Introduction
Chronic hepatitis B and C are caused by hepatitis B
virus (HBV) and hepatitis C virus (HCV), respectively. Both cause
liver cancer, but affected patients have few subjective symptoms
(1-3).
However, appropriate antiviral treatment can lower the risks
associated with these conditions (4-6).
Antiviral agents continue to improve, and a high percentage of
patients with hepatitis C can achieve a sustained virological
response with minimal adverse effects by using direct-acting
antivirals (DAAs) (7-10).
Additionally, in patients with hepatitis B, the levels of HB
surface antigen (HBsAg) and HBV-DNA can be reduced using pegylated
interferon or nucleotide analogues (11-14).
Therefore, to prevent an increase in liver cancer, it is essential
to improve the screening rates for viral hepatitis and administer
appropriate antiviral treatment to patients with hepatitis. The
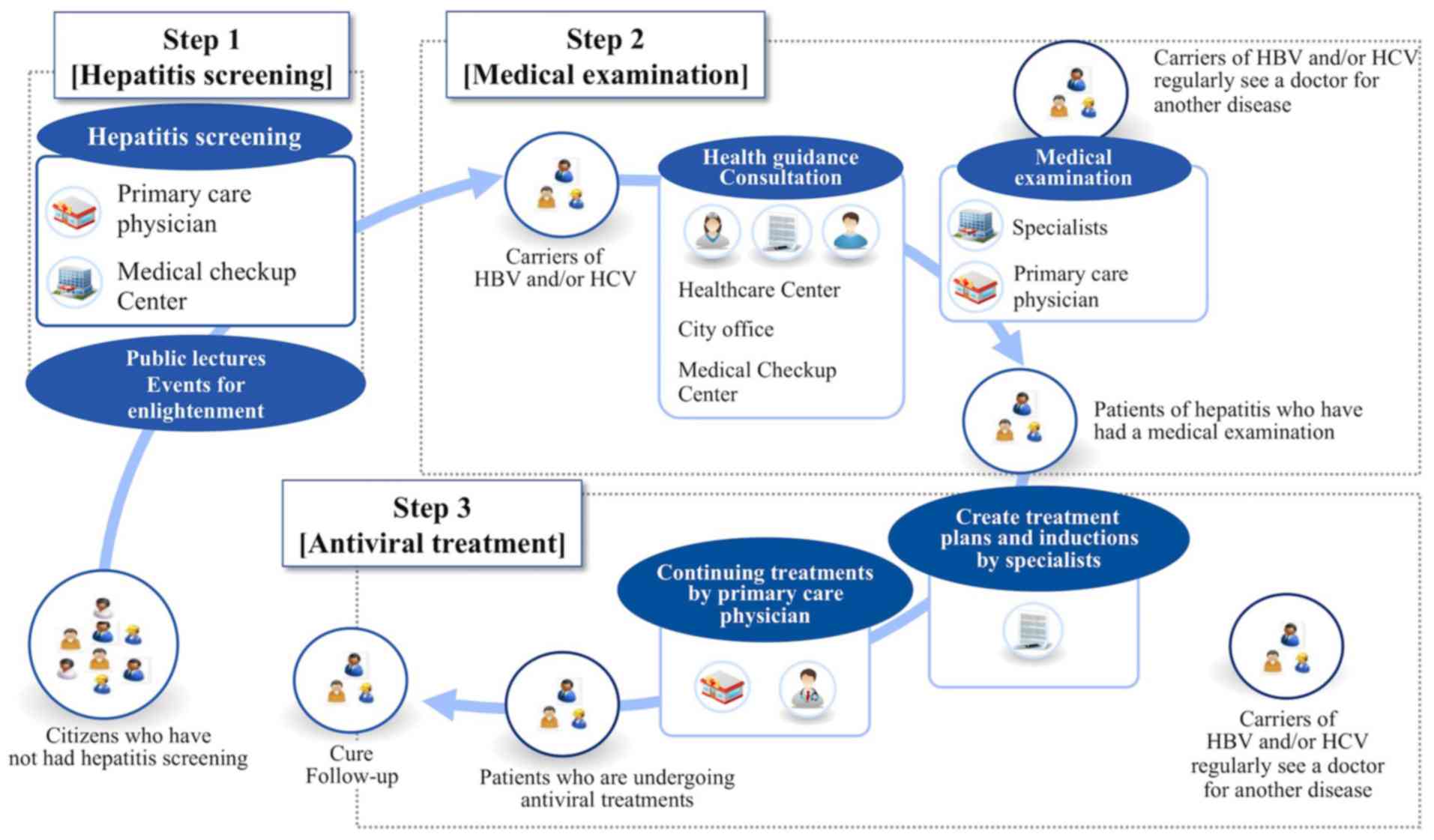
following three steps are typically followed for comprehensive
antiviral treatment: hepatitis screening, in which HBsAg and HCV
antibody (HCVAb) are examined; medical examination for viral
carriers, in which HBV-DNA and/or HCV-RNA are measured and imaging
as abdominal ultrasonography is performed; and administration of
individualized antiviral treatment or liver-supporting therapy
(Fig. 1) (15).
As of 2011 in Saga prefecture, the rates of HBsAg
and HCVAb positivity were 1.05 and 1.18%, respectively (16), which are higher than those of first
blood donors from all of Japan (0.25-0.29% and 0.10-0.21%,
respectively) (17). A rough
estimate of the liver cancer rates in Saga prefecture for the
16-year period beginning in 1999 stands as the worst consecutive
time period for liver cancer in all of Japan (18). Thus, reducing liver cancer mortality
rates is one of the most important issues for Saga prefecture.
