Introduction
Inflammatory bowel disease (IBD), including
ulcerative colitis (UC) and Crohn's disease, is characterized by
chronic relapsing inflammation of the gastrointestinal (GI) tract
(1). Over the past 20 years, the
incidence and prevalence of IBD has risen sharply in developing
areas of the world, including in Asia, South America, the Middle
East and Africa (2). In China, the
total number of IBD cases between 2005 and 2014 was ~350,000 and it
has been predicted that the number of patients with IBD will reach
1.5 million by 2025(2). IBD is
primarily caused by the aberrant activation of the immune system in
response to abnormal alterations to the gut environment (3). Furthermore, the diverse microbiota in
the GI tract serves a critical role in the development of IBD
(4). In a previous study,
significantly increased levels of Fusobacterium spp. and
Enterrococcus faecalis were identified in the feces of
patients with IBD compared with healthy controls (5). IBD has also been reported to be
correlated with decreased levels of Erysipelotrichales,
Bacteroidales, Clostridiales and Faecalibacterium
prausnitzii, and an increased abundance of
Enterobacteriaceae, Pasteurellacaea, Veillonellaceae,
Proteobacter and Fusobacteriaceae (6).
The treatment options for UC are based on a variety
of parameters, including patient age, severity of disease, relapse
frequency and disease course (7). At
present, the standard treatment strategies for UC include
anti-inflammatory therapeutics, systemic administration of
steroids, immunosuppressants and biologics, and even surgery
(8). However, a substantial
proportion of patients with UC are resistant or intolerant to the
standard treatment strategies, therefore, the manipulation of
enteric microbiota has become a focus for the treatment of UC
(6).
Fecal microbiota transplantation (FMT) is a
therapeutic process in which the fecal microbiota of a healthy
donor is transplanted into a diseased recipient to reconstruct the
gut microbial community and restore microbial homeostasis (9). Based on a previous study that reported
an overall cure rate of 90% for refractory or recurrent
Clostridium difficile (C. difficile) infection with
FMT, researchers are investigating the use of FMT in intestinal
diseases, including in IBD (10).
Previous studies have demonstrated the therapeutic effects of FMT
in IBD (11,12); however, its effects on UC have not
been investigated extensively.
The safety and efficacy of FMT in patients with UC
has been assessed in three small, randomized controlled clinical
trials (RCTs) (13-15).
The three studies differed in terms of infusion protocol, weekly
treatment and clinical outcomes. The study conducted by Moayyedi
et al (13) reported that FMT
induced remission in patients with active UC. A total of 70
patients with active UC were treated weekly with FMT or water enema
(placebo) for 6 weeks. The remission rate (full Mayo score ≤2;
endoscopic Mayo score=0) in the FMT group was significantly higher
compared with the placebo group (24% vs. 5%, respectively). A
recently published systematic review conducted by Costello et
al (16) meta-analyzed 14 cohort
studies and 4 RCTs, including 308 FMT-treated patients with UC. In
these meta-analyses of RCTs, it was reported that FMT effectively
treated UC with a clinical remission rate of 28% (39/140) in
patients treated with FMT, compared with 9% (13/137) in patients
treated with the placebo. Furthermore, clinical response was
achieved in 49% (69/140) of patients treated with FMT compared with
28% (38/137) of patients treated with the placebo. In the 14 cohort
studies, 24% (39/168) of patients treated with FMT achieved
clinical remission.
Although a number of studies have reported the
beneficial effect of FMT for UC, it is still unclear how the GI
microbiota impacts UC status. In the present study, the efficacy
and safety of FMT was assessed in patients with mild to moderate
active UC by assessing clinical responses and identifying
associated components in the fecal microbiota.
Materials and methods
Study design and patients.
The present study was conducted to assess the
efficacy and safety of FMT in patients who were treated for mild to
moderate active UC at Guangzhou First People's Hospital between
January 2017 and December 2017 and approved by the Ethics Committee
of Guangzhou First People's Hospital (approval no. K-2017-103-02).
In total, 47 patients (age, 18-75 years) were enrolled over this
period. The baseline demographic and clinical characteristics of
the patients are summarized in Table
I. Written informed consent was obtained from all participants.
UC was diagnosed based on clinical, endoscopic and histological
criteria, including clinically and endoscopically active UC, a
total Mayo score of 3-10, a Mayo endoscopy subscore ≥1 and a
Physician's Global Assessment subscore ≤2(17). Mild activity was defined as a total
Mayo score of 3-5 and moderate activity was defined as a score of
6-10. The exclusion criteria for patients with UC were as follows:
Severe disease activity (total Mayo clinical score ≥10);
indeterminate colitis; co-morbid chronic disease; food allergy;
irritable bowel syndrome; history of bowel cancer, pregnancy or
other severe diseases, including diabetes and cancer; GI surgery
(except for appendicectomy) during the 3 months before enrollment;
used antibiotics or probiotics during the 4 weeks before
enrollment; and had been followed up for <8 weeks. Furthermore,
patients with GI infections, including parasitic and C.
difficile infections, were excluded from the study. During the
current study, concomitant treatments using 5-aminosalicylic acid,
immunomodulators or anti-tumor necrosis factor (TNF) agents were
permitted, as long as the dose was stable prior to enrollment.
Furthermore, patients were not allowed to take antibiotics,
probiotics or corticosteroids during the present study, and
patients who were previously on steroid treatment (corticosteroids)
were taken off of the treatment 1 month prior to enrollment.
 | Table IBaseline patient characteristics. |
Table I
Baseline patient characteristics.
| Parameters | Data |
|---|
| Total patients | 44.0 |
| Age (years) | 44.4±15.5 |
| Sex | |
|
Male | 25.0 (57%) |
|
Female | 19.0 (43%) |
| Smoke | |
|
Smoker | 9.0 (20%) |
|
Non-smoker | 35.0 (80%) |
| Disease extent | |
|
Proctitis | 10.0 (23%) |
|
Left-sided | 24.0 (54%) |
|
Extensive | 10.0 (23%) |
| Disease duration
(months) | 55.7±25.3 |
| Concomitant
drugs | |
|
None | 4.0 (9%) |
|
Oral
5-aminosalicylate | 28.0 (64%) |
|
Oral
immunomodulator (azathioprine, cyclosporine, methotrexate) | 10.0 (23%) |
|
Oral
steroids | 11.0 (25%) |
| Previous anti-TNF
therapy | 4.0 (9%) |
| Total Mayo
score | 5.9±2.0 |
| Mayo endoscopic
subscore | 1.9±0.7 |
|
1 | 13.0 (30%) |
|
2 | 23.0 (52%) |
|
3 | 8.0 (18%) |
| UCEIS score | 4.4±2.1 |
| Mayo clinical
score | 4.0±1.5 |
| IBDQ score | |
|
ESR
(mm/h) | 25.5±20.6 |
|
CRP
(mg/l) | 3.0±0.7 |
|
PCT
(ng/ml) | 0.1±0.0 |
|
White blood
cell count (x109 cells/l) | 7.3±1.9 |
|
Red blood
cell count (x1012 cells/l) | 4.4±0.7 |
|
Hemoglobin
(g/l) | 121.5±24.8 |
|
Platelet
count (x109 cells/l) | 309.3±112.7 |
|
Albumin
(g/l) | 37.8±4.4 |
Healthy stool donors were recruited and screened
using previously described criteria (18). Healthy donors (3; sex, male; age,
24-29 years; median age, 25 years) were selected from volunteers
able to attend Guangzhou First People's Hospital, who were not
pregnant and had good dietary and sleep habits (reported sleep of
7-8 h per day; reported taking part in physical exercise >3
times per week; did not eat fast food; did not smoke; did not drink
alcohol). Prior to sample donation, the following laboratory tests
were performed: Blood (complete blood count), erythrocyte
sedimentation rate (ESR), procalcitonin (PCT), C-reactive protein
(CRP), biochemical, hepatitis A, hepatitis B, hepatitis C, human
immunodeficiency virus (HIV), syphilis and stool tests (stool
parasites, ova and culture). Donor exclusion criteria were as
follows: Infectious diseases (HIV, hepatitis B or C); high-risk
sexual behavior; use of illicit drugs; communicable disease (for
example, upper respiratory tract infection); GI co-morbidities
(history of or current IBD, irritable bowel syndrome, chronic
constipation, chronic diarrhea, intrinsic GI illness/condition,
history of or current GI malignancy or polyposis, family history of
colorectal cancer or history of major GI surgery); use of
antimicrobials, probiotics or systemic antineoplastic agents during
the 12 weeks prior to sample collection; other conditions,
including systemic autoimmunity, metabolic syndrome, obesity (body
mass index ≥30) or moderate to severe undernutrition/malnutrition;
history of malignant illness; and ongoing oncologic therapy.
