Introduction
Idiopathic membranous nephropathy (IMN) is an
organ-specific autoimmune inflammatory disease of the kidneys
(1). IMN is the primary glomerular
disease affecting adults >60 years of age and is a common cause
of adult-onset nephropathy syndrome (NS) (1-3).
IMN is characterized by thickening of the basement membrane
(4) with subepithelial deposits of
immune complexes mostly composed of immunoglobulin (Ig)G and the
complement protein C3 (5,6), which are detectable using
immunofluorescence or electron microscopy. Clinical presentations
of IMN vary from subnephropathy range proteinuria to NS with heavy
proteinuria, also including hypertension, renal insufficiency and
microscopic hematuria (7). Previous
studies of the natural history of IMN have reported that 5-30 and
40% of patients with IMN have spontaneous complete or partial
remission after 5 years, respectively, whereas IMN in 30-40% of
patients progresses to end-stage renal disease (ESRD) within 5-15
years (8,9). Among hospitalized patients with primary
glomerular nephropathy, a decreasing trend in IgA nephropathy and
an increasing trend of IMN were reported by some researchers
(10). Globally, hypertension acts
as one of the leading etiologies for chronic kidney disease (CKD)
(11). Moreover, as some patients
have CKD due to high blood pressure, hypertension is considered
secondary to renal diseases (12). A
previous study reported that high blood pressure during renal
biopsy is a poor prognostic factor of patients with IMN (13). The association between IMN and
hypertension is not fully understood up to now. The present study
hypothesized that hypertension worsens IMN prognosis and serves a
central role in IMN disease progression. The present study
investigated whether hypertension was associated with clinical
parameters in IMN and examined IMN prognosis. Therefore, these
clinicians can monitor and treat patients with PMN who may be
inclined to hypertension, as to reduce occurrence of patients with
PMN with hypertension and delay disease progression.
Materials and methods
Study population
The present retrospective study recruited patients
with IMN from The First Affiliated Hospital of Nanchang University
(Nanchang, China) between January 2010 and June 2018. NS was
defined according to the standard criteria used in Japan (14): i) Urine protein excretion (3.5
g/day); ii) serum albumin (3.0 g/dl) or serum total protein (6.0
g/dl); iii) presence of edema; and iv) total cholesterol (250
mg/dl). The first and second criteria are considered necessary to
diagnose IMN (15). According to the
Kidney Disease Improving Global Outcomes (KDIGO) clinical practice
guidelines for glomerulonephritis, IMN can be diagnosed by kidney
biopsy (16). Patients with systemic
diseases, such as rheumatic diseases, malignant tumors, hepatitis B
or C virus infection, tuberculosis and other kidney diseases were
excluded from the study (17-20).
Patients <18 years old were also excluded. A total of 220
patients (male, 137; female, 83; age, 18-75 years; mean age
51.21±12.78 years) with previously diagnosed IMN were included in
the study. The inclusion criteria were as follows: i) Age, 18-75
years; ii) MN diagnosed by renal biopsy; iii) informed consent form
signed voluntarily by the participant; and iv) antihypertensive
drugs were administered, but no statins or other drugs were
received. The exclusion criteria were as follows: i) Patients with
infections, malignant tumors, tuberculosis or other serious kidney
diseases; ii) patients with insufficient follow-up; and iii)
patients determined to be inappropriate for participation in the
study by an investigator. The study was reviewed and approved by
The Ethics Committee of The Nanchang University Hospital. All
patients signed a patient consent form.
Study design
The enrolled patients were divided into two groups
based on the criteria for hypertension: Hypertension and
non-hypertension groups. Hypertension was defined as average
systolic blood pressure (SBP) ≥140 mmHg or average diastolic blood
pressure (DBP) ≥90 mmHg (21).
Patients with a history of hypertension whose BP was not above the
mentioned level following hospitalization with antihypertensive
drugs were also classified under the hypertension group. BP was
measured by an experienced physician using a mercury
sphygmomanometer and an appropriately sized cuff applied to the
right arm at heart level after ≥5 min of rest in a seated position.
The present study examined 100 patients with IMN and hypertension
(hypertension group) and 120 patients with IMN without hypertension
(non-hypertension group) who underwent renal biopsy at The First
Affiliated Hospital of Nanchang University.
