Introduction
Myositis ossificans (MO) is a disease featuring
heterotropic bone formation within a muscle or any other type of
soft tissue (tendons, ligament, fascia and connective tissue). MO
is divided into two categories as follows: MO progressiva (MOP) or
fibrodysplasia ossificans progressive (FOP) and MO traumatica (MOT)
(1,2).
MO encountered in the masticatory muscles is not
frequently reported and its most common symptom is trismus
(3,4). A case of MO in the masticatory muscles
was described in the present study that was performed over three
generations.
Case report
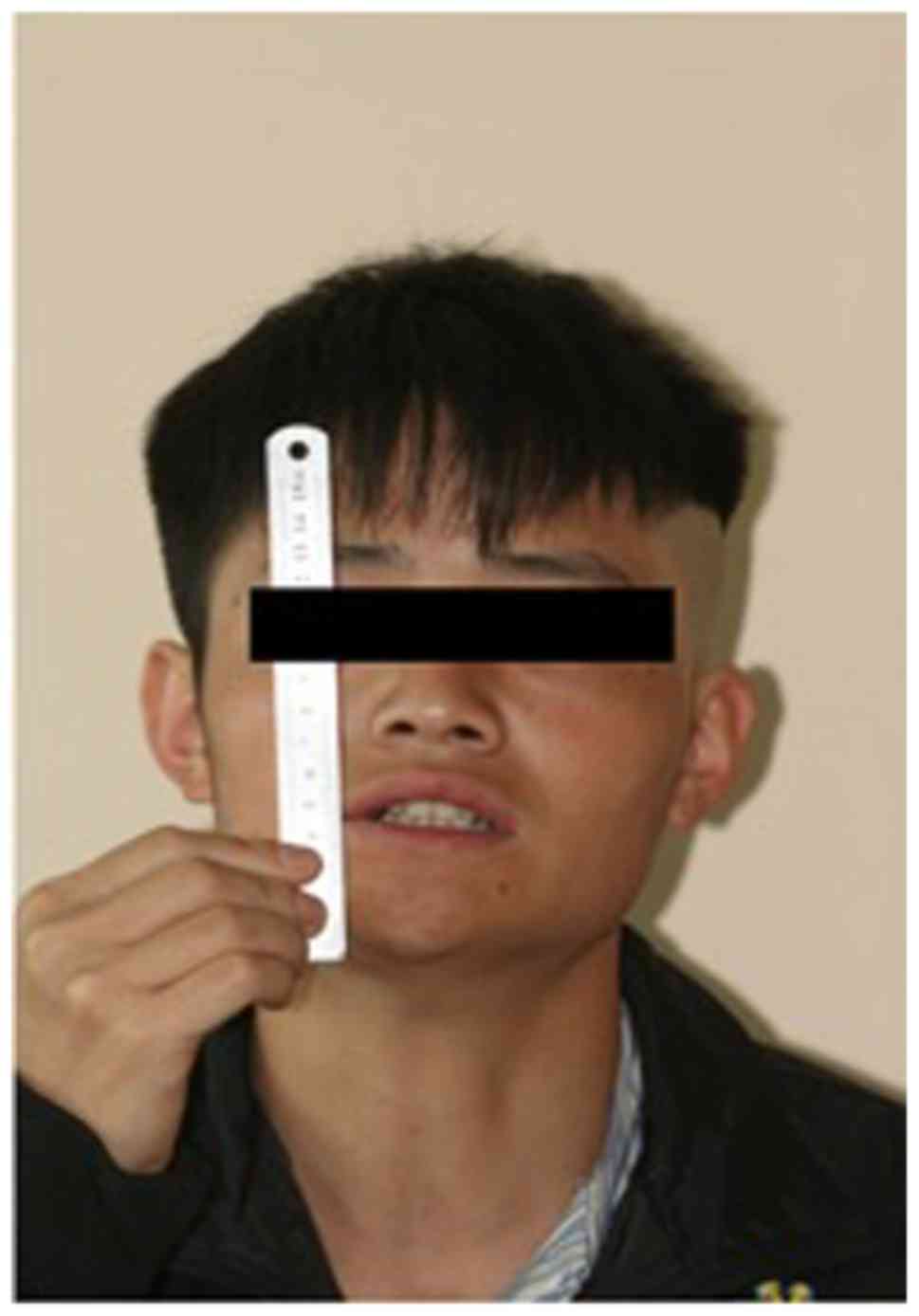
A 22-year-old male was admitted to the Department of
Oral Surgery, Ninth People's Hospital, Shanghai Jiao Tong
University School of Medicine (Shanghai, China) in March 2013 with
an inability to open his mouth which had been developing gradually
for >3 years. The patient was previously diagnosed with
pericoronitis of the lower left wisdom tooth. Following treatment
for this condition, the patient noted a progressive decrease in the
ability to open his mouth, resulting in almost complete trismus. In
December 2011, coronoidectomy on the left side and extraction of
the left lower and upper molars were performed. The patient's
active maximal incisal opening (MIO) gradually decreased
post-operatively and trismus reappeared following 1 month from the
date of the surgery (Fig. 1).