Transendoscopic enteral tubing (TET)
tube insertion.
Standard bowel preparation (19) was performed using polyethylene
glycol-electrolyte solution and subsequently, a colonoscopy was
performed in each patient to examine the whole colon and distal
ileum. A TET (FMT Medical Co., Ltd.) tube was inserted via the anus
as far as the terminus of the ileum, using an endoscope, and while
the head of the TET tube was kept stable, the endoscope was
carefully removed. The endoscope was reinserted and the head of the
TET was fixed with a clamp that was attached to the intestinal wall
(Fig. S1A). An additional two loops
of the TET were fixed with clamps to the intestinal wall while
removing the endoscope (Fig. S1B
and C). The end of the TET was
fixed with tape to the sacral skin (Fig. S1D), as previously described
(20,21). The appearance of the TET tube is
detailed in Fig. S2.
Feces preparation and
intervention.
The fresh fecal samples were collected in a clean
room next to the FMT operating room. The feces was inspected
visually by examining the form and the presence of blood and/or
mucous. Within 0.5 h of collection, ~150-200 g donated fresh feces
was dissolved in 1000 ml physiological saline and was purified
using the GenFMTer automatic purification system (FMT Medical Co.,
Ltd.), which performs microfiltration and centrifugation steps to
obtain a centrifuged microbiota sample (according to the
manufacturers protocol). At 1 day post-TET insertion, 150 ml
physiological saline containing ~50 cm3 centrifuged
microbiota was infused into the entire colon of the patient via the
TET tube. Patients were required to remain in the right lateral
position for ≥30 min after FMT (to maintain the largest contact
area between microbiota and intestines) and were allowed to eat 2 h
later. The FMT procedure was repeated every other day for a total
of 3 treatments, with each patient only receiving FMT obtained from
the same donor (20). The FMT of
each donor was batched and fecal samples were stored at -80˚C until
further use.
Follow-up.
FMT follow-up visits were scheduled at weeks 1, 4
and 12. At each visit, bowel frequency, bleeding, GI symptoms,
adverse events (AEs), changes in medication and quality of life of
the patient were assessed using the Inflammatory Bowel Disease
Questionnaire (IBDQ) (22). The
partial Mayo scores were calculated based on bowel frequency,
rectal bleeding and the Physician's Global Assessment score. At
weeks 4 and 12, blood and stool samples were collected for blood
tests and the determination of ESR, CRP and PCT values. Molecular
microbiological analyses were performed using fecal samples
obtained from patients 1 day prior to FMT, and 4 and 12 weeks after
FMT.
Extraction of genomic DNA.
Total genomic DNA was extracted from the fecal
samples using the cetyl trimethylammonium bromide/sodium dodecyl
sulfate method and DNA concentration and purity were monitored by
gel electrophoresis using 1% agarose gels, as previously described
(23). DNA was diluted to 1 ng/µl
with sterile water before loading on the gel.
Amplicon generation.
16S ribosomal (r)RNA/18S rRNA/internal transcribed
spacer (ITS) genes in different regions (16S V4, 16S V3-V4, 16S
V4-V5, 18S V4, 18S V9, ITS1 and ITS2) were amplified using the
following primers: 16S V4, 515 forward, 5'-GTGCCAGCMGCCGCGGTAA-3'
and 806 reverse, 5'-GGACTACHVGGGTWTCTAAT-3'; 16S V3-V4, 341
forward, 5'-CCTAYGGGRBGCASCAG-3' and 806 reverse,
5'-GGACTACNNGGGTATCTAAT-3'; 16S V4-V5, 515 forward,
5'-GTGCCAGCMGCCGCGGTAA-3' and 907 reverse,
5'-CCGTCAATTCCTTTGAGTTT-3';18S V4 528 forward,
5'-GCGGTAATTCCAGCTCCAA-3' and 706 reverse,
5'-AATCCRAGAATTTCACCTCT-3'; 18S V9 1380 forward,
5'-CCCTGCCHTTTGTACACAC-3' and 1510 reverse,
5'-CCTTCYGCAGGTTCACCTAC-3'; ITS1 1F forward,
5'-CTTGGTCATTTAGAGGAAGTAA-3' and 1F reverse,
5'-GCTGCGTTCTTCATCGATGC-3'; ITS2 ITS3-2024 forward,
5'-GCATCGATGAAGAACGCAGC-3' and ITS4-2409 reverse,
5'-TCCTCCGCTTATTGATATGC-3'. All PCR reactions were carried out in
30 µl reactions with 15 µl of Phusion® High-Fidelity PCR
Master Mix (New England Biolabs, Inc.); 0.2 µM of forward and
reverse primers, and ~10 ng template DNA. Thermal cycling consisted
of initial denaturation at 98˚C for 1 min, followed by 30 cycles of
denaturation at 98˚C for 10 sec, annealing at 50˚C for 30 sec and
elongation at 72˚C for 30 sec. Final extension was at 72˚C for 5
min. Subsequently, the PCR products were mixed with the same volume
of 1X Loading Buffer (Takara Bio, Inc.) and visualized by gel
electrophoresis on a 2% agarose gel. The PCR products were excised
and purified from the agarose gel using the GeneJETTM Gel
Extraction kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Library preparation and
sequencing.
Sequencing libraries were generated from the
amplified genomic DNA using the Ion Plus Fragment Library kit (48
rxns; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Library quality was assessed on the
Qubit® 2.0 Fluorometer (Thermo Fisher Scientific, Inc.).
The library was sequenced on the Ion S5™ XL system
(Thermo Fisher Scientific, Inc.) and single-end reads (400/600 bp)
were generated.
Sequence analyses.
Sequence analyses were performed using Uparse
software (version 7.0.1001; drive5.com/uparse) (24). Sequences with ≥97% similarity were
assigned to the same operational taxonomic units (OTUs).
Representative sequences from each OTU were screened for further
annotation. After the samples were rarefied to the same sequencing
depth, and α and β diversities, differential OTU abundance analyses
were performed using Uparse and Quantitative Insights Into
Microbial Ecology software (version 1.7.0; qiime.org/) (25). Shannon's diversity index was used to
display the diversity of the gut microbiota. Chao1 estimator was
used to display the richness of the microbiota. Metastats analysis
was used to analyze the diversity of bacteria in different groups
(26).
Outcomes.
The primary outcomes referred to as steroid-free
clinical responses at week 4 after FMT were as follows: A decrease
of ≥3 points in the Mayo score, a reduction ≥50% in total Mayo
subscores of rectal bleeding plus stool frequency, or both. The
secondary outcomes were as follows: Steroid-free clinical remission
(total Mayo subscore ≤1 for rectal bleeding plus stool frequency),
steroid-free remission (total Mayo score ≤2 with no individual
subscore ≥1, and mucosal healing defined by a Mayo endoscopy
subscore ≤1), quality of life (assessed with the IBDQ) and safety
(assessed by AEs) (27). At week 12
after FMT, the patients were categorized into responder and
non-responder groups, a responder was defined by a decrease of ≥3
points in the Mayo score, a reduction ≥50% in total Mayo subscores
of rectal bleeding plus stool frequency, or both; a non-responder
was defined by a decrease of <3 points in the Mayo score and a
reduction <50% in total Mayo subscores of rectal bleeding plus
stool frequency.
Statistical analysis.
Data are presented as the percentage of patients or
the mean ± standard deviation. The χ2 test, unpaired
t-test, Fisher's exact test or Wilcoxon signed rank test were used
to compare data containing two groups. One-way ANOVA followed by
Tukey's post hoc test was used to compare data containing ≥3
groups. All statistical analyses were performed using SPSS software
(version 23.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical results.
Between January 2017 and December 2017, a total of
78 patients were recruited, however, only 47 were enrolled in the
present study, as some did not meet the inclusion criteria/met one
of the exclusion criteria. After FMT, three patients dropped out of
the study before week 4, therefore, only 44 patients were included
in the analyses at week 4 and 12. The baseline demographic and
clinical characteristics of the patients are summarized in Table I, and the FMT outcomes are summarized
in Table II. At week 4,
steroid-free clinical response and steroid-free clinical remission
were observed in 37 (84.1%) and 31 (70.5%) patients, respectively.
