Introduction
Acute pancreatitis (AP), which manifests as
abdominal pain and elevated pancreatic enzymes, occurs due to
premature activation of pancreatic enzymes in the pancreas
(1). Hypertriglyceridemia (HTG),
gallstones and alcohol consumption have been associated with the
pathogenesis of AP (2). Changes in
lifestyle and diet have contributed to the increased incidence of
HTG-induced AP (HTGAP) in recent decades (3). A previous study published in 2016
revealed that HTGAP accounted for 2-5% of AP cases in the US
(2). The morbidity rate of severe
pancreatitis was estimated to be 12-38% among patients with HTGAP
(4). HTGAP poses a serious threat to
human health, as it features a young age at onset, serious
complications and poor prognosis (5). HTGAP is caused by the release of
excessive free fatty acids and lysolecithin from lipoprotein
substrates in pancreatic cells, which damage the acinar cells and
the microvascular membranes when albumin exceeds the carrying
capacity of the pancreas. This results in systemic inflammatory
response syndrome (SIRS) (6),
pancreatic cell damage (7) and
possibly multiple organ dysfunction syndromes (8). A previous study demonstrated that
elevated serum triglyceride (TG) is associated with the severity of
pancreatitis, and that the progression of HTGAP may be prevented if
serum TG levels drop to <5.56 mmol/l (9). Therefore, controlling the serum TG
levels in patients with pancreatitis may mitigate the progression
of the disease.
In addition to fasting, analgesia, enteral
nutrition, fluid replacement and antibiotics, studies have reported
that intensive insulin therapy (IIT) (10,11) and
plasma exchange (PE) (9,12,13) may
be effective methods for reducing serum TG levels (11). Insulin has an important role in
enhancing lipoprotein lipase (LPL) activity and lowering serum TG
levels. However, other studies revealed that the clinical
applications of IIT were limited by neurological complications
caused by severe hypoglycemia and did not significantly improve the
prognosis of critically ill patients (11,14,15). At
present, non-intensive insulin therapy (NIIT) is widely used as a
therapeutic strategy for patients with HTGAP in China, and is able
to reduce the risk of severe hypoglycemia and associated
complications. PE is a procedure that removes plasma from the blood
and replaces it with new plasma. PE rapidly removes TG and
inflammatory mediators from the blood, and is widely used in the
treatment of HTGAP. However, previous studies have revealed that PE
is associated with complications, including allergy, infection,
unstable circulation and abnormal blood coagulation. In addition,
PE has a relatively high treatment cost and is highly dependent on
blood products (9,16). To the best of our knowledge, no
previous study has systematically compared IIT, PE and NIIT.
Therefore, the aim of the present study was to
compare the efficacy of the aforementioned TG-lowering therapies to
improve the prognosis of patients with HTGAP, as well as their
associated complications.
Patients and methods
Patients
The present study retrospectively screened 132
patients with HTGAP admitted to the Departments of Emergency,
Gastroenterology and International Medicine and the Medical
Intensive Care Unit of Peking Union Medical College Hospital
(PUMCH; Beijing, China) and the Department of Emergency of The
Second Hospital of Hebei Medical University (Shijiazhuang, China)
between January 2013 and August 2018. The inclusion criteria were
as follows: i) Confirmed diagnosis of HTGAP; ii) serum TG level
≥11.3 mmol/l; iii) no relevant treatment prior to admission; and
iv) age ≥18 years. The exclusion criteria were as follows: i)
Biliary, alcoholic, toxic, immune, idiopathic or chronic
pancreatitis, or pancreatic tumors; ii) chronic diseases, including
malignant tumors, chronic organ failure and nephrotic syndrome;
iii) incomplete clinical data; iv) patients receiving TG-lowering
agents, including heparin and oral TG-lowering drugs; and vi)
insulin allergy. A number of baseline clinical indicators,
including age, sex, serum amylase level and the presence of severe
AP (SAP) or SIRS were noted.
Diagnostic criteria
The diagnostic criteria for patients were based on
the 2012 classification of the International Association of
Pancreatology (IAP) (17), according
to which patients must exhibit two of the following three
characteristics to be diagnosed with AP: i) Abdominal pain
consistent with AP; ii) activity of serum amylase and/or lipase at
least 3 times higher than the upper limit of normal; and iii)
abdominal imaging consistent with changes in AP. HTG was defined as
fasting serum TG >11.3 mmol/l or serum TG levels 5.65-11.3
mmol/l and visible chylomicrons in the blood. Patients were
diagnosed with HTGAP according to the aforementioned criteria,
after excluding biliary diseases, as well as the effects of
alcohol, drugs and other factors.