Unfortunately, hepatitis screening rates remained low even in 2011:
26.3% for HBV and 47.0% for HCV. Additionally, 63.6% of viral
carriers received a detailed examination, and 40.0% of both HBV and
HCV carriers were accepted into antiviral treatment (16). In 2011, Saga prefecture established
institutional protocols for the purpose of tracking a particular
viral liver disease and established a Liver Center within Saga
University Hospital in January 2012(15). The Liver Center disseminates medical
information to the public using various media. However, these
enlightenment activities take time and are expensive. Therefore, it
is essential to identify effective information sources (factors)
that help patients to make better decisions.
Each of the three above-mentioned steps is expected
to contribute to an increase in patients who reach the subsequent
step and to expose which information sources may have led those
patients to each step. The aim of our study was to determine which
factors are effective in patients' decision-making processes from
initial screening to receiving antiviral treatment.
Materials and methods
Study design and participants
During the 5-month period from March to August 2013,
we recruited patients receiving antiviral treatment from one of the
11 medical institutions specializing in hepatitis treatment in Saga
prefecture. Participants underwent face-to-face interviews by the
hepatitis coordinator, who asked them about information sources
that had affected their decision-making process, and filled in the
unsigned questionnaire. Specifically, the questionnaire asked the
participants which of 18 sources of information they had accessed
(multiple choice answer) in each of the three steps mentioned
above; which of the 18 information sources had been most
influential (single answer) in each of the three steps; sex; age
group; residential area; viral type; time of hepatitis screening;
time of medical examination; and time of antiviral treatment. The
hepatitis coordinators were paramedical staff members actively
engaged in the field of liver disease, such as hospital nurses,
public care nurses, pharmacists, and nutritionists.
In each of the three steps, we divided the number of
participants who received all 18 information sources by the total
number of participants to determine the participants' recognition
of each information source. Then, to determine the influence of
each information source (i.e., the direct influence that it
provided each participant to reach the subsequent step), we divided
the number of participants who answered, ‘This information was most
influential’ by the number of participants who received each source
of information.
The 18 sources of information that we investigated
comprised 7 human factors, 10 public relations sources (social
factors), and 1 other source. The seven human factors comprised
recommendations from ‘a primary care physician,’ ‘a hospital
nurse,’ ‘a public health nurse (PHN),’ ‘a hepatitis coordinator,’
‘a pharmacist,’ ‘friends or family,’ and ‘work colleagues’ The 10
social factors consisted of ‘posters for enlightenment,’ ‘direct
mail,’ ‘PR brochures from a city,’ ‘3-minute TV programs for
enlightenment,’ ‘a feature TV program about viral hepatitis and
liver cancer,’ ‘a TV commercial message for enlightenment,’
‘newspapers,’ ‘magazines,’ ‘websites about liver diseases,’ and
‘public lectures about liver diseases.’ We defined ‘hepatitis
screening’ as examinations that included tests for HBsAg and HCVAb,
‘medical examination’ as examinations that tested HBV-DNA or
HCV-RNA and included imaging such as abdominal ultrasonography, and
‘antiviral treatment’ as a treatment plan that included pegylated
interferon or nucleotide analogues for patients with HBV and
pegylated interferon, ribavirin, and DAA for patients with HCV.
The present study was approved by the Institutional
Review Board and Ethics Committee of Saga University Hospital (No.
2014-10-10) and conformed to the ethical guidelines of the 8th
version of the Declaration of Helsinki (October 2013). We performed
data extraction with consideration of the protection and privacy of
our participants' personal information.
Statistical analysis
Microsoft Excel 2011 (Microsoft, Redmond, WA, USA)
was used to aggregate the survey data and depict the scatter
diagrams. Continuous variables are presented as mean (standard
deviation) or median. Proportions and categorical variables were
assessed using the χ2 test or Fisher's exact test and
residual analysis. P<0.05 was considered to indicate a
statistically significant difference. All analyses were carried out
using IBM SPSS (v.21.0; SPSS, Tokyo, Japan).
Results
Background of participants
Table I shows the characteristics (including
demographics) of the 182 participants who answered the
questionnaire. They ranged in age from 20 to >80, and most were
in their 60s. Of these participants, 93 (51.1%) were male and 153
(84.1%) were HCV carriers. We obtained questionnaire data from
participants in all districts except the eastern district of the
prefecture because there is no specialized medical institution in
that region. Table II shows the
time it took participants to progress from hepatitis screening
through medical examination to antiviral treatment. Some
participants could not answer this section because they forgot
either the time at which they underwent hepatitis screening or the
time at which they underwent medical examination. The median and
mean periods of time between undergoing a hepatitis screening and
undergoing a medical examination were 0.0 and 19.6 months,
respectively, while the median and mean periods of time between
undergoing a hepatitis screening and receiving an antiviral
treatment were 8.0 and 56.2 months, respectively. Importantly,
patients with HCV were classified into two groups: Those who had
been receiving treatment for more than 10 years since their initial
hepatitis screening, and those who had been treated for a short
time since their initial hepatitis screening. Data regarding the
specific characteristics of these two groups are not shown.
 | Table IITime interval from hepatitis screening
to antiviral treatment. |
Table II
Time interval from hepatitis screening
to antiviral treatment.