Clinical parameters
Basic demographic data included age, sex, course of
the disease, edema, hypertension history, SBP, DBP and mean
arterial pressure (MAP). The present study measured red blood cell
count (RBC), hemoglobin (Hb), total protein (TP), albumin, serum
creatinine (Scr), uric acid (UA), potassium (K), calcium (Ca),
phosphorus (P), triglycerides (TG), total cholesterol (TC),
high-density lipoprotein (HDL), low-density lipoprotein (LDL) and
calculated estimated glomerular filtration rate (e-GFR) were
collected from 220 patients with IMN.The e-GFR was calculated using
the CKD-EPI creatinine equation recommended by the KDIGO and was
expressed as ml/min/1.73 m2 of body surface area
(22). In addition, 24 h urine
protein was measured.
Histopathologic parameters
The kidney biopsy specimens frozen as 5 µm sections,
dried at room temperature for 30 min, fixed using acetone at 4˚C
for 10 min and washed three times using PBS. Fluorescein labeled
antibody [Rabbit Anti Human IgG/FITC; cat. no. GF020229; 1:80;
Rabbit Anti Human IgA/FITC; cat. no. GF020429; 1:40; Rabbit Anti
Human IgM/FITC cat. no. GF020329; 1:40; Rabbit Anti Human
Fibrinogen/FITC; cat. no. GF011129; 1:40; Rabbit Anti Human C1q
Complement/FITC; cat. no. GF025429; 1:40; Rabbit Anti Human C3c
Complement/FITC; cat. no. GF020129; 1:20; all, Gene Tech (Shanghai)
Co., Ltd.] was used and incubate with 37˚C for 30 min. Tissues were
watched with PBS three times and sealed using glycerin. Tissues
were then observed using fluorescence microscope (magnification,
x100; eyepiece, magnification, x10; objective magnification, x10).
Renal biopsy specimens including ≥10 glomeruli were analyzed. All
renal biopsies were processed according to the standard techniques
of light microscopy (magnification, x400), immunofluorescence
microscopy (magnification, x400) and electron microscopy
(magnification, x2,000) (7). To
confirm the pathology, all samples were reviewed by two
pathologists in The First Affiliated Hospital of Nanchang
University. The following parameters were assessed by
histopathology: Glomerular sclerosis, segmental sclerosis, ischemic
sclerosis, crescents, mesangial cells and matrix hyperplasia. In
addition, mesangial hypercellularity, interstitial fibrosis,
tubular atrophy and vascular lesions were measured. These
parameters was analyzed using SPSS software (version 22.0; IBM
Corp.).
End-point of the study
Disease progression was defined as a ≥50% decline in
the baseline e-GFR, doubling of Scr levels, diagnosis of ESRD and
requiring renal replacement therapy after follow-up (23), including hemodialysis, peritoneal
dialysis and kidney transplant, and death. The present study
reviewed the medical record of each patient retrospectively from
the date of renal biopsy to death, the development of ESRD or the
last clinical visit (November 15, 2018). The average follow-up was
35.70 months.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 22.0; IBM Corp.). Continuous data are presented
as the mean ± SD, whereas categorical data are presented as
frequencies and percentages. Differences in continuous variables
between the two groups were assessed using independent t-tests. A
comparison of univariate predictors of clinical outcomes between
the groups was performed using χ2 test for categorical
variables. The renal progression-free rates were calculated using
the Kaplan-Meier analysis and comparisons between the groups were
performed using the log-rank test. Multivariate Cox proportional
hazard regression analysis was performed to determine independent
variables associated with the renal outcomes. The results are
presented as hazard ratio (H) with a 95% CI. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of demographic and
laboratory parameters between the two groups
IMN is known to be a common cause of primary
glomerulopathy (10). The present
study examined 100 patients with IMN associated with hypertension
(hypertension group) and 120 patients with IMN without hypertension
(non-hypertension group) that underwent renal biopsy at The First
Affiliated Hospital of Nanchang University. In the present study,
the prevalence of hypertension was 45.45% among the patients.
Baseline characteristics of patients with and without hypertension
are summarized in Table I. Among the
patients, 137 (62.2%) were male and 83 (37.7%) were female, with a
male to female ratio of 1.65:1. The mean age of patients with IMN
was 51.21±12.78 years. A significant difference was found in the
age (P=0.001), hypertension history (P<0.0001), SBP
(P<0.0001), DBP (P<0.0001), MAP (P<0.0001), albumin
(P=0.046), Scr (P=0.001), LDL (P=0.018), 24 h urine protein
(P=0.028) and e-GFR (P=0.013) between the groups with and without
hypertension. There were no significant differences in the
following parameters between the two groups: Sex, course of the
disease, edema, RBC, Hb, TP, UA, K, Ca, P, TG, TC and HDL.
 | Table IComparison of demographic and
laboratory parameters between the two groups. |
Table I
Comparison of demographic and
laboratory parameters between the two groups.