TG-lowering interventions
Following hospital admission, the patients received
routine treatment, including fasting, pain management,
gastrointestinal decompression, fluid resuscitation, nutritional
support and antibiotics, and were put on a TG-lowering regimen. The
TG-lowering strategies used were as follows: i) For IIT, patients
were treated with a continuous intravenous infusion of 0.1-0.3
U/kg/h insulin and 10% glucose to prevent hypoglycemia. ii) For
NIIT, patients continuously received an intravenous injection of
insulin and 5 or 10% glucose, at a ratio of 1:4 or 1:6. iii) For
PE, a double-lumen venous catheter was inserted into patients
without vascular puncture contraindications, and PE was performed
using a Gambro AK10 dialysis machine with a PF 2000 N fiber plasma
filter. Patients received the first PE procedure within 24 h of
admission, then treated PE every other day. During each treatment,
1.5-2.0 plasma volumes were replaced with 5% albumin solution and
fresh frozen plasma. Citrate anticoagulation was used during the
procedure. The blood glucose levels were maintained at 6.7-11.1
mmol/l in all patients receiving insulin and serum TG levels were
monitored every 4 h.
Outcome
Therapeutic indicators, including the 24-h TG
clearance rate, onset to treatment time (OTT), time required to
reach the target TG levels and the length of stay (LOS), as well as
outcome indicators, including mortality, local complications,
requirement for surgery and therapy-associated complications
(hypokalemia, hypoglycemia, pipeline congestion, shiver, skin rash
and deep vein thrombosis), were monitored. Serum TG levels ≤5.65
mmol/l served as the treatment endpoint in the three groups.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 24.0; IBM Corp.). Continuous data were expressed
as the mean ± standard deviation or median and interquartile range,
and the significance of differences between groups was assessed
using analysis of variance or the Kruskal-Wallis test followed by
Bonferroni's post-hoc test. Categorical data were presented as n or
n (%) and comparisons were performed using the χ2 or
Fisher's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
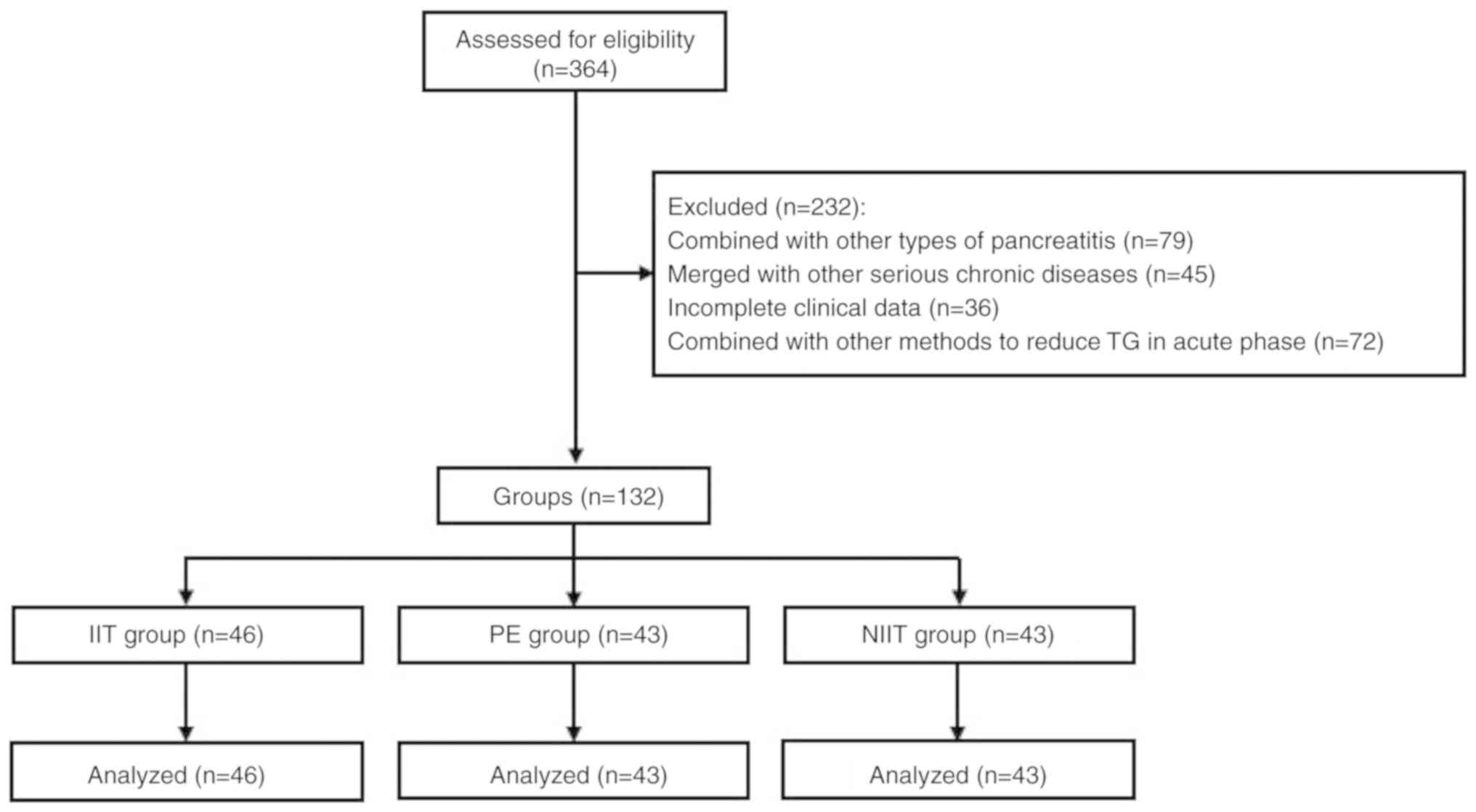
A flow chart illustrating the patient selection
process for the present study is provided in Fig. 1. A total of 132 patients with HTGAP