Physical examination revealed a well-nourished male
without apparent developmental abnormalities. The MIO at
presentation was 0 mm. The patient was unable to protrude his mouth
or produce any excursive movements. Palpation of the masseter and
temporalis muscles did not reveal any abnormality. Intraoral
examination indicated normal mucosa with no evidence of submucous
fibrosis.
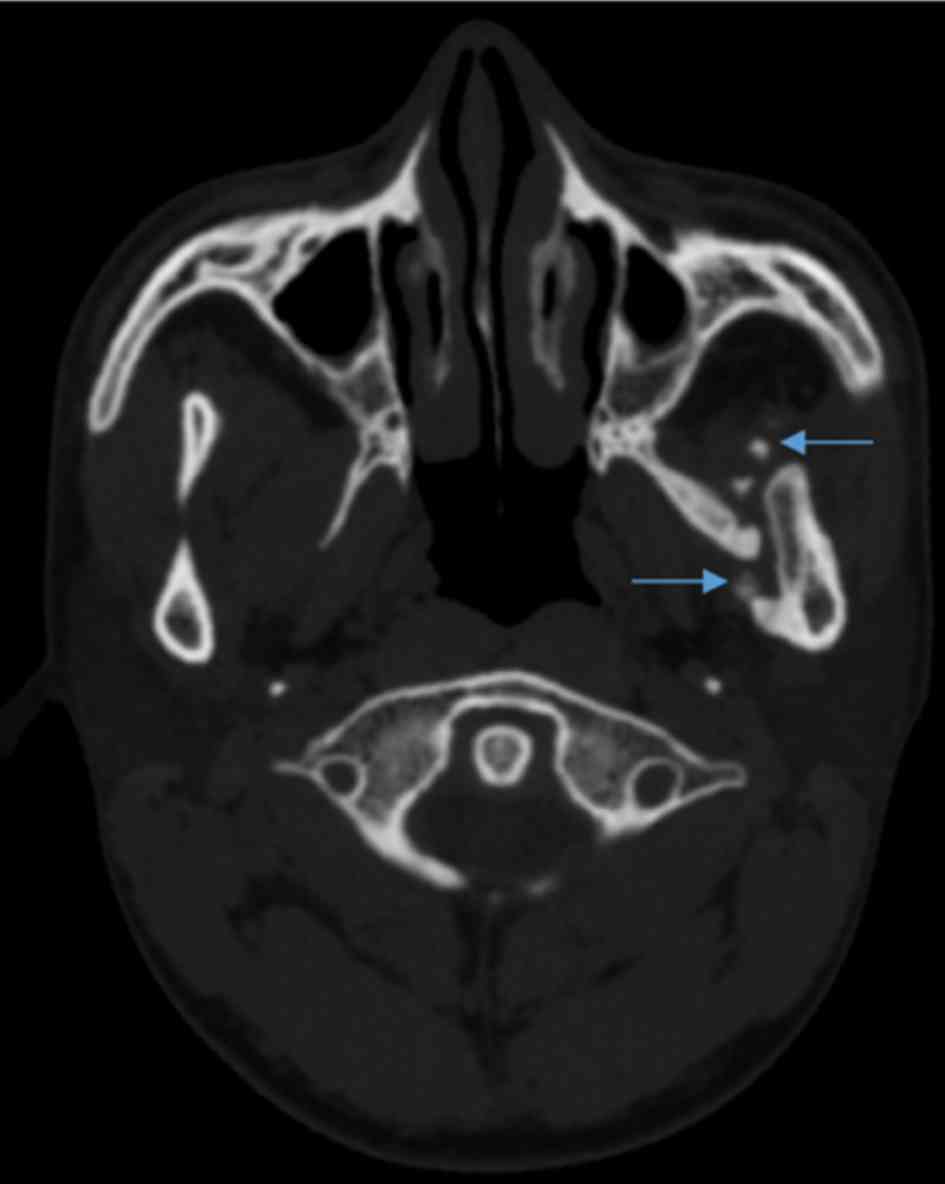
CT scans indicated a region of high attenuation
within the left lateral pterygoid muscle, extending from the left
lateral pterygoid plate to the medial surface of the left mandible
(Fig. 2). Laboratory test results,
including calcium, phosphate and parathyroid hormone, were within
normal limits.
