to be further
examined.
Overall, transcriptome sequencing and bioinformatics
analysis determined the genetic changes of MMP-related genes in
FJOA and demonstrated the implications of the extracellular matrix
and MMPs in FJOA from a molecular aspect. The present results
revealed the roles of the extracellular matrix and MMPs in FJOA,
contributing to the understanding of the underlying mechanisms
behind FJOA. This might contribute to the investigation of novel
therapeutic targets for FJOA treatment.
Acknowledgements
Not applicable.
Funding
This work was supported by supported by the Jiangsu
Provincial Scientific Research Projects of ‘333 Project’ (grant no.
BRA2017204) and the Jiangsu Provincial Youth Medical Talents
Subsidy Project (grant no. QNRC2016413).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC conceived and designed the experiments. CC and GX
performed the experiments. CC, GX, ZC and YS analyzed the data. CC
and ZC wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by the
Human Ethics Committee of the Second Affiliated Hospital of Nantong
University. All participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schroeppel JP, Crist JD, Anderson HC and
Wang J: Molecular regulation of articular chondrocyte function and
its significance in osteoarthritis. Histol Histopathol. 26:377–394.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
García-Carbajal ZY, Garciadiego-Cázares D,
Parra-Cid C, Aguilar-Gaytan R, Velasquillo C, Ibarra C and Castro
Carmona JS: Cartilage tissue engineering: The role of extracellular
matrix (ECM) and novel strategies. 2013 DOI: 10.5772/55917.
|
|
3
|
Sophia Fox AJ, Bedi A and Rodeo SA: The
basic science of articular cartilage: Structure, composition, and
function. Sports Health. 1:461–468. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Newman AP: Articular cartilage repair. Am
J Sports Med. 26:309–324. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Martel-Pelletier J, Boileau C, Pelletier
JP and Roughley PJ: Cartilage in normal and osteoarthritis
conditions. Best Pract Res Clin Rheumatol. 22:351–384.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim SH, Turnbull J and Guimond S:
Extracellular matrix and cell signalling: The dynamic cooperation
of integrin, proteoglycan and growth factor receptor. J Endocrinol.
209:139–1351. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maldonado M and Nam J: The role of changes
in extracellular matrix of cartilage in the presence of
inflammation on the pathology of osteoarthritis. Biomed Res Int.
2013(284873)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Prasadam I, Farnaghi S, Feng JQ, Gu W,
Perry S, Crawford R and Xiao Y: Impact of extracellular matrix
derived from osteoarthritis subchondral bone osteoblasts on
osteocytes: Role of integrinbeta1 and focal adhesion kinase
signaling cues. Arthritis Res Ther. 15(R150)2013.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Kapoor C, Vaidya S, Wadhwan V; Hitesh,
Kaur G and Pathak A: Seesaw of matrix metalloproteinases (MMPs). J
Cancer Res Ther. 12:28–35. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Steffensen B, Häkkinen L and Larjava H:
Proteolytic events of wound-healing-coordinated interactions among
matrix metalloproteinases (MMPs), integrins, and extracellular
matrix molecules. Crit Rev Oral Biol Med. 12:373–398.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang J, Zhang J, Wu C, Guo X, Chen C, Bao
G, Sun Y, Chen J, Xue P, Xu G and Cui Z: Up-regulation of TRAF2
inhibits chondrocytes apoptosis in lumbar facet joint
osteoarthritis. Biochem Biophys Res Commun. 503:1659–1665.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gellhorn AC, Katz JN and Suri P:
Osteoarthritis of the spine: The facet joints. Nat Rev Rheumatol.
9:216–224. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nakamura A, Rampersaud YR, Sharma A, Lewis
SJ, Wu B, Datta P, Sundararajan K, Endisha H, Rossomacha E, Rockel
JS, et al: Identification of microRNA-181a-5p and microRNA-4454 as
mediators of facet cartilage degeneration. JCI Insight.
1(e86820)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen C, Bao GF, Xu G, Sun Y and Cui ZM:
Altered Wnt and NF-κB Signaling in Facet Joint Osteoarthritis:
Insights from RNA deep sequencing. Tohoku J Exp Med. 245:69–77.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kettler A and Wilke HJ: Review of existing
grading systems for cervical or lumbar disc and facet joint
degeneration. Eur Spine J. 15:705–718. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim D, Pertea G, Trapnell C, Pimentel H,
Kelley R and Salzberg SL: TopHat2: Accurate alignment of
transcriptomes in the presence of insertions, deletions and gene
fusions. Genome Biol. 14(R36)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mortazavi A, Williams BA, McCue K,
Schaeffer L and Wold B: Mapping and quantifying mammalian
transcriptomes by RNA-Seq. Nat Methods. 5:621–628. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC and Lempicki RA:
DAVID bioinformatics resources: Expanded annotation database and
novel algorithms to better extract biology from large gene lists.
