Introduction
Hemangiomas (HAs) remains the most common benign
vascular tumors among infants, with an annual frequency of 5-10%
(1). Its appearance is similar to
infantile hemangiomas and congenital hemangiomas, but their
clinical presentations are considered to be different (1). The life cycle of HA consists of three
stages: Proliferation, quiescence and involution (2). As stem/progenitor cells, angiogenesis
and pre-existing vessels are sufficient to induce vasculogenesis,
HA proliferation is likely to occur (3). HA-derived endothelial cells (HDECs),
progenitor/stem cells and perivascular cells are three
well-characterized cells isolated from Has (4,5).
However, the mechanisms underlying the development and progression
of HA are not yet fully understood.
MicroRNAs (miRNAs/miRs) are short regulatory RNAs
usually consisting of ~22 nucleotides (6). These genes function as important
regulators that mediate mRNA degradation or protein-coding
silencing in a sequence-dependent manner (7). miRNAs serve a critical role in cancer
cell growth, apoptosis, epithelial-mesenchymal transition, therapy
response and clinical outcome (8).
Several miRNAs, including miR-126(9), miR-424(10), miR-143(11) and let-7(12) have been detected in resected tumor
tissues or cell lines, and have been demonstrated to tightly
regulate the development of HA. Previous reports have revealed
frequent miR-4458 downregulation in lung cancer (13), hepatocellular carcinoma (HCC)
(14) and colon cancer (15). miR-4458 acts as a tumor suppressor
and decreases the proliferation of lung cancer cells (13). It also functions as a potential
predictor of HCC (14) and guides
glycolysis and lactate production in colon cancer cells (15). However, the role of miR-4458 in HA is
yet to be elucidated.
Receptor tyrosine kinase insulin-like growth factor
1 receptor (IGF1R), located on 15q26.3, is expressed ubiquitously
on cell surfaces and the binding of its ligands (IGF1, IGF2 and
insulin) triggers the tyrosine kinase activity of the receptor
(16,17). Abnormalities of the IGF1R signaling
pathway are associated with growth, progression and disorders of
metabolism in a range of different types of cancer (18,19).
Hyperexpression of the IGF1R gene is frequently observed in breast
cancer (18), penile cancer
(19) and epithelial ovarian cancer
(20). Numerous studies have
demonstrated that IGF1R induces proliferation, survival, cell cycle
progression and a series of malignant behaviors in cancer cells
(20-22).
A previous study provided novel insight into the biological
function of another IGF receptor, IGF2R, in HA (23). Silencing of IGFR2 abrogated the
proliferation of HDECs and promoted apoptosis in vitro and
in vivo (23). miR-4458 has
been implicated in IGF1R signaling, which regulates the development
of lumbar disc degeneration (24).
Therefore, these results may suggest a potential implication for
IGF1R in the development of HA.
In the present study, the cellular functions of
miR-4458 and one of its key targets, IGF1R, were determined. The
potential molecular mechanisms of action were validated in
vitro, thus, highlighting the potential of targeting the
miR-4458/IGF1R axis in patients with HAs.
Materials and methods
Cell culture and transfection
Primary HDECs derived from proliferating-phase HA
tumors were purchased from the Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences. Human umbilical vein
endothelial cells (HUVECs) were purchased from the American Type
Culture Collection. The cell lines were cultured in DMEM (Thermo
Fisher Scientific Inc.) with 10% heat-inactivated FBS (Thermo
Fisher Scientific Inc.) in a humidified atmosphere containing 5%
CO2 at 37˚C.
The miR-4458 mimics, miR-4458 inhibitor and
miR-negative control (NC) were purchased from Guangzhou RiboBio
Co., Ltd. Small interfering (si)RNA targeting IGF1R (si-IGF1R) and
si-NC were synthesized by Shanghai GenePharma Co., Ltd. The
experimental design was adapted from previously described
literature (11,25,26).