were included in the present study and divided into following three
groups: i) The IIT group (n=46), ii) the PE group (n=43) and the
NIIT group (n=43). The mean age of patients in the IIT group was
39.00±11.01 years including 30 males and 16 females. In the PE
group, the average age was 35.72±8.95 years, and the number of male
and female patients was 29 and 14, respectively. There were 31 male
and 12 female patients in the NIIT group with the mean age of
37.49±9.66 years. Clinical parameters, including sex, age, serum TG
and amylase levels, SAP and SIRS, were not significantly different
between the three groups (P>0.05; Table I).
 | Table ICharacteristics of patients under
different treatments. |
Table I
Characteristics of patients under
different treatments.
| Variable | PE (n=43) | IIT (n=46) | NIIT (n=43) | χ2/F | P-value |
|---|
| Male gender | 29 (67.44) | 30 (65.22) | 31 (72.09) | 0.50 | 0.779 |
| Age (years) | 35.72±8.95 | 39.00±11.01 | 37.49±9.66 | 1.49 | 0.229 |
| TG (mmol/l) | 23.10
(16.62-47.11) | 28.22
(23.58-38.90) | 26.22
(18.84-40.45) | 0.23 | 0.893 |
| Amylase (U/l) | 569.0
(338.0-1104.0) | 495.50
(209.0-784.0) | 737.0
(292.0-1145.0) | 4.47 | 0.107 |
| SAP | 18 (41.86) | 20 (43.48) | 17 (39.53) | 0.14 | 0.931 |
| SIRS | 33 (76.74) | 36 (78.26) | 32 (62.79) | 0.19 | 0.912 |
Effects of the TG-lowering
therapies
As presented in Table
II, the 24-h TG clearance rate (χ2=7.74, P=0.021),
OTT (χ2=14.50, P<0.001) and the time required to
reach the target TG level (χ2=6.12, P=0.047) were
significantly different among the three groups. The 24-h TG
clearance rate in patients in the NIIT group was significantly
lower than that in the PE group (P<0.05). However, there were no
significant differences between the IIT and NIIT groups or the IIT
and PE groups with this regard (P>0.05). The time required to
reach the target TG level in patients in the NIIT group was longer
than that in the PE group (P<0.05), while there were no such
differences between the IIT and NIIT groups or the IIT and PE
groups (P>0.05). The OTT of the patients in the PE group was
obviously increased compared with that in the IIT and NIIT groups
(P<0.05); however, no statistically significant difference in
the OTT was observed between the IIT and NIIT groups (P>0.05).
