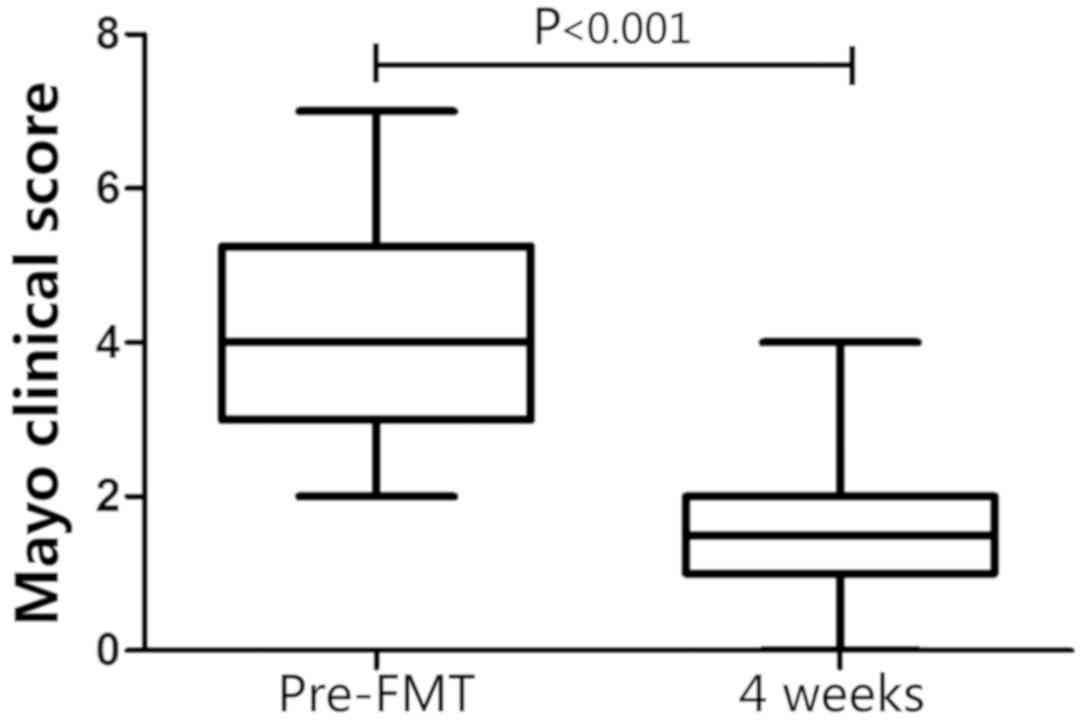
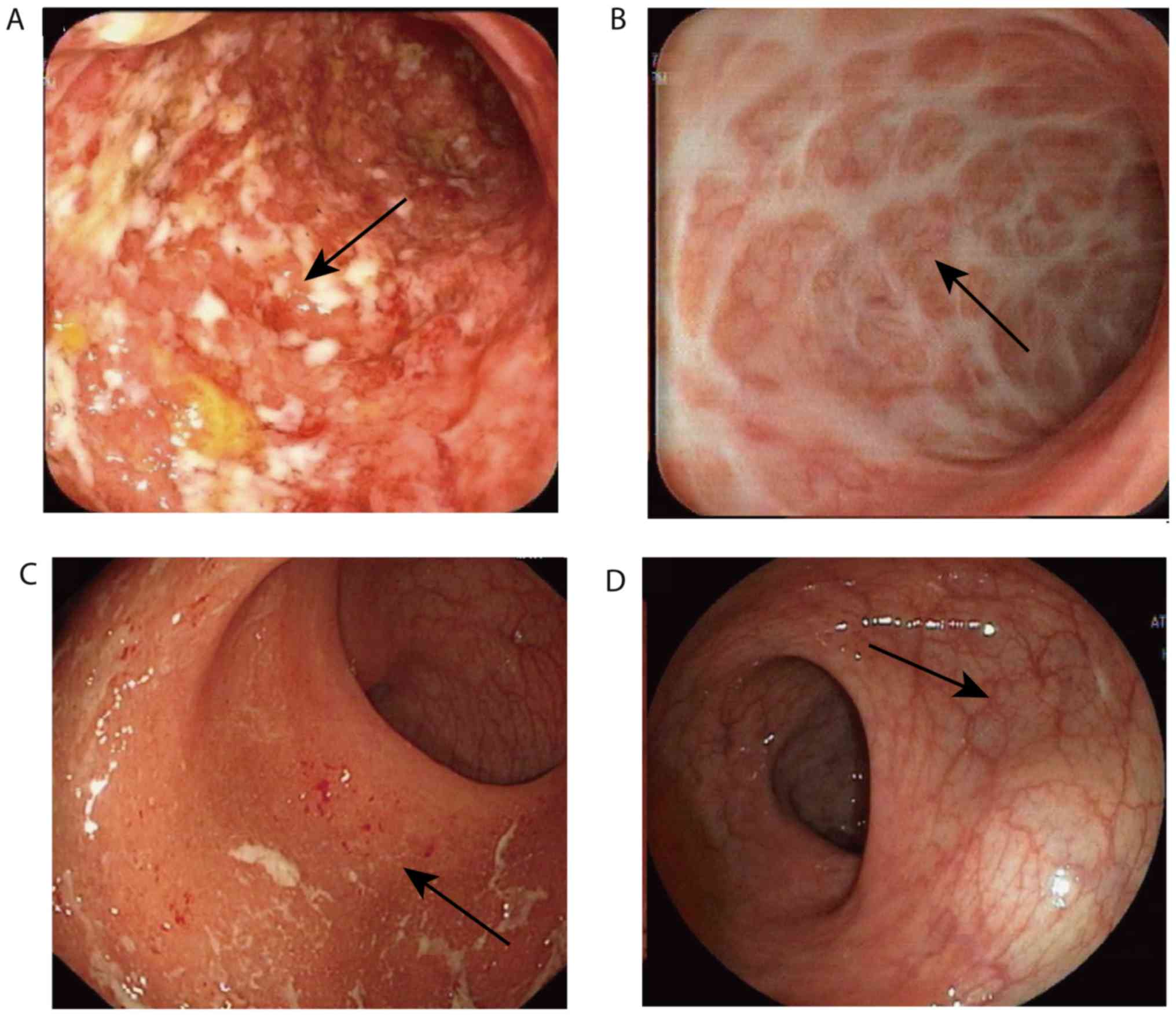
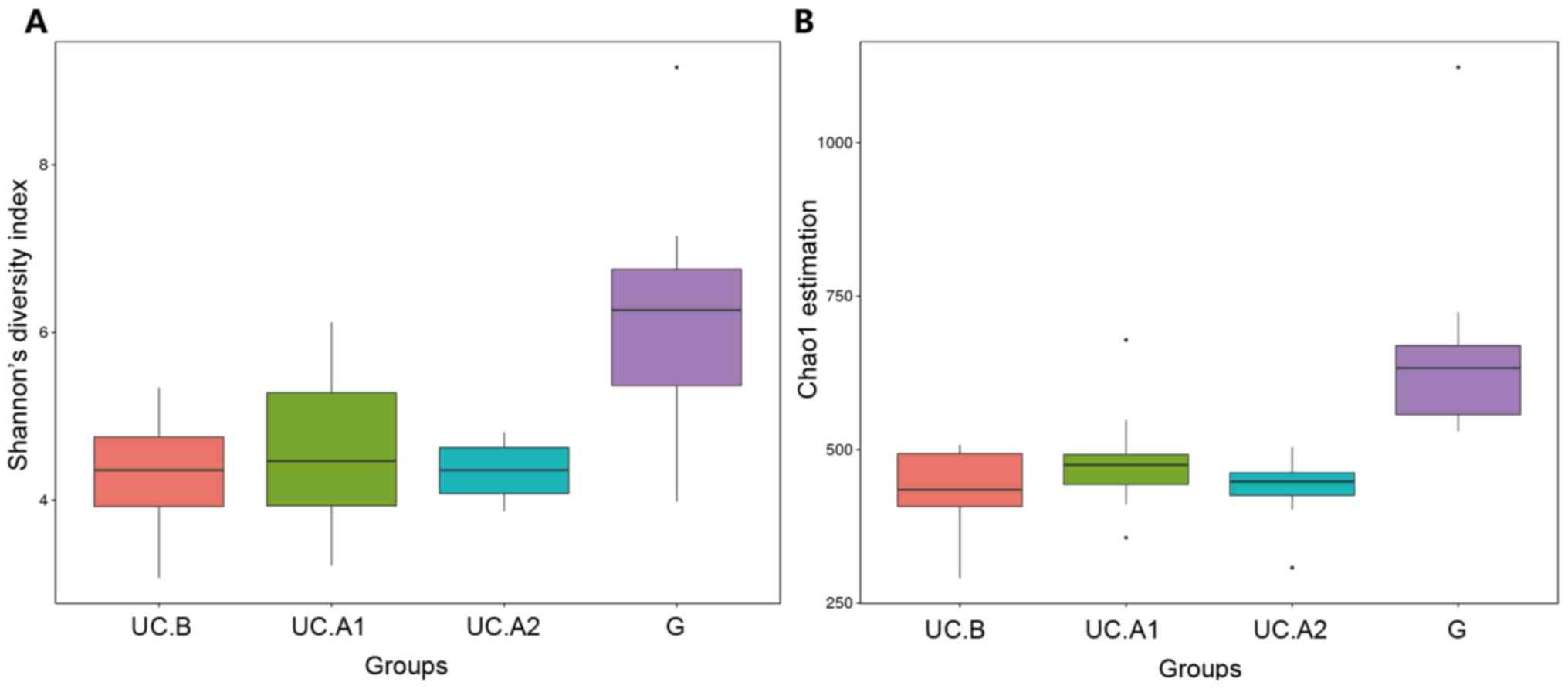
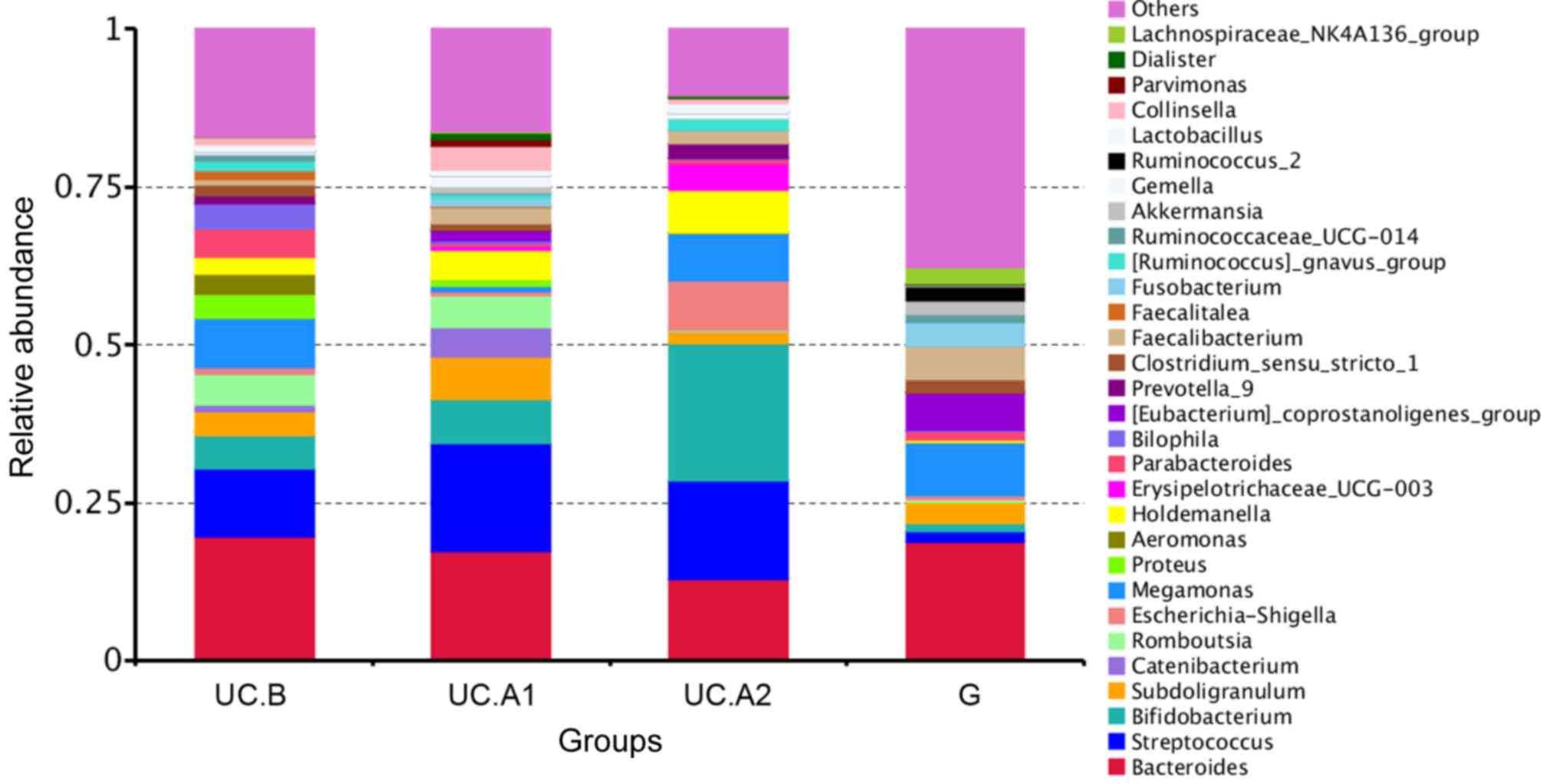
|
1
|
Feuerstein JD and Cheifetz AS: Ulcerative
colitis: Epidemiology, diagnosis, and management. Mayo Clin Proc.
89:1553–1563. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kaplan GG: The global burden of IBD: From
2015 to 2025. Nat Rev Gastroenterol Hepatol. 12:720–727.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Loftus EV Jr: Clinical epidemiology of
inflammatory bowel disease: Incidence, prevalence, and
environmental influences. Gastroenterology. 126:1504–1517.
