Introduction
From the pathological standpoint, in several
diseases, including spondylosis, deformity, tumor, infection,
fracture and instability, it is necessary to reconstruct a stable
structure and correct an abnormal relationship between adjacent
vertebral structures (1). Spinal
fusion may enhance the mechanical stability of the spine via
reconstruction and stabilization of the vertebral column;
therefore, spinal fusion is currently one of the main treatment
options for the aforementioned diseases (2,3). Until
now, autografts have been the gold standard for use as spinal
fusion materials; however, limited bone graft sources and
donor-site morbidity hinder their extensive use, especially for
those cases in which large amounts of bone graft material are
required. By contrast, allograft and xenogenic bone, and other
potential options for bone graft substitution or supplementation,
including ceramics, calcium phosphate compounds, collagen gel and
demineralized bone matrix, have shown significant variability in
osteoinductive properties and clinical efficacy (4-6). Certain biological
factors, such as bone morphogenetic protein have shown similar or
improved fusion rates compared with autografts; however, potential
safety concerns require further clarification (5). With the development of tissue
engineering as an alternative approach for spinal fusion, bone
tissue engineering has become a topic of particular interest
(7,8).
The elements of tissue engineering comprise seed
cells, biological scaffolding and growth factors. Seed cells serve
an important role in the effects of tissue engineering technology
(9). As classical seed cells, bone
marrow mesenchymal stem cells (BM-MSCs) have frequently been
employed in bone tissue engineering due to their multi-lineage
differentiation potential and rapid in vitro amplification
(10,11). MSCs have been reported to exist in
many types of mesenchymal tissue, and different tissue-derived MSCs
differ from each other in properties including proliferation and
differentiation potential, and tissue regeneration capacity
(12,13). Therefore, specific types of MSCs
should be chosen as appropriate for the intended tissue engineering
application.
The intervertebral disc (IVD) is composed of annulus
fibrosus (AF), nucleus pulposus (NP) and cartilage endplate (CEP).
Previous studies reported that different types of MSCs exist in AF
and NP regions (14,15). The present research team identified
MSCs in CEP, which they designated as CEP-derived stem cells
(CESCs), and found that CESCs share similar morphology,
proliferation rate, cell cycle, immunophenotype and stem cell gene
expression with BM-MSCs (16).
Furthermore, CESCs have exhibited superior chondrogenic and
osteogenic potentials compared with BM-MSCs in vitro
(16,17). In an in vivo study, CESCs
showed more powerful NP regeneration potential compared with
AF-derived stem cells, NP-derived stem cells and BM-MSCs derived
from the same patient following transplantation into the rabbit
IVD, and displayed no obvious immune rejection as heterografts
(18). However, the osteogenic
characteristics of CESCs in vivo are unclear. Large
quantities of CEP samples that are usually discarded as clinical
waste in spinal fusion surgeries could be collected and reused for
the extraction of CESCs, and may serve as an adequate seed cell
source for experimental or clinical studies. Therefore, it is
necessary to investigate the in vivo bone formation capacity
of CESCs, and explore whether they have the potential to serve as
seed cells for bone tissue engineering.
In the present study, CESCs and BM-MSCs were
harvested from the same donors who received a lumbar spinal fusion
procedure. After culturing and expanding, the cells were each
seeded into porous hydroxyapatite (PHA). After 14 days in
vitro induction, the cell/PHA composites were tested to
determine the difference in osteogenic mRNA expression between the
two types of seed cells. In addition, cell/PHA composites were
implanted into a rabbit lumbar intertransverse process fusion model
after 3 days in vitro induction. Eight weeks later, those
grafts were gross observed, palpated and inspected with
three-dimensional (3D) computed tomography (CT) reconstruction,
micro-CT and quantitative histology to obtain bone formation
indices for the comparison of in vivo osteogenic
capacity.
Materials and methods
Ethics statement
All procedures were approved by the Institutional
Review Board of Xinqiao Hospital and the patients provided written
informed consent in the study before surgery. All animal
experiments were also approved by the Xinqiao Hospital Committee on
Ethics for the Care and Use of Laboratory Animals.
Isolation and culture of CESCs
The procedures for the isolation and culture of
CESCs were performed as previously described (17,18). CEP
samples were derived from 11 patients (age range: 37.9-61.2 years)
who received lumbar fusion surgery at Xinqiao Hospital (Chongqing,
China) between June 2015 and August 2016. The severity of CEP
damage was determined as described by Rajasekaran et al
(19). The characteristics of the
patients and the tests in which their CESCs were used are shown in
Table I.
 | Table ICharacteristics of the patients
enrolled in the study. |
Table I
Characteristics of the patients
enrolled in the study.
| Case no. | Age (years) | Sex | Symptoms | Diagnosis | Disc level | CEPDT | Test item |
|---|
| 1 | 54 | F | BP-RP |
Spondylolisthesis | L5/S1 | VI | FC |
| 2 | 57 | M | BP-RP | Lumbar disc
herniation | L5/S1 | V | 3D culture |
| 3 | 56 | F | BP-RP | Lumbar disc
herniation | L4/5 | V | 3D culture |
| 4 | 61 | M | BP |
Spondylolisthesis | L5/S1 | V | FC |
| 5 | 62 | F | BP |
Spondylolisthesis | L4/5 | VI | In vivo |
| 6 | 56 | F | BP | Lumbar discogenic
pain | L4/5 | V | In vivo |
| 7 | 51 | F | BP |
Spondylolisthesis | L5/S1 | V | In vivo |
| 8 | 58 | M | BP-RP |
Spondylolisthesis | L5/S1 | VI | FC |
| 9 | 56 | F | BP-RP |
Spondylolisthesis | L4/5 | V | 3D culture |
| 10 | 54 | F | BP-RP |
Spondylolisthesis | L5/S1 | VI | In vivo |
| 11 | 51 | F | BP-RP |
Spondylolisthesis | L5/S1 | V | In vivo |
Isolation and culture of BM-MSCs
Bone marrow samples were obtained from the
aforementioned patients. Isolation and culture procedures for
BM-MSCs were performed as previously described (20,21). In
brief, 6 ml bone marrow was aspirated from the iliac crest and
centrifuged at 900 x g for 25 min at 20˚C with an equal volume of
1.073 g/ml Percoll solution (Sigma-Aldrich; Merck KGaA).
Mononuclear cells were carefully extracted and rinsed twice with
PBS. Finally, the cells were suspended with DMEM/F12 (Hyclone)
supplemented with 10% fetal calf serum (FCS; Hyclone) and 100 U/ml
penicillin-streptomycin (Hyclone), then cultured in
25-cm2 cell culture flasks (Costar; Corning, Inc.) with
an atmosphere of 5% CO2 at 37˚C. Thereafter, the culture
medium was refreshed every 3 days. When 90% confluence was reached,
the cells were passaged.