Nucleic Acids Res. 35:W169–W175. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reich M, Liefeld T, Gould J, Lerner J,
Tamayo P and Mesirov JP: GenePattern 2.0. Nat Genet. 38:500–501.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Verma RP and Hansch C: Matrix
metalloproteinases (MMPs): Chemical-biological functions and
(Q)SARs. Bioorg Med Chem. 15:2223–2268. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wasilewska A, Taranta-Janusz K,
Zoch-Zwierz W, Rybi-Szumińska A and Kolodziejczyk Z: Role of matrix
metalloproteinases (MMP) and their tissue inhibitors (TIMP) in
nephrology. Przegl Lek. 66:485–490. 2009.(In Polish). PubMed/NCBI
|
|
27
|
White JM: ADAMs: Modulators of Cell-cell
and cell-matrix interactions. Curr Opin Cell Biol. 15:598–606.
2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Theocharis AD, Skandalis SS, Gialeli C and
Karamanos NK: Extracellular matrix structure. Adv Drug Deliv Rev.
97:4–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Rozario T and DeSimone DW: The
extracellular matrix in development and morphogenesis: A dynamic
view. Dev Biol. 341:126–140. 2010.
|
|
31
|
Engler AJ, Sen S, Sweeney HL and Discher
DE: Matrix elasticity directs stem cell lineage specification.
Cell. 126:677–689. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rohani MG and Parks WC: Matrix remodeling
by MMPs during wound repair. Matrix Biol. 44-46:113–121.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Apte SS and Parks WC: Metalloproteinases:
A parade of functions in matrix biology and an outlook for the
future. Matrix Biol. 44-46:1–6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bertrand J, Cromme C, Umlauf D, Frank S
and Pap T: Molecular mechanisms of cartilage remodelling in
osteoarthritis. Int J Biochem Cell Biol. 42:1594–1601.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Arpino V, Brock M and Gill SE: The role of
TIMPs in regulation of extracellular matrix proteolysis. Matrix
Biol. 44-46:247–254. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zeng GQ, Chen AB, Li W, Song JH and Gao
CY: High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis.
Genet Mol Res. 14:14811–14822. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lipari L and Gerbino A: Expression of
gelatinases (MMP-2, MMP-9) in human articular cartilage. Int J
Immunopathol Pharmacol. 26:817–823. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lim NH, Meinjohanns E, Meldal M,
Bou-Gharios G and Nagase H: In vivo imaging of MMP-13 activity in
the murine destabilised medial meniscus surgical model of
osteoarthritis. Osteoarthritis Cartilage. 22:862–868.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim JS, Ali MH, Wydra F, Li X, Hamilton
JL, An HS, Cs-Szabo G, Andrews S, Moric M, Xiao G, et al:
Characterization of degenerative human facet joints and facet joint
capsular tissues. Osteoarthritis Cartilage. 23:2242–2251.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lu P, Takai K, Weaver VM and Werb Z:
Extracellular matrix degradation and remodeling in development and
disease. Cold Spring Harb Perspect Biol. 3(pii:
a005058)2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wolfsberg TG, Straight PD, Gerena RL,
Huovila AP, Primakoff P, Myles DG and White JM: ADAM, a widely
distributed and developmentally regulated gene family encoding
membrane proteins with a disintegrin and metalloprotease domain.
Dev Biol. 169:378–383. 1995.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kerna I, Kisand K, Suutre S, Murde M and
Tamm A, Kumm J and Tamm A: The ADAM12 is upregulated in synovitis
and postinflammatory fibrosis of the synovial membrane in patients
with early radiographic osteoarthritis. Joint Bone Spine. 81:51–16.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lv ZT, Liang S, Huang XJ, Cheng P, Zhu WT
and Chen AM: Association between ADAM12 Single-nucleotide
polymorphisms and knee osteoarthritis: A Meta-analysis. Biomed Res
Int. 2017(5398181)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Poonpet T, Tammachote R, Tammachote N,
Kanitnate S and Honsawek S: Association between ADAM12 polymorphism
and knee osteoarthritis in Thai population. Knee. 23:357–361.
2016.PubMed/NCBI View Article : Google Scholar
|