| Total | HBV infected
patient | HCV infected
patient |
|---|
| Variables | n | % | n | % | n | % |
|---|
| Hepatitis screening
to medical examination (months) |
|
0-3 | 76 | 73.8 | 12 | 92.3 | 63 | 70.8 |
|
4-6 | 5 | 4.9 | 1 | 7.7 | 4 | 4.5 |
|
7-12 | 6 | 5.8 | 0 | 0 | 6 | 6.7 |
|
12-36 | 4 | 3.9 | 0 | 0 | 4 | 4.5 |
|
36-120 | 6 | 5.8 | 0 | 0 | 6 | 6.7 |
|
>120 | 6 | 5.8 | 0 | 0 | 6 | 6.7 |
|
Median | 0 | | 0 | | 0 | |
|
Mean
(SD) | 19.6 (53.2) | | 0.7 (1.2) | | 22.6 (56.6) | |
| Medical examination
to antiviral treatment (months) |
|
0-3 | 68 | 59.1 | 5 | 71.4 | 61 | 57.5 |
|
4-6 | 13 | 11.3 | 0 | 0 | 13 | 12.3 |
|
7-12 | 6 | 5.2 | 0 | 0 | 6 | 5.7 |
|
12-36 | 12 | 10.4 | 2 | 28.6 | 10 | 9.4 |
|
36-120 | 4 | 3.5 | 0 | 0 | 4 | 3.8 |
|
>120 | 12 | 10.4 | 0 | 0 | 12 | 11.3 |
|
Median | 0 | | 0 | | 0 | |
|
Mean
(SD) | 27.7 (67.6) | | 8.1 (11.7) | | 29.4 (70.1) | |
| Hepatitis screening
to antiviral treatment (months) |
|
0-3 | 32 | 32 | 3 | 42.9 | 28 | 30.4 |
|
4-6 | 16 | 16 | 0 | 0 | 16 | 17.4 |
|
7-12 | 7 | 7 | 1 | 14.3 | 6 | 6.5 |
|
12-36 | 9 | 9 | 1 | 14.3 | 8 | 8.7 |
|
36-120 | 16 | 16 | 1 | 14.3 | 15 | 16.3 |
|
>120 | 20 | 20 | 1 | 14.3 | 19 | 20.7 |
|
Median | 8 | | 7 | | 8 | |
|
Mean
(SD) | 56.2 (90.0) | | 70.9 (132.1) | | 56.3 (86.2) | |
Recognition and impact in the
hepatitis screening step
Table III shows the number of participants who
received information sources and who acted by direct influence of
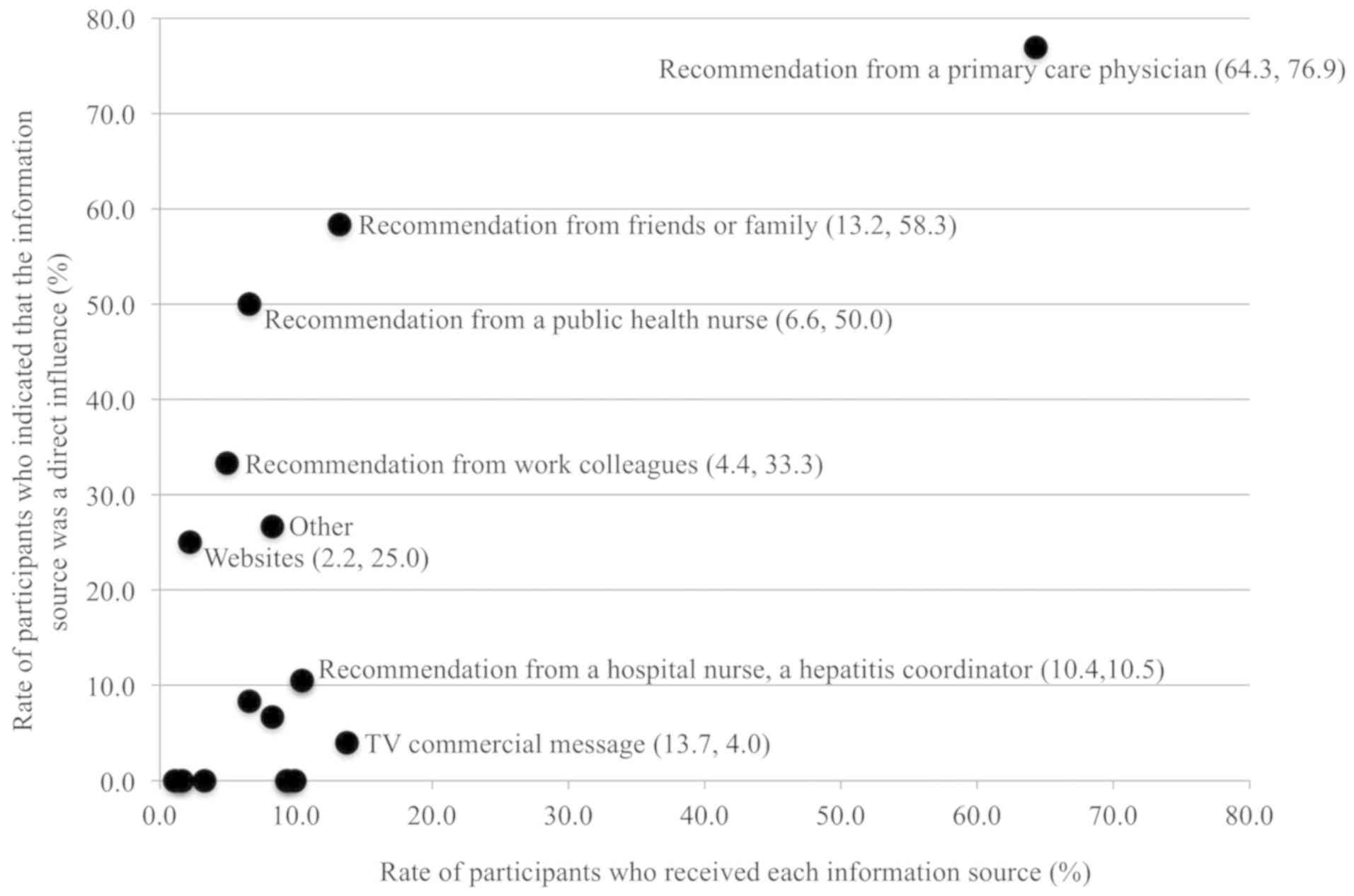
the information source at each of the three steps. Figs. 2-4 show the former and latter
percentages in two dimensions for each of the three steps. In the
first step (hepatitis screening), 117 (64.3%) participants received
recommendations from their primary care physician, and 90 (76.9%)
of these participants indicated that those recommendations were the
most influential in their decision to undergo hepatitis screening
(Fig. 2 and Table III). Another 13.2% of participants