2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takahashi K, Nishida A, Fujimoto T, Fujii
M, Shioya M, Imaeda H, Inatomi O, Bamba S, Sugimoto M and Andoh A:
Reduced abundance of butyrate-producing bacteria species in the
fecal microbial community in Crohn's disease. Digestion. 93:59–65.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou Y, Chen H, He H, Du Y, Hu J, Li Y, Li
Y, Zhou Y, Wang H, Chen Y and Nie Y: Increased Enterococcus
faecalis infection is associated with clinically active Crohn
disease. Medicine (Baltimore). 95(e5019)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kostic AD, Xavier RJ and Gevers D: The
microbiome in inflammatory bowel disease: Current status and the
future ahead. Gastroenterology. 146:1489–1499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jackson B and De Cruz P: Algorithms to
facilitate shared decision-making for the management of
mild-to-moderate ulcerative colitis. Expert Rev Gastroenterol
Hepatol. 12:1079–1100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Coskun M, Vermeire S and Nielsen OH: Novel
targeted therapies for inflammatory bowel disease. Trends Pharmacol
Sci. 38:127–142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
D'Haens GR and Jobin C: Fecal microbial
transplantation for diseases beyond recurrent clostridium difficile
infection. Gastroenterology. 157:624–636. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sbahi H and Di Palma JA: Faecal microbiota
transplantation: Applications and limitations in treating
gastrointestinal disorders. BMJ Open Gastroenterol 3.
e000087:2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gordon H and Harbord M: A patient with
severe Crohn's colitis responds to Faecal Microbiota
Transplantation. J Crohns Colitis. 8:256–257. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cao Y, Zhang B, Wu Y, Wang Q, Wang J and
Shen F: The value of fecal microbiota transplantation in the
treatment of ulcerative colitis patients: A systematic review and
meta-analysis. Gastroenterol Res Pract 2018.