Determination of the cell surface
antigen profile
BM-MSCs and CESCs from 3 patients were analyzed to
determine their respective surface immunophenotypes by flow
cytometry. Cells were washed with PBS twice and fixed with 4%
paraformaldehyde at 4˚C for 10 min, then were incubated in the dark
for 20 min with fluorescein isothiocyanate (FITC)-coupled
monoclonal antibodies: CD11b-FITC, CD34-FITC, CD45-FITC, CD90-FITC,
and CD105-FITC. The cells were then washed with PBS twice and
re-suspended in 200 µl PBS. Finally, the cell suspension was passed
through a Flow Cytometer, and the antigen phenotype was analyzed
using Flow Jo software (version 7.5, Flow Jo LLC). Mouse isotype
antibodies were used as controls.
Stem cell seeding in the PHA
graft
BM-MSCs and CESCs derived from 8 patients were
trypsinized, rinsed and re-suspended in fresh medium. After
microscopic counting, 3x106 cells were dropped into PHA
(1.0x1.0x3.0 cm; porosity 42.2±1.8%; average pore diameter 180±60
µm) and centrifuged at 80 x g for 1 min at 20˚C. Cells/PHA grafts
were incubated in an incubator with 5% CO2 at 37˚C for
24 h. Then, they were induced for 2 weeks with basal medium
supplemented with 100 nM dexamethasone, 0.2 mM ascorbate and 10 mM
β-glycerophosphate (Sigma-Aldrich; Merck KGaA). During the
induction period, the osteogenic medium was changed every 3
days.
Quantitative assay of alkaline
phosphatase (ALP) activity
To quantify the ALP activity of the in vitro
cultured grafts, a modified procedure was used (22). After induction for 1 or 2 weeks in
the osteogenic medium, the grafts were washed with PBS, and then
incubated in 1.0 ml lysis solution comprising 10 mM Tris-HCl, 1 mM
MgCl2 and 1% Triton X-100 at 4˚C. The supernatant was
transferred to a 96-well plate (50 µl/well), and incubated with 100
µl substrate (p-nitrophenyl phosphate; 6.7 mM/l) at room
temperature for 10 min. Then, 100 µl NaOH (0.1 M) was added to stop
the reaction. The optical density (OD) at 405 nm (OD405)
was measured using a spectrophotometer. The OD405 value
of a PHA graft containing no cells served as control, and each
sample was tested in triplicate.
Reverse transcription-quantitative
reverse transcriptase polymerase chain reaction (RT-qPCR)
assay
To evaluate the expression of osteogenic-specific
genes in cells/PHA grafts in vitro, RT-qPCR was used
(23). Stem cells harvested from 3
patients were each assigned to a PHA graft. After 2 weeks of
induction, total RNA was extracted from each cell/PHA graft using a
Total RNA Extraction kit (Qiagen GmbH) (24). RNA concentration and quality were
evaluated on the basis of the OD 260/280 ratio. The mRNA (1.0 µl)
was reversely transcribed to cDNA using a First Strand cDNA kit
(Qiagen GmbH) according to the manufacturer's instructions. A total
reaction volume of 25 µl containing SYBR-Green Master Mix reagent
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was amplified
via qPCR (ABI Prism 7000; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 30 sec; 40 cycles of 95˚C for 5 sec and
60˚C for 32 sec; and a final dissociation stage at 95˚C for 15 sec,
60˚C for 60 sec and 95˚C for 15 sec. The osteogenic-specific genes
and reaction conditions are shown in Table II, and β-actin served as an internal
control to normalize the expression of the mRNA of these genes in
different cell types. The quantitative determination of target mRNA
expression was conducted according to the 2-ΔΔCq method
as previously described (24,25).
 | Table IIPrimer sequences and procedure
parameters used in the qPCR analysis. |
Table II
Primer sequences and procedure
parameters used in the qPCR analysis.
| Gene name | Primer sequences
(5' to 3') | Ta (˚C) | Cycles (n) |
|---|
| β-actin |
GTGGGGCGCCCCAGGCACCA (forward) | 56 | 42 |
| |
CTTCCTTAATGTCACGCACGATTTC (reverse) | | |
| OC |
ATGAGAGCCCTCACACTCCTC (forward) | 60 | 28 |
| |
GCCGTAGAAGCGCCGATAGGC (reverse) | | |
| Runx2 | ACGACAACCGCACCATGGT
(forward) | 60 | 28 |
| |
CTGTAATCTGACTCTGTCCT (reverse) | | |
| ALP |
TGGAGCTTCAGAAGCTCAACACCA (forward) | 58 | 30 |
| |
ATCTCGTTGTCTGAGTACCAGTCC (reverse) | | |
| OPN |
AGAATGCTGTGTCCTCTGAAG (forward) | 59 | 29 |
| |
GTTCGAGTCAATGGAGTCCTG (reverse) | | |
| BSP |
AAGGCTACGATGGCTATGATGGT (forward) | 61 | 30 |
| |
AATGGTAGCCGGATGCAAAG (reverse) | | |
Animal model
An animal model was produced using previously
reported methods (26,27). A total of 24 New Zealand white
rabbits (The Third Military Medical University; age range 8-12
weeks), of mixed sexes (13 mala and 11 female) weighing 2.0-2.5 kg
were used in the study. Rabbits were fed with rabbit pellets and
drinking water ad libitum and reared in a constant
temperature room at 20˚C with 50±5% humidity, 0.03% CO2
and 12-h light/dark cycles. The rabbits were randomly divided into
3 groups (each n=8) as follows: BM-MSCs/PHA grafts; CESCs/PHA
grafts; and PHA only grafts containing no cells to serve as a
control. The rabbits were anesthetized with sodium pentobarbital
(30 mg/kg) via intravenous injection. Following removal of all the
soft tissues, decortication of transverse process L4-L5 was
performed to provide the fusion bed. Then, grafts that had
undergone 3 days in vitro induction were implanted into
bilateral sides of the intertransverse process interval, in
parallel with the spine. Finally, the surgical incision was closed
layer by layer.
Spiral CT scanning
To evaluate bone formation and fusion conditions,
spiral CT scanning was conducted at 8 weeks after implantation
surgery. The lumbar spine segment L3-L6 was scanned at 1-mm slice
thickness and reconstructed into 3D images (SOMATOM Emotion;
Siemens Healthineers). To observe the fusion conditions, 5 axial
slices were scanned at positions containing the L4 and L5
transverse process attachments to the graft, and three intermediate
regions, respectively.