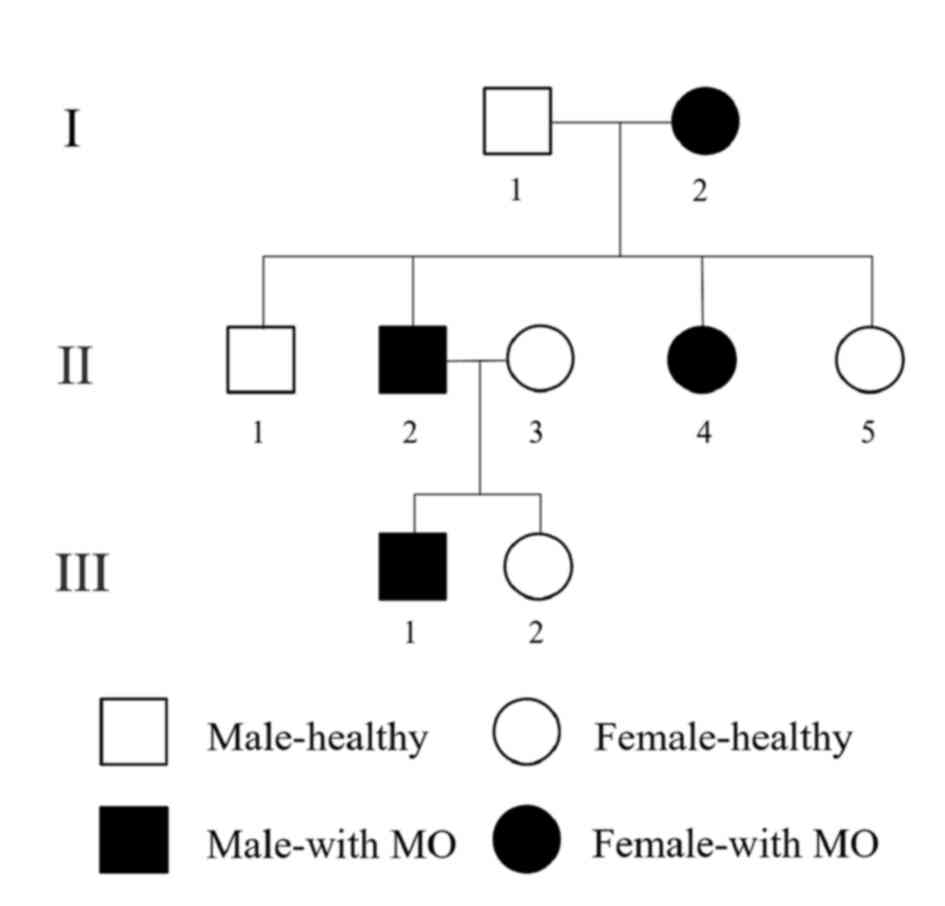
Following review of the patient's family history, a
pedigree was established in which the possible occurrence of the
disease was depicted over three generations (Fig. 3). Apart from the proband, three other
members of the family had been similarly affected. The involvement
was exclusively localized to the maxillofacial region, while hands
and feet were normal. However, imaging characteristics, treatment
options and prognosis were different among the patients. The
patient's grandmother and aunt noted limited mouth opening without
apparent trauma or infection and the sign spontaneously resolved