(5480961)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moayyedi P, Surette MG, Kim PT, Libertucci
J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch
W and Lee CH: Fecal microbiota transplantation induces remission in
patients with active ulcerative colitis in a randomized controlled
trial. Gastroenterology. 149(102.e6-109.e6)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rossen NG, Fuentes S, van der Spek MJ,
Tijssen JG, Hartman JH, Duflou A, Löwenberg M, van den Brink GR,
Mathus-Vliegen EM, de Vos WM, et al: Findings from a randomized
controlled trial of fecal transplantation for patients with
ulcerative colitis. Gastroenterology.
149(110.e4-118.e4)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Paramsothy S, Kamm MA, Kaakoush NO, Walsh
AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W,
Paramsothy R, et al: Multidonor intensive faecal microbiota
transplantation for active ulcerative colitis: A randomised
placebo-controlled trial. Lancet. 389:1218–1228. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Costello SP, Soo W, Bryant RV, Jairath V,
Hart AL and Andrews JM: Systematic review with meta-analysis:
Faecal microbiota transplantation for the induction of remission
for active ulcerative colitis. Aliment Pharmacol Ther. 46:213–224.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schroeder KW, Tremaine WJ and Ilstrup DM:
Coated oral 5-aminosalicylic acid therapy for mildly to moderately
active ulcerative colitis. A randomized study. N Engl J Med.
317:1625–1629. 1987.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paramsothy S, Borody TJ, Lin E, Finlayson
S, Walsh AJ, Samuel D, van den Bogaerde J, Leong RW, Connor S, Ng
W, et al: Donor recruitment for fecal microbiota transplantation.
Inflamm Bowel Dis. 21:1600–1606. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hassan C, Bretthauer M, Kaminski MF,
Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green
S, Kuiper T, et al: Bowel preparation for colonoscopy: European
Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy.
45:142–150. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cui B, Li P, Xu L, Zhao Y, Wang H, Peng Z,
Xu H, Xiang J, He Z, Zhang T, et al: Step-up fecal microbiota
transplantation strategy: A pilot study for steroid-dependent
ulcerative colitis. J Transl Med. 13(298)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peng Z, Xiang J, He Z, Zhang T, Xu L, Cui
B, Li P, Huang G, Ji G, Nie Y, et al: Colonic transendoscopic
enteral tubing: A novel way of transplanting fecal microbiota.
Endosc Int Open. 4(E610-E613)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guyatt G, Mitchell A, Irvine EJ, Singer J,
Williams N, Goodacre R and Tompkins C: A new measure of health
status for clinical trials in inflammatory bowel disease.
Gastroenterology. 96:804–810. 1989.PubMed/NCBI
|
|
23
|
Cui B, Gai Z, She X, Wang R and Xi Z:
Effects of chronic noise on glucose metabolism and gut
microbiota-host inflammatory homeostasis in rats. Sci Rep.
6(36693)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Edgar RC: UPARSE: Highly accurate OTU
sequences from microbial amplicon reads. Nat Methods. 10:996–998.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Caporaso JG, Kuczynski J, Stombaugh J,
Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich
JK, Gordon JI, et al: QIIME allows analysis of high-throughput
community sequencing data. Nat Methods. 7:335–336. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
White JR, Nagarajan N and Pop M:
Statistical methods for detecting differentially abundant features
in clinical metagenomic samples. PLoS Comput Biol.
5(e1000352)2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Feagan BG, Patel H, Colombel JF, Rubin DT,
James A, Mody R and Lasch K: Effects of vedolizumab on
health-related quality of life in patients with ulcerative colitis:
Results from the randomised GEMINI 1 trial. Aliment Pharmacol Ther.