HDECs were seeded in 24-well plates at a density of
5x104 cells per well, and when the cells had reached 80%
confluency, they were transfected with 50 nM of mimics or
inhibitor, or 10 µg of the si-IFG1R or si-NC plasmids for 48 h
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. To quantify
miR-4458 expression, RT-qPCR was performed in triplicate on a 7300
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using a TaqMan microRNA Reverse Transcription kit (Thermo
Fisher Scientific, Inc.) and TaqMan gene expression master mix
(Thermo Fisher Scientific, Inc.). The temperature conditions for
reverse transcription were as follows: 37˚C for 15 min and 85˚C for
5 sec. For the detection of IGF1R, RT-qPCR was performed using
PrimeScript RT Master mix (Takara Bio, Inc.) and a QuantiNova SYBR
Green PCR kit (Qiagen China Co., Ltd.) according to the
manufacturer's protocol. The sequences of the primers used were as
follows: miR-4458 forward, 5'-AGAGGTAGGTGTGGAAGAA-3' and reverse,
5'-GCGAGCACAGAATTAATACGAC-3; U6 forward, 5'-CTCGCTTCGGCAGCACA-3'
and reverse, 5'-AACGCTTCACGAATTTGCGT-3'; IGF1R forward,
5'-TCGACATCCGCAACGACTATC-3' and reverse,
5'-TCGACATCCGCAACGACTATC-3'; GAPDH forward,
5'-ATCACCATCTTCCAGGAGCGA-3' and reverse,
5'-CCTTCTCCATGGTGGTGAAGAC-3'. The data were analyzed using the
comparative Cq method (2-∆∆Cq)
(27). The expressions of miR-4458
and IGF1R were normalized to those of U6 small nuclear RNA and
GAPDH, respectively.
Cell proliferation assay
A cell proliferation assay was performed using an
MTT and 5-ethynyl-2'-deoxyuridine (EdU) incorporation assay. For
the MTT assay, cells at a density of 5x103 cells/well
were incubated in 96-well plates in triplicate and cultured at 37˚C
for five days. At the timepoints of 1, 2, 3, 4 and 5 days, cells
were treated with 10 µl MTT (0.5 mg/ml; Sigma-Aldrich; Merck KGaA)
for 2 h at 37˚C, followed by incubation with 100 µl of DMSO for 2 h
at 37˚C. The absorbance was recorded at 595 nm using an enzyme
immunoassay analyzer (Bio-Rad Laboratories, Inc.). For the EdU
labeling assay, a Cell-Light EdU DNA cell kit (Guangzhou RiboBio
Co., Ltd.) was used. Transfected cells were seeded into 96-well
plates (1x104 cells/well) and cultured at 37˚C for 48 h.
Subsequently, cells were washed twice with PBS and permeabilized
using 0.5% Triton X-100 for 10 min. Subsequently 50 mM EdU solution
was added to each well for 2 h, and the cells were fixed and
stained using 1x Apollo567 for 30 min in darkness at 37˚C. The
nucleus was counterstained with DAPI (Sigma-Aldrich; Merck KGaA) at
37˚C for 30 min. Stained cells were examined using a fluorescence
microscope (magnification, x200; Nikon Corporation). The proportion
of EdU-positive nuclei (red) to blue fluorescent nuclei was
calculated by counting six microscopic fields randomly for each
well in five separate experiments.
Analysis of cell cycle and apoptosis
using flow cytometry
To detect cell cycle progression, transfected cells
were collected, washed twice with cold PBS and fixed with 70%
ethanol overnight at 4˚C. Subsequently, cells were washed with PBS
twice and stained with 50 mg/ml propidium iodide (PI;
Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. The
percentage of cells in the G0/G1, S and G2/M phase was determined
using flow cytometry (BD Biosciences). For apoptosis analysis,
transfected cells were trypsinized, washed with cold PBS and
resuspended in fluorescence-activated cell-sorting (FACS) buffer
containing 2% FBS and treated with the FITC-Annexin V Apoptosis
Detection kit with 7-AAD (BioLegend, Inc.) for 30 min at 4˚C. Early
apoptosis (Annexin V+/7-AAD-) and late
apoptosis (Annexin V+/7-AAD+) were analyzed
using flow cytometry and Cell Quest software (version 5.1; BD
Biosciences). Three separate experiments were performed for each
sample.