received recommendations from friends or family, and of those,
58.3% took the opportunity to act directly and get screened. From
the information category of social factors, 13.7% of participants
accessed a relevant TV commercial message; however, only 4.0% of
this group took direct influence to undergo hepatitis
screening.
 | Table IIINumbers of participants who received
information sources and who acted in direct opportunity to the
information source at each of the three steps. |
Table III
Numbers of participants who received
information sources and who acted in direct opportunity to the
information source at each of the three steps.
| Step 1 hepatitis
screening | Step 2 medical
examination | Step 3 antiviral
treatment |
|---|
| Source of
information | Participants who
received information (%)a | Direct influence
(%)b | Participants who
received information (%)a | Direct influence
(%)b | Participants who
received information (%)a | Direct influence
(%)b |
|---|
| Recommendations
from human |
|
Recommendation
from a primary care physician | 117 (64.3) | 90 (76.9c) | 141 (77.5) | 103 (73.0c) | 138 (75.8) | 107 (77.5c) |
|
Recommendation
from a hospital nurse | 19 (10.4) | 2 (10.5) | 36 (19.8) | 6 (16.7) | 25 (13.7) | 4 (16.0) |
|
Recommendation
from a public health nurse | 12 (6.6) | 6 (50.0) | 19 (10.4) | 5 (26.3) | 14 (7.7) | 9 (64.3) |
|
Recommendation
from a hepatitis coordinator | 19 (10.4) | 2 (10.5) | 31 (17.0) | 3 (9.7c) | 24 (13.2) | 2 (8.3c) |
|
Recommendation
from a pharmacist | 3 (1.6) | 0 | 9 (4.9) | 0 | 5 (2.7) | 0 |
|
Recommendation
from friends or family | 24 (13.2) | 14 (58.3) | 36 (19.8) | 14 (38.9) | 24 (13.2) | 14 (58.3) |
|
Recommendation
from work colleagues | 9 (4.9) | 3 (33.3) | 6 (3.3) | 2 (33.3) | 7 (3.8) | 3 (42.9) |
| Information
provided passively |
|
Posters for
enlightenment | 15 (8.2) | 1 (6.7) | 20 (11.0) | 0 | 11 (6.0) | 0 |
|
Direct
Mail | 3 (1.6) | 0 | 2 (1.1) | 0 | 3 (1.6) | 0 |