| Parameters | HG | NHG | T or
χ2 | P-value |
|---|
| Age, years | 54.34±13.10 | 48.60±11.94 | -3.4 | 0.001 |
| Sex | | | 0.04 | 0.839 |
|
Male | 63 (28.60) | 74 (33.60) | | |
|
Female | 37 (16.80) | 46 (20.90) | | |
| Course of
disease | 5.81±8.55 | 6.4±11.29 | 0.43 | 0.668 |
| Edema | | | 0.82 | 0.499 |
|
Yes | 92 (41.80) | 106 (48.20) | | |
|
No | 8 (3.60) | 14 (6.40) | | |
| Hypertension
history | | | 48.26 | P<0.001 |
|
Yes | 34 (15.50) | 0 (0.00) | | |
|
No | 66 (30.00) | 120 (54.50) | | |
| SBP, mmHg | 144.07±19.28 | 115.74±12.92 | -12.54 | P<0.001 |
| DBP, mmHg | 90.66±13.08 | 74.22±8.95 | -10.66 | P<0.001 |
| MAP, mmHg | 108.46±13.31 | 88.06±8.97 | -13.05 | P<0.001 |
|
RBCx1012/l | 4.35±0.67 | 4.38±0.71 | 0.31 | 0.758 |
| Hb, g/l | 129.19±22.21 | 128.35±19.35 | -0.3 | 0.765 |
| TP, g/l | 46.96±8.48 | 47.35±8.02 | 0.35 | 0.727 |
| Alb, g/l | 23.50±6.75 | 25.20±6.01 | 2.01 | 0.046 |
| Scr, g/l | 91.1±46.68 | 74.25±24.93 | -3.25 | 0.001 |
| UA, µmol/l | 371.56±78.67 | 367.34±93.28 | -0.36 | 0.72 |
| K, mmol/l | 4.00±0.54 | 4.01±0.45 | 0.23 | 0.818 |
| Ca, mmol/l | 2.02±0.18 | 2.06±0.16 | 1.63 | 0.104 |
| P, mmol/l | 1.18±0.19 | 1.14±0.22 | -1.13 | 0.26 |
| TG, mmol/l | 3.15±2.55 | 2.57±2.11 | -1.85 | 0.065 |
| TC, mmol/l | 7.25±2.01 | 6.78±1.91 | -1.79 | 0.075 |
| HDL, mmol/l | 1.48±0.59 | 1.59±0.68 | 1.3 | 0.196 |
| LDL, mmol/l | 5.03±2.14 | 4.40±1.73 | -2.38 | 0.018 |
| 24 h UP g/24 h | 4.56±2.52 | 3.83±2.35 | -2.21 | 0.028 |
| e-GFR | 81.83±35.32 | 94.09±36.65 | 2.51 | 0.013 |
Comparison of pathological parameters
between the two groups
Analysis of pathological parameters between the
hypertension and non-hypertension groups is shown in Table II. Ischemic sclerosis and vascular
lesions were associated with hypertension in IMN. The present
univariate analysis results suggested that glomerular sclerosis,
segmental sclerosis, ischemic sclerosis, interstitial fibrosis,
tubular atrophy and vascular lesions were associated with the
primary outcome in patients with IMN and hypertension.
 | Table IIComparison of pathological parameters
between the two groups. |
Table II
Comparison of pathological parameters
between the two groups.