Bioinformatics and luciferase reporter
assay
Online miRNA target prediction algorithms, including
PicTar4 (pictar.mdc-berlin.de), TargetScan
(targetscan.org/vert_71/) and miRanda (microrna.org) were used to
predict the potential target genes of miR-4458. For the luciferase
reporter assay, a fragment of IGF1R 3' untranslated region (UTR)
containing the putative binding site of miR-4458 was amplified and
inserted into the luciferase reporter vector psiCHECK-2 (Promega
Corporation) between the XhoI and NotI sites to
construct wild-type IGF1R reporter vector (Wt IGF1R). The
nucleotides which paired with miR-4458 were mutated by
site-directed mutagenesis and also cloned into psiCHECK-2 vector to
construct mutated IGF1R 3'UTR reporter vector (Mut IGF1R). HDECs
were co-transfected with 20 µg of Wt IGF1R or Mut IGF1R with 200 ng
of miR-4458 mimic or miR-NC using Lipofectamine® 2000.
After 48 h of transfection, luciferase activity was measured using
the Dual-luciferase Reporter assay system (Promega Corporation)
according to the manufacturer's instructions. Firefly luciferase
activity was normalized to Renilla luciferase activity.
Western blotting
Total proteins were extracted from HDECs using a
RIPA lysis (Beyotime Institute of Biotechnology) and quantified
using a Pierce bicinchoninic acid protein assay kit (Thermo Fisher
Scientific Inc.). Protein samples (30 µg) were resolved using 12%
SDS-PAGE and transferred onto a PVDF membrane (EMD Millipore). The
membrane was blocked with 5% skim milk powder for 1 h at room
temperature and subsequently incubated with a primary antibody
against IGF1R (cat. no. ab131476; 1:2,000; Abcam) or GAPDH (cat.
no. 10494-1-AP; 1:500,000; ProteinTech Group, Inc.) overnight at
4˚C. Following incubation with the primary antibodies, membranes
were incubated with the goat anti-rabbit immunoglobulin G (HRP)
secondary antibody (cat. no. ab205718; 1:5,000; Abcam) at room