|
PR brochures
from a city | 12 (6.6) | 1 (8.3) | 14 (7.7) | 2 (14.3) | 13 (7.1) | 2 (15.4) |
|
3-minute TV
programs for enlightenment | 18 (9.9) | 0 | 31 (17.0) | 0 | 10 (5.5) | 0 |
|
Feature TV
program about viral hepatitis and | 17 (9.3) | 0 | 19 (10.4) | 0 | 20 (11.0) | 0 |
|
hepatocellular
carcinoma |
|
TV
commercial message for enlightenment | 25 (13.7) | 1 (4.0c) | 37 (20.3) | 2 (5.4c) | 19 (10.4) | 0 |
|
Newspapers | 17 (9.3) | 0 | 26 (14.3) | 1 (3.8c) | 14 (7.7) | 0 |
|
Magazines | 6 (3.3) | 0 | 10 (5.5) | 0 | 5 (2.7) | 0 |
| Information
obtained actively |
|
Websites
about liver diseases | 4 (2.2) | 1 (25.0) | 9 (4.9) | 1 (11.1) | 6 (3.3) | 0 |
|
Public
lectures about liver diseases | 2 (1.1) | 0 | 6 (3.3) | 1 (16.7) | 1 (0.5) | 0 |
| Others | 15 (8.2) | 4 (26.7) | 15 (8.2) | 1 (6.7) | 9 (4.9) | 3 (33.3) |
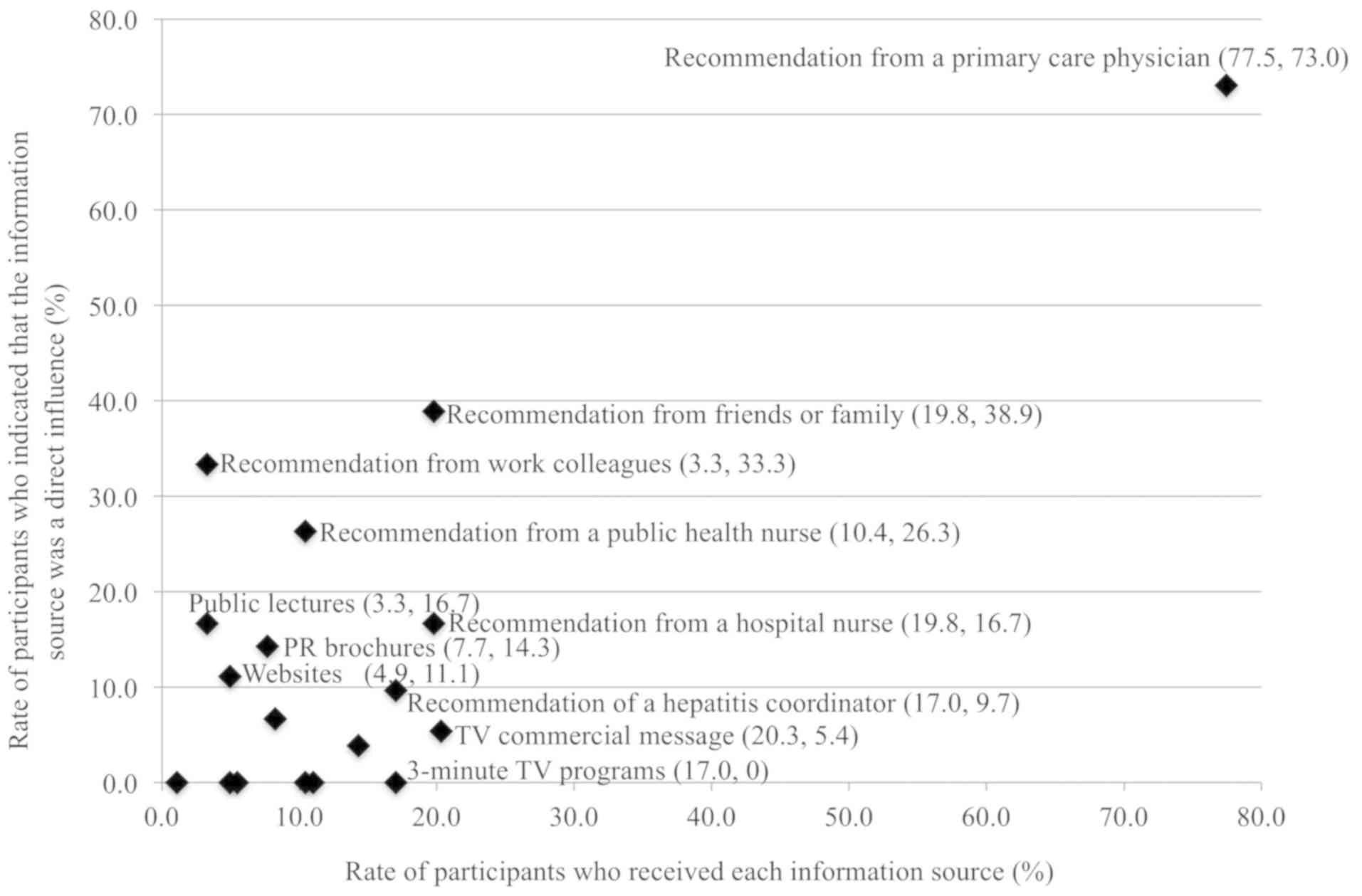
Recognition and impact in the medical
examination step
In the second step (medical examination), 77.5% of
participants received recommendations from their primary care
physician, of which 73.0% took direct action and underwent a
medical examination (Fig. 3 and
Table III). Recommendations from
friends and family had a higher recognition rate than from PHC
nurses (19.8% vs. 10.4%, respectively), but direct influence was
not significant (38.9% vs. 26.3%, respectively; P=0.58). In the
information category of enlightenment activities, exposure to a TV
commercial (20.3%) was higher than exposure to recommendations from
humans except for those from primary care physicians; however, the
relative impact of a TV commercial (5.4%) was significantly lower.
Exposure to other social factors comprised public lectures (3.3%
received, of which 16.7% took action), PR brochures (7.7% received,
of which 14.3% took action), and websites (4.9% received, of which
11.1% took action); these sources might have served as direct
opportunities for participants to some extent.