Manual palpation
At 8 weeks after implantation, animals were
sacrificed with an overdose of sodium pentobarbital. The objective
lumbar spine (L4-L5 processes) was exposed after the removal of
soft tissues, then manually palpated as previously described
(28,29). Only if no movement was detected in
the L4-L5 segment, and was confirmed by two checkers in a blinded
manner, was the implanted graft considered as fused.
Micro-CT analysis
To assess the quality of the newly formed bone in
the grafts, micro-CT was used (29,30). At
8 weeks after implantation, all the extraneous vertebrae and soft
tissues were dissected, and the implants were scanned using
micro-CT (GE Healthcare, Canada) using the following parameters: 60
kV; 0.6 mm aluminum filter; 800 µA; number of players=150. More
than 1,000 axial images were obtained from each graft at the
threshold of 1,200 HU. The region of interest was chosen
symmetrically in the left and right grafts as a cylinder
(0.5x0.5x0.5 cm3) at rdifferent coronary positions. The
grafts were equally portioned into five segments by 4
cross-sections. To evaluate osteogenesis, six morphometric indices
were measured as follows: i) bone mineral density (BMD); ii) bone
mineral content (BMC); iii) tissue mineral density (TMD); iv)
tissue mineral content (TMC); v) bone volume fraction (BVF); vi)
bone volume (BV) (30,31). PHA containing no stem cells served as
control. Two photographers analyzed the data in a blinded
manner.
Histological analysis
Animals were sacrificed with an overdose of sodium
pentobarbital at 8 weeks after implantation. The graft specimen was
harvested, fixed in 10% neutral buffered formalin for 24 h at 20˚C
and sequentially dehydrated in ethanol solutions. Then, the graft
was embedded in polymethylmethacrylate solution for 1 week. Grafts
were sectioned to 50 µm using a diamond saw (Leica Microsystems
GmbH, Germany). The slices were stained with Villanueva-Goldner's
trichrome (VG) at 20˚C for 30 min and observed with a light
microscope (Olympus Corporation) to evaluate osteogenesis by two
pathologists in a blinded manner. A total of 9 sections from 3
grafts, with 3 random sections obtained from each graft, were
quantitatively analyzed for newly formed bone and collagen I in VG
staining using Image-Pro Plus software 6.0 (Media Cybernetics,
Inc.). Osteogenesis was quantified on the basis of the area volume
of light blue and red staining, which represented collagen I and
newly formed trabecular bone, respectively (32).
Statistical analysis
SPSS version 13.0 software (IBM Corp.) was used for
statistical analysis. All data are presented as the mean ± standard
deviation. The two-tailed Student's t-test was used when comparing
only two groups, and one-way ANOVA followed by Fisher's Least
Significant Difference or Bonferroni's correction post-hoc tests
were used to analyze differences among three groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell morphology and antigenic
phenotype
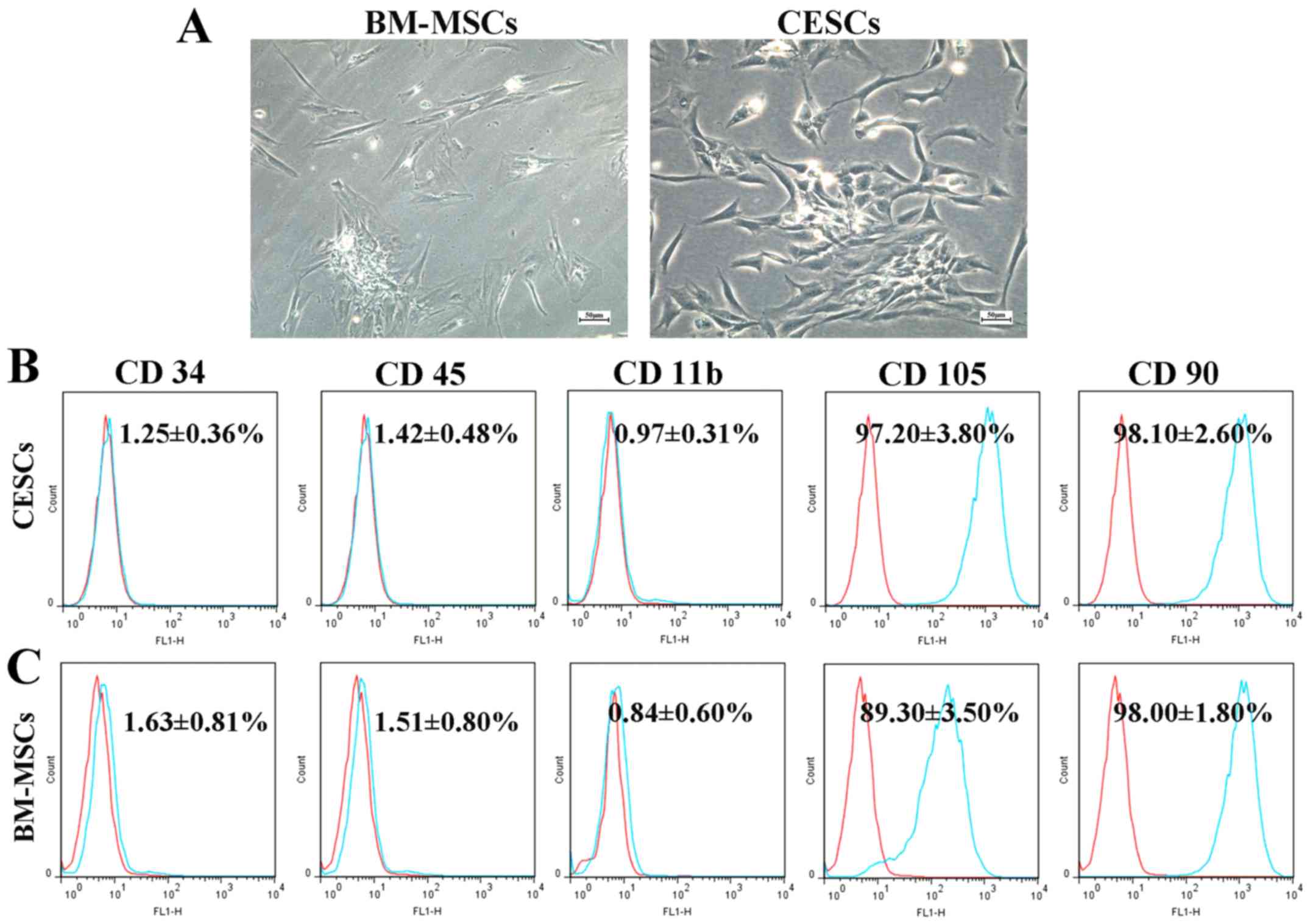
The BM-MSCs and CESCs exhibited a similar
spindle-shaped appearance while in culture (passage 2; Fig. 1A). Flow cytometric analysis indicated
that CESCs and BM-MSCs shared an analogous antigenic phenotype
(Fig. 1B and C). Both cell types were negative for CD34,
CD11b and CD45 (<2%), positive for CD90, and moderately positive
for CD105. No marked differences were detected in the expression
levels of CD11b, CD90, CD34 and CD45 between the two cell
types.