45:264–275. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cold F, Browne PD, Günther S, Halkjaer SI,
Petersen AM, Al-Gibouri Z, Hansen LH and Christensen AH: Multidonor
FMT capsules improve symptoms and decrease fecal calprotectin in
ulcerative colitis patients while treated-an open-label pilot
study. Scand J Gastroenterol. 54:289–296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sood A, Mahajan R, Singh A, Midha V, Mehta
V, Narang V, Singh T and Singh Pannu A: Role of faecal microbiota
transplantation for maintenance of remission in patients with
ulcerative colitis: A pilot study. J Crohns Colitis. 13:1311–1317.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tian Y, Zhou Y, Huang S, Li J, Zhao K, Li
X, Wen X and Li XA: Fecal microbiota transplantation for ulcerative
colitis: A prospective clinical study. BMC Gastroenterol.
19(116)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang F, Zhang T, Zhu H and Borody TJ:
Evolution of fecal microbiota transplantation in methodology and
ethical issues. Curr Opin Pharmacol. 49:11–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eiseman B, Silen W, Bascom GS and Kauvar
AJ: Fecal enema as an adjunct in the treatment of pseudomembranous
enterocolitis. Surgery. 44:854–859. 1958.PubMed/NCBI
|
|
33
|
Schwan A, Sjölin S, Trottestam U and
Aronsson B: Relapsing clostridium difficile enterocolitis cured by
rectal infusion of homologous faeces. Lancet. 2(845)1983.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cammarota G, Ianiro G, Tilg H,
Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P,
Pintus C, Hart A, et al: European consensus conference on faecal
microbiota transplantation in clinical practice. Gut. 66:569–580.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ott SJ, Waetzig GH, Rehman A,
Moltzau-Anderson J, Bharti R, Grasis JA, Cassidy L, Tholey A,
Fickenscher H, Seegert D, et al: Efficacy of sterile fecal filtrate
transfer for treating patients with clostridium difficile
infection. Gastroenterology. 152(799.e7-811.e7)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ramai D, Zakhia K, Ofosu A, Ofori E and
Reddy M: Fecal microbiota transplantation: Donor relation, fresh or
frozen, delivery methods, cost-effectiveness. Ann Gastroenterol.
32:30–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hui W, Li T, Liu W, Zhou C and Gao F:
Fecal microbiota transplantation for treatment of recurrent C.
difficile infection: An updated randomized controlled trial
meta-analysis. PLoS One. 14(e0210016)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hamilton MJ, Weingarden AR, Sadowsky MJ
and Khoruts A: Standardized frozen preparation for transplantation
of fecal microbiota for recurrent Clostridium difficile infection.
Am J Gastroenterol. 107:761–767. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hamilton MJ, Weingarden AR, Unno T,
Khoruts A and Sadowsky MJ: High-throughput DNA sequence analysis
reveals stable engraftment of gut microbiota following
transplantation of previously frozen fecal bacteria. Gut Microbes.
4:125–135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nishida A, Imaeda H, Ohno M, Inatomi O,
Bamba S, Sugimoto M and Andoh A: Efficacy and safety of single
fecal microbiota transplantation for Japanese patients with mild to
moderately active ulcerative colitis. J Gastroenterol. 52:476–482.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Allegretti JR, Fischer M, Sagi SV, Bohm
ME, Fadda HM, Ranmal SR, Budree S, Basit AW, Glettig DL, de la
Serna EL, et al: Fecal microbiota transplantation capsules with
targeted colonic versus gastric delivery in recurrent clostridium
difficile infection: A comparative cohort analysis of high and lose
dose. Dig Dis Sci. 64:1672–1678. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
El-Salhy M, Hausken T and Hatlebakk JG:
Increasing the dose and/or repeating faecal microbiota
transplantation (FMT) increases the response in patients with
irritable bowel syndrome (IBS). Nutrients. 11:2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kelly CR, Kahn S, Kashyap P, Laine L,
Rubin D, Atreja A, Moore T and Wu G: Update on fecal microbiota
transplantation 2015: Indications, methodologies, mechanisms, and
outlook. Gastroenterology. 149:223–237. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bafeta A, Yavchitz A, Riveros C, Batista R
and Ravaud P: Methods and reporting studies assessing fecal
microbiota transplantation: A systematic review. Ann Intern Med.