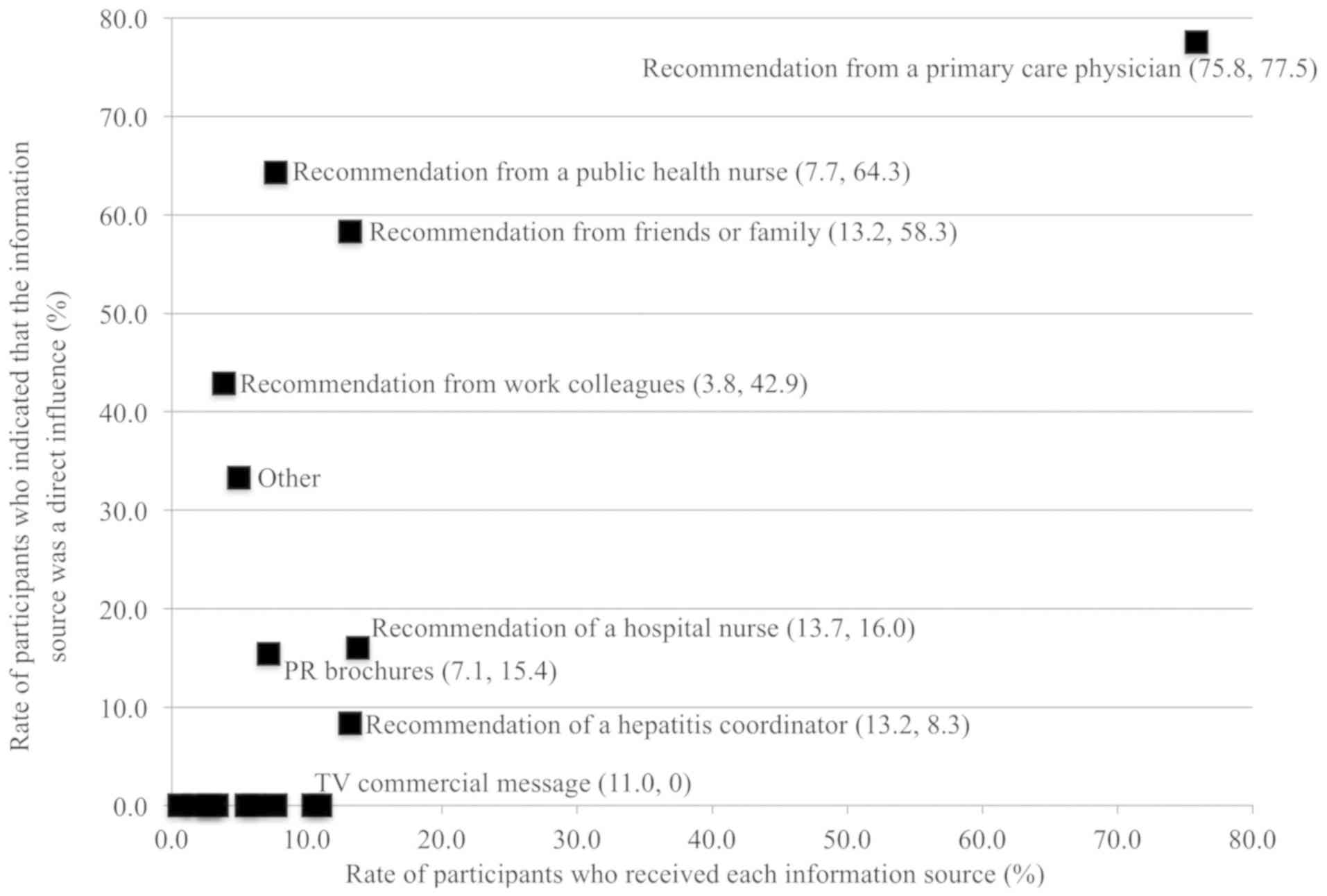
Recognition and impact in the
antiviral treatment step
In the third step (antiviral treatment), more
participants (75.8%) obtained recommendations from their primary
care physician than from any other information source; this source
also had the strongest impact (77.5%) of any information source
(Fig. 4 and Table III). Although recommendations from
PHNs (7.7%), friends and family (13.2%), and work colleagues (3.8%)
represented a small proportion of participants' exposure to
information, these sources had a high impact (64.3, 58.3, and
42.9%, respectively) on participants' decisions to accept antiviral
treatment. In the category of social factors, despite wide
exposure, no source except PR brochures (7.1% received, of which
15.4% took action) evidenced an impact that led patients to accept
antiviral treatment.
Recognition and impact after February
2013
Table IV shows the recognition and impact of
participants who first accessed each step after February 2013, when
the Liver Center began conducting enlightenment activities. In this
period, recommendations from their primary care physician had a
high impact in each of the three steps (100, 90.0, and 76.7%,
respectively). Although relatively few participants received other
sources of information, the recommendations that they received from
friends and family also showed a high impact in each of the three
steps (100, 100, and 50%, respectively). Recognition of a TV
commercial in this period was significantly higher than that during
the whole study period for step 3 (28.6% vs. 10.4%); however, no
more than 10.0% of this group took direct influence to receive
antiviral treatment.
Differences in sex, age group, and
viral type
We found no significant differences in the results
in any of the three steps according to sex, age group, or viral
type. We also found no significant differences in the results
between the two groups of patients with HCV classified by the time
from hepatitis screening to antiviral treatment.
Discussion
The present study is the first to assess factors
that influence citizens' recognition and decisions about receiving
antiviral treatment for viral liver disease in Japan. Most patients
in this study received recommendations from a primary care
physician and were strongly influenced by this factor in their
decisions. Therefore, we believe that primary care physicians who
promote appropriate antiviral therapy can be an important factor in
the prevention and management of liver cancer. Furthermore,
recommendations from friends, family, or work colleagues appear to
have as much power as those from a PHN to move patients toward a
decision. Within each of the three steps, hospital nurses had many
opportunities for contact with patients, but PHNs were more
effective in taking advantage of opportunities to persuade the
patient directly. With respect to educational activities, the
majority of participants were most aware of TV commercials.
However, public lectures, PR brochures, and websites might also
have persuasive power with patients.