over 1 year. The patient's sister has never suffered from limited
mouth opening. In comparison, the situation was different in the
patient's father: First, the wisdom tooth extraction was performed
due to symptoms associated with MIO occurring, which then further
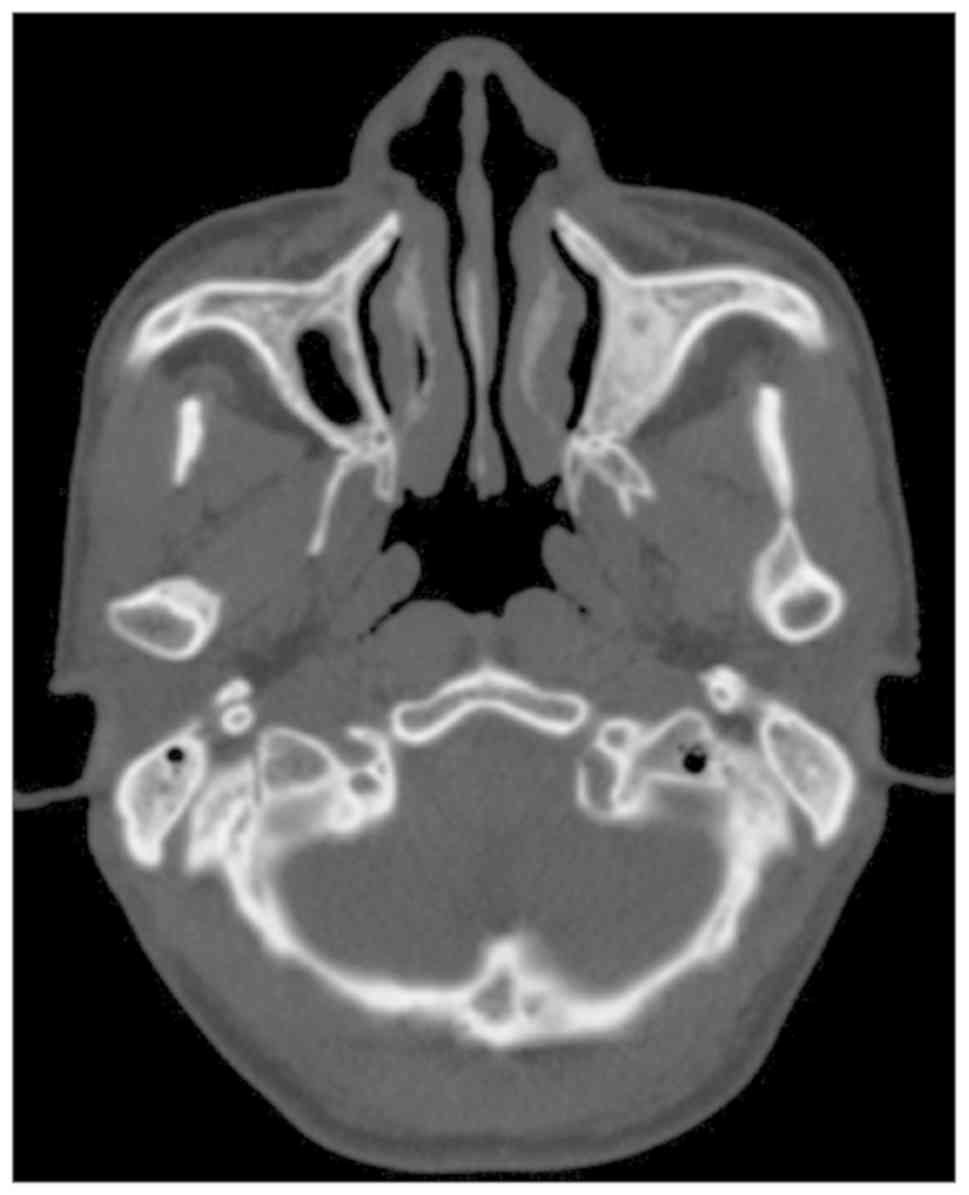
worsened. Furthermore, the lesion did not regress and it became a
functional handicap-trismus. In addition, no calcifications were
observed in the masticatory muscles as determined by CT scans
(Fig. 4) and histology. The MIO of
the proband's father was 2.5 cm following coronoidectomy and
masticatory muscle stripping.