| Parameter | HG | NHG | T or
χ2 | P-value |
|---|
| GS, case (%) | 31 (14.09) | 33 (15.00) | 0.778 | 0.036 |
| PGS (%) | 0.06±0.11 | 0.04±0.09 | -1.32 | 0.188 |
| SS, case (%) | 3 (1.36) | 4 (1.82) | -0.15 | 0.048 |
| PSS (%) | 0.004±0.02 | 0.004±0.02 | -0.08 | 0.939 |
| IS, case (%) | 27 (12.27) | 28 (12.73) | -0.822 | 0.011 |
| PIS (%) | 0.04±0.10 | 0.05±0.13 | 0.14 | 0.886 |
| Crescent, case
(%) | 8 (3.64) | 7 (3.18) | -0.65 | 0.515 |
| Percentage of
crescents (%) | 0.02±0.08 | 0.004±0.02 | -1.56 | 0.122 |
| MC/MAH | | | 2.351 | 0.314 |
|
0 | 15 (6.82) | 19 (8.64) | | |
|
<25% | 60 (27.27) | 81 (36.82) | | |
|
25-49% | 25 (11.36) | 20 (9.09) | | |
|
50-74% | 0 (0.00) | 0 (0.00) | | |
|
>75% | 0 (0.00) | 0 (0.00) | | |
| MEH | | | 5.915 | 0.2 |
|
0 | 15 (6.82) | 23 (10.45) | | |
|
<25% | 23 (10.45) | 24 (10.91) | | |
|
25-49% | 52 (23.64) | 69 (31.36) | | |
|
50-74% | 8 (3.64) | 2 (0.91) | | |
|
>75% | 2 (0.91) | 2 (0.91) | | |
| IF | | | 5.399 | 0.038 |
|
0 | 61 (27.73) | 76 (34.55) | | |
|
<25% | 7 (3.18) | 6 (2.73) | | |
|
25-49% | 26 (11.82) | 36 (16.36) | | |
|
50-74% | 4 (1.82) | 0 (0.00) | | |
|
>75% | 2 (0.91) | 2 (0.91) | | |
| TA | | | 9.449 | 0.041 |
|
0 | 18 (8.18) | 25 (11.36) | | |
|
<25% | 20 (9.09) | 29 (13.18) | | |
|
25-49% | 53 (24.09) | 64 (29.09) | | |
|
50-74% | 7 (3.18) | 0 (0.00) | | |
|
>75% | 2 (0.91) | 2 (0.91) | | |
| VL | | | 1.107 | 0.023 |
|
Yes | 74 (33.64) | 81 (36.82) | | |
|
No | 26 (11.82) | 39 (17.73) | | |
Survival analysis of cumulative renal
survival rate of hypertension and non-hypertension groups, and risk
factors associated with patients with IMN and hypertension
developing into ESRD
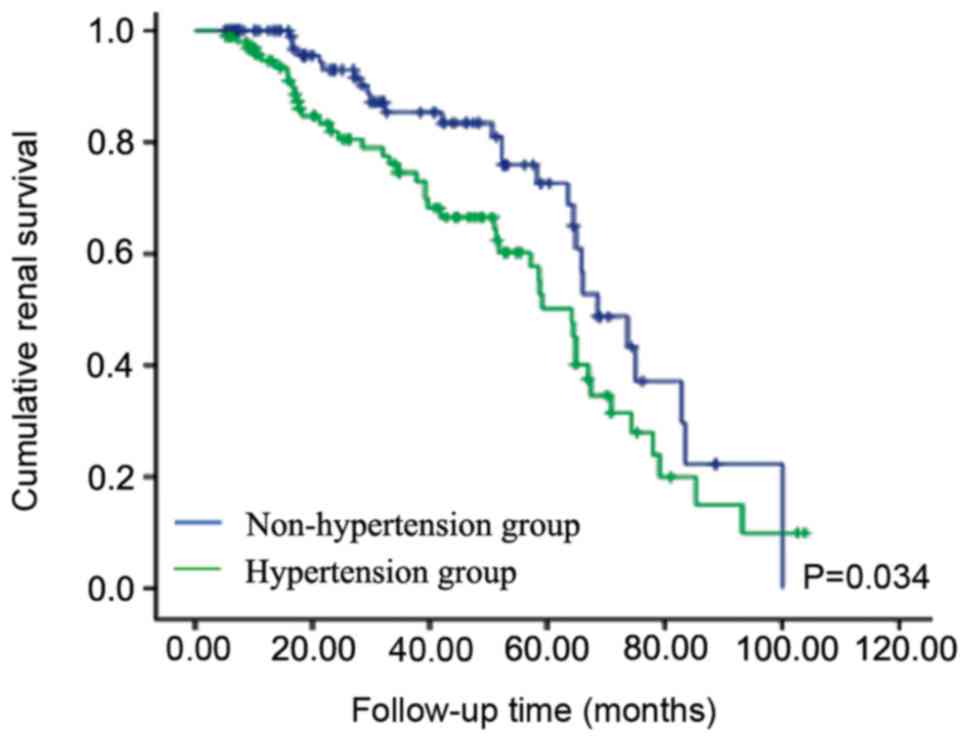
Follow-up data were available for 220 patients with
IMN with and without hypertension. Average observation time was
35.70 months (range, 5.10-103.77 months). In total, 54 patients
reported a 50% decline in e-GFR or doubling of Scr levels. During
follow-up, in eight patients IMN had progressed to ESRD. There were
nine cases of mortality and the cause of death was unknown. The
renal survival rates in patients with hypertension were
significantly reduced compared with patients without hypertension
(log-rank test, P=0.034; Fig. 1).