temperature for 2 h. After washing with PBS, protein bands were
visualized using enhanced chemiluminescence reagent (GE
Healthcare).
Statistical analysis
All quantitative data were expressed as the mean ±
standard deviation from three independent experiments. Differences
between two groups were compared using a Student's t-test and
differences between three or more were analyzed using one-way ANOVA
followed by post hoc Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-4458 significantly decreases cell
proliferation in HDECs
To determine the biological role of miR-4458 in HAs,
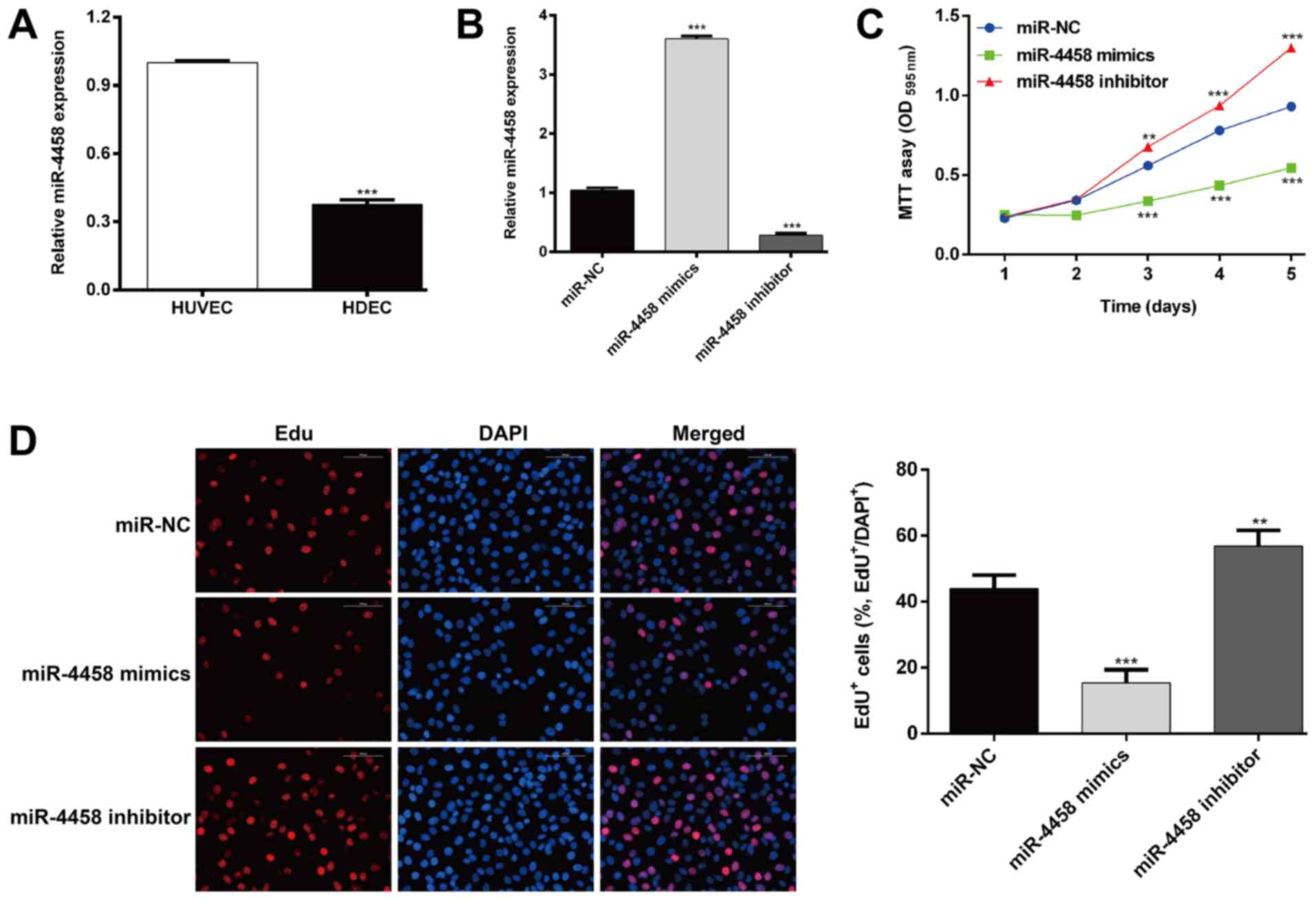
the expression of miR-4458 in HDECs and HUVECs was determined. As
presented in Fig. 1A, the expression
of miR-4458 was significantly lower in HDECs compared with HUVECs
(P<0.001). HDECs were subsequently transfected with miR-4458
mimics or an inhibitor to upregulate or downregulate the expression
of miR-4458, respectively. The efficiency of transfection was
determined using RT-qPCR (Fig. 1B;
P<0.001). The effects of miR-4458 on the cell proliferation of
HDECs was then determined. As presented in Fig. 1C, MTT assay results revealed that
cell growth rate was significantly decreased in cells transfected
with miR-4458 mimics (P<0.001 after 3 days), and enhanced in
cells transfected with miR-4458 inhibitors (P<0.01 after 3 days)
when compared with cells transfected with miR-NC. The EdU
incorporation assay (Fig. 1D)
demonstrated that the percentage of EdU-positive cells was
significantly reduced in cells transfected with miR-4458 mimics
(P<0.001) compared with cells transfected with miR-NC.
Transfection with of miR-4458 inhibitor significantly increased the
percentage of EdU-positive cells compared with miR-NC treated cells
(P<0.01). These data suggested that miR-4458 may be a negative
regulator of proliferation in HDEC.