The ossification of the lateral pterygoid muscle of
the proband examined in the present study led to the diagnosis of
MO. However, the classification of the patient's MO type (MOP or
MOT) remained undetermined. Mutational analysis is usually applied
to confirm the diagnosis of MOP. It may aid the early diagnosis of
this disease and is valuable for cases of suspected variants. The
proband, the proband's father and the proband's sister were willing
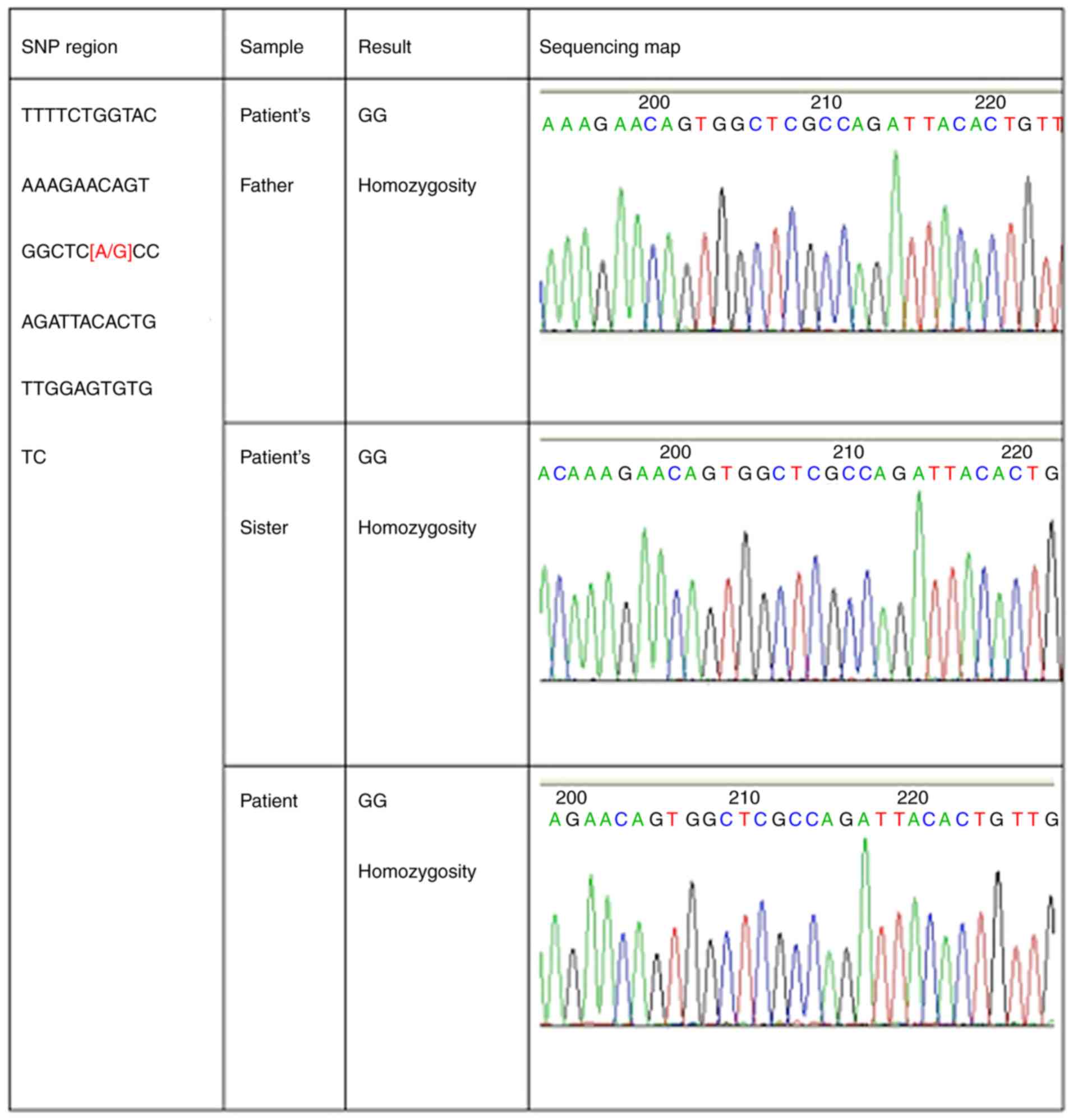
to participate and genomic DNA was examined from their blood.
Mutations in the activin A receptor type 1 (ACVR1) gene may be
screened by PCR amplification of 12 exons containing protein-coding
sequences. The results indicated a mutation but it was after the
stop-codon in the non-coding region (Fig. 5). However, the disease was finally
diagnosed as MOP due to the patients' medical and family history,
as well as clinical, radiological and pathological results.