167:34–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li N, Tian H, Ma C, Ding C, Ge X, Gu L,
Zhang X, Yang B, Hua Y, Zhu Y and Zhou Y: Efficacy analysis of
fecal microbiota transplantation in the treatment of 406 cases with
gastrointestinal disorders. Zhonghua Wei Chang Wai Ke Za Zhi.
20:40–46. 2017.(In Chinese). PubMed/NCBI
|
|
46
|
Li N, Tian HL, Chen QY, Yang B, Ma CL, Lin
ZL, Zhang XY, Zhao D, Huang ZX, Jiang J and Qin HL: Efficacy
analysis of fecal microbiota transplantation in the treatment of
2010 patients with intestinal disorders. Zhonghua Wei Chang Wai Ke
Za Zhi. 22:861–868. 2019.(In Chinese; Abstract available in Chinese
from the publisher). PubMed/NCBI
|
|
47
|
Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S,
Luo WW, Tan B and Wang XY: Relationship between intestinal
microbiota and ulcerative colitis: Mechanisms and clinical
application of probiotics and fecal microbiota transplantation.
World J Gastroenterol. 24:5–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Paramsothy S, Paramsothy R, Rubin DT, Kamm
MA, Kaakoush NO, Mitchell HM and Castaño-Rodríguez N: Faecal
microbiota transplantation for inflammatory bowel disease: A
systematic review and meta-analysis. J Crohns Colitis.
11:1180–1199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lopez-Siles M, Duncan SH, Garcia-Gil LJ
and Martinez-Medina M: Faecalibacterium prausnitzii: From
microbiology to diagnostics and prognostics. ISME J. 11:841–852.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Duncan SH, Hold GL, Harmsen HJ, Stewart CS
and Flint HJ: Growth requirements and fermentation products of
Fusobacterium prausnitzii, and a proposal to reclassify it
as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst
Evol Microbiol. 52:2141–2146. 2002.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Falony G, Joossens M, Vieira-Silva S, Wang
J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M,
Vandeputte D, et al: Population-level analysis of gut microbiome
variation. Science. 352:560–564. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Qin J, Li R, Raes J, Arumugam M, Burgdorf
KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al: A
human gut microbial gene catalogue established by metagenomic
sequencing. Nature. 464:59–65. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhou Y, Xu ZZ, He Y, Yang Y, Liu L, Lin Q,
Nie Y, Li M, Zhi F, Liu S, et al: Gut microbiota offers universal
biomarkers across ethnicity in inflammatory bowel disease diagnosis
and infliximab response prediction. mSystems. 3:2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hourigan SK, Ahn M, Gibson KM,
Pérez-Losada M, Felix G, Weidner M, Leibowitz I, Niederhuber JE,
Sears CL, Crandall KA and Oliva-Hemker M: Fecal transplant in
children with clostridioides difficile gives sustained reduction in
antimicrobial resistance and potential pathogen burden. Open Forum
Infect Dis. 6(ofz379)2019. View Article : Google Scholar
|
|
55
|
Costello SP, Hughes PA, Waters O, Bryant
RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J,
Campaniello MA, Mavrangelos C, et al: Effect of fecal microbiota
transplantation on 8-week remission in patients with ulcerative
colitis: A randomized clinical trial. JAMA. 321:156–164.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang JW, Wang YK, Zhang F, Su YC, Wang JY,
Wu DC and Hsu WH: Initial experience of fecal microbiota
transplantation in gastrointestinal disease: A case series.
Kaohsiung J Med Sci. 35:566–571. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Long C, Yu Y, Cui B, Jagessar SAR, Zhang
J, Ji G, Huang G and Zhang F: A novel quick transendoscopic enteral
tubing in mid-gut: Technique and training with video. BMC
Gastroenterol. 18(37)2018.PubMed/NCBI View Article : Google Scholar
|