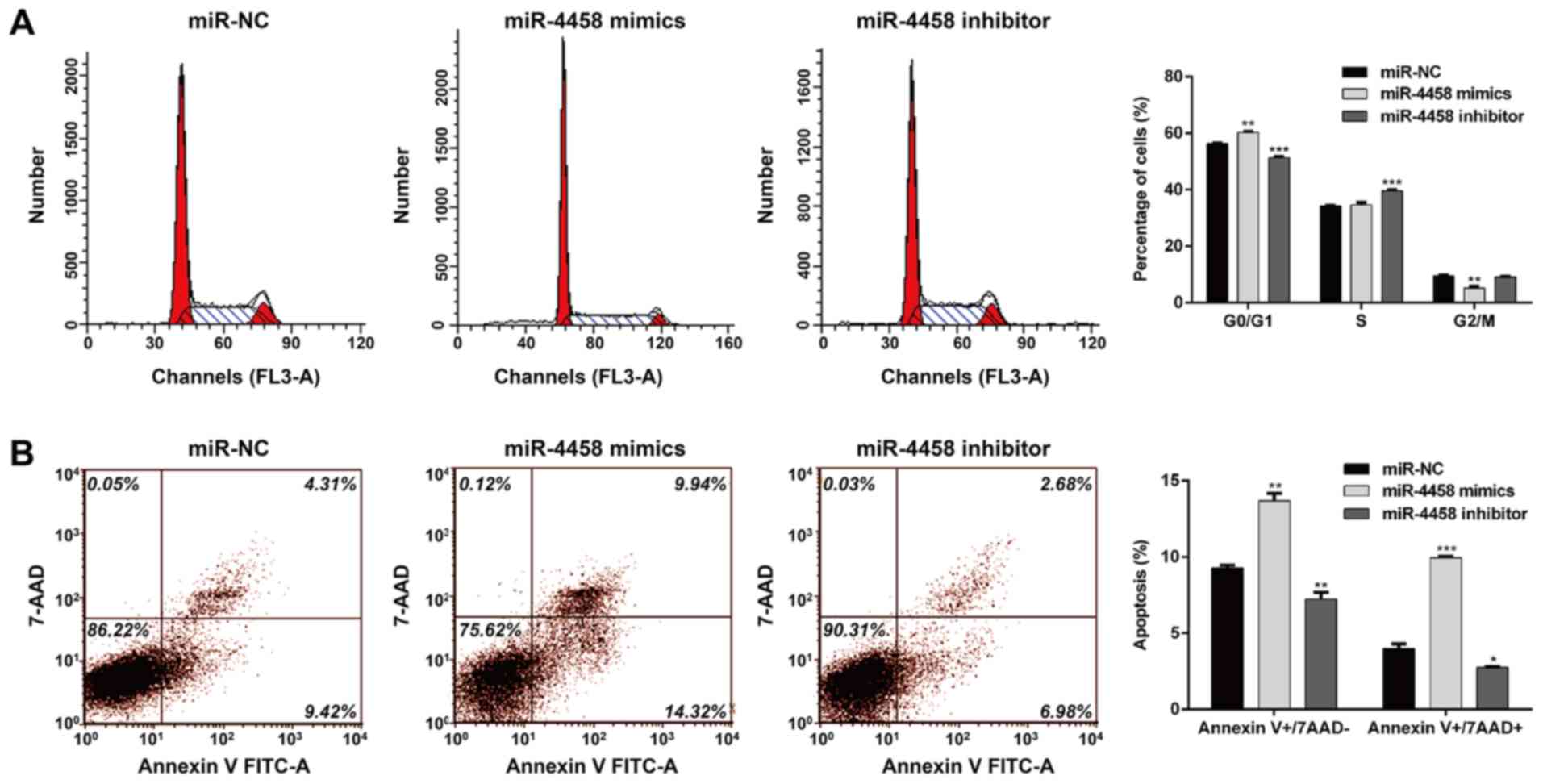
miR-4458 induces cell cycle arrest and
apoptosis in HDECs
The effects of miR-4458 on HDECs cell cycle
distribution and apoptosis were determined. As presented in
Fig. 2A, the percentage of cells in
G0/G1 were significantly increased following transfection miR-4458
mimics (P<0.01) and decreased in cells transfected with the
miR-4458 inhibitor (P<0.001) when compared with miR-NC treated
cells. Similarly, miR-4458 overexpression significantly reduced the
percentage of cells in G2/M (P<0.01) and miR-4458 knockdown
increased the percentage of cells at S phase (P<0.001) compared
with cells transfected with miR-NC. These results suggested that
miR-4458 may induce cell cycle G0/G1 phase arrest in HDECs. In
addition, flow cytometry analysis further demonstrated that
miR-4458 overexpression promoted cell early apoptosis (P<0.01;
7AAD-) and late apoptosis (P<0.001;
7ADD+), and the percentage of cells in both early
(P<0.01) and late (P<0.05) was significantly reduced in cells
transfected with the miR-4458 inhibitors in HDECs compared with
cells transfected with miR-NC (Fig.