During surgery of the proband in 2013, the calcified
lesion was excised and the masticatory muscle was removed. The
excised site was filled with a buccal fat pad. Post-operative
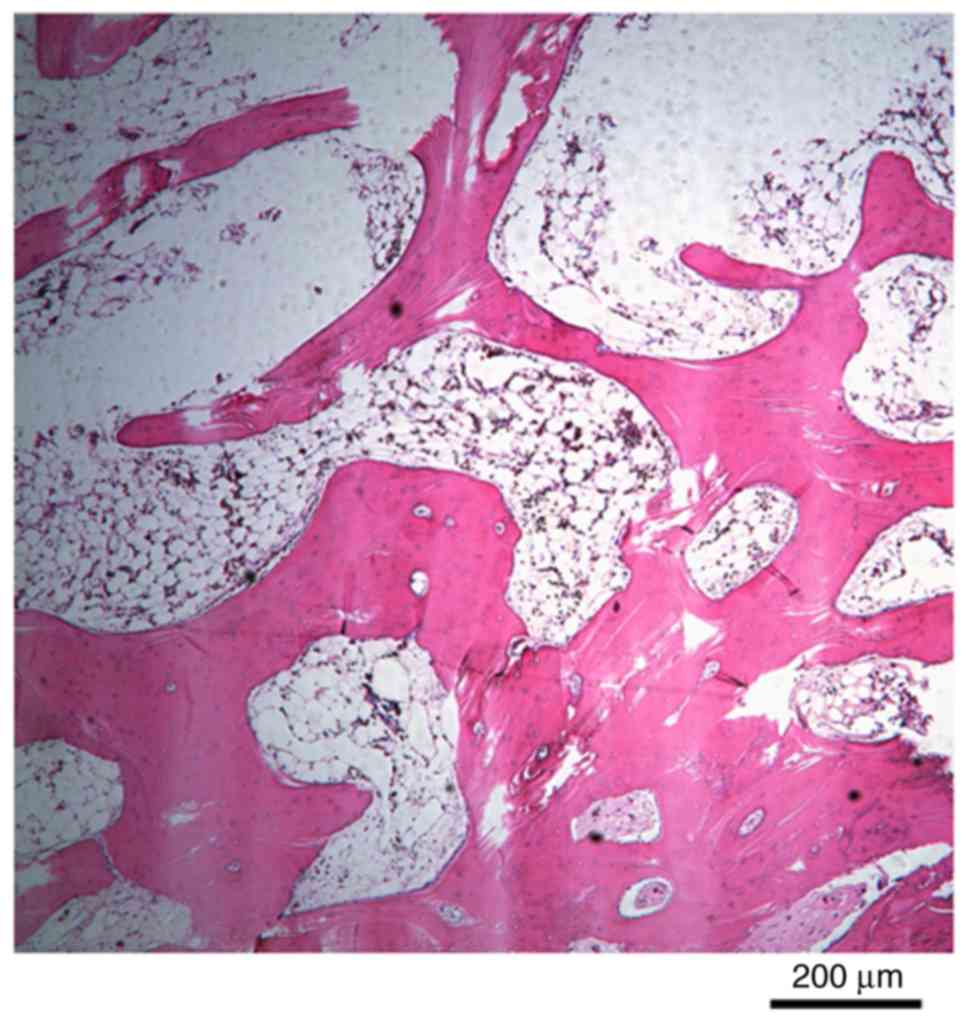
pathology indicated that bone tissue was formed and that the
trabeculae of the bone tissues contained osteocytes and numerous
reversal lines (Fig. 6). CT
examination of the pterygoid lamina to the internal ramus indicated
no calcification and band formation at 2 months after the surgery.
At 2 years after surgery, the patient's MIO is maintained at 1.5 cm
(Table I).
 | Table IDetails of the proband and the
proband's father. |
Table I
Details of the proband and the
proband's father.
| | | | | | | MIO | |
|---|
| Patient | Age
(years)/gender | Lesion location | Chief complaint | History of
trauma | Treatment | Pre-operative
(mm) | Intraoperative
(mm) | Post-operative
(mm) | Outcome |
|---|
| Proband | 22/M | Lateral
pterygoid | Trismus | None | i) Coronoidectomy +
extraction of third molars (other hospital) | | | | |
| | | | | | ii) Excision +
coronoidecomy + BFP flap | 0 | 45 | 15 mm (2 years) | No recurrence |
| Proband's father | 49/M | Masseter | Swelling,
trismus | Tooth extraction | Excision +
coronoidectomy | 0 | 45 | 25 mm (2 years) | No recurrence |
Discussion
MO of masticatory muscles is not frequently reported
in the literature with the most common symptom of being trismus.