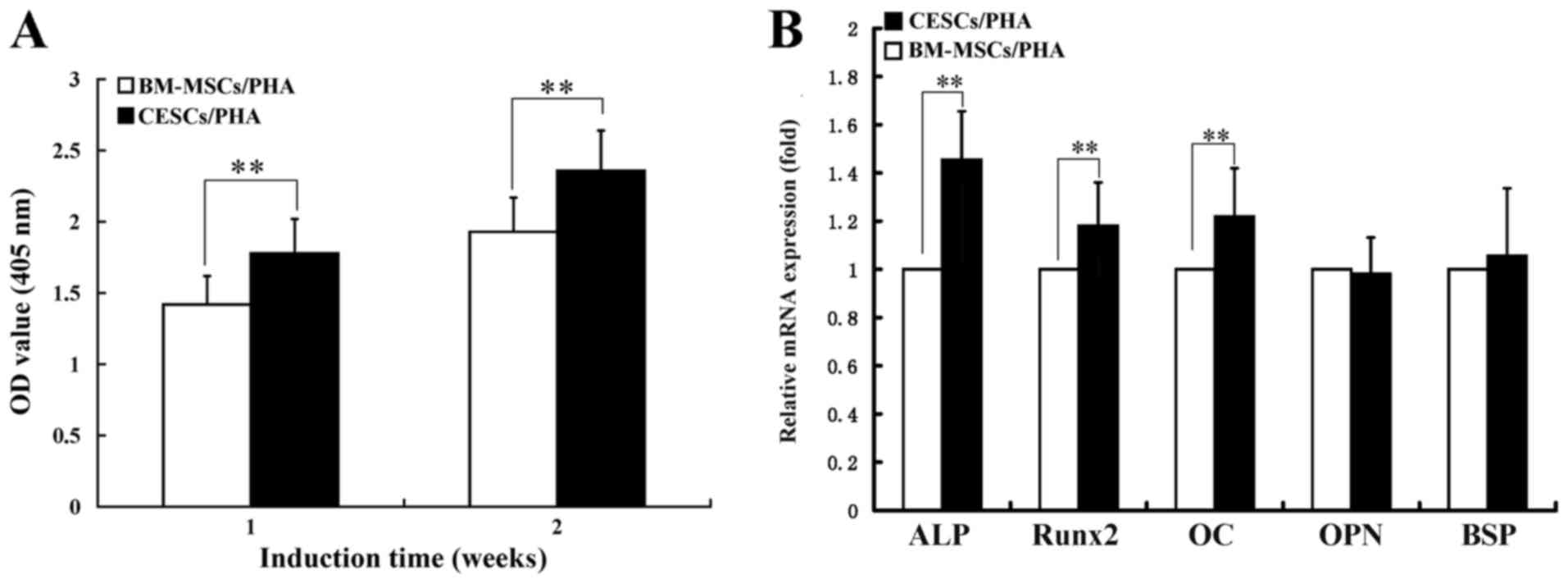
ALP activity
The ALP activity results indicated that the
OD405 values of both two cell types contained in the
grafts increased from 1 to 2 weeks of induction (Fig. 2A). At the 1-week culture time point,
the OD405 value of the CESCs/PHA group was significantly
higher compared with that of the BM-MSCs/PHA group (1.80±0.26 vs.
1.47±0.24; P<0.01; Fig. 2A). A
significant difference was also detected between the two groups at
2 weeks (2.36±0.28 vs. 1.92±0.25; P<0.01; Fig. 2A).
Osteogenic capacity in 3D culture
According to the RT-qPCR assay results, after 2
weeks of induction, the CESCs/PHA group exhibited a significantly
higher expression level of ALP mRNA compared with the BM-MSCs/PHA
group (1.45±0.20 vs. 1.0-fold; P<0.01; Fig. 2B). For Runx2 and osteocalcin (OC)
mRNA, significantly higher expression was also observed in the
CESCs/PHA group compared with the BM-MSCs/PHA group (1.19±0.18 vs.
1.0-fold for Runx2; 1.24±0.20 vs. 1.0 for OC; P<0.01; Fig. 2B). However, both groups exhibited
comparable expression levels for osteopontin and bone sialoprotein
mRNA (P>0.05; Fig. 2B).
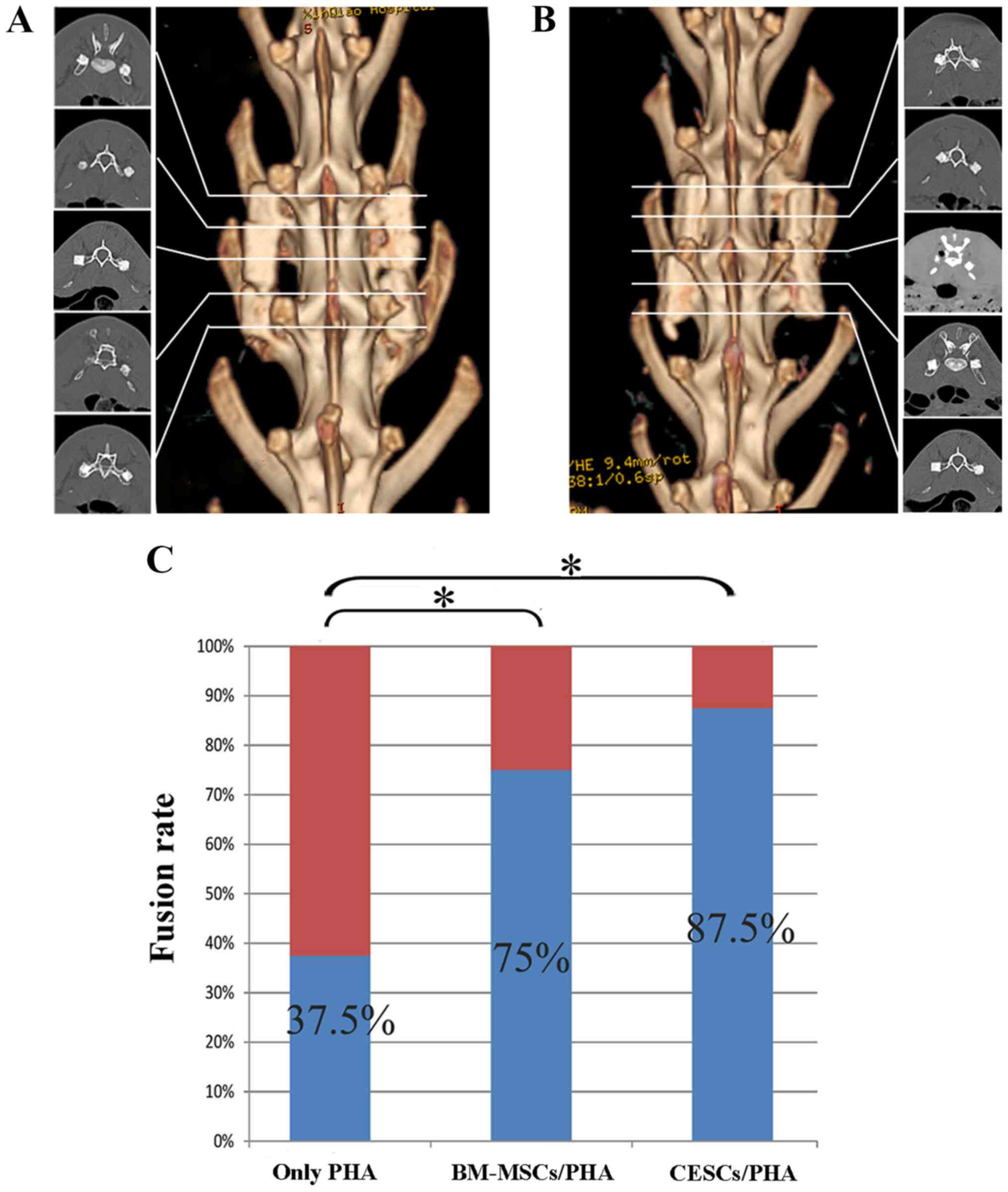
3D CT evaluation and fusion
status
Although all grafts broke into several parts during
surgery, at 8 weeks after implantation, reconstructed 3D CT
demonstrated bony healing of the fractured segments and definite
fusion existing between the transverse processes (L4 and L5) and
the graft in the majority of the cell-containing grafts (Fig. 3A and B).
Analysis using manual palpation revealed that 7/8
animals (87.50%) in the CESCs/PHA group and 6/8 animals (75.0%) in
the BM-MSCs/PHA group achieved fusion; however, fusion was obtained
in only 3/8 samples (37.5%) for the graft comprising only PHA. The
fusion rate in the control group was lower compared with the
CESCs/PHA and BM-MSCs/PHA groups (P<0.05). Furthermore, no
significant difference in fusion rate was detected between the
CESCs/PHA and BM-MSCs/PHA groups (P>0.05; Fig. 3C).
Bone formation analysis by
micro-CT
According to the micro-CT data, all the osteogenesis
indices in the CESCs/PHA group had higher values compared with
those of the BM-MSCs/PHA group (Fig.
4). Significant differences were observed between the two stem
cell-containing groups for BV, BVF, BMC, BMD and TMD (P<0.01;
Fig. 4B).
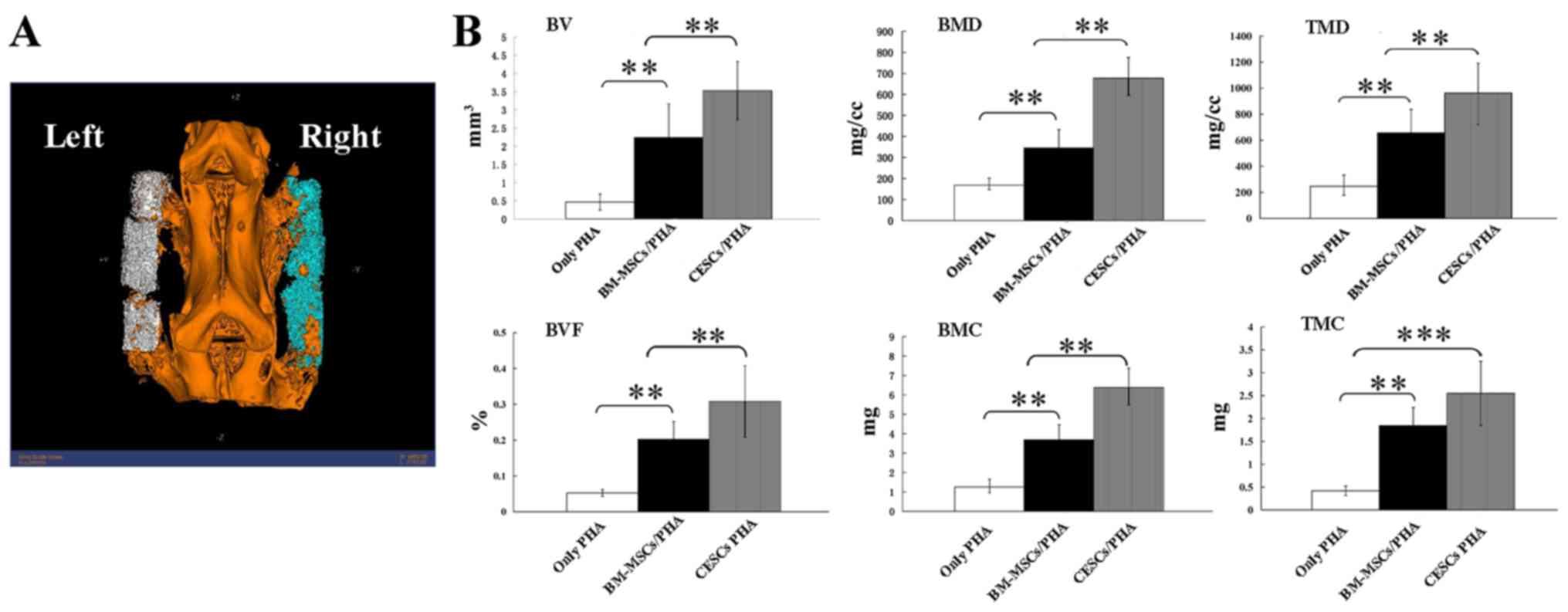 | Figure 4Assessment of newly formed bone
quality using micro-CT. (A) Reconstructed three dimensional
schematic diagram obtained by micro-CT. (B) Significant differences
existed between the BM-MSCs/PHA and CESCs/PHA groups for BV, BVF,
BMC, BMD and TMD (P<0.01). **P<0.01,
***P<0.001; n=8. PHA, porous
hydroxyapatite; CESCs, cartilage endplate-derived stem cells;
BM-MSCs, bone marrow mesenchymal stem cells; BV, bone volume; BVF,
bone volume fraction; BMC, bone mineral content; BMD, bone mineral
density; TMD, tissue mineral density; TMC, tissue mineral
content. |
Histological assessment
In VG stained slices, collagen I, newly formed
trabecular bone and PHA were stained as blue, red and black,
respectively (Fig. 5A-F). The
CESCs/PHA grafts exhibited more newly formed collagen I and
trabecular bone than the BM-MSCs/PHA group. For the only PHA graft,
the content of collagen I and trabecular bone was clearly the
lowest. Quantitative data indicated that the CESCs/PHA group had
740±62 µm2 newly formed trabecular bone and 863±84
µm2 of collagen I, whereas the respective values in the
BM-MSCs/PHA group were 381±36 and 740±54 µm2,
respectively (Fig. 5G). Significant
differences were detected between each pair of the three groups for
collagen I and trabecular bone (P<0.01).
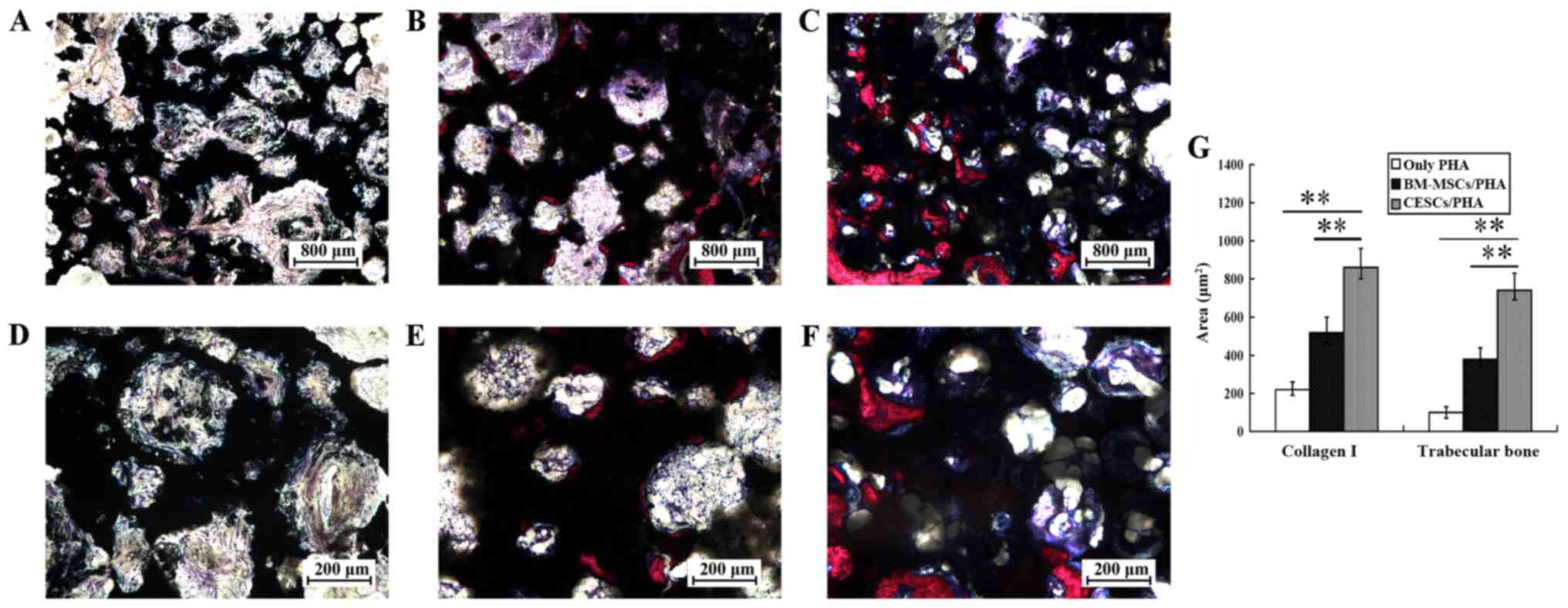 | Figure 5VG stained sections of grafts at 8
weeks after implantation. (A-F) Collagen I, trabecular bone and PHA
are indicated by blue, red and black staining, respectively.
Representative images of (A and D) only PHA, (B and E) BM-MSCs/PHA
and (C and F) CESCs/PHA grafts are shown. The CESCs/PHA grafts
exhibited higher volumes of collagen I and newly trabecular bone
than the BM-MSCs/PHA grafts. The only PHA grafts served as control.
(G) Quantitative data indicated that the CESCs/PHA group had 740±62
µm2 newly formed trabecular bone and 863±84
µm2 collagen I, whereas the respective areas for the
BM-MSCs/PHA group were 381±36 and 740±54 µm2,
respectively. **P<0.01. n=8. VG,
Villanueva-Goldner's trichrome; PHA, porous hydroxyapatite; CESCs,
cartilage endplate-derived stem cells; BM-MSCs, bone marrow
mesenchymal stem cells. |
Discussion
MSCs are an attractive cell population for use in
the regeneration of various tissues due to their multilineage
differentiation potential (10).
Studies have indicated the extensive use of MSCs, especially
BM-MSCs, in bone tissue engineering (8). The MSCs used in the present study were
obtained using previously described methods (10,18). In
addition, the cell surface antigen profiles were also basically
consistent with those in previous studies, and indicate that the
cells used in the present study possess the basic characteristics
of MSCs described by the International Society for Cellular Therapy
(18,33).
Generally, the degenerative status of NP and CEP is
hemi-quantitatively judged by magnetic resonance imaging (34,35). In
the present study, CESCs were obtained from human degenerated CEP
of types V and VI according to the previously described
classification (19). Whether the
bone formation ability correlates with the degeneration level of
the extracted sample remains to be elucidated. The possible
discrepant biological characteristics and bone formation potential
of the CESCs derived from clinical CEP samples with different
degenerative degrees merits investigation in future studies.
Unlike 2D culturing, the 3D culture environment
closely resembles the in vivo environment. Different growing
conditions may lead to differences in biological characteristics.
ALP mRNA expression and ALP activity were significantly upregulated
in CESCs as compared with BM-MSCs in the present study, and were
accordant in 2D and 3D culturing environments (16). OC as a marker for the late stage of
osteoblast differentiation was expressed at a significantly highly
level in CESCs/PHA compared with BM-MSCs/PHA, which was consistent
with previous 2D culture data (16,36). In
addition, significantly higher expression of Runx2, another
specific matrix protein expression marker for bone maturation, was
observed in CESCs/PHA compared with BM-MSCs/PHA in the 3D
environment, whereas no significant difference was detected between
CESCs and BM-MSCs in 2D culture (16,37). It
is hypothesized that the aforementioned differences may be
partially attributed to the favorable cell-cell and
cell-extracellular matrix (ECM) interactions in a 3D
multilayered-cell environment.
PHA is a classical scaffold material with good
biocompatibility, bone induction and bone conduction properties,
and is often used in bone tissue engineering research (38). The PHA used in the present study had
a porosity of 42.2±1.8%, an average pore diameter of 180±60 µm, and
a 3D framework in which cellular proliferation and differentiation,
and ECM deposition may occur. However, the weak fracture resistance
of PHA predisposes it to break under torsion or shear force
(39). In the present study, nearly
all PHA grafts broke into two to four parts following
transplantation into the intertransverse process. However, the
broken grafts were restored to integrity by the newly formed bone
that gradually bridged the defects of the broken material.
Furthermore, the grafts with CESCs or BM-MSCs demonstrated
significantly higher fusion rates compared with the PHA only
control. Generally, in the present study, PHA breakage during the
experimental process did not affect the evaluation of bone
formation capacity. Instead, it further certified that the
implanted stem cells, especially CESCs, provided a stronger
osteogenic and repair capacity, even under the challenging
environment caused by PHA breakage.
Micro-CT is a reliable in vivo method for the
quantitative and qualitive evaluation of newly formed bone without
physical disruption of the sample. Indices such as BV, BVF, BMC,
BMD, TMC and TMD indirectly reflect fusion quality (40). In the CESCs/PHA group, BV, BVF, BMC,
BMD and TMD values were significantly higher compared with those of
the BM-MSCs/PHA group; only the TMC values exhibited no significant
differences between these two groups. These results indicate that
the CESCs/PHA complex was able to induce bone regeneration more
efficiently. In addition, quantitative histology complemented the
micro-CT data by revealing that the volume of collagen I, the main
organic component of bone (41), and
newly regenerated trabecular bone in the CESCs/PHA group were
significantly higher than those in the BM-MSCs/PHA group.
Therefore, the results of micro-CT and histological analysis
confirmed that the CESCs/PHA composite enhanced the quantity and
quality of bone formation.
However, the present study has certain limitations.
Firstly, rabbits are relatively small in size, and the mechanical
stress that the implanted grafts endured in rabbits are likely to
differ from those in the human spine. Different mechanical factors
might have a profound effect on the biological characteristics of
seed cells, including their osteogenic capacities. Therefore, in
subsequent studies, larger animals such as goats or nonhuman
primates might be used to provide a more restrictive biomechanical
environment that is more analogous to that of the human spine.
Secondly, autologous bone grafting was not set as the gold standard
in this study due to the small number of experimental animals;
therefore, the final fusion efficacy that CESCs could achieve
relative to the gold standard remains unknown. Thirdly, no
biomechanical tests were performed to further assess the quality of
the newly formed bones due to the small size of the rabbit spine.
To address this issue, larger animals should be included in future
studies.
To the best of our knowledge, the present study was
the first to use stem cells derived from human degenerated CEP as
seed cells for tissue-engineered bone products, and compare their
osteogenesis with the traditional seed cells, BM-MSCs, in the 3D
in vitro environment and in vivo rabbit spinal fusion
model. Although the present study yielded encouraging results, the
definite osteogenic efficacy relative to the gold standard and the
long-term safety for in vivo implantation require further
investigation prior to clinical application. In addition, PHA
should be improved to enhance its fracture resistance, or an
alternative scaffold with fine biocompatibility and mechanical
properties should be considered for further study.
In conclusion, the present study preliminarily
compared the osteogenic capacity between CESCs and BM-MSCs derived
from the same donors in the rabbit lumbar intertransverse process
fusion model. CESCs exhibited superior bone formation ability than
BM-MSCs when used with PHA under a 3D environment in vitro
and in vivo. The results indicate that CESCs have potential
as an efficient and sufficient seed cell source for bone tissue
engineering, and CESC-based products show promise as superior
candidates for future clinical application in spinal fusion or
other bone regeneration and repair issues.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81472076 and 81560369).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW performed the experiments, wrote the manuscript
and collected, analyzed and interpreted data. YZ contributed to
study design and conception, wrote the manuscript and analysed and
interpreted data. CQL, TWC and JW performed the experiments and
obtained clinical samples. BH contributed to study design and
conception, analysed and interpreted data, and wrote and gave final
approval of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Institutional
Review Board of Xinqiao Hospital. All patients provided written
informed consent for participation in the study. All animal
experiments were approved by the Xinqiao Hospital Committee on
Ethics for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Kawakami N, Tsuji T, Imagama S, Lenke LG,
Puno RM and Kuklo TR: Spinal Deformity Study Group. Classification
of congenital scoliosis and kyphosis: A new approach to the
three-dimensional classification for progressive vertebral
anomalies requiring operative treatment. Spine (Phila Pa 1976).
34:1756–1765. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
An H, Boden SD, Kang J, Sandhu HS, Abdu W
and Weinstein J: Summary statement: Emerging techniques for
treatment of degenerative lumbar disc disease. Spine (Phila Pa
1976). 28 (Suppl 15):S24–S25. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hidaka C, Goshi K, Rawlins B,
Boachie-Adjei O and Crystal RG: Enhancement of spine fusion using
combined gene therapy and tissue engineering BMP-7-expressing bone
marrow cells and allograft bone. Spine (Phila Pa 1976).
28:2049–2057. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boden SD: Overview of the biology of
lumbar spine fusion and principles for selecting a bone graft
substitute. Spine (Phila Pa 1976). 27 (Suppl 1):S26–S31.
2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rihn JA, Kirkpatrick K and Albert TJ:
Graft options in posterolateral and posterior interbody lumbar
fusion. Spine (Phila Pa 1976). 35:1629–1639. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Minamide A, Yoshida M, Kawakami M,
Yamasaki S, Kojima H, Hashizume H and Boden SD: The use of cultured
bone marrow cells in type I collagen gel and porous hydroxyapatite
for posterolateral lumbar spine fusion. Spine (Phila Pa 1976).
30:1134–1138. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Szpalski C, Barbaro M, Sagebin F and
Warren SM: Bone tissue engineering: Current strategies and
techniques-part II: Cell types. Tissue Eng Part B Rev. 18:258–269.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Seong JM, Kim BC, Park JH, Kwon IK,
Mantalaris A and Hwang YS: Stem cells in bone tissue engineering.
Biomed Mater. 5(062001)2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bruder SP and Fox BS: Tissue engineering
of bone: Cell based strategies. Clin Orthop Relat Res. (Suppl
367):S68–S83. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD and Moorman MA: Multilineage potential of
adult human mesenchymal stem cells. Science. 284:143–147.
1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jones E and McGonagle D: Human bone marrow
mesenchymal stem cells in vivo. Rheumatology. 47:126–131. 2008.
View Article : Google Scholar
|
|
12
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Blanco JF, Graciani IF, Sanchez-Guijo FM,
Muntion S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado
MV, Cruz G, Gutierrez-Cosío S, et al: Isolation and
characterization of mesenchymal stromal cells from human
degenerated nucleus pulposus: Comparison with bone marrow
mesenchymal stromal cells from the same subjects. Spine (Phila Pa
1976). 35:2259–2265. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Feng G, Yang X, Shang H, Marks IW, Shen
FH, Katz A, Arlet V, Laurencin CT and Li X: Multipotential
differentiation of human anulus fibrosus cells: An in vitro study.
J Bone Joint Surg Am. 92:675–685. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu LT, Huang B, Li CQ, Zhuang Y, Wang J
and Zhou Y: Characteristics of stem cells derived from the
degenerated human intervertebral disc cartilage endplate. PLoS One.
6(e26285)2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang B, Liu LT, Li CQ, Zhuang Y, Luo G,
Hu SY and Zhou Y: Study to determine the presence of progenitor
cells in the degenerated human cartilage endplates. Eur Spine J.
21:613–622. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang H, Zhou Y, Huang B, Liu LT, Liu MH,
Wang J, Li CQ, Zhang ZF, Chu TW and Xiong CJ: Utilization of stem
cells in alginate for nucleus pulposus tissue engineering. Tissue
Eng Part A. 20:908–920. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rajasekaran S, Venkatadass K, Naresh Babu
J, Ganesh K and Shetty AP: Pharmacological enhancement of disc
diffusion and differentiation of healthy, ageing and degenerated
discs: Results from in-vivo serial post-contrast MRI studies in 365
human lumbar discs. Eur Spine J. 17:626–643. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bilic G, Zeisberger SM, Mallik AS,
Zimmermann R and Zisch AH: Comparative characterization of cultured
human term amnion epithelial and mesenchymal stromal cells for
application in cell therapy. Cell Transplant. 17:955–968.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lubis AM, Sandhow L, Lubis VK, Noor A,
Gumay F, Merlina M, Yang W, Kusnadi Y, Lorensia V, Sandra F and
Susanto NH: Isolation and cultivation of mesenchymal stem cells
from iliac crest bone marrow for further cartilage defect
management. Acta Med Indones. 43:178–184. 2011.PubMed/NCBI
|
|
22
|
Jackson WM, Aragon AB, Bulken-Hoover JD,
Nesti LJ and Tuan RS: Putative heterotopic ossification progenitor
cells derived from traumatized muscle. J Orthop Res. 27:1645–1651.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ripamonti U, Klar RM, Renton LF and
Ferretti C: Synergistic induction of bone formation by hOP-1,
hTGF-beta3 and inhibition by zoledronate in macroporous
coral-derived hydroxyapatites. Biomaterials. 31:6400–6410.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS
and Lee SH: In vivo bone formation following transplantation of
human adipose-derived stromal cells that are not differentiated
osteogenically. Tissue Eng Part A. 14:1285–1294. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Alanay A, Chen C, Lee S, Murray SS,
Brochmann EJ, Miyazaki M, Napoli A and Wang JC: The adjunctive
effect of a binding peptide on bone morphogenetic protein enhanced
bone healing in a rodent model of spinal fusion. Spine (Phila Pa
1976). 33:1709–1713. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Boden SD, Schimandle JH and Hutton WC: An
experimental lumbar intertransverse process spinal fusion model.
Radiographic, histologic, and biomechanical healing
characteristics. Spine (Phila Pa 1976). 20:412–420. 1995.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grauer JN, Bomback DA, Lugo R, Troiano NW,
Patel TC and Friedlaender GE: Posterolateral lumbar fusions in
athymic rats: Characterization of a model. Spine J. 4:281–286.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miyazaki M, Zuk PA, Zou J, Yoon SH, Wei F,
Morishita Y, Sintuu C and Wang JC: Comparison of human mesenchymal
stem cells derived from adipose tissue and bone marrow for ex vivo
gene therapy in rat spinal fusion model. Spine (Phila Pa 1976).
33:863–869. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sheyn D, Rüthemann M, Mizrahi O, Kallai I,
Zilberman Y, Tawackoli W, Kanim LE, Zhao L, Bae H, Pelled G, et al:
Genetically modified mesenchymal stem cells induce mechanically
stable posterior spine fusion. Tissue Eng Part A. 16:3679–3686.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hildebrand T, Laib A, Müller R, Dequeker J
and Rüegsegger P: Direct three-dimensional morphometric analysis of
human cancellous bone: Microstructural data from spine, femur,
iliac crest, and calcaneus. J Bone Miner Res. 14:1167–1174.
1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cinotti G, Patti AM, Vulcano A, Della
Rocca C, Polveroni G, Giannicola G and Postacchini F: Experimental
posterolateral spinal fusion with porous ceramics and mesenchymal
stem cells. J Bone Joint Surg Br. 86:135–142. 2004.PubMed/NCBI
|
|
33
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kuisma M, Karppinen J, Haapea M,
Lammentausta E, Niinimaki J and Tervonen O: Modic changes in
vertebral endplates: A comparison of MR imaging and multislice CT.
Skeletal Radiol. 38:141–147. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang F, Yuan PW, Hao YQ and Lu ZM: Emodin
enhances osteogenesis and inhibits adipogenesis. BMC Complement
Altern Med. 14(74)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hassan MQ, Tare RS, Lee SH, Mandeville M,
Morasso MI, Javed A, van Wijnen AJ, Stein JL, Stein GS and Lian JB:
BMP2 commitment to the osteogenic lineage involves activation of
Runx2 by DLX3 and a homeodomain transcriptional network. J Biol
Chem. 281:40515–40526. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Oryan A, Alidadi S, Moshiri A and Maffulli
N: Bone regenerative medicine: Classic options, novel strategies,
and future directions. J Orthop Surg Res. 9(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee JH, Yu CH, Yang JJ, Baek HR, Lee KM,
Koo TY, Chang BS and Lee CK: Comparative study of fusion rate
induced by different dosages of Escherichia coli-derived
recombinant human bone morphogenetic protein-2 using hydroxyapatite
carrier. Spine J. 12:239–248. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schulte FA, Lambers FM, Kuhn G and Muller
R: In vivo micro-computed tomography allows direct
three-dimensional quantification of both bone formation and bone
resorption parameters using time-lapsed imaging. Bone. 48:433–442.
2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Miller A: Collagen: The organic matrix of
bone. Philos Trans R Soc Lond B Biol Sci. 304:455–477.
1984.PubMed/NCBI View Article : Google Scholar
|