Steroid-free remission was not achieved in any patient (Table II). Accordingly, the patients were
categorized into responder and non-responder groups. These two
groups displayed no significant differences in their baseline
demographic and clinical characteristics (Table III). The clinical response rate was
higher in patients with mild active UC (18/23, 78%) compared with
patients with moderate active UC (16/21, 76%), although no
statistical significance was observed between these two groups. The
clinical response rate in patients with a lower Mayo endoscopic
subscore (10/13, 77%) was similar compared with patients with a
higher subscore (24/31, 77%; Table
III). Furthermore, the UC endoscopic index of severity scores
suggested a similar outcome to the Mayo endoscopic subscores
(Table III). The association
between the clinical response rate and clinical parameters,
including age, sex, smoking, disease extent, disease duration, IBDQ
scores, concomitant drug use and donors, was also assessed, and no
significant associations were identified (P>0.05; Table III). The Mayo clinical score was
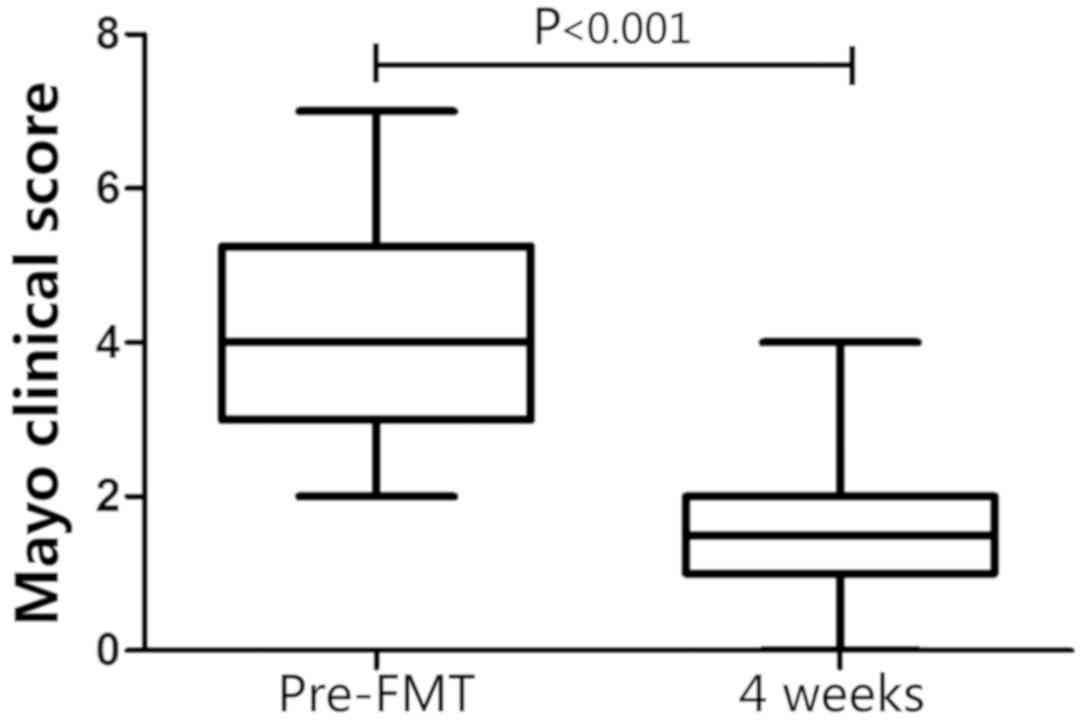
significantly decreased at week 4 compared with the baseline score
in the FMT group (4.02±1.47 at baseline; 1.91±1.07 at week 4;
P<0.001; Fig. 1). At week 4, the
mucosal activity was reassessed by colonoscopy in 10 responders.
All 10 responders displayed a steroid-free endoscopic response
(Mayo endoscopy subscore ≤1 with a reduction of ≥1 point from the
baseline) and none achieved steroid-free endoscopic remission (Mayo
endoscopy subscore=0; Table II).
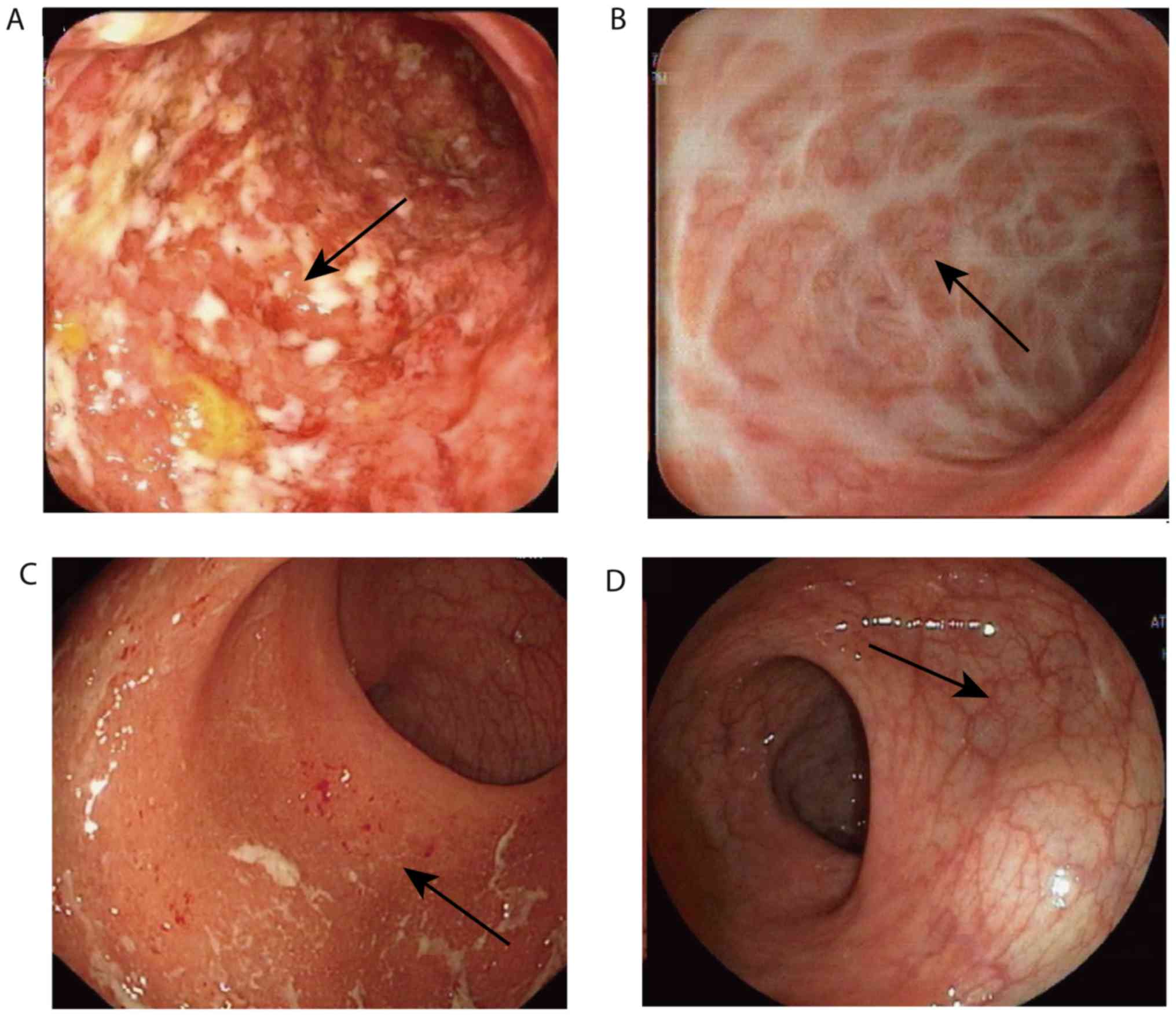
Representative images of the colons of two responders displaying
steroid-free endoscopic remission are presented in Fig. 2. No serious complications, including
anaphylactic shock or septicemia, occurred in 41/44 patients
following FMT or during the 12-week follow-up. Increased diarrhea
frequency was reported in three patients within 24 h of FMT, and
two patients experienced abdominal pain within 6 h of FMT. These
AEs were short-term, and disappeared within 1 day without any
medical intervention. Of the patients with UC, 94% (32/34) of
responders and 60% (6/10) of non-responders were willing to undergo
FMT again (data not shown).
 | Table IIOutcomes of fecal microbiota
transplantation. |
Table II
Outcomes of fecal microbiota
transplantation.