With respect to the influence a primary care
physician can have on patient decision-making, a previous study
showed that primary care physicians' encouragement of patients to
undergo cancer screening did not increase the number of examinees
(19). In contrast, another study
showed that the primary reason patients with arthritis chose not to
use disease-modifying anti-rheumatic drugs was ‘because the doctor
did not recommend it’ (20). One
study regarding the influence of others on patient decision-making
revealed that family and friends were involved in the
decision-making process around active surveillance (i.e., actively
awaiting treatment) in patients with prostate cancer (21). However, these findings do not specify
their impact. Another study showed that nurses affect the
decision-making of patients with breast cancer (22). Our study is the first to examine
factors that influence the treatment-related decisions of patients
with viral liver disease.
In agreement with previous research, our findings
indicate that primary care physicians are a strong force in
patients' health-related decision-making processes and can thus
improve the likelihood of a patient receiving detailed examinations
and antiviral therapy for viral liver disease. Furthermore, this
study shows that it is possible to quantify that influence on
participants. Importantly, although recommendations from family,
friends, and work colleagues affected a high percentage of the
decision-making process of patients with viral liver disease, we do
not consider them to be strong factors because very few
participants accessed them. PHNs might have more influence on
patients than hospital nurses; a difference in job descriptions was
considered one of the reasons. That is, a PHN's focus is the
prevention of disease progression, while a hospital nurse's main
work is caring for patients. Of all social factors that we
investigated, websites and public lectures were the factors that
participants searched and visited independently. Thus, it is
understandable that these two sources became influential factors in
interested participants' decision-making process. In contrast,
other factors were observed regardless of a participant's
intentions.
Because the primary care physician has a strong
influence on the decision-making of the patient, an increase in the
number of both liver disease specialists and primary care
physicians with an interest in liver disease is important for the
prevention and management of liver cancer. Few participants
received a recommendation from family, friends, or work colleagues;
however, because those particular sources have considerable
influence in moving patients toward healthy decisions, increasing
the opportunities for patients to access those factors could be an
effective strategy. Finally, although participants were not moved
directly to make decisions about liver screening or therapy based
on information gleaned from the media, their awareness of this
source was high. Given that the provision of appropriate
information is assumed to improve the right knowledge of citizens,
it might also improve the right knowledge of other persons involved
in the patient's life, and then their recommendations would
influence the patients. We suggest that synergy of human and media
sources may contribute to effective management of viral hepatitis
and liver cancer.
Our study of patients with HCV involved two groups:
Patients who had been receiving treatment for more than 10 years
since their initial hepatitis screening and those who had been
treated for a short time since their hepatitis screening. There
were no significant differences in the characteristics of the two
groups, such as sex, age group, residential area, or information
sources. Further investigation into the former group might
contribute to the identification of different factors that urge
HCV-positive persons to undergo antiviral treatment.
We assessed the recognition of aggressive
enlightenment activities and their impact on participants during
the period when these activities were conducted. During this
period, human factors might have influenced the decision-making
process of the patient. Recognition of TV commercials increased,
and the results showed that TV commercials were watched. However,
it was difficult for commercials to directly influence the decision
making of the patient. The benefit of media is the ability to send
information to people who are not interested and allow them to
recognize information regarding diseases. However, a very large
amount of information is presented by the media; people recognize
information regarding diseases, but they receive it as only one
aspect of a very large amount of information. This is why it may be
difficult for the media to influence patients' decision making. In
addition, use of the media is associated with certain costs, and we
did not examine outcomes and cost-effectiveness in the present
analysis.
One limitation of this study was the small sample
size. Furthermore, the etiology of liver disease among the enrolled
patients included both HBV and HCV because there were fewer
patients with HBV than HCV. In fact, these etiologies differ, and
patients with HBV and HCV should ideally be analyzed separately.
Moreover, sex- and age-related differences might be revealed in a
larger sample of patients. Another limitation was the data
collection method. The hepatitis coordinators collected the data
retrospectively. Thus, information bias and selection bias may have
been present. Furthermore, the hepatitis coordinators comprised
paramedical staff members, so human factors were duplicated. We
might not have obtained accurate results. It should also be noted
that this study was conducted during a time when interferon-based
treatment was mainstream; had DAA treatment been mainstream,
different results may have been obtained. Finally, we did not
analyze sex- or age-related differences in the participants'
decisions to take subsequent steps. The older citizens become, the
more they may go to the hospital, and they tend to receive
recommendations from their primary care physician more often as
they become older. Practically, clinical doctors have the right of
final decisions regarding examinations. Additional analysis of such
factors could lead to better insights into more effective
strategies in patients' decision-making process.
Acknowledgments
The authors thank Dr Soichi Tamai (McKinsey &
Company) for his excellent advice.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contribution
MO, NK and YE designed the present study. MO, SO,
SI, YK and KM acquired, analyzed and interpreted the data, and
drafted the manuscript. SK, YT and JF performed statistical
analysis. HT and KA developed the study protocol, generated the
original database and supervised the current study. KA and YE gave
final approval of the version to be published. MO and NK analyzed
data using various software applications. SO, SI and YE assessed
data integrity and the accuracy of data analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board and Ethics Committee of Saga University Hospital
(approval no. 2014-10-10) and conformed to the ethical guidelines
of the 8th version of the Declaration of Helsinki (October 2013).