Boffano et al (5) reviewed 42
cases of MO of the masticatory muscles, among which the masseter
was the most frequently involved muscle (25 patients, 47%),
followed by the medial pterygoid muscle (14 patients, 26%), the
lateral pterygoid muscle (9 patients, 17%) and the temporalis
muscle (5 patients, 10%).
CT is considered the best way to characterize the
calcification zone pattern and may be used prior to determination
of the characteristic calcification mode on radiography (6). Early lesions of the axial skeleton have
been reported to appear as amorphous calcifications within the soft
tissue. The accumulation of mature lesions may appear
well-circumscribed with a ring of calcification surrounding a
relatively radiolucent central portion (7). Therefore, long-standing lesions may
appear with diffuse calcification (7).
The histological course of MO progresses from an
immature, highly cellular fibroblastic lesion to a mature mass with
a peripheral lamellar bone. The hallmark of MO is the manifestation
of the zonal architecture with peripheral ossification and the
presence of a central cellular region. The outer zone is composed
of a mature lamellar bone with active osteoclasts, whereas the
intermediate zone occasionally includes osteoid tissue, cartilage
or woven bone formation and active osteoblasts. The central zone is
composed of loose fibrovascular tissue resembling granulation
tissue, containing spindle cells and prominent giant mesenchymal
cells (1,8).
In 1998, Debeney-Bruyerre et al (9) reported similar cases to those of the
present study. The proband's mouth opening was limited and devoid
of congenital malformations. A CT scan demonstrated the formation
of bilateral masticatory muscle calcification and similar symptoms
were noted in the proband's pedigree for five consecutive
generations. The diagnosis of this disease was FOP. Unfortunately,
no subsequent genetic linkage studies were performed, since the
patient and his relatives did not cooperate.
In 2006, Shore et al (10) successfully mapped FOP to chromosome
2q23-24. Typical patients with FOP (congenital double-knee
malformation and progressive heterotopic ossification) developed
this disease due to mutations in the ACVR1 gene, which affected the
bone morphogenetic protein (BMP) type I receptor. When the 617th
guanine is mutated into adenine (617G→A), arginine becomes
histidine during protein translation, exposing the original
phosphorylation site of this protein. BMP binds to the mutated
residue, which in turn activates the intracellular signaling
pathway and promotes the formation of heterotopic bone.
Subsequently, Kaplan et al (11) performed a DNA sequence analysis of
112 patients with FOP and demonstrated that in addition to the
classical frequent mutation sites reported previously, new mutation
sites of ACVR1 were present in a limited number of atypical
patients with FOP.
A combined analysis of the clinical characteristics
of this proband with previous data from cases reported in the
literature was performed in order to confirm the diagnosis.
Mutational analysis of the ACVR1 gene was performed. Pathogenic
gene analysis was performed using the samples from the patient's
father and the patient's sister. Written informed consent was
provided by all participants of the study.
ACVR1 is divided into four parts, namely the ligand
region, the transmembrane region, the glycine-serine enrichment
region and the kinase region. Initially, blood samples from the
patient, the patient's father and the patient's sister were
analyzed for frequent mutation sites of the ACVR1 gene. The test
results indicated no mutation in the 617th guanine of these three
subjects. Subsequently, the entire exon of the ACVR1 gene was
expanded and a hybrid region including thymine and cytosine was
identified in exon 12; this mutation was located in the non-coding
region following the stop codon. Whether the mutation has an impact
on the disease reported in the current study remains elusive and
may be worthy of investigation in the future. Therefore, mutations
in the ACVR1 gene were not detected in the pedigree analyzed in the
present study. Whole-genome sequencing, including coding and
non-coding regions, will be performed in future studies.
The treatment of MO is controversial and
challenging. The patient of the present case report underwent two
surgeries as follows: Coronoidectomy on the left side in 2011 and
excision of calcified lesion and buccal fat pad reconstruction in
2013. Complete excision of the ossified mass appears to be a
generally accepted treatment for this disease (12). Following removal of the ossified
mass, inter-positional fat graft is used as a means of
reconstruction by a limited number of surgeons (13). Active physiotherapy may be initiated
in the immediate post-operative period to sustain the mouth opening
and prevent scar formation (14).