2B).
miR-4458 directly targets IGF1R in
HDECs
To dissect the molecular mechanism by which miR-4498
regulates cell proliferation, cell cycle and apoptosis,
bioinformatics analysis was performed to predict the target gene of
miR-4458. Among these predicted genes, IGF1R was considered to be a
potential target gene of miR-4458. The fragment of IGF1R 3' UTR
containing the putative binding site of miR-4458 is presented in
Fig. 3A. A dual-luciferase assay was
performed to confirm whether IGF1R was a target gene of miR-4458.
The results revealed that when the reporter vector containing the
Wt IGF1R was transfected with miR-4458 mimics, the luciferase
expression was significantly decreased when compared with the
miR-NC (Fig. 3B; P<0.001),
whereas mutation of the target region completely abolished this
observation. Therefore, the expression of IGF1R in HDECs was
determined and it was demonstrated that IGF1R mRNA expression was
significantly higher in HDECs compared with HUVECs (Fig. 3C; P<0.001). RT-qPCR analysis
(Fig. 3D; P<0.001) and western
blotting analyses (Fig. 3E) further
demonstrated that miR-4458 mimics decreased the expression of
IGF1R, whereas the miR-4458 inhibitor increased its expression in
HDECs. These results indicated that miR-4458 may negatively
regulate the expression of IGF1R by directly targeting its 3'-UTR
in HDECs.
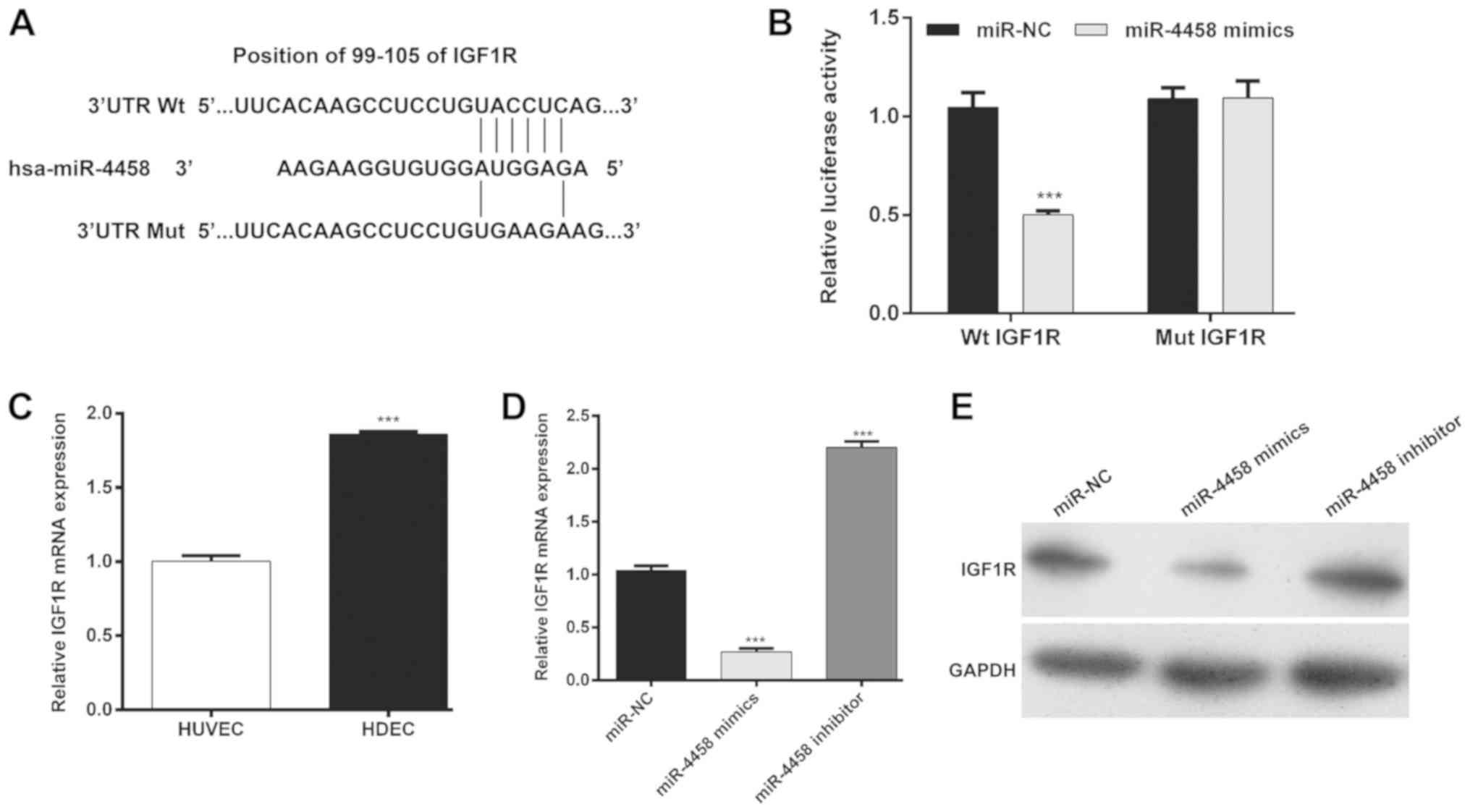 | Figure 3IGF1R is a target gene of miR-4458 in
HDECs. (A) miR-4458 binding sites in the 3'-UTR of IGF1R mRNA were
predicted. IGF1R mutations were constructed by mutating the base
pairing site between miR-4458 and its target in the seed regions.