After the TG-lowering therapies, the 24-h amylase levels were not
significantly different among the three groups (P>0.05).
 | Table IIComparison of the effects of
TG-lowering therapies between different treatment groups. |
Table II
Comparison of the effects of
TG-lowering therapies between different treatment groups.
| Variable | PE (n=43) | IIT (n=46) | NIIT (n=43) |
χ2/H | P-value |
|---|
| 24-h TG clearance
rate (%) | 0.71
(0.62-0.84) | 0.68
(0.56-0.79) | 0.62
(0.47-0.76)a | 7.74 | 0.021 |
| 24-h amylase levels
(U/l) | 243.0
(130.0-458.0) | 231.50
(86.50-350.75) | 492.0
(208.50-912.50) | 0.93 | 0.628 |
| OTT (h) | 41.0
(28.0-61.00) | 31.0
(25.0-39.0)a | 26.0
(17.0-36.0)a | 14.50 | <0.001 |
| Time required to
reach target TG levels (h) | 44.0
(21.0-68.0) | 49.0
(32.0-120.0) | 72.0
(33.0-120.0)a | 6.12 | 0.047 |
Complications in the treatment
groups
There were no statistically significant differences
in mortality, local complications, requirement for surgery, SIR and
cure rate among the three treatment groups (P>0.05). The
incidence of therapy-associated complications in the PE group
(30.23%) was significantly higher than that in the IIT (2.17%) and
NIIT (4.65%) groups, while there was no overall difference in the
incidence of complications between the IIT and NIIT groups
(P>0.05). The incidence of skin rash in the PE group was higher
than that in the IIT and NIIT groups (P<0.05), while no obvious
difference regarding skin rash was present between the IIT and NIIT
groups (P>0.05). The LOS in the PE group was increased compared
with that in the IIT and NIIT groups (χ2=7.05,
P<0.05); however, there was no significant difference in the LOS
between the IIT and NIIT groups (P>0.05; Table III).
 | Table IIIComparison of clinical outcomes
between different treatment groups. |
Table III
Comparison of clinical outcomes
between different treatment groups.
| Variable | PEa (n=43) | IIT (n=46) | NIIT (n=43) | χ2b | P-value |
|---|
| Mortality | 3 (6.98) | 3 (6.52) | 2 (4.65) | - | 1.0 |
| Local
complications | 20 (46.51) | 19 (41.30) | 13 (30.23) | 2.50 | 0.287 |
| Requirement for
surgery | 4 (9.30) | 6 (13.04) | 7 (16.28) | 0.93 | 0.627 |
| Therapy-associated
complications |
|
None | 30 | 45 | 41 | - | <0.001 |
|
Hypokalemia | 0 | 1 | 0 | - | 0.351 |
|
Hypoglycemia | 0 | 0 | 2 | - | 0.210 |
|
Pipeline
congestion | 1 | 0 | 0 | - | 0.326 |
|
Shiver | 2 | 0 | 0 | - | 0.104 |
|
Skin
rash | 9 | 0 | 0 | - | <0.001 |
|
Deep vein
thrombosis | 1 | 0 | 0 | - | 0.325 |
| LOS (days) | 22.0
(14.0-30.0) | 16.50
(12.00-27.0) | 14.0
(7.0-22.0)c | 7.05 | 0.030 |
| SIRS | 15 (29.40) | 19 (37.30) | 17 (37.30) | 0.41 | 0.815 |
| Cure | 32 (74.42) | 34 (73.91) | 31 (74.42) | 0.07 | 0.967 |
Discussion
AP is one of the most common gastrointestinal
diseases that require hospitalization, with >270,000 cases
reported annually in the US (18).
The clinical characteristics of AP are easily distinguished from
other relatively mild and self-limiting illnesses and may lead to
persistent or multisystem organ failure. Enhanced serum TG levels
are a risk factor for AP and HTG is observed in up to 10% of
patients with AP (7). Compared with
other causes of AP, HTGAP is associated with an increased incidence
of complications (7,19). It has been demonstrated that serum
TG, which is hydrolyzed by pancreatic lipase into toxic free fatty
acids, is associated with the occurrence and development of HTGAP
(9) and serum TG levels in patients
with pancreatitis have been reported to be as high as 21%
(11.3-22.6 mmol/l) (4,20). Therefore, rapid reduction of serum TG
levels during the early onset of the disease and the maintenance of
serum TG at levels <5.65 mmol/l may prevent the progression of
HTGAP (9). In current clinical
practice, insulin and/or PE are commonly used as TG-lowering
therapies for HTGAP (21,22); however, to the best of our knowledge,
there are currently no uniform, standardized and comprehensive
therapeutic guidelines for patients with HTGAP.