An opt-out approach was used to obtain informed consent from the
patients and personal information was protected during data
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y,
Yoshizawa K, Nakano Y, Furuta S, Akahane Y, Nishioka K, Purcell RH,
et al: Interrelationship of blood transfusion, non-A, non-B
hepatitis and hepatocellular carcinoma: Analysis by detection of
antibody to hepatitis C virus. Hepatology. 12:671–675.
1990.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fattovich G, Bortolotti F and Donato F:
Natural history of chronic hepatitis B: Special emphasis on disease
progression and prognostic factors. J Hepatol. 48:335–352.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cardoso AC, Moucari R, Figueiredo-Mendes
C, Ripault MP, Giuily N, Castelnau C, Boyer N, Asselah T,
Martinot-Peignoux M, Maylin S, et al: Impact of peginterferon and
ribavirin therapy on hepatocellular carcinoma: Incidence and
survival in hepatitis C patients with advanced fibrosis. J Hepatol.
52:652–657. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ikeda K, Saitoh S, Arase Y, Chayama K,
Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H
and Kawanishi M: Effect of interferon therapy on hepatocellular
carcinogenesis in patients with chronic hepatitis type C: A
long-term observation study of 1,643 patients using statistical
bias correction with proportional hazard analysis. Hepatology.
29:1124–1130. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kasahara A, Hayashi N, Mochizuki K,
Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A,
Kiyosawa K, Okuda M, et al: Risk factors for hepatocellular
carcinoma and its incidence after interferon treatment in patients
with chronic hepatitis C. Osaka Liver Disease Study Group.
Hepatology. 27:1394–1402. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kumada H, Suzuki Y, Ikeda K, Toyota J,
Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et
al: Daclatasvir plus asunaprevir for chronic HCV genotype 1b
infection. Hepatology. 59:2083–2091. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeuzem S, Dusheiko GM, Salupere R, Mangia
A, Flisiak R, Hyland RH, Illeperuma A, Svarovskala E, Brainard DM,
Symonds WT, et al: Sofosbuvir and ribavirin in HCV genotypes 2 and
3. N Engl J Med. 370:1993–2001. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Afdhal N, Zeuzem S, Kwo P, Chojkier M,
Gitlin N, Puoti M, Romero-Gomes M, Zarski JP, Agarwal K, Buggisch
P, et al: Ledipasvir and sofosbuvir for untreated HCVgenotype 1
infection. N Engl J Med. 370:1889–1898. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Feld JJ, Kowdley KV, Coakley E, Sigal S,
Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias
T, et al: Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir
with ribavirin. N Engl J Med. 370:1594–1603. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Piratvisuth T, Lau G, Chao YC, Jin R,
Chutaputti A, Zhang QB, Tanwandee T, Button P and Popescu M:
Sustained response to peginterferon alfa-2a (40 kD) with or without
lamivudine in Asian patients with HBeAg-positive and HBeAg-negative
chronic hepatitis. B Hepatol Int. 2:102–110. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ono A, Suzuki F, Kawamura Y, Sezaki H,
Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitou S, Arase Y, et al:
Long-term continuous entecavir therapy in nucleos(t)ide-naive
chronic hepatitis B patients. J Hepatol. 57:508–514.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM,
Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, et al: Entecavir treatment
reduces hepatic events and deaths in chronic hepatitis B patients
with liver cirrhosis. Hepatology. 58:1537–1547. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Marcellin P, Heathcote EJ, Buti M, Gane E,
de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, et
al: Tenofovir disoproxil fumarate versus adefovir dipivoxil for
chronic hepatitis B. N Engl J Med. 359:2442–2455. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eguchi Y, Maeyama K, Ozaki I and Hrai K:
Medical system for liver disease treatment in response to basic act
on hepatitis measures. Nihon Naikagakkai Zasshi. 103:11–18.
2014.(In Japanese). PubMed/NCBI View Article : Google Scholar
|
|
16
|
SagaPrefectural Government: Saga Liver
Disease Measures promotion Plan (In Japanese). Available at:
http://www.pref.saga.lg.jp/kiji00334103/3_34103_1_20134893825.pdf.
Accessed January 15, 2017.
|
|
17
|
Ministry of Health, Labor and Welfare,
Japan. Investigation Committee for blood business sectional meeting
security technology 2011 first (In Japanese) Available at:
http://www.mhlw.go.jp/stf/shingi/2r98520000020hlt.html.
Accessed January 15, 2017.
|
|
18
|
Cancer Information and Service National
Cancer Center Japan. Cancer Registry and Statistics (In Japanese).
Available at: http://ganjoho.jp/reg_stat/statistics/dl/index.html.
Accessed January 15, 2017.
|
|
19
|
Tateno Y, Miyazaki Y, Tsuboi S and Uehara
R: Can screening invitations from primary care physicians increase
participation in cancer screenings on remote islands? General
Medicine. 14:40–47. 2013.
|
|
20
|
Lacaille D and Pam R: Why are people with
rheumatoid arthritis (RA) not using DMARDs? Understanding gaps in
care. J Rheumatol. 46(1218)2008.
|
|
21
|
Penson DF: Factors influencing patients'
acceptance and adherence to active surveillance. J Natl Cancer Inst
Monogr. 2012:207–212. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
McAllister KA and Schmitt ML: Impact of a
nurse navigator on genomic testing and timely treatment decision
making in patients with breast cancer. Clin J Oncol Nurs.
19:510–512. 2015.PubMed/NCBI View Article : Google Scholar
|