(B) HDECs were co-transfected with miR-4458 mimics or miR-NCs, and
Mut or Wt IGF1R 3'-UTR-luciferase reporters. Luciferase activity of
the reporter genes containing either Wt IGF1R or Mut 3'-UTRs was
determined after 48 h.
***P<0.001 vs. miR-NC. (C)
RT-qPCR was performed to determine the expression of miR-4458 in
HUVEC and HDEC cells.
***P<0.001 vs. miR-NC. (D)
RT-qPCR and (E) western blotting results of IGF1R expression in
HDECs following transfection with miR-4458 mimics, inhibitor or
miR-NC are presented.
***P<0.001 vs. miR-NC. IGF1R,
insulin-like growth factor 1 receptor; miR, microRNA; HDEC,
hemangioma derived epithelial cells; UTR, untranslated region; NC,
negative control; reverse transcription-quantitative PCR; Mut,
mutant; Wt, wild-type; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
IGF1R regulates miR-4458-mediated cell
proliferation, cell cycle arrest and apoptosis
Since IGF1R was negatively regulated by miR-4458, it
was speculated that miR-4458 also regulated cell proliferation,
cell cycle progression and apoptosis by targeting IGF1R. To
validate this hypothesis, HDECs were transfected with si-IGF1R,
miR-4458 mimics or co-transfected with si-IGF1R and miR-4458
mimics, respectively. As shown in Fig.
4A, the expression of IGF1R was significantly decreased after
si-IGF1R transfection when compared with si-NC transfection, and
further notably decreased after co-transfection with miR-4458
mimics + si-IGF1R when compared with miR-4458 mimics transfection
alone. As presented in Fig. 4B,
IGF1R knockdown significantly decreased cell proliferation
(P<0.001) when compared with si-NC transfection. IGF