LPL is widely expressed in muscle and capillary
endothelial cells in adipose tissue, and serves an important role
in fat metabolism by catalyzing the breakdown of chylomicrons and
low-density lipoproteins into glycerol and free fatty acids
(23). Previous studies have
demonstrated that insulin reduces chylomicron levels in the blood
by enhancing the activity of LPL and inhibits hormone-sensitive
lipase in lipocytes to decrease the breakdown of lipocytes and the
production of TG (11). Furthermore,
insulin promotes the degradation of chylomicrons and decreases the
serum levels of TG, which is beneficial in patients with poorly
controlled diabetes or diabetic ketoacidosis (24-26).
A previous study reported that continuous intravenous infusion of
insulin reduced serum TG levels by 40% within 24 h (27), which is similar to the results
obtained in the present study. While insulin therapy is widely
utilized in early HTGAP due to its ease of use, fewer complications
and cost-effectiveness compared with PE treatment, excessive
insulin administration may lead to neurological complications
caused by severe hypoglycemia and does not significantly improve
the prognosis of critically ill patients (28).
Betteridge et al (29) first described the use of PE for the
treatment of HTGAP. PE reduces serum TG levels, eliminates excess
proteases and supplements protease inhibitors. Several studies have
demonstrated that PE decreases TG serum levels by 70-89% (13,30). The
results obtained in the present study further demonstrated the
efficacy of PE in significantly decreasing TG serum levels. In
addition to effectively removing TG from the plasma of patients
with HTGAP, PE eliminates pro-inflammatory cytokines, including
interleukin-1 and tumor necrosis factor-α (31), and decreases neutrophil extracellular
traps. However, previous studies have revealed that PE therapy is
limited due to the demand for vascular puncture and indwelling
intravenous catheters, and is associated with allergy, infection,
unstable circulation and abnormal blood coagulation in patients
with HTGAP. Furthermore, PE has a relatively high treatment cost
and is highly dependent on blood products (17,32). In
2016, the American Society for Apheresis suggested that HTGAP
should only be regarded as a Class III indication for PE (33).
The present study demonstrated that the OTT of
patients in the IIT and NIIT groups was decreased compared with
that in the PE group. PE treatment requires additional time to
prepare the equipment and materials, perform the vascular puncture
and to measure coagulation function. In the present study, the LOS
in the NIIT group was not significantly longer than that in the PE
group, as NIIT induced fewer complications to shorten the inpatient
time for HTGAP patients. The complications in the IIT group were
similar to those in the NIIT group and significantly less than
those in the PE group. The 24-h TG clearance rate in the PE group
was 71%, which was higher compared with that in the NIIT group.
Furthermore, the incidence of therapy-associated complications in
the PE group was higher compared with that in the other two groups.
As a type of blood purification treatment, PE sifts out the
majority of non-cellular components from the blood. A large-pore
filter rapidly and effectively removes TG and their metabolites
from the plasma of patients with HTGAP and reduces the levels of
pancreatic enzymes. However, repeated PE treatments are not
feasible due to the risk of complications, increased demand for
blood products and treatment cost (17). In the present study, no significant
differences in the OTT, the 24-h TG clearance rate and the
incidence of therapy-associated complications were obtained between
the IIT and NIIT groups.
To the best of our knowledge, the present study was
the first to compare the efficacy of IIT, NIIT and PE in patients
with HTGAP. The results demonstrated that patients in the NIIT and
IIT groups exhibited a similar OTT, 24-h TG clearance rate,
incidence of therapy-associated complications and LOS. Furthermore,
patients in the NIIT and IIT groups had fewer complications
compared with patients in the PE group. The present study had a
retrospective design. A limitation was that only data of patients
from northern China were analyzed, as data of patients from
southern China were not available. As there may be differences in
lipid metabolism between northern and southern populations in China
(34), a randomized controlled trial
is required to further verify these results.