| Outcome | Week 4 | Week 12 |
|---|
| Steroid-free
clinical remission | 31 (70.5%) | 30 (68.2%) |
| Steroid-free
clinical response | 37 (84.1%) | 34 (77.3%) |
| Steroid-free
remission | 0 | 0 |
 | Table IIIBaseline characteristics of
responders and non-responders. |
Table III
Baseline characteristics of
responders and non-responders.
| Parameters | Responders
(n=34) | Non-responders
(n=10) | P-value |
|---|
| Age (years) | 42.7±14.5 | 49.1±14.4 | 0.25 |
| Sex | | | 0.30 |
|
Male | 22.0 (62%) | 4.0 (40%) | |
|
Female | 12.0 (38%) | 6.0 (60%) | |
| Smoke | | | 0.69 |
|
Smoker | 6.0 (18%) | 3.0 (30%) | |
|
Non-smoker | 28.0 (82%) | 7.0 (70%) | |
| Disease extent | | | 0.93 |
|
Proctitis | 8.0 (24%) | 2.0 (20%) | |
|
Left-sided | 18.0 (52%) | 6.0 (60%) | |
|
Extensive | 8.0 (24%) | 2.0 (20%) | |
| Disease duration
(months) | 56.4±24.4 | 53.3±29.5 | 0.74 |
| Concomitant
drugs | | | |
|
None | 3.0 (9%) | 1.0 (10%) | |
|
Oral
5-aminosalicylate | 21.0 (62%) | 7.0 (70%) | 0.92 |
|
Oral
immunomodulator | 8.0 (24%) | 2.0 (20%) | 0.84 |
|
Oral
steroids | 9.0 (26%) | 2.0 (20%) | 1.00 |
| Previous anti-TNF
therapy | 3.0 (9%) | 1.0 (10%) | |
| Total Mayo
score | 5.9±2.0 | 5.8±2.0 | 0.84 |
|
3-5 | 18.0 (53%) | 5.0 (50%) | |
|
6-10 | 16.0 (47%) | 5.0 (50%) | |
| Mayo endoscopic
subscore | | | 0.98 |
|
1 | 10.0 (29%) | 3.0 (30%) | |
|
2 | 18.0 (53%) | 5.0 (50%) | |
|
3 | 6.0 (18%) | 2.0 (20%) | |
| UCEIS score | 4.3±2.1 | 4.5±2.2 | 0.81 |
| Mayo clinical
score | 4.1±1.5 | 3.9±1.5 | 0.77 |
| IBDQ score | | | |
|
ESR
(mm/h) | 22.4±18.4 | 35.8±25.1 | 0.07 |
|
CRP
(mg/l) | 2.7±3.5 | 5.1±2.0 | 0.42 |
|
PCT
(ng/ml) | 0.1±0.1 | 0.1±0.0 | 0.85 |
|
White blood
cell count (x109 cells/l) | 7.1±1.9 | 8.0±1.5 | 0.13 |
|
Red blood
cell count (x1012 cells/l) | 4.4±0.7 | 4.4±0.6 | 0.82 |
|
Hemoglobin
(g/l) | 123.0±23.7 | 116.5±29.2 | 0.74 |
|
Platelet
count (x109 cells/l) | 308.5±118.8 | 358.6±57.8 | 0.13 |
|
Albumin
(g/l) | 38.2±4.3 | 36.6±5.0 | 0.33 |
| Donor identifier
no. | | | 0.30 |
|
1 | 17.0 (50%) | 3.0 (30%) | |
|
2 | 1.0 (3%) | 1.0 (10%) | |
|
3 | 16.0 (47%) | 6.0 (60%) | |
Microbiome results.
Fecal samples obtained 1 day before FMT, and at
weeks 4 and 12 post-FMT were evaluated. Microbiota analyses were
performed in 32 fecal samples from 12 patients, who were all
responders, and 12 donors. The fecal samples were categorized into
four groups: Pre-FMT (UC.B), week 4 (UC.A1), week 12 (UC.A2) and
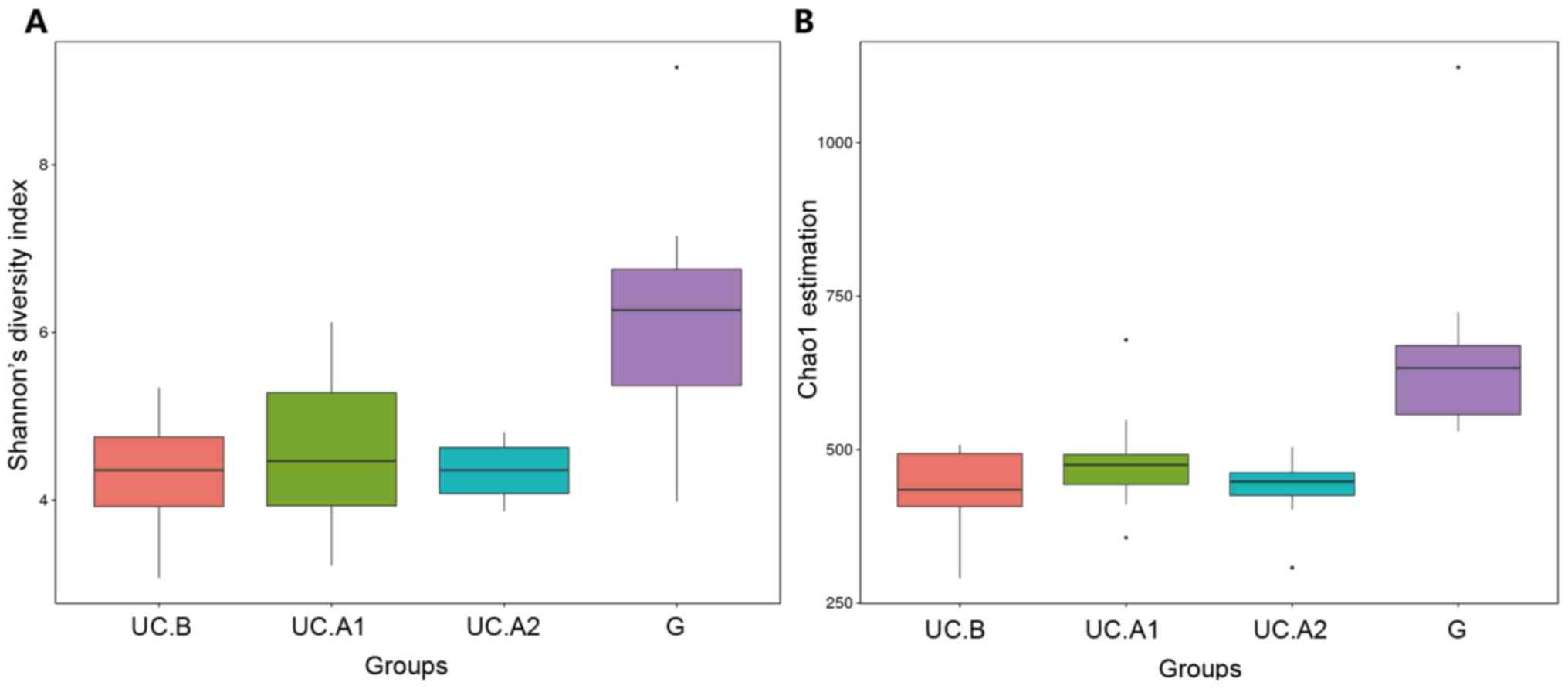
donors (G). The diversity (Shannon's diversity index) and richness
of the fecal microbiota (Chao1 estimator) were markedly decreased
in patients with UC pre-FMT compared with healthy donors
(P<0.05; Fig. 3A and B). After FMT, these two indicators
suggested that the diversity and richness of the fecal microbiota
were increased at week 4 post-FMT but then slightly decreased at
week 12 post-FMT (Fig. 3A and
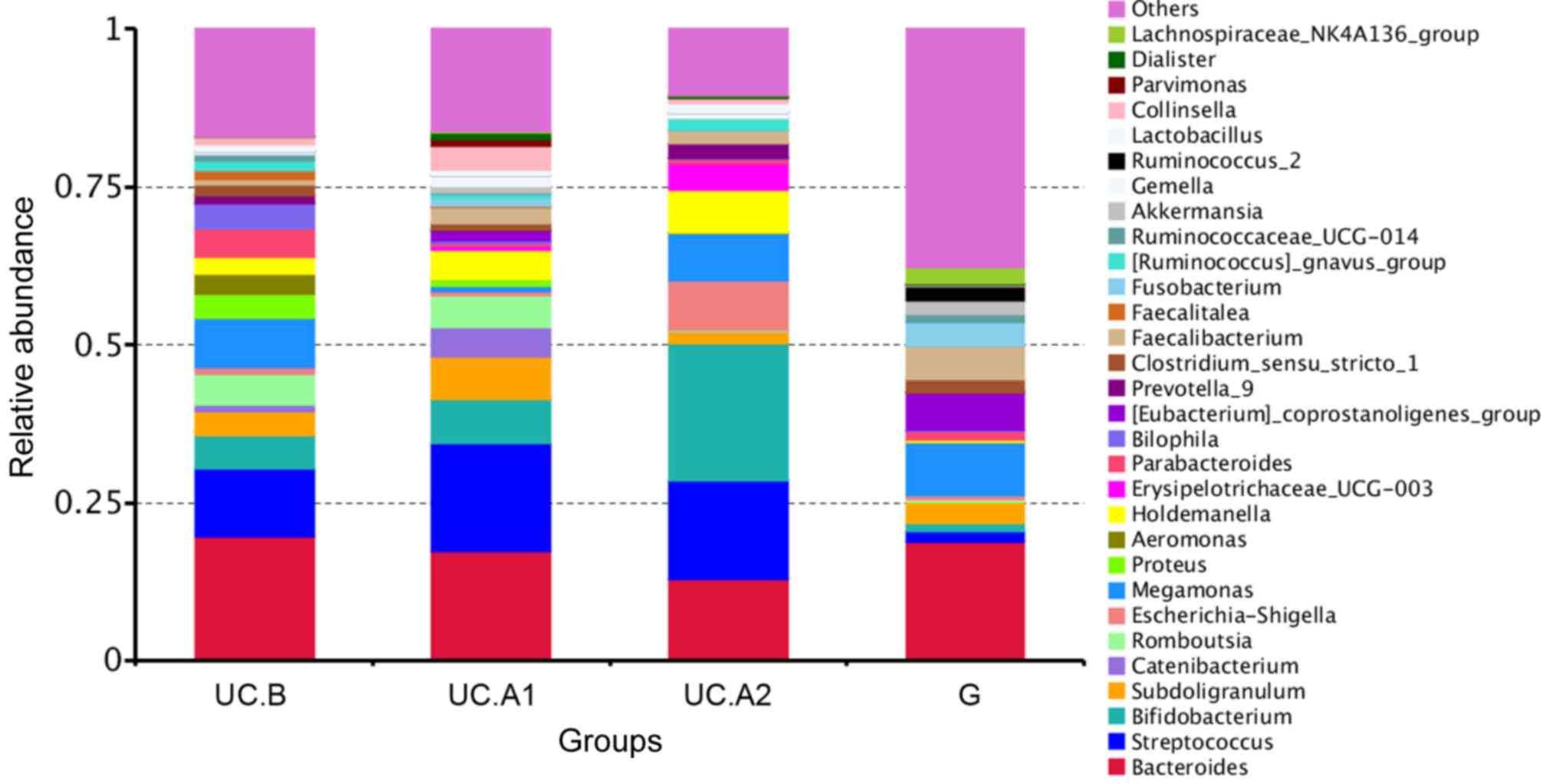
B). Patients with UC displayed a
decreased abundance in Ruminococcus_2 and
Faecalibacterium, and an increased abundance in
Bifidobacterium, Escherichia-Shigella,
Faecalitalea, Streptococcus, Aeromonas and
Proteobacter (Fig. 4). After
FMT, some alterations in the abundance of microbiota in patients
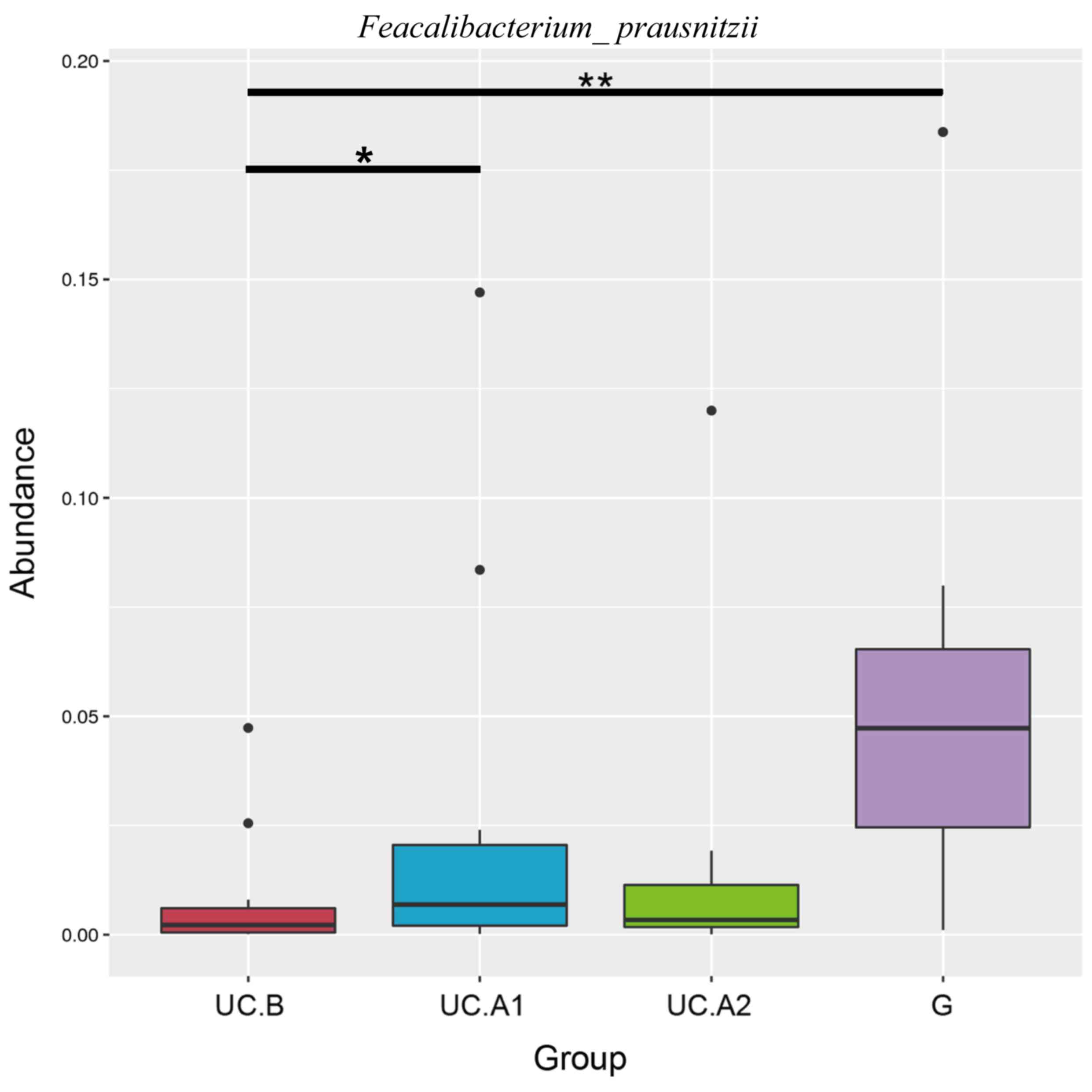
with UC were markedly similar to that of healthy donors. F.
prausnitzii abundance displayed a significant decrease in
patients with UC compared with healthy donors (P<0.01; Fig. 5). At week 4 post-FMT, F.
prausnitzii abundance was significantly increased (P<0.05)
in patients with UC compared with the baseline abundance, and was
decreased compared with healthy donors (P<0.05; Fig. 5).
Discussion
The present study demonstrated that three rounds of
FMT effectively treated mild to moderate active UC, as evidenced by
the steroid-free clinical responses that occurred in 77.3% (34/44)
of patients at 12 weeks post-FMT. The Mayo clinical score
significantly decreased at week 4 compared with the baseline in FMT
treated patients (1.91±1.07 vs. 4.02±1.47; P<0.001). No AEs
occurred in 93.2% (41/44) of patients after FMT or during the 12
weeks of follow-up (data not shown). The results suggested an
improved effect of FMT in patients with UC compared with previous
studies (13,28-30). The improved efficacy displayed in the present
study may be explained by the modified FMT procedures used,
including stool preparation (feces exposure to air for <30 min),
microbiota resource (fresh; all FMTs for one patient were derived
from the same donor), delivery route (via TET tube; remained in the
right lateral position for 30 min to maintain the largest contact
area between microbiota and intestines), dosage (150 ml to avoid
inducing intestinal peristalsis) and intensity (three times within
one week).
Fresh donor feces have to be diluted and homogenized
to an administrable form before FMT can be performed (31). In the majority of previous studies
(32-35),
donor stool was mixed in normal sterile, non-bacteriostatic saline,
which is presumed to guarantee the quality of microbiota.
Subsequently, the mixture was homogenized, often manually, and
filtered using a gauze, strainer or coffee filter. This
purification process may alter the bacterial levels in the fecal
suspension. In the present study, the fresh donor feces were
microfiltered and centrifuged using an automatic purification
system, which ensured the quality of fecal microbiota in the
suspension.
It is not clear whether fresh or frozen-thawed
microbiota improves the efficiency of FMT; however, frozen-thawed
microbiota is a more convenient method (36,37).
Hamilton et al (38) reported
the successful use of standardized, partially purified and frozen
fecal microbiota to treat C. difficile infection.
High-throughput 16S rRNA gene sequencing has displayed stable
engraftment of gut microbiota following FMT (39). Moayyedi et al (13) reported efficacy of frozen-thawed
stool in FMT for active UC. Furthermore, other previous studies
have reported that both frozen (15)
and fresh (13,14) donor stool were effective for UC.
However, Nishida et al (40)
doubted the efficacy of using fresh stool for FMT in patients with
UC, but the present study further suggested that this application
was efficacious in UC. Another debate regarding FMT is whether
pooled or single donor stool results in the highest efficacy. In a
study including 81 patients who received FMT or placebo enemas for
5 days a week for 8 weeks, Paramsothy et al (15) used pooled donor stool. The aim when
using pooled donor stool was to increase the diversity of
microorganisms in the stool suspension. Cao et al (12) reported that 27% of the patients in
the FMT group displayed steroid-free clinical and endoscopic
remission at week 8 compared with 8% in the placebo group (P=0.02).
However, in a study conducted by Rossen et al (14), no statistically significant
difference in clinical and endoscopic remission between the FMT and
control groups of patients with mild to moderate active UC was
reported. However, the microbiota of the responders displayed
distinct features compared with the non-responders. A key advantage
of a pooled stool is that it increases the chances of transmitting
key bacteria to the recipient; however, it is not clear whether
this hypothesis is translated into real efficacy (41,42).
The frequency and duration between each FMT also
impacts the outcomes. Paramsothy et al (15) used a large dose over a long duration
(5 days per week for 8 weeks; 40 doses; 1500 g), and Moayyedi et
al (13) delivered 8.3 g stool
per week for 6 weeks via enema (6 doses; a total of 49.8 g). These
two aforementioned studies achieved similar remission rates,
therefore, it could be hypothesized that moderate dose and frequent
application may improve the efficiency of FMT.
A number of systematic reviews have reported various
approaches of FMT for the treatment of IBD, including colonoscopy,
retention enema, nasoduodenal tube, pills or a combination
(43,44). Paramsothy et al (15) used the retention enema approach to
perform FMT in patients with UC, while Gordon et al
(11) employed a nasoduodenal tube
approach, and Cold et al (28) investigated the use of FMT capsules.
According to two retrospective studies (45,46),
nasointestinal delivery can be uncomfortable and result in a number
of AEs, including severe nausea, vomiting, reduced food intake and
sensory discomfort; delivery of the fecal microbiota suspension to
the cecum may be difficult with the retention enema method;
furthermore, the FMT capsule preparation increases the exposure
time of the microbiota to the air, thus affecting bacterial
activity. In the present study, a TET fixed to the cecum was used
for the delivery of the fecal microbiota suspension to ensure the
infusion of fecal microbiota into the whole colon. The high
remission rate in the present study may be explained by the
standardized and automatic purification process, fresh microbiota,
appropriate dosage and intensity, and use of TET.
The gut microbiota is involved in the development of
intestinal inflammation and UC. Therefore, microbial manipulation
could be an alternative therapeutic approach for UC (47). FMT may serve as an effective
treatment strategy for UC, as it is able to correct the altered gut
microbial community and restore microbial homeostasis (48). In the present study, both the
diversity (Shannon's diversity index) and abundance (Chao1
estimator) of the fecal microbiota were markedly decreased in
patients with UC compared with healthy donors. However, both
indexes at pre-FMT, and weeks 4 and 12 post-FMT displayed no
significant difference. The results of the present study suggested
that the majority of patients with UC achieved steroid-free
clinical response or remission, but no steroid-free remission,
after receiving FMT, indicating that FMT may ameliorate UC, but did
not cure the disease. Therefore, it could be suggested that
patients with UC may require repeated FMT to treat the disease.
Consistent with previous studies, the present study
suggested that alterations to the enteric microbiota following FMT
were primarily manifested by the decrease of pathogenic bacteria
(for example, Faecalitalea and Proteobacter) and the
increase of probiotic bacteria (for example,
Bifidobacterium, Ruminococcus and
Faecalibacterium). However, pathogenic bacterium
Escherichia-Shigella decreased at 4 weeks post-FMT but then
increased at 12 weeks post-FMT compared with pre-FMT, possibly due
to a reduction in the inhibitory effect of probiotics on pathogens
over time. The present study focused on alterations to the
abundance of F. prausnitzii, which is a type of
anti-inflammatory and health-promoting probiotic (49). In the intestine, F.
prausnitzii produce butyrate, a major energy source for
colonocytes to fight against IBD (50). In addition, butyrate can reduce
intestinal mucosa inflammation by inhibiting NF-κβ transcription
factor activation, upregulating peroxisome proliferator-activated
receptor-γ expression and inhibiting interferon-γ expression
(51). F. prausnitzii
abundance is correlated with various IBD-related signaling pathway
mediators, including T helper 17 cells/interleukin (IL)-17,
forkhead box 3-T regulatory-transforming growth factor-β/IL-10 and
IL-23(52). A decreased abundance of
F. prausnitzii is associated with the development and
recurrence of UC (49). Similarly,
it has been suggested that F. prausnitzii can be used as a
biomarker for the diagnosis and treatment of IBD (53). In the present study, the abundance of
F. prausnitzii was significantly decreased in patients with
UC compared with healthy donors. After FMT, the levels of F.
prausnitzii were significantly increased at week 4 compared
with the baseline, but remained lower compared with healthy donors.
F. prausnitzii abundance is strongly correlated with the
diagnostic and therapeutic effectiveness of FMT (54).
The present study had a number of limitations.
Firstly, the present study was an open-label study and not a
double-blind RCT; therefore, the results may have included
potential bias from the researchers and patients, and the placebo
effect cannot be ruled out. Secondly, the limitations of TET are as
follows: i) Patients had to take purgatives to clean the colon,
which may have influenced the gut flora; ii) although the TET tube
was maintained in the same position for repeated FMT delivery, the
tube usually fell out of place spontaneously at ~1 week, therefore
it may be difficult to use the TET method for long-term delivery;
and iii) the TET tube was positioned using colonoscopy, therefore
it could be suggested that the method is only suitable for diseases
that occur near to the anus, and is not ideal for diseases
localized in the small intestine. Thirdly, partial Mayo scores were
used as important criteria for classifying disease severity,
however, these scores are primarily focused on clinical symptoms.
Furthermore, the mucosal activity was only determined by
colonoscopy in 10 responders and not in all patients. Therefore,
endoscopic remission or responses could not be investigated in the
present study. Fourthly, the ESR and other related inflammatory
indicators in non-responders were slightly increased after FMT. It
was hypothesized that FMT could introduce a large number of
exogenous gut microbiota into the patient's intestine, which might
increase intestinal immunity. However, in the present study
individual differences were not excluded and a larger sample size
is required to further investigate the efficiency of FMT for UC.
Finally, the observation period was only 12 weeks; therefore,
future studies are required to assess the long-term outcomes of the
TET therapeutic strategy for UC.
The therapeutic role of FMT in UC varies in
different reports, as evidenced by the rate of clinical response
ranging between 39 and 55% in four RCTs investigating the use of
FMT in UC (55). Ramai et al
(36) reported that the clinical
response in patients at 1 month and 3 months after FMT was 74.3 and
51.4%, respectively. A pilot study in India reported 87.1% clinical
response, 58.1% endoscopic remission and 45.2% histological
remission at week 48 post-FMT (29).
The patients with UC treated with FMT in the present study
displayed a steroid-free clinical response rate of 84.1 and 77.3%
at week 4 and week 12, respectively. The high response rate may
have been due to the following reasons: i) The modified FMT
procedures, including stool preparation, microbiota resource,
delivery route, dosage, and intensity; ii) certain patients had an
irregular medical history of taking antibiotics and hormones due to
repeated enteritis, which may have been a reason for repeated
episodes of UC; iii) the donors in the present study were young and
had good habits and lifestyles, making the composition of the gut
microbiota active and compatible. However, the FMT method requires
further investigation into the effects of antibiotic use before
FMT, fresh or frozen administration, and the location (upper GI or
lower GI tract) and frequency of administration. In the present
study, the TET procedure, frequency of administration and use of
150-200 g fresh donated feces were based on previous studies
investigating the use of FMT treatment for UC (21,31,56,57). In
addition, the 16S sequencing results of the present study suggested
that the relative abundance of F. prausnitzii significantly
increased at 4 weeks post-FMT, which had not been reported in
previous FMT studies. F. prausnitzii is an important
short-chain fatty acid bacteria in the intestine; therefore, it has
been suggested that FMT should focus on the separation and
cultivation of functional bacteria (49).
In conclusion, the results of the present study
suggested that the delivery of fresh microbiota suspension via TET
was an effective and safe method for patients with mild to moderate
active UC. Furthermore, F. prausnitzii may serve as a
diagnostic and therapeutic biomarker of FMT in patients with UC.
Additionally, the results suggested that the donor selection, stool
preparation, delivery route, dosage and intensity of FMT should be
standardized. Further investigation using larger multi-center
studies with a longer follow up period and data analysis on
histology, endoscopy and the microbiome are required to confirm the
efficacy and safety of FMT for UC remission.
Supplementary Material
TET insertion images. A TET tube was
inserted via the anus into the cecum, as far as the terminus of the
ileum, using an endoscope, and while the head of the TET tube was
kept stable, the endoscope was carefully removed. (A) The endoscope
was reinserted and the head of the TET was fixed with a clamp that
was attached to the intestinal wall. (B and C) A further two loops
of the TET were fixed with clamps to the intestinal wall while
removing the endoscope. (D) The end of the TET tube was fixed with
tape to the outside of the skin at the lower back. TET,
transendoscopic enteral tubing.
Transendoscopic enteral tubing
tube.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Guangzhou
General Science and Technology Project of Health and Family
Planning (grant nos. 20181A011007 and 20191A011001), the National
Natural Science Foundation of China (grant no. NSFC 81871905), the
Natural Science Foundation of Guangdong Province (grant. no.
2018A030313676) and the Guangzhou Planned Project of Science and
Technology (grant nos. 201707010275 and 201904010132).
Availability of data and materials
The datasets analyzed during the present study are
not publicly available due to patient privacy concerns but are
available from the corresponding author on reasonable request.
Authors' contributions
HTC contributed to the design of the study,
recruited the patients and drafted the manuscript. HLH performed
the statistical analysis, interpreted the data and drafted the
manuscript. HMX and QLL performed the sample collection and
performed DNA extraction. JH collected the clinical information and
performed the follow-up examinations. YQL and YLZ prepared the
fecal samples into filtrate for administration during the FMT
procedure. YQN and YJZ designed and supervised the study,
interpreted the data and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guangzhou First People's Hospital (approval no.
K-2017-103-02). Written informed consent was obtained from all
patients.
Patient consent for publication
All patients provided written consent for
publication of all figures/pictures including endoscopy and patient
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feuerstein JD and Cheifetz AS: Ulcerative
colitis: Epidemiology, diagnosis, and management. Mayo Clin Proc.
89:1553–1563. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kaplan GG: The global burden of IBD: From
2015 to 2025. Nat Rev Gastroenterol Hepatol. 12:720–727.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Loftus EV Jr: Clinical epidemiology of
inflammatory bowel disease: Incidence, prevalence, and
environmental influences. Gastroenterology. 126:1504–1517.
2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takahashi K, Nishida A, Fujimoto T, Fujii
M, Shioya M, Imaeda H, Inatomi O, Bamba S, Sugimoto M and Andoh A:
Reduced abundance of butyrate-producing bacteria species in the
fecal microbial community in Crohn's disease. Digestion. 93:59–65.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou Y, Chen H, He H, Du Y, Hu J, Li Y, Li
Y, Zhou Y, Wang H, Chen Y and Nie Y: Increased Enterococcus
faecalis infection is associated with clinically active Crohn
disease. Medicine (Baltimore). 95(e5019)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kostic AD, Xavier RJ and Gevers D: The
microbiome in inflammatory bowel disease: Current status and the
future ahead. Gastroenterology. 146:1489–1499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jackson B and De Cruz P: Algorithms to
facilitate shared decision-making for the management of
mild-to-moderate ulcerative colitis. Expert Rev Gastroenterol
Hepatol. 12:1079–1100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Coskun M, Vermeire S and Nielsen OH: Novel
targeted therapies for inflammatory bowel disease. Trends Pharmacol
Sci. 38:127–142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
D'Haens GR and Jobin C: Fecal microbial
transplantation for diseases beyond recurrent clostridium difficile
infection. Gastroenterology. 157:624–636. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sbahi H and Di Palma JA: Faecal microbiota
transplantation: Applications and limitations in treating
gastrointestinal disorders. BMJ Open Gastroenterol 3.
e000087:2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gordon H and Harbord M: A patient with
severe Crohn's colitis responds to Faecal Microbiota
Transplantation. J Crohns Colitis. 8:256–257. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cao Y, Zhang B, Wu Y, Wang Q, Wang J and
Shen F: The value of fecal microbiota transplantation in the
treatment of ulcerative colitis patients: A systematic review and
meta-analysis. Gastroenterol Res Pract 2018.
(5480961)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moayyedi P, Surette MG, Kim PT, Libertucci
J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch
W and Lee CH: Fecal microbiota transplantation induces remission in
patients with active ulcerative colitis in a randomized controlled
trial. Gastroenterology. 149(102.e6-109.e6)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rossen NG, Fuentes S, van der Spek MJ,
Tijssen JG, Hartman JH, Duflou A, Löwenberg M, van den Brink GR,
Mathus-Vliegen EM, de Vos WM, et al: Findings from a randomized
controlled trial of fecal transplantation for patients with
ulcerative colitis. Gastroenterology.
149(110.e4-118.e4)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Paramsothy S, Kamm MA, Kaakoush NO, Walsh
AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W,
Paramsothy R, et al: Multidonor intensive faecal microbiota
transplantation for active ulcerative colitis: A randomised
placebo-controlled trial. Lancet. 389:1218–1228. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Costello SP, Soo W, Bryant RV, Jairath V,
Hart AL and Andrews JM: Systematic review with meta-analysis:
Faecal microbiota transplantation for the induction of remission
for active ulcerative colitis. Aliment Pharmacol Ther. 46:213–224.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schroeder KW, Tremaine WJ and Ilstrup DM:
Coated oral 5-aminosalicylic acid therapy for mildly to moderately
active ulcerative colitis. A randomized study. N Engl J Med.
317:1625–1629. 1987.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paramsothy S, Borody TJ, Lin E, Finlayson
S, Walsh AJ, Samuel D, van den Bogaerde J, Leong RW, Connor S, Ng
W, et al: Donor recruitment for fecal microbiota transplantation.
Inflamm Bowel Dis. 21:1600–1606. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hassan C, Bretthauer M, Kaminski MF,
Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green
S, Kuiper T, et al: Bowel preparation for colonoscopy: European
Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy.
45:142–150. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cui B, Li P, Xu L, Zhao Y, Wang H, Peng Z,
Xu H, Xiang J, He Z, Zhang T, et al: Step-up fecal microbiota
transplantation strategy: A pilot study for steroid-dependent
ulcerative colitis. J Transl Med. 13(298)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peng Z, Xiang J, He Z, Zhang T, Xu L, Cui
B, Li P, Huang G, Ji G, Nie Y, et al: Colonic transendoscopic
enteral tubing: A novel way of transplanting fecal microbiota.
Endosc Int Open. 4(E610-E613)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guyatt G, Mitchell A, Irvine EJ, Singer J,
Williams N, Goodacre R and Tompkins C: A new measure of health
status for clinical trials in inflammatory bowel disease.
Gastroenterology. 96:804–810. 1989.PubMed/NCBI
|
|
23
|
Cui B, Gai Z, She X, Wang R and Xi Z:
Effects of chronic noise on glucose metabolism and gut
microbiota-host inflammatory homeostasis in rats. Sci Rep.
6(36693)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Edgar RC: UPARSE: Highly accurate OTU
sequences from microbial amplicon reads. Nat Methods. 10:996–998.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Caporaso JG, Kuczynski J, Stombaugh J,
Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich
JK, Gordon JI, et al: QIIME allows analysis of high-throughput
community sequencing data. Nat Methods. 7:335–336. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
White JR, Nagarajan N and Pop M:
Statistical methods for detecting differentially abundant features
in clinical metagenomic samples. PLoS Comput Biol.
5(e1000352)2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Feagan BG, Patel H, Colombel JF, Rubin DT,
James A, Mody R and Lasch K: Effects of vedolizumab on
health-related quality of life in patients with ulcerative colitis:
Results from the randomised GEMINI 1 trial. Aliment Pharmacol Ther.
45:264–275. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cold F, Browne PD, Günther S, Halkjaer SI,
Petersen AM, Al-Gibouri Z, Hansen LH and Christensen AH: Multidonor
FMT capsules improve symptoms and decrease fecal calprotectin in
ulcerative colitis patients while treated-an open-label pilot
study. Scand J Gastroenterol. 54:289–296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sood A, Mahajan R, Singh A, Midha V, Mehta
V, Narang V, Singh T and Singh Pannu A: Role of faecal microbiota
transplantation for maintenance of remission in patients with
ulcerative colitis: A pilot study. J Crohns Colitis. 13:1311–1317.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tian Y, Zhou Y, Huang S, Li J, Zhao K, Li
X, Wen X and Li XA: Fecal microbiota transplantation for ulcerative
colitis: A prospective clinical study. BMC Gastroenterol.
19(116)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang F, Zhang T, Zhu H and Borody TJ:
Evolution of fecal microbiota transplantation in methodology and
ethical issues. Curr Opin Pharmacol. 49:11–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eiseman B, Silen W, Bascom GS and Kauvar
AJ: Fecal enema as an adjunct in the treatment of pseudomembranous
enterocolitis. Surgery. 44:854–859. 1958.PubMed/NCBI
|
|
33
|
Schwan A, Sjölin S, Trottestam U and
Aronsson B: Relapsing clostridium difficile enterocolitis cured by
rectal infusion of homologous faeces. Lancet. 2(845)1983.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cammarota G, Ianiro G, Tilg H,
Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P,
Pintus C, Hart A, et al: European consensus conference on faecal
microbiota transplantation in clinical practice. Gut. 66:569–580.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ott SJ, Waetzig GH, Rehman A,
Moltzau-Anderson J, Bharti R, Grasis JA, Cassidy L, Tholey A,
Fickenscher H, Seegert D, et al: Efficacy of sterile fecal filtrate
transfer for treating patients with clostridium difficile
infection. Gastroenterology. 152(799.e7-811.e7)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ramai D, Zakhia K, Ofosu A, Ofori E and
Reddy M: Fecal microbiota transplantation: Donor relation, fresh or
frozen, delivery methods, cost-effectiveness. Ann Gastroenterol.
32:30–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hui W, Li T, Liu W, Zhou C and Gao F:
Fecal microbiota transplantation for treatment of recurrent C.
difficile infection: An updated randomized controlled trial
meta-analysis. PLoS One. 14(e0210016)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hamilton MJ, Weingarden AR, Sadowsky MJ
and Khoruts A: Standardized frozen preparation for transplantation
of fecal microbiota for recurrent Clostridium difficile infection.
Am J Gastroenterol. 107:761–767. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hamilton MJ, Weingarden AR, Unno T,
Khoruts A and Sadowsky MJ: High-throughput DNA sequence analysis
reveals stable engraftment of gut microbiota following
transplantation of previously frozen fecal bacteria. Gut Microbes.
4:125–135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nishida A, Imaeda H, Ohno M, Inatomi O,
Bamba S, Sugimoto M and Andoh A: Efficacy and safety of single
fecal microbiota transplantation for Japanese patients with mild to
moderately active ulcerative colitis. J Gastroenterol. 52:476–482.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Allegretti JR, Fischer M, Sagi SV, Bohm
ME, Fadda HM, Ranmal SR, Budree S, Basit AW, Glettig DL, de la
Serna EL, et al: Fecal microbiota transplantation capsules with
targeted colonic versus gastric delivery in recurrent clostridium
difficile infection: A comparative cohort analysis of high and lose
dose. Dig Dis Sci. 64:1672–1678. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
El-Salhy M, Hausken T and Hatlebakk JG:
Increasing the dose and/or repeating faecal microbiota
transplantation (FMT) increases the response in patients with
irritable bowel syndrome (IBS). Nutrients. 11:2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kelly CR, Kahn S, Kashyap P, Laine L,
Rubin D, Atreja A, Moore T and Wu G: Update on fecal microbiota
transplantation 2015: Indications, methodologies, mechanisms, and
outlook. Gastroenterology. 149:223–237. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bafeta A, Yavchitz A, Riveros C, Batista R
and Ravaud P: Methods and reporting studies assessing fecal
microbiota transplantation: A systematic review. Ann Intern Med.
167:34–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li N, Tian H, Ma C, Ding C, Ge X, Gu L,
Zhang X, Yang B, Hua Y, Zhu Y and Zhou Y: Efficacy analysis of
fecal microbiota transplantation in the treatment of 406 cases with
gastrointestinal disorders. Zhonghua Wei Chang Wai Ke Za Zhi.
20:40–46. 2017.(In Chinese). PubMed/NCBI
|
|
46
|
Li N, Tian HL, Chen QY, Yang B, Ma CL, Lin
ZL, Zhang XY, Zhao D, Huang ZX, Jiang J and Qin HL: Efficacy
analysis of fecal microbiota transplantation in the treatment of
2010 patients with intestinal disorders. Zhonghua Wei Chang Wai Ke
Za Zhi. 22:861–868. 2019.(In Chinese; Abstract available in Chinese
from the publisher). PubMed/NCBI
|
|
47
|
Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S,
Luo WW, Tan B and Wang XY: Relationship between intestinal
microbiota and ulcerative colitis: Mechanisms and clinical
application of probiotics and fecal microbiota transplantation.
World J Gastroenterol. 24:5–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Paramsothy S, Paramsothy R, Rubin DT, Kamm
MA, Kaakoush NO, Mitchell HM and Castaño-Rodríguez N: Faecal
microbiota transplantation for inflammatory bowel disease: A
systematic review and meta-analysis. J Crohns Colitis.
11:1180–1199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lopez-Siles M, Duncan SH, Garcia-Gil LJ
and Martinez-Medina M: Faecalibacterium prausnitzii: From
microbiology to diagnostics and prognostics. ISME J. 11:841–852.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Duncan SH, Hold GL, Harmsen HJ, Stewart CS
and Flint HJ: Growth requirements and fermentation products of
Fusobacterium prausnitzii, and a proposal to reclassify it
as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst
Evol Microbiol. 52:2141–2146. 2002.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Falony G, Joossens M, Vieira-Silva S, Wang
J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M,
Vandeputte D, et al: Population-level analysis of gut microbiome
variation. Science. 352:560–564. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Qin J, Li R, Raes J, Arumugam M, Burgdorf
KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al: A
human gut microbial gene catalogue established by metagenomic
sequencing. Nature. 464:59–65. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhou Y, Xu ZZ, He Y, Yang Y, Liu L, Lin Q,
Nie Y, Li M, Zhi F, Liu S, et al: Gut microbiota offers universal
biomarkers across ethnicity in inflammatory bowel disease diagnosis
and infliximab response prediction. mSystems. 3:2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hourigan SK, Ahn M, Gibson KM,
Pérez-Losada M, Felix G, Weidner M, Leibowitz I, Niederhuber JE,
Sears CL, Crandall KA and Oliva-Hemker M: Fecal transplant in
children with clostridioides difficile gives sustained reduction in
antimicrobial resistance and potential pathogen burden. Open Forum
Infect Dis. 6(ofz379)2019. View Article : Google Scholar
|
|
55
|
Costello SP, Hughes PA, Waters O, Bryant
RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J,
Campaniello MA, Mavrangelos C, et al: Effect of fecal microbiota
transplantation on 8-week remission in patients with ulcerative
colitis: A randomized clinical trial. JAMA. 321:156–164.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang JW, Wang YK, Zhang F, Su YC, Wang JY,
Wu DC and Hsu WH: Initial experience of fecal microbiota
transplantation in gastrointestinal disease: A case series.
Kaohsiung J Med Sci. 35:566–571. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Long C, Yu Y, Cui B, Jagessar SAR, Zhang
J, Ji G, Huang G and Zhang F: A novel quick transendoscopic enteral
tubing in mid-gut: Technique and training with video. BMC
Gastroenterol. 18(37)2018.PubMed/NCBI View Article : Google Scholar
|