The present Cox proportional hazard regression results suggested
that DBP may be an independent risk factor for IMN progression (H,
5.160; CI, 0.865-0.989; P=0.023). The present results suggested
that age (H, 4.839; CI, 1.008-1.142; P=0.028), sex (H, 5.680; CI,
0.031-0.714; P=0.017), Scr (H, 20.920; CI, 1.035-1.089;
P<0.001), UA (H, 4.783; CI, 0.982-0.0.999; P=0.029), 24 h urine
protein (H, 6.318; CI, 1.079-0.1.850; P=0.012) and e-GFR (H, 4.008;
CI, 1.001-1.062; P=0.045) were significant and independent risk
factors in patients with IMN with hypertension. Glomerular
sclerosis (H, 8.722; CI, 1.860-21.559; P=0.003), segmental
sclerosis (H, 7.737; CI, 7.770-13.219; P=0.005), percentage of
ischemic sclerosis (H, 4.729; CI, 1.444-11.945; P=0.030), crescents
(H, 5.938; CI, 0.003-0.526; P=0.015), interstitial fibrosis (H,
8.128; CI, 0.005-1.052; P=0.043), and vascular lesions (H, 4.049;
CI, 1.030-9.766; P=0.044) were also identified as significant and
independent risk factors in patients with IMN with hypertension
(Table III).
 | Table IIIMultivariate Cox hazard regression
analysis of the disease risk factors and patients with idiopathic
membranous nephropathy with hypertension developing into end-stage
renal disease. |
Table III
Multivariate Cox hazard regression
analysis of the disease risk factors and patients with idiopathic
membranous nephropathy with hypertension developing into end-stage
renal disease.
| | | | | | | 95.0% CI |
|---|
| Parameter | B | SE | HR | P-value | Exp (B) | Lower | Upper |
|---|
| Age, years | 0.070 | 0.032 | 4.839 | 0.028 | 1.073 | 1.008 | 1.142 |
| Sex | -1.899 | 0.797 | 5.680 | 0.017 | 0.150 | 0.031 | 0.714 |
| DBP, mmHg | -0.078 | 0.034 | 5.160 | 0.023 | 0.925 | 0.865 | 0.989 |
| Scr, µmol/l | 0.060 | 0.013 | 20.920 | <0.001 | 1.062 | 1.035 | 1.089 |
| UA, µmol/l | -0.010 | 0.004 | 4.783 | 0.029 | 0.990 | 0.982 | 0.999 |
| 24 h urine protein,
g | 0.346 | 0.138 | 6.318 | 0.012 | 1.413 | 1.079 | 1.850 |
| e-GFR | 0.030 | 0.015 | 4.008 | 0.045 | 1.031 | 1.001 | 1.062 |
| Glomerular
sclerosis | 1.846 | 0.625 | 8.722 | 0.003 | 6.333 | 1.860 | 21.559 |
| Segmental
sclerosis | 6.942 | 2.496 | 7.737 | 0.005 | 1034.718 | 7.770 | 13.219 |
| Percentage of
IS | 3.722 | 1.711 | 4.729 | 0.030 | 41.328 | 1.444 | 11.945 |
| Crescents | -3.286 | 1.348 | 5.938 | 0.015 | 0.037 | 0.003 | 0.526 |
| Interstitial
fibrosis | 0.010 | 0.013 | 8.128 | 0.043 | 1.023 | 0.005 | 1.052 |
| Vascular
lesion | 1.154 | 0.574 | 4.049 | 0.044 | 3.172 | 1.030 | 9.766 |
Discussion
IMN is the most common primary glomerular disease in
individuals >60 years old (24).
In patients with IMN, one-third of patients with spontaneous
remission report progressive renal failure, whereas the remaining
patients report stable renal function (25). Nephritic syndrome, massive
proteinuria, hematuria, impaired renal function and hypertension
are common in IMN (26). The results
from previous studies on IMN prognostic factors are highly variable
(12). In addition, whether
demographic parameters, laboratory parameters and histological
lesions contribute to the improvement of renal function in patients
with hypertension is not fully understood.
The present retrospective study investigated the
prognosis and risk factors for renal survival in patients with IMN
with hypertension. In the present study, the majority of patients
with IMN were >40 years. A higher incidence of hypertension
among elderly patients compared with adult patients has previously
been reported (27). The present
results are consistent with the results of the previous studies
(28,29). Moreover, the present study identified
that Scr, LDL and 24 h urine protein were increased, and serum
albumin and e-GFR were decreased in patients with IMN with
hypertension. Huh et al (18)
reported that low serum albumin levels at the onset of the disease
were associated with poor renal prognosis of patients with IMN.
Another previous study reported that high levels of Scr at
diagnosis are major predictors of the progression of IMN to ESRD
(30). Proteinuria has also been
used as a major predictor for renal prognosis of IMN in a
conventional predictive model (31).
Patients with limited proteinuria are deemed to have a better
prognosis (31). Previous studies
identified lower e-GFR to be significantly associated with the risk
of progression of IMN to ESRD (32,33). The
present results suggested that hypertension may be associated with
the severity of IMN, which is consistent with results from previous
studies. In the majority of studies, hypertension was not indicated
to be an independent predictor of MN (34,35). To
the best of our knowledge, the present study was the first to
identify primary factors for hypertension development in patients
with IMN using a retrospective study design, measuring parameters
such as uric acid, age and sex (36).
The mechanism of hypertension and disease severity,
and whether hypertension can exacerbate kidney disease is not fully
understood. The present results suggested that age and sex were
statistically significant between the hypertension and
non-hypertension groups, which were in line with findings from a
previous study (37). Uric acid was
previously reported to be independently associated with prevalent
CKD and hypertension (36,38). Hypertension can cause
nephroangiosclerosis (39). The
severity of renal histological lesions, particularly of
interstitial fibrosis, glomerular sclerosis and vasculopathy, are
considered negative prognostic indicators for IMN (40). The present results suggested that
interstitial fibrosis, glomerular sclerosis, vasculopathy and
crescents were significantly different between the hypertension and
non-hypertension groups. However, other parameters were not
statistically significant in the present study, which may be
attributed to the small sample size. The present results suggested
that the accumulated survival rate was significantly higher in
patients with IMN without hypertension compared with patients with
hypertension. In the present study, a multivariate Cox proportional
hazards regression analysis was performed to investigate the
association of DBP with renal outcomes. A previous study showed
that patients with IMN suffered from CKD as a result of
hypertension, and that hypertension was secondary to renal disease
(18). Therefore, monitoring BP
during diagnosis in patients with IMN may facilitate prognosis.
The present study had several limitations, such as a
small number of patients with IMN, which limited the statistical
power of the study. Therefore, future studies with larger sample
sizes and longer periods of follow-up are required to investigate
the influence of BP in patients with IMN. In addition, the present
study did not analyze antibodies against phospholipase A2 receptors
and thrombospondin type I domain-containing 7A, which have been
suggested to be correlated with IMN disease severity (41). Finally, the primary outcome of MN can
be complete and partial remission, including remission of
proteinuria (42); however, in the
present study, quantitative proteinuria was not followed up at the
end-point of the study.
In conclusion, the present results suggested that
patients with IMN with hypertension reported worse
clinicopathological features and lower cumulative renal survival
rate compared with patients without hypertension. The present
results suggested that DBP may be an independent risk factor for
the development of IMN with hypertension. Early detection and
correction of hypertension could help delay the deterioration of
renal function and improve prognosis of patients with IMN.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL wrote the manuscript. WL, SG and JL collected and
analyzed the study data. SG performed the histological examination
of the kidney. YW conceived and designed the study, proofread the
manuscript and revised the manuscript for impoartant intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The First Affiliated Hospital of Nanchang University.
Patients who participated in this research had complete clinical
data. Signed informed consents were obtained from the patients or
guardians (when the patient was incapacitated).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beck LH Jr and Salant DJ: Membranous
nephropathy: From models to man. J Clin Invest. 124:2307–2314.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Sekula P, Li Y, Stanescu HC, Wuttke M,
Ekici AB, Bockenhauer D, Walz G, Powis SH, Kielstein JT, Brenchley
P, et al: Genetic risk variants for membranous nephropathy:
Extension of and association with other chronic kidney disease
aetiologies. Nephrol Dial Transplant. 32:325–332. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pozdzik A, Brochériou I, David C, Touzani
F, Goujon JM and Wissing KM: Membranous nephropathy and
anti-podocytes antibodies: Implications for the diagnostic workup
and disease management. Biomed Res Int.
2018(6281054)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ronco P and Debiec H: Pathogenesis of
membranous nephropathy: Recent advances and future challenges. Nat
Rev Nephrol. 8:203–213. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sinico RA, Mezzina N, Trezzi B, Ghiggeri
GM and Radice A: Immunology of membranous nephropathy: From animal
models to humans. Clin Exp Immunol. 183:157–165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Larsen CP, Messias NC, Silva FG, Messias E
and Walker PD: Determination of primary versus secondary membranous
glomerulopathy utilizing phospholipase A2 receptor staining in
renal biopsies. Mod Pathol. 26:709–715. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang XD, Cui Z, Zhang MF, Wang J, Zhang
YM, Qu Z, Wang X, Huang J, Wang F, Meng LQ, et al: Clinical
implications of pathological features of primary membranous
nephropathy. BMC Nephrol. 19(215)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Beck Lh Jr, Bonegio RG, Lambeau G, Beck
DM, Powell DW, Cummins TD, Klein JB and Salant DJ: M-type
phospholipase A2 receptor as target antigen in idiopathic
membranous nephropathy. N Engl J Med. 361:11–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van den Brand JA, Hofstra JM and Wetzels
JF: Low-molecular-weight proteins as prognostic markers in
idiopathic membranous nephropathy. Clin J Am Soc Nephrol.
6:2846–2853. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu X, Wang G, Chen N, Lu T, Nie S, Xu G,
Zhang P, Luo Y, Wang Y, Wang X, et al: Long-term exposure to air
pollution and increased risk of membranous nephropathy in China. J
Am Soc Nephrol. 27:3739–3746. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chiu HF, Chen H, Lu KC and Shu KH: Taiwan
Society of Nephrology: Distribution of glomerular diseases in
Taiwan: Preliminary report of national renal biopsy
registry-publication on behalf of Taiwan society of nephrology. BMC
Nephrol. 19(6)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
da Silva AQB, de Sandes-Freitas TV, Mansur
JB, Medicina-Pestana JO and Mastroianni-Kirsztajn G: Clinical
presentation, outcomes, and treatment of membranous nephropathy
after transplantation. Int J Nephrol. 2018(3720591)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tu WH, Petitti DB, Biava CG, Tulunay O and
Hopper J Jr: Membranous nephropathy: Predictors of terminal renal
failure. Nephron. 36:118–124. 1984.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saito T, Iwano M, Matsumoto K, Mitarai T,
Yokoyama H, Yorioka N, Nishi S, Yoshimura A, Sato H, Ogahara S, et
al: Mizoribine therapy combined with steroids and mizoribine blood
concentration monitoring for idiopathic membranous nephropathy with
steroid-resistant nephrotic syndrome. Clin Exp Nephrol. 21:961–970.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kida H, Asamoto T, Yokoyama H, Tomosugi N
and Hattori N: Long-term prognosis of membranous nephropathy. Clin
Nephrol. 25:64–69. 1986.PubMed/NCBI
|
|
16
|
Tang Z, Wang Y, Tao L, Guo Y, Zheng Y and
Zheng D: The elevated levels of urinary angiotensinogen are
correlated with the severity of idiopathic membranous nephropathy.
BMC Nephrol. 19(357)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Di J, Qian Q, Yang M, Jiang Y, Zhou H, Li
M and Zou Y: Efficacy and safety of long-course tacrolimus
treatment for idiopathic membranous nephropathy. Exp Ther Med.
16:979–984. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huh H, Lee H, Lee JP, Kim DK, Oh S, Oh YK,
Kim YS and Lim CS: Factors affecting the long-term outcomes of
idiopathic membranous nephropathy. BMC Nephrol.
18(104)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu YH, Chen CH, Chen SY, Lin YJ, Liao WL,
Tsai CH, Wan L and Tsai FJ: Association of phospholipase A2
receptor 1 polymorphisms with idiopathic membranous nephropathy in
Chinese patients in Taiwan. J Biomed Sci. 17(81)2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu NX, Xie QH, Sun ZX, Wang J, Li Y, Wang
L, Liu SJ, Xue J and Hao CM: Renal phospholipase A2 receptor and
the clinical features of idiopathic membranous nephropathy. Chin
Med J (Engl). 130:892–898. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
1999 World Health
Organization-International society of hypertension guidelines for
the management of hypertension. Guidelines subcommittee. J
Hypertens. 17:151–183. 1999.
|
|
22
|
Cattran DC, Feehally J and Terence Cook H:
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group:
KDIGO2012 clinical practice guideline for the evaluation and
management of chronic kidney disease. Kidney Int (Suppl 3).