Non-surgical treatments include non-steroidal anti-inflammatory
drugs, magnesium, bisphosphonates and warfarin. Furthermore,
low-dose radiation therapy, immobilization, ice, elevation,
ultrasound, cold laser and iontophoresis may be applied (15).
Acknowledgements
Not applicable.
Funding
No funding was received.
Data availability
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QJ and CL wrote the manuscript. WQ and CY were
responsible for patient diagnosis and treatment. CY, MC and YQ
performed the surgery. MC revised the manuscript. CL has made
substantial contributions to analysis and interpretation of medical
records, including genomic DNA examination; and has drafted the
manuscript for important intellectual content, including case
report, discussion and literature review. QJ has made substantial
contributions to conception and design, and acquisition of medical
records.
Ethics approval and consent to
participate
Written informed consent was provided by all
participants of the study.
Patient consent for publication
The patients provided their consent for the
publication of the data and images in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walczak BE, Johnson CN and Howe BM:
Myositis ossificans. J Am Acad Orthop Surg. 23:612–622.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Smith R: Myositis ossificans progressiva:
A review of current problems. Semin Arthritis Rheum. 4:369–380.
1975.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Godhi SS, Singh A, Kukreja P and Singh V:
Myositis ossificans circumscripta involving bilateral masticatory
muscles. J Craniofac Surg. 22:e11–e13. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Parkash H and Goyal M: Myositis ossificans
of medial pterygoid muscle. A cause for temporomandibular joint
ankylosis. Oral Surg Oral Med Oral Pathol. 73:27–28.
1992.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boffano P, Zavattero E, Bosco G and
Berrone S: Myositis ossificans of the left medial pterygoid muscle:
Case report and review of the literature of myositis ossificans of
masticatory muscles. Craniomaxillofac Trauma Reconstr. 7:43–50.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shehab D, Elgazzar AH and Collier BD:
Heterotopic ossification. J Nucl Med. 43:346–353. 2002.PubMed/NCBI
|
|
7
|
Kim DD, Lazow SK, Har-El G and Berger JR:
Myositis ossificans traumatica of masticatory musculature: A case
report and literature review. J Oral Maxillofac Surg. 60:1072–1076.
2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Saka B, Stropahl G and Gundlach KK:
Traumatic myositis ossificans (ossifying pseudotumor) of temporal
muscle. Int J Oral Maxillofac Surg. 31:110–111. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Debeney-Bruyerre C, Chikhani L, Lockhart
R, Favre-Dauvergne E, Weschler B, Bertrand JC and Guilbert F:
Myositis ossificans progressiva: Five generations where the disease
was exclusively limited to the maxillofacial region. A case report.
Int J Oral Maxillofac Surg. 27:299–302. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shore EM, Xu M, Feldman GJ, Fenstermacher
DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, et
al: A recurrent mutation in the BMP type I receptor ACVR1 causes
inherited and sporadic fibrodysplasia ossificans progressiva. Nat
Genet. 38:525–527. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Kaplan FS, Xu M, Seemann P, Connor JM,
Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ,
Gillessen-Kaesbach G, et al: Classic and atypical fibrodysplasia
ossificans progressiva (FOP) phenotypes are caused by mutations in
the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum
Mutat. 30:379–390. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hanisch M, Hanisch L, Fröhlich LF,
Werkmeister R, Bohner L and Kleinheinz J: Myositis ossificans
traumatica of the masticatory muscles: Etiology, diagnosis and
treatment. Head Face Med. 14(23)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rattan V, Rai S and Vaiphei K: Use of
buccal pad of fat to prevent heterotopic bone formation after
excision of myositis ossificans of medial pterygoid muscle. J Oral
Maxillofac Surg. 66:1518–1522. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kayal L, Manoharan GVMG and Joshi B:
Myositis ossificans of the masseter muscle. Saudi J Med Med Sci.
6:119–120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Thangavelu A, Vaidhyanathan A and Narendar
R: Myositis ossificans traumatica of the medial pterygoid. Int J
Oral Maxillofac Surg. 40:545–549. 2011.PubMed/NCBI View Article : Google Scholar
|