In conclusion, the present study analyzed the
effects of three TG-lowering therapies on the prognosis of patients
with HTGAP. Parameters including the OTT, 24-h TG clearance rate,
incidence of therapy-associated complications and LOS were
compared. Patients who received NIIT and IIT had a similar
prognosis and exhibited fewer complications compared with patients
treated with PE. NIIT and IIT may perform favorably if administered
shortly after symptom onset. To ensure appropriate use of resources
and limit medical costs, NIIT may be used to treat patients with
HTGAP requiring further clinical assessment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the CAMS
Innovation Fund for Medical Sciences (grant no.
2016-I2M-1-003).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JX, XY and SY conceptualized and developed the study
design. DY, XL, KJ, YF and DL aquired, analyzed and interpreted the
data. SY and DL discussed the results and wrote the manuscript. LZ,
XS and JY provided comments, suggested appropriate modifications
and made corrections to the study. SY, JX and XY revised the
manuscript. All authors read and approved the final manuscript.
Ethical approval and consent to
participate
This study was approved by the institutional review
boards of PUMCH (approval no. S-K554) and the Second Hospital of
Hebei Medical University (approval no. 2018-P042).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frossard JL, Steer ML and Pastor CM: Acute
pancreatitis. Lancet. 371:143–152. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Forsmark CE, Vege SS and Wilcox CM: Acute
pancreatitis. N Engl J Med. 375:1972–1981. 2016. View Article : Google Scholar
|
|
3
|
Kitagawa S and Sawai K:
Hypertriglyceridemia-induced acute pancreatitis with normal
pancreatic enzymes. Am J Med. 131:e299–e300. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Berglund L, Brunzell JD, Goldberg AC,
Goldberg IJ, Sacks F, Murad MH and Stalenhoef AF: Endocrine
society: Evaluation and treatment of hypertriglyceridemia: An
endocrine society clinical practice guideline. J Clin Endocrinol
Metab. 97:2969–2989. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Adiamah A, Psaltis E, Crook M and Lobo DN:
A systematic review of the epidemiology, pathophysiology and
current management of hyperlipidaemic pancreatitis. Clin Nutr.
37:1810–1822. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang CL, Liu J, Lu Y, Fan J, Wang X, Liu
J, Zhang W and Zeng Y: Clinical features and treatment of
hypertriglyceridemia-induced acute pancreatitis during pregnancy: A
retrospective study. J Clin Apher. 31:571–578. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsuang W, Navaneethan U, Ruiz L, Palascak
JB and Gelrud A: Hypertriglyceridemic pancreatitis: Presentation
and management. Am J Gastroenterol. 104:984–991. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li X, Ke L, Dong J, Ye B, Meng L, Mao W,
Yang Q, Li W and Li J: Significantly different clinical features
between hypertriglyceridemia and biliary acute pancreatitis: A
retrospective study of 730 patients from a tertiary center. BMC
Gastroenterol. 18(89)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kadikoylu G, Yukselen V, Yavasoglu I,
Coşkun A, Karaoglu AO and Bolaman Z: Emergent therapy with
therapeutic plasma exchange in acute recurrent pancreatitis due to
severe hypertriglyceridemia. Transfus Apher Sci. 43:285–289.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hamza E, Hakim KA, Bousselmi K, Alromaihi
D and Sharif O: Effectiveness of intensive insulin therapy in the
management of acute necrotizing pancreatitis induced by very severe
hypertriglyceridemia. Bahrain Med Bull. 39:62–65. 2017. View Article : Google Scholar
|
|
11
|
van den Berghe G, Wouters P, Weekers F,
Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P,
Lauwers P and Bouillon R: Intensive insulin therapy in critically
ill patients. N Engl J Med. 345:1359–1367. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wassay SAM, Dar FJ, Saleh AK and Mansoor
I: Role of therapeutic plasma exchange in the treatment of severe
hypertriglyceridemia: An experience. Ther Adv Endocrinol Metab.
8:169–172. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ramírez-Bueno A, Salazar-Ramírez C,
Cota-Delgado F, de la Torre-Prados MV and Valdivielso P:
Plasmapheresis as treatment for hyperlipidemic pancreatitis. Eur J
Intern Med. 25:160–163. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Van den Berghe G, Wilmer A and Bouillon R:
Intensive insulin therapy in the medical ICU-Reply. New Engl J Med.
354:2070–2071. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
NICE-SUGAR Study Investigators. Finfer S,
Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook
D, Dodek P, et al: Intensive versus conventional glucose control in
critically ill patients. N Engl J Med. 360:1283–1297.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McLeod BC: Therapeutic apheresis: Use of
human serum albumin, fresh frozen plasma and cryosupernatant plasma
in therapeutic plasma exchange. Best Pract Res Clin Haematol.
19:157–167. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group: Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Peery AF, Dellon ES, Lund J, Crockett SD,
McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K,
Morgan DR, et al: Burden of gastrointestinal disease in the United
States: 2012 update. Gastroenterology. 143:1179–1187.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nawaz H, Koutroumpakis E, Easler J, Slivka
A, Whitcomb DC, Singh VP, Yadav D and Papachristou GI: Elevated
serum triglycerides are independently associated with persistent
organ failure in acute pancreatitis. Am J Gastroenterol.
110:1497–1503. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ivanova R, Puerta S, Garrido A, Cueto I,
Ferro A, Ariza MJ, Cobos A, Gonzalea-Santos P and Valdivielso P:
Triglyceride levels and apolipoprotein E polymorphism in patients
with acute pancreatitis. Hepatobiliary Pancreat Dis Int. 11:96–101.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Garg R and Rustagi T: Management of
hypertriglyceridemia induced acute pancreatitis. Biomed Res Int.
2018(4721357)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Joglekar K, Brannick B, Kadaria D and
Sodhi A: Therapeutic plasmapheresis for
hypertriglyceridemia-associated acute pancreatitis: Case series and
review of the literature. Ther Adv Endocrinol Metab. 8:59–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dijk W, Ruppert PMM, Oost LJ and Kersten
S: Angiopoietin-like 4 promotes the intracellular cleavage of
lipoprotein lipase by PCSK3/furin in adipocytes. J Biol Chem.
293:14134–14145. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Scherer J, Singh VP, Pitchumoni CS and
Yadav D: Issues in hypertriglyceridemic pancreatitis: An update. J
Clin Gastroenterol. 48:195–203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Skrypnik D, Ratajczak M, Karolkiewicz J,
Mądry E, Pupek-Musialik D, Hansdorfer-Korzon R, Walkowiak J,
Jakubowski H and Bogdański P: Effects of endurance and
endurance-strength exercise on biochemical parameters of liver
function in women with abdominal obesity. Biomed Pharmacother.
80:1–7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stepien M, Kujawska-Luczak M, Szulinska M,
Kregielska-Narozna M, Skrypnik D, Suliburska J, Skrypnik K, Regula
J and Bogdanski P: Beneficial dose-independent influence of
Camellia sinensis supplementation on lipid profile, glycemia, and
insulin resistance in an NaCl-induced hypertensive rat model. J
Physiol Pharmacol. 69:2018.doi: 10.26402/jpp.2018.2. PubMed/NCBI View Article : Google Scholar
|
|
27
|
Thuzar M, Shenoy VV, Malabu UH, Schrale R
and Sangla KS: Extreme hypertriglyceridemia managed with insulin. J
Clin Lipidol. 8:630–634. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kelly JL: Continuous insulin infusion:
When, where, and how? Diabetes Spectr. 27:218–223. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Betteridge DJ, Bakowski M, Taylor KG,
Reckless JP, de Silva SR and Galton DJ: Treatment of severe
diabetic hypertriglyceridaemia by plasma exchange. Lancet.
1(1368)1978.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Biberci Keskin E, Koçhan K, Köker İH,
Gülen B, İnce AT and Şentürk H: The role of plasma exchange in
hypertriglyceridemia-induced acute pancreatitis. Eur J
Gastroenterol Hepatol. 31:674–677. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Norman J: The role of cytokines in the
pathogenesis of acute pancreatitis. Am J Surg. 175:76–83.
1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yeh JH, Chen JH and Chiu HC:
Plasmapheresis for hyperlipidemic pancreatitis. J Clin Apher.
18:181–185. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schwartz J, Padmanabhan A, Aqui N, Balogun
RA, Connelly-Smith L, Delaney M, Dunbar NM, Witt V, Wu Y and Shaz
BH: Guidelines on the use of therapeutic apheresis in clinical
practice-evidence-based approach from the writing committee of the
American society for apheresis: The seventh special issue. J Clin
Apher. 1:149–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Song PK, Man QQ, Li H, Pang SJ, Jia SS, Li
YQ, He L, Zhao WH and Zhang J: Trends in lipids level and
dyslipidemia among Chinese adults, 2002-2015. Biomed Environ Sci.
32:559–570. 2019.PubMed/NCBI View Article : Google Scholar
|