S1–S150. 2013. View Article : Google Scholar
|
|
23
|
Nair V, Robinson-Cohen C, Smith MR,
Bellovich KA, Bhat ZY, Bobadilla M, Brosius F, de Boer IH, Essioux
L, Formentini I, et al: Growth differentiation factor-15 and risk
of CKD progression. J Am Soc Nephrol. 28:2233–2240. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cattran DC and Brenchley PE: Membranous
nephropathy: Integrating basic science into improved clinical
management. Kidney Int. 91:566–574. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Q, Huang B, Liu X, Liu B, Zhang Y,
Zhang Z, Hua J, Fan Y, Hu L, Meng M, et al: Ultrasensitive
quantitation of anti-phospholipase A2 receptor antibody as a
diagnostic and prognostic indicator of idiopathic membranous
nephropathy. Sci Rep. 7(12049)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roy S, Korula A, Basu G, Jacob S,
Varughese S and Tamilarasi V: Immunohistochemical glomerular
expression of phospholipase A2 receptor in primary and secondary
membranous nephropathy: A retrospective study in an Indian cohort
with clinicopathological correlations. Nephron Extra. 7:1–9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Calvo-Río V, Loricera J, Mata C, Martín L,
Ortiz-Sanjuán F, Alvarez L, González-Vela MC, González-Lamuño D,
Rueda-Gotor J, Fernández-Llaca H, et al: Henoch-Schönlein purpura
in northern Spain: Clinical spectrum of the disease in 417 patients
from a single center. Medicine (Baltimore). 93:206–113.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ayalon R and Beck LH Jr: Membranous
nephropathy: Not just a disease for adults. Pediatr Nephrol.
30:31–39. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Couser WG: Primary membranous nephropathy.
Clin J Am Soc Nephrol. 12:983–997. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gupta S, Connolly J, Pepper RJ, Walsh SB,
Yaqoob MM, Kleta R and Ashman N: Membranous nephropathy: A
retrospective observational study of membranous nephropathy in
north east and central London. BMC Nephrol. 18(201)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yamaguchi M, Ando M, Katsuno T, Tsuboi N
and Maruyama S: Urinary protein and renal prognosis in idiopathic
membranous nephropathy: A multicenter retrospective cohort study in
Japan. Ren Fail. 40:435–441. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Heeringa SF, Branten AJ, Deegens JK,
Steenbergen E and Wetzels JF: Focal segmental glomerulosclerosis is
not a sufficient predictor of renal outcome in patients with
membranous nephropathy. Nephrol Dial Transplant. 22:2201–2207.
2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y,
Xu JS, Huang SM, Wang LN, Huang W, et al: Modified glomerular
filtration rate estimating equation for Chinese patients with
chronic kidney disease. J Am Soc Nephrol. 17:2937–2944.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Reidy K and Kaskel FJ: Pathophysiology of
focal segmental glomerulosclerosis. Pediatr Nephrol. 22:350–354.
2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Troyanov S, Roasio L, Pandes M, Herzenberg
AM and Cattran DC: Renal pathology in idiopathic membranous
nephropathy: A new perspective. Kidney Int. 69:1641–1648.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kuwabara M, Hisatome I, Niwa K, Hara S,
Roncal-Jimenez CA, Bjornstad P, Nakagawa T, Andres-Hernando A, Sato
Y, Jensen T, et al: Uric acid is a strong risk marker for
developing hypertension from prehypertension: A 5-year Japanese
cohort study. Hypertension. 71:78–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang BO, Cheng M, Yang M, Han S, Zhang
YH, Shi HG, Zhu L and Zhao XZ: Analysis of the prognostic risk
factors of idiopathic membranous nephropathy using a new surrogate
end-point. Biomed Rep. 4:147–152. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jolly SE, Mete M, Wang H, Zhu J, Ebbesson
SO, Voruganti VS, Comuzzie AG, Howard BV and Umans JG: Uric acid,
hypertension, and chronic kidney disease among Alaska Eskimos: The
genetics of coronary artery disease in alaska natives (GOCADAN)
study. J Clin Hypertens (Greenwich). 14:71–77. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ihm CG: Hypertension in chronic
glomerulonephritis. Electrolyte Blood Press. 13:41–45.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ponticelli C and Glassock RJ: Glomerular
diseases: Membranous nephropathy-a modern view. Clin J Am Soc
Nephrol. 9:609–616. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tomas NM, Beck LH Jr, Meyer-Schwesinger C,
Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U,
Dabert-Gay AS, et al: Thrombospondin type-1 domain-containing 7A in
idiopathic membranous nephropathy. N Engl J Med. 371:2277–2287.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Beck LH Jr, Fervenza FC, Beck DM, Bonegio
RG, Malik FA, Erickson SB, Cosio FG, Cattran DC and Salant DJ:
Rituximab-induced depletion of anti-PLA2R autoantibodies predicts
response in membranous nephropathy. J Am Soc Nephrol. 22:1543–1550.
2011.PubMed/NCBI View Article : Google Scholar
|