Introduction
The harmful effects of cigarette smoking have been
known for decades. Chronic active and passive exposition to smoke
increases the risk of pulmonary diseases, lung fibrosis, asthma,
cancers, cardiovascular and metabolic diseases (1-4).
The harmful effects of cigarette smoking are predominantly
associated with nicotine, which is the most biologically active
component of the smoke (3). The
nicotine receptors are dispersed within the whole body, so the
effects on human health are complex (1,5). Most of
the inhaled nicotine is absorbed into the blood stream by lungs'
cells, and therefore its impact on the lungs themselves is
significant (6,7). Additionally, the cigarette smoke
deposits in the lung tissue causing chronic inflammation (4,8).
The vapour of electronic cigarettes (e-cig) does not
undergo the combustion and is free of tar substances. That is why
it is believed to constitute a safer form of ‘smoking’, also in the
treatment of the addiction to conventional cigarettes (3,9).
Moreover, since the usage of e-cigs is similar to that of
conventional cigarettes, the withdrawal from smoking seems easier
than when nicotine gums or patches are used (9-12).
E-cig liquids contain different substances which after heating form
aerosol (vapour), imitating smoke (13). Except from nicotine, one can find
there humectants (propylene glycol, glycerine), concentrated
flavours (extracts from herbs or plants) and other mood boosting
substances (14). In recent years,
some countries have introduced legal regulations referring to the
liquid contents e.g. it is forbidden to add both psychoactive
substances and sildenafil (15-17).
What is more, the regulations additionally limit the access to the
electronic cigarettes for adolescents. Nevertheless, these products
can be easily purchased on the Internet and are frequently used by
minors and young adults (18-20).
The data concerning the safety of e-cig usage are
inconsistent. Although it was initially presumed that the e-cig
usage was completely safe, subsequent observations proved that
vapers alike smokers suffer from numerous disorders and therefore
further investigations were conducted (2,21,22).
Such problems as pain, dizziness, fever, vomiting, gingivitis,
cough, throat irritation as well as severe organizing pneumonia
with hypoxemic respiratory failure were reported in e-cig users
(18,10,23,24). Vapour may contain additional toxic substances
similar to those in conventional cigarettes such as formaldehyde,
carbonyls and nitrosamines (8).
Those harmful effects may be also caused by trace metals such as
nickel, chromium, tin, aluminium, all of which are leached from
e-cig core assembly (25,26).
Yet, the effect of vapour on lung tissue has not
been evaluated. Clinical symptoms of lungs function disorders
observed in e-cig users may be the consequences of lung tissue
histopathological changes. The aim of the current study was to
assess the safety of e-cigs in comparison to the conventional
cigarettes in an animal model.
Materials and methods
Experiments
Our experiment was conducted on 30 male Wistar rats
of average body weight 187,82±12,56 g at the beginning of the
study. The animals were divided into three groups: A, B, C. The
animals in group A were exposed to scent free e-cig vapour. During
the 10 min exposition, the rats were placed in a 0.1 m3
PCV cage and were exposed to the vapour with the use of the pump
(0.18 kW; 1.4/1.6 A; 230 V; 50/60 Hz). It was installed on one side
of the box, while e-cigarette aerosol was puffed on the other. This
mechanism generates airflow into the cage. The cage containing 5
animals at a time was hermetically sealed with the two holes (e-cig
and pump connection points) that were left open. Animals were
exposed in order to consume 0.6 ml/day of e-liquid containing 12
mg/ml of nicotine, propylene glycol and water produced by Inawera
Dot Com Sp. Z o.o. Sp. K. One cycle of treatment consisted of 5 min
puff followed by 20 min stop. During the experiment the e-cig
voltage was set at 5.5 V. At the end of the cycle the animals were
transferred to a clean cage. Animals were subjected to 1 cycle/day
for 5 consecutive days/week, and for 6 consecutive weeks (27) The rats in group B were exposed to the
smoke from 10 traditional cigarettes with the same total nicotine
dose as in group A. In total, one group of animals was exposed to
210 mg of nicotine. The concentration of nicotine in serum of each
rat was not tested. Therefore, it is not possible to precisely
define the dose of nicotine received by single rat. Our study is
based on the official list of the substances in the liquid as
reported by the manufacturer. No additional tests were performed to
assess the liquid composition.
The rats in group C constituted the control group
and they were exposed to the same inhalation-related stress that
other rats but without the nicotine element. The animals were
decapitated without anaesthesia 24 h after last exposition and
their lungs were dissected. Body weight of rats at the time of
sacrifice was: 290.73 SD 15,69 g (group A), 287.67 SD 21.34 g
(group B), 324.38 SD 19, 16 g (group C).
Our study involves histological analysis of multiple
organs as well as some biochemical and molecular markers
measurement by ELISA. Therefore, we had to carefully choose the
right euthanasia method, so that it would not affect our results.
Due to multiple concerns, we have chosen the decapitation without
prior anesthaesia. It was performed by an experienced worker of the
Experimental Medicine Facility of the Medical University of Lublin.
The CO2 euthanasia is known to negatively affect the
lungs, the study of which are important part of our project
(28,29). Pentobarbital on the other hand
significantly changes the biochemistry of brain, including the
acetylcholinesterase activity (30).
Moreover, similarly to CO2 it also alters the blood
biochemical markers (31).
Enflurane, halothane, isoflurane and sevoflurane negatively affect
rat sperm (32). Moreover,
isoflurane euthanasia significantly impact the metabolism of liver,
possibly changing the glycogen levels (33). The cervical dislocation was rejected
as it requires prior anesthesia. Concussion on the other hand could
significantly damage the brain, therefore, it was also not
considered.
To conclude, decaptation was chosen because contrary
to other methods accepted by EU Directive 2010/63/EU it does not
induce significant changes to any organs except for the neck and
does not alter the biochemistry. Bearing the responsibility, we
have obtained the necessary approval of Bioethical Committee for
the whole experiment, including the decapitation.
After fixing the material in 10% buffered formalin,
the organs were embedded in paraffin blocks. The material was then
tailored into 5-µm-thick sections. The experiment was conducted
with the formal approval of the local animal care committees:
‘Local Ethics Committee for Animal Experiments’ by University of
Life Sciences in Lublin (30/2015). The study was carried out in
accordance with Directive 2010/63/Eu of The European Parliament and
of The Council of 22 September 2010 on The Protection of Animals
Used For Scientific Purposes.
Hematoxylin and eosin (H&E),
periodic Acid-Schiff (PAS) and Masson's trichrome staining
The histomorphological evaluation of the tissues
stained with H&E was performed with the use of a light
microscope using lenses x10, x40 and x100. Fifty fields of view
from each animal were analysed. Samples were stained by Masson's
Trichrome method to assess collagen deposition and fibrosis. PAS
staining was done to visualise the blood-air barrier. We used a
standard procedure of described stainings. The thickness of the
membrane forming blood-air barrier was measured using a microscope
with digital camera Olympus BX41, lens x100 and CellSense software
in the PAS stained sections. Optical density of the picture of the
trichrome-stained area was outlined and quantified using ImageJ
software and its associated colour deconvolution plugin as
described previously (34,35).
Immunohistochemical (IHC) and orcein
stainings
This immunohistochemical staining was performed with
the use of antibodies directed against α-Smooth Muscle Actin
(α-SMA, Elabscience, polyclonal rabbit anti-human, -mouse and -rat
antibody, E-AB-33323, dilution 1:200, previously used by Li et
al (36) to assess
myofibroblasts that are responsible for collagen fiber production
and form blood vessel walls. The exposure of the antigenic sites
was performed thermally by incubation in citrate buffer solution
with pH=6, in a microwave oven at 800 W, for 3 cycles lasting 5 min
each. In order to inhibit endogenous peroxidase activity slides
were incubated in 0.3% perhydrol (H2O2) in
methanol for 30 min. The samples were incubated in normal serum for
1 h to block the non-specific bindings. The material was incubated
at 4˚C overnight (17 h) in diluted primary antibody. The
diaminobenzidine solution (DAB) and hematoxylin colouration were
used to visualize the reaction (5 min). Negative controls were
prepared in a similar manner, only the specific primary antibody
was omitted. The material was evaluated with a light microscope
using lenses x10 and x40. Quantification of the vessel number was
determined by the visualization of the α-SMA muscle actin in the
vessel wall cells. Blood vessels were counted in 50 fields of view
under 100x lens. Elastic fibers were evaluated in orcein
staining.
Statistical analysis
The obtained test results were statistically
analysed with Statistica 13.0 (StatSoft). The Shapiro-Wilk test was
used to assess the data distribution and the Kruskal-Wallis test
was used to calculate the P-values. In this regard, a probability
(P-value) <0.05 was considered statistically significant.
Results
H&E staining
A collapse of parenchyma, hyperhagia, hyperplasia of
type II of pneumocytes, and an increased number of macrophages was
observed in both experimental groups (Fig. 1). Furthermore, in the e-cig group
eosinophils and mononuclear cells infiltration was noted, as well
as thickened alveolar septa, hyperaemia, intrabronchiolar
erythrocytes and the increase of mucus production. In the
conventional cigarette group irregularity of alveolar lumen was
observed, as well as features of emphysema, vacuolization of cells
in the alveolar septa, mucus intrabronchioles and hemorrhage into
bronchiole and alveolar lumen.
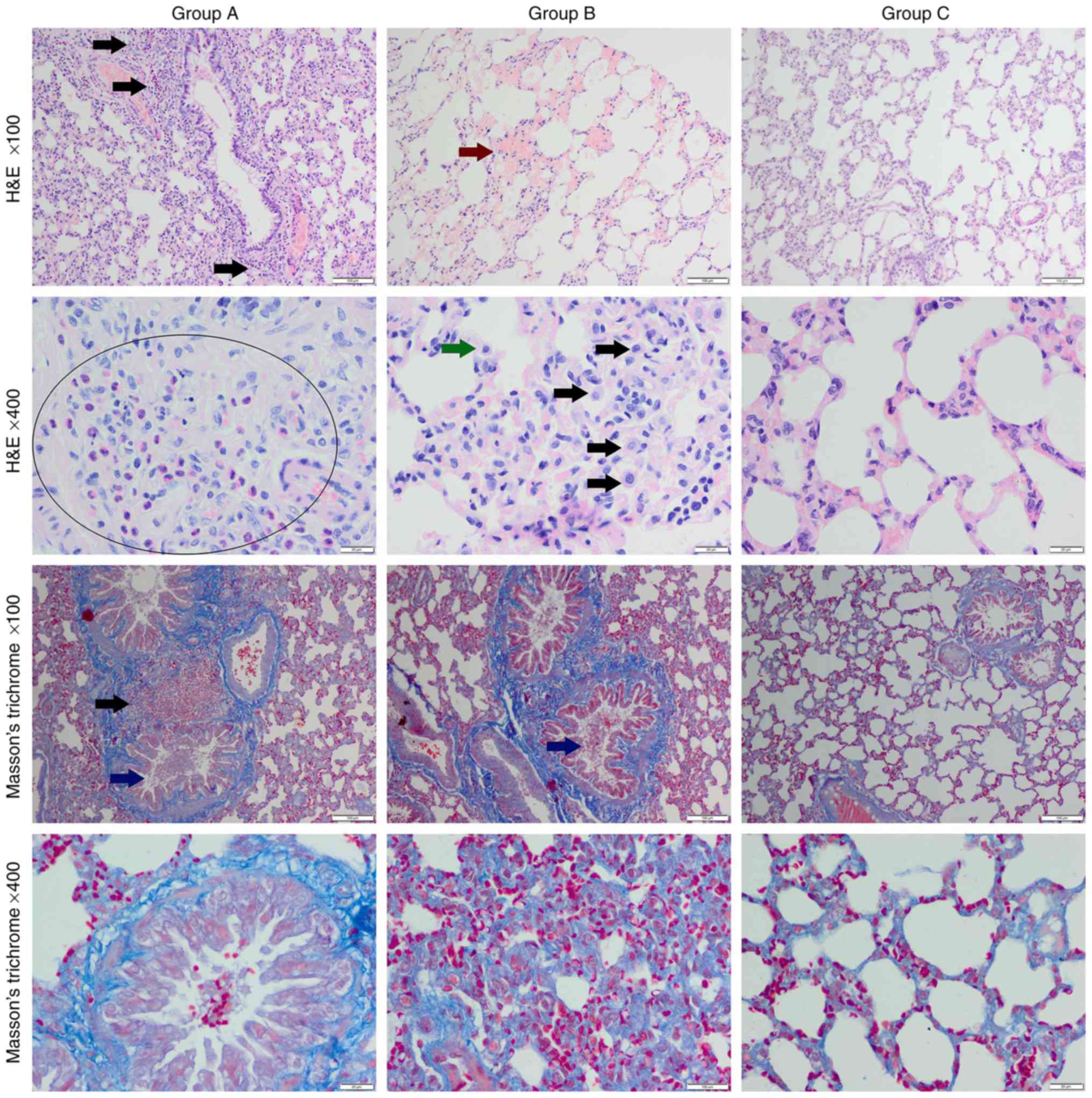 | Figure 1Histological structure of tested
lungs. Normal arrangement of the lung tissue with collagen fibers
surrounding the bronchioles could be seen in the control group
(group C). The e-cig group (group A) exhibited infiltration of
eosinophils, erythrocytes and mononuclear cells (black arrows),
thickened alveolar septa, and hyperaemia. Collagen depositions
within alveolar septa and in peribronchiolar area could also be
observed. In the conventional cigarette group (group B),
haemorrhage (red arrow), thickened alveolar septa, macrophages
(green and black arrows), collagen deposition within thickened
alveolar septa, erythrocytes and mucus intrabronchioles could be
observed (blue arrow). Magnification, x100 or x400. H&E,
hematoxylin and eosin. |
Masson's Trichrome staining
In the Masson's Trichrome staining, sections of
increased collagen deposition within thickened alveolar septa and
initial fibrosis was observed in both experimental groups (Fig. 1). The highest optical density score
was noted in the conventional cigarette group (Table I). In the control group, normal
appearance of the lung tissue and collagen fibres was noted, the
latter only surrounded the bronchioles.
 | Table IOptical Density score of Masson's
trichrome staining, thickness of blood-air barrier forming membrane
measured in PAS staining and number of blood vessels observed in
one field of view at 100X magnification. |
Table I
Optical Density score of Masson's
trichrome staining, thickness of blood-air barrier forming membrane
measured in PAS staining and number of blood vessels observed in
one field of view at 100X magnification.
| | Group | |
|---|
| Measured
parameter | A (n=500) | B (n=500) | C (n=500) | P-value in U
test |
|---|
| Optical sensity
score of Masson staining's trichrome | 0.18±0.05 | 0.22±0.06 | 0.13±0.02 | A:C, P=0.0002;B:C,
P<0.0001; A:B, P=0.0080 |
| Thickness of
blood-air barrier forming membrane measured in PAS staining,
µm | 0.40±0.08 | 0.44±0.19 | 0.20±0.06 | A:C, P=0.0300; B:C,
P=0.0200; A:B, P=0.4000 |
| Number of blood
vessels observed in one field of view at x100 magnification | 6.17±2.04 | 7.86±2.6 | 3.38±1.36 | A:C, P=0.0009; B:C,
P<0.0001; A:B, P=0.1200 |
PAS and orcein staining
In the PAS staining, an increase of the blood-air
barrier-forming membrane thickness was observed in both
experimental groups (Table I and
Fig. 2). In the orcein staining
delicate elastin fibres were noted in the control group. An initial
elastolysis was observed in both experimental groups-4 elastic
fibres were disrupted, sparse, irregular and thickened (Fig. 2).
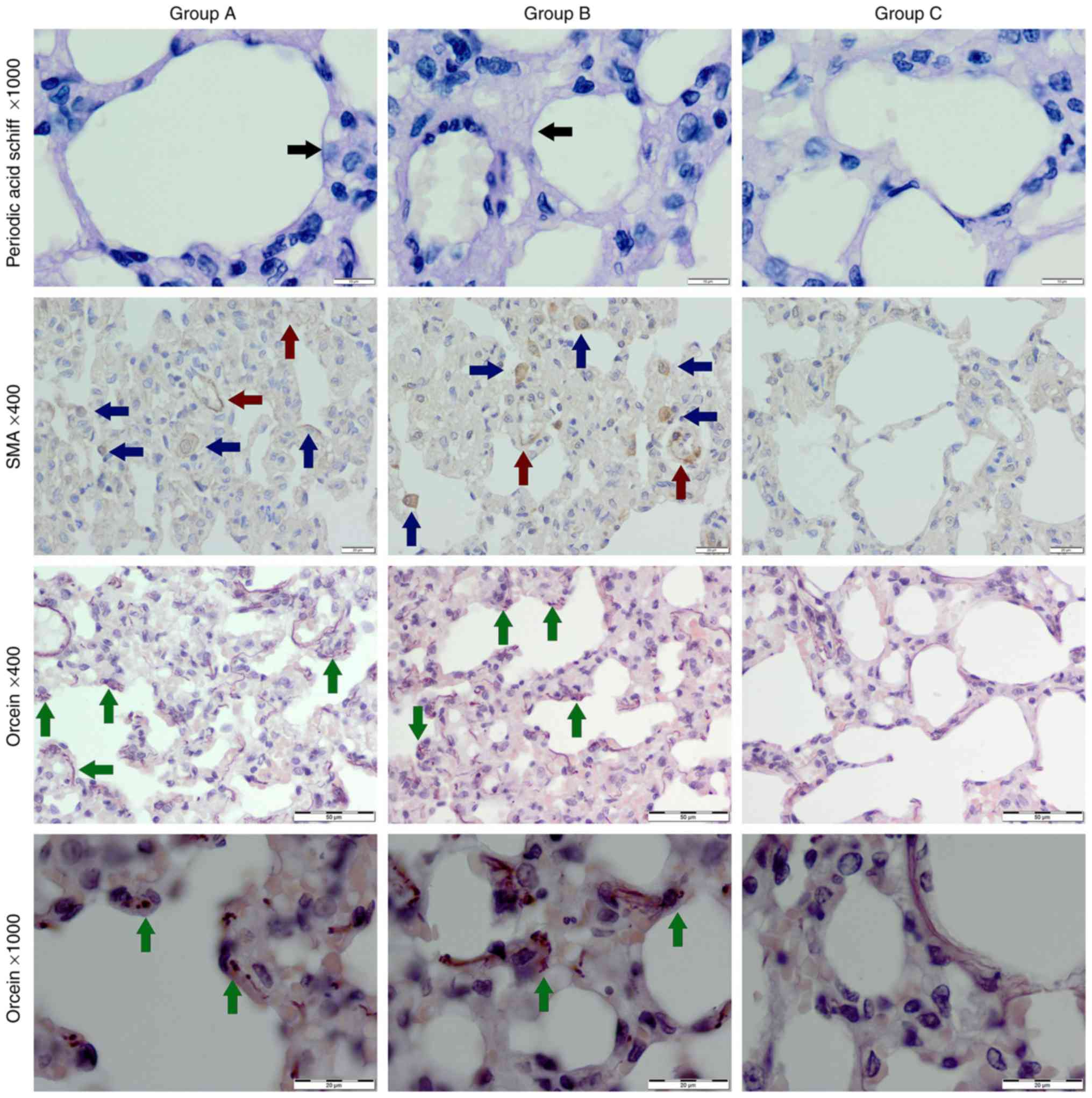 | Figure 2Control group (group C) exhibited
normal alveoli, single myofibroblast and blood vessels with
positive expression of α-SMA, and delicate normal elastic fibers
within alveolar septa in orcein staining (green arrow). In the
e-cig group (group A) and the conventional cigarette group (group
B), thickened basement membrane (black arrows), vacuolization of
cells in alveolar septa, more numerous α-SMA positive
myofibroblasts (blue arrows) and blood vessels (red arrows), and
thicker, disrupted, sparse elastic fibers (green arrows) were
observed. Original magnification, x400 or x1,000. SMA, smooth
muscle actin. |
Immunohistochemical staining,
α-SMA
The highest expression of α-SMA was seen in the
conventional cigarette group B (Fig.
2). The most numerous myofibroblasts were within parenchyma of
that same group (20±5 in one field of view), fewer in e-cig group A
(17±6 in one field of view) and just single ones in the control
group. Numbers of α-SMA positive blood vessels were the highest in
the conventional cigarette group (Table
I).
Discussion
Never-smokers including adolescents and young adults
reach out for e-cigarettes more and more frequently. Their
knowledge concerning these products is at best insufficient
(18,37-39). What is more, the use of e-cigs may also lead to
nicotine dependence and have harmful impact on health (22,40,41). The
exposition to vapours produced by e-cig has caused morphological
alterations in the human lung fibroblasts, induced the oxidative
stress and inflammatory response in the lungs of mice (7,14,21). A
case of acute alveolitis with intra-alveolar fibrosis, infiltration
of macrophages, eosinophils and neutrophils has been described by
Itoh et al (42). The vapour
generated by e-cig probably affects the gene expression of the
circadian rhythm-related proteins in healthy and sick people
(43). Among others, the exposure to
e-cig may cause the induction of genes involved in oxidative and
xenobiotic stress pathways, increased reactive oxygen species
production, decreased expression of genes involved in cilia
assembly and movement in the human bronchial epithelial cells
(44).
In the current study we used rat that is a
representative model for human exposure to e-cigarette vapour and
conventional cigarette smoke. The current study utilised similar
methodology as that proposed by Canistro et al (27) in terms of animal exposure to
e-cigarette vapour.
To the best of the authors' knowledge, the present
study is the first to demonstrate that the exposition to vapour or
smoke disrupts the structure of the lung tissue. Numerous
pathological changes such as the bronchial haemorrhage and the
increase of alveolar septa thickness, infiltration of eosinophils
and macrophages were observed in the lungs of rats exposed to
vapour. Yet, more significant destructive changes within the
features of the emphysema and fibrosis were present in the lungs of
animals exposed to conventional smoke. The elastic fibres
responsible for the regulation of alveolar thickness as well as the
thickness of bronchiole lumen, were disrupted in both experimental
groups.
Our results are in line with the study of Valença
et al (45), in which rats
receiving nicotine intraperitoneally had disorganised parenchyma
and inflammatory cells infiltration. Moreover, elastic fibres were
disrupted, the thickness of alveolar septa and the number of
parenchyma vessels was increased (45). The alveolar septa thickening may
depend on the increased volume of blood capillaries, inflammatory
infiltration and oedema. Moreover, cigarette smoking has activated
the neutrophil and macrophage elastases which damage the elastic
fibres and lead to emphysema (45).
It is presumed that the observed disruptive changes
of the lung tissues are probably associated with oxidative stress
and the dysfunction of blood-air barrier caused by the toxic
substances present in smoke or vapour (3,46). The
study conducted by Schweitzer et al (46) has shown that nicotine contained in
e-cig liquid triggered the disruption of endothelial barrier in the
cultured cell monolayers and increased lung inflammation by the
induction of oxidative stress in mice. Other study proved that
nicotine applied subcutaneously in a daily dose of 1.5 mg/kg for 4
weeks may damage rat lung tissue and may lead to the
intraparenchymal haemorrhage, respiratory epithelial proliferation,
extensive interstitial fibrosis and empchysematous changes
(3). Reinikovaite et al
(47) showed that vapour of e-cig
can damage lung tissue comparably to conventional cigarette smoke.
On the contrary to our study, the emphysematous changes and the
decrease of lung capillaries number was observed in rats exposed to
either smoke or vapour similarly to the rats exposed to nicotine
administered subcutaneously (47).
The acute disruption and inflammation as well as
ineffectiveness of regeneration mechanisms caused a chronic
condition leading to the alterations of lung architecture and
pulmonary fibrosis. The nicotine stimulates fibroblast
proliferation and collagen type I production (5). Additionally, nicotine may cause the
induction of the fibroblasts differentiation into myofibroblasts.
The latter are responsible for the pro-fibrotic extracellular
matrix proteins secretion. Consequently, the accumulation of
collagen within lung tissue leads to fibrosis and decreases the gas
exchange area (22,48).
In the current study, the expression of α-SMA, a
marker for myofibroblasts, was overexpressed in both experimental
groups, but to a higher extent in the conventional cigarette group.
In conclusion, the current study proves that usage of e-cigarettes
leads to milder pathological changes when compared to the smoking
of conventional cigarettes. However, e-cigs cannot be considered to
be completely safe (38,49). Apparently, the exposure to nicotine
in the form of e-cig leads to the degeneration of the lung tissue,
formation of collagen deposits, the activation of eosinophils,
myofibroblasts and angiogenesis. Furthermore, those changes may
lead to the alterations of lung architecture which additionally
hinders the gas exchange in areas.
The current study was conducted with the use of
laboratory animals for a limited period of six weeks. Only male
rats were used to retain homogeneity of studied groups-further
studies should include the intersex comparison.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant of the
Minister of Science and Higher Education (grant no. MNmb 246).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
EWG and PCW conceived and designed the study. EWG
and MKZ conducted experiments. EWG, MKZ and BJJ performed
histological analysis. BJJ and PCW contributed reagents. EWG and KC
analyzed data. EWG, MKZ and KC wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The experiment was conducted with the formal
approval of the Local Ethics Committee for Animal Experiments of
the University of Life Sciences in Lublin (approval no.
30/2015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mishra A, Chaturvedi P, Datta S, Sinukumar
S, Joshi P and Garg A: Harmful effects of nicotine. Indian J Med
Paediatr Oncol. 36:24–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ratajczak A, Feleszko W, Smith DM and
Goniewicz M: How close are we to definitively identifying the
respiratory health effects of e-cigarettes? Expert Rev Respir Med.
12:549–556. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Al-Obaidi S, Mathew TC and Dean E:
Exercise may offset nicotine-induced injury in lung tissue: A
preliminary histological study based on a rat model. Exp Lung Res.
38:211–221. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Avino P, Scungio M, Stabile L, Cortellessa
G, Buonanno G and Manigrasso M: Second-hand aerosol from tobacco
and electronic cigarettes: Evaluation of the smoker emission rates
and doses and lung cancer risk of passive smokers and vapers. Sci
Total Environ. 642:137–147. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vicary GW, Ritzenthaler JD, Panchabhai TS,
Torres-González E and Roman J: Nicotine stimulates collagen type I
expression in lung via α7 nicotinic acetylcholine receptors. Respir
Res. 18(115)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Benowitz NL, Hukkanen J and Jacob P III:
Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp
Pharmacol. 192:29–60. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lerner CA, Sundar IK, Yao H, Gerloff J,
Ossip DJ, McIntosh S, Robinson R and Rahman I: Vapours produced by
electronic cigarettes and e-juices with flavourings induce
toxicity, oxidative stress, and inflammatory response in lung
epithelial cells and in mouse lung. PLoS One.
10(e0116732)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu Q, Jiang D, Minor M and Chu HW:
Electronic cigarette liquid increases inflammation and virus
infection in primary human airway epithelial cells. PLoS One.
9(e108342)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yong HH, Hitchman SC, Cummings KM, Borland
R, Gravely SM, McNeill A and Fong GT: Does the regulatory
environment for E-cigarettes influence the effectiveness of
E-cigarettes for smoking cessation?: Longitudinal findings from the
ITC four country survey. Nicotine Tob Res. 19:1268–1276.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mohamed MH, Rahman A, Jamshed S and
Mahmood S: Effectiveness and safety of electronic cigarettes among
sole and dual user vapers in Kuantan and Pekan, Malaysia: A
six-month observational study. BMC Public Health.
18(1028)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McRobbie H, Bullen C, Hartmann-Boyce J and
Hajek P: Electronic cigarettes for smoking cessation and reduction.
Cochrane Database Syst Rev: Dec 17, 2014 (Epub ahead of print).
doi: 10.1002/14651858.CD010216.pub2.
|
|
12
|
Hartmann-Boyce J, Chepkin SC, Ye W, Bullen
C and Lancaster T: Nicotine replacement therapy versus control for
smoking cessation. Cochrane Database Syst Rev.
5(CD000146)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wagener TL, Floyd EL, Stepanov I, Driskill
LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N and
Queimado L: Have combustible cigarettes met their match? The
nicotine delivery profiles and harmful constituent exposures of
second-generation and third-generation electronic cigarette users.
Tob Control. 26:e23–e28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Verhaegen A and Van Gaal L: Do
E-cigarettes induce weight changes and increase cardiometabolic
risk? A signal for the future. Obes Rev. 18:1136–1146.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Camenga DR, Kong G, Cavallo DA and
Krishnan-Sarin S: Current and former Smokers' use of electronic
cigarettes for quitting smoking: An exploratory study of
adolescents and young adults. Nicotine Tob Res. 19:1531–1535.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Buu A, Hu YH, Piper ME and Lin HC: The
association between e-cigarette use characteristics and combustible
cigarette consumption and dependence symptoms: Results from a
national longitudinal study. Addict Behav. 84:69–74.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sassano MF, Davis ES, Keating JE, Zorn BT,
Kochar TK, Wolfgang MC, Glish GL and Tarran R: Evaluation of
e-liquid toxicity using an open-source high-throughput screening
assay. PLoS Biol. 16(e2003904)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Richmond SA, Pike I, Maguire JL and
Macpherson A: E-cigarettes: A new hazard for children and
adolescents. Paediatr Child Health. 23:255–259. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Greenhill R, Dawkins L, Notley C, Finn MD
and Turner JJD: Adolescent awareness and use of electronic
cigarettes: A review of emerging trends and findings. J Adolesc
Health. 59:612–619. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McMillen R, Tanski S, Wilson K, Klein JD
and Winickoff JP: Adolescent use of different E-cigarette products.
Pediatrics. 142(pii: e20180260)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Glynos C, Bibli SI, Katsaounou P, Pavlidou
A, Magkou C, Karavana V, Topouzis S, Kalomenidis I, Zakynthinos S
and Papapetropoulos A: Comparison of the effects of e-cigarette
vapor with cigarette smoke on lung function and inflammation in
mice. Am J Physiol Lung Cell Mol Physiol. 315:L662–L672.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Javed F, Kellesarian SV, Sundar IK,
Romanos GE and Rahman I: Recent updates on electronic cigarette
aerosol and inhaled nicotine effects on periodontal and pulmonary
tissues. Oral Dis. 23:1052–1057. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khan MS, Khateeb F, Akhtar J, Khan Z, Lal
A, Kholodovych V and Hammersley J: Organizing pneumonia related to
electronic cigarette use: A case report and review of literature.
Clin Respir J. 12:1295–1299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Walele T, Bush J, Koch A, Savioz R, Martin
C and O'Connell G: Evaluation of the safety profile of an
electronic vapour product used for two years by smokers in a
real-life setting. Regul Toxicol Pharmacol. 92:226–238.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gaur S and Agnihotri R: Health effects of
trace metals in electronic cigarette Aerosols-A systematic review.
Biol Trace Elem Res. 188:295–315. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Williams M, Villarreal A, Bozhilov K, Lin
S and Talbot P: Metal and silicate particles including
nanoparticles are present in electronic cigarette cartomizer fluid
and aerosol. PLoS One. 8(e57987)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Canistro D, Vivarelli F, Cirillo S, Babot
Marquillas CB, Buschini A, Lazzaretti M, Marchi L, Cardenia V,
Rodriguez-Estrada MT, Lodovici M, et al: E-cigarettes induce
toxicological effects that can raise the cancer risk. Sci Rep.
7(2028)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Burkholder TH, Niel L, Weed JL, Brinster
LR, Bacher JD and Foltz CJ: Comparison of carbon dioxide and argon
euthanasia: Effects on Behavior, Heart Rate, and respiratory
lesions in rats. J Am Assoc Lab Anim Sci. 49:448–453.
2010.PubMed/NCBI
|
|
29
|
Fawell JK, Thomson C and Cooke L:
Respiratory artefact produced by carbon dioxide and pentobarbitone
sodium euthanasia in rats. Lab Anim. 6:321–326. 1972.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Novotný L, Misík J, Karasová J, Kuča K and
Bajgar J: Influence of different ways of euthanasia on the activity
of cholinesterases in the rat. J App Biomedicine. 7:133–136.
2009.
|
|
31
|
Pierozan P, Jernerén F, Ransome Y and
Karlsson O: The choice of euthanasia method affects metabolic serum
biomarkers. Basic Clin Pharmacol Toxicol. 121:113–118.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Stutler SA, Johnson EW, Still KR,
Schaeffer DJ, Hess RA and Arfsten DP: Effect of method of
euthanasia on sperm motility of mature Sprague-Dawley rats. J Am
Assoc Lab Anim Sci. 46:13–20. 2007.PubMed/NCBI
|
|
33
|
Brooks DM and Hand WR Jr: A Cost analysis:
General endotracheal versus regional versus monitored anesthesia
care. Mil Med. 164:303–305. 1999.PubMed/NCBI
|
|
34
|
Varghese F, Bukhari AB, Malhotra R and De
A: IHC profiler: An open source plugin for the quantitative
evaluation and automated scoring of immunohistochemistry images of
human tissue samples. PLoS One. 9(e96801)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hernández-Morera P, Castaño-González I,
Travieso-González CM, Mompeó-Corredera B and Ortega-Santana F:
Quantification and statistical analysis methods for vessel wall
components from stained images with Masson's Trichrome. PLoS One.
11(e0146954)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Z, Liu X, Wang B, Nie Y, Wen J, Wang Q
and Gu C: Pirfenidone suppresses MAPK signalling pathway to reverse
epithelial-mesenchymal transition and renal fibrosis. Nephrology
(Carlton). 22:589–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jongenelis MI, Brennan E, Slevin T,
Kameron C, Rudaizky D and Pettigrew S: Differences in use of
electronic nicotine delivery systems by smoking status and
demographic characteristics among Australian young adults. Health
Promot J Austr. 30:207–211. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Rohde JA, Noar SM, Horvitz C, Lazard AJ,
Cornacchione Ross J and Sutfin EL: The role of knowledge and risk
beliefs in adolescent E-Cigarette use: A pilot study. Int J Environ
Res Public Health. 15(pii: E830)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Melin K, Conte-Schmidt N, Martínez-Arroyo
K, Rosa-Pérez K, Soto-Avilés AE and Hernández-Muñoz JJ: Knowledge
and perceptions of E-cigarettes and the motivations for their use:
Talking to smokers (E-cigarettes and/or Conventional Cigarettes)
and Non-smokers in Puerto Rico. P R Health Sci J. 37:148–154.
2018.PubMed/NCBI
|
|
40
|
Hughes JR and Callas PW: Prevalence of
withdrawal symptoms from electronic cigarette cessation: A
cross-sectional analysis of the US Population Assessment of Tobacco
and Health. Addict Behav. 91:234–237. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Case KR, Mantey DS, Creamer MR, Harrell
MB, Kelder SH and Perry CL: E-cigarette-specific symptoms of
nicotine dependence among Texas adolescents. Addict Behav.
84:57–61. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Itoh M, Aoshiba K, Herai Y, Nakamura H and
Takemura T: Lung injury associated with electronic cigarettes
inhalation diagnosed by transbronchial lung biopsy. Respirol Case
Rep. 6(e00282)2017.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Lechasseur A, Jubinville É, Routhier J,
Bérubé JC, Hamel-Auger M, Talbot M, Lamothe J, Aubin S, Paré MÈ,
Beaulieu MJ, et al: Exposure to electronic cigarette vapors affects
pulmonary and systemic expression of circadian molecular clock
genes. Physiol Rep. 5(e13440)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Moses E, Wang T, Corbett S, Jackson GR,
Drizik E, Perdomo C, Perdomo C, Kleerup E, Brooks D, O'Connor G, et
al: Molecular Impact of electronic cigarette aerosol exposure in
human bronchial epithelium. Toxicol Sci. 155:248–257.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Valença SS, de Souza da Fonseca A, da Hora
K, Santos R and Porto LC: Lung morphometry and MMP-12 expression in
rats treated with intraperitoneal nicotine. Exp Toxicol Pathol.
55:393–400. 2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schweitzer KS, Chen SX, Law S, Van Demark
M, Poirier C, Justice MJ, Hubbard WC, Kim ES, Lai X, Wang M, et al:
Endothelial disruptive proinflammatory effects of nicotine and
e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol.
309:L175–L187. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Reinikovaite V, Rodriguez IE, Karoor V,
Rau A, Trinh BB, Deleyiannis FW and Taraseviciene-Stewart L: The
effects of electronic cigarette vapour on the lung: Direct
comparison to tobacco smoke. Eur Respir J.
51(1701661)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Blaauboer ME, Boeijen FR, Emson CL, Turner
SM, Zandieh-Doulabi B, Hanemaaijer R, Smit TH, Stoop R and Everts
V: Extracellular matrix proteins: A positive feedback loop in lung
fibrosis? Matrix Biol. 34:170–178. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Carson JL, Zhou L, Brighton L, Mills KH,
Zhou H, Jaspers I and Hazucha M: Temporal structure/function
variation in cultured differentiated human nasal epithelium
associated with acute single exposure to tobacco smoke or
E-cigarette vapor. Inhal Toxicol. 29:137–144. 2017.PubMed/NCBI View Article : Google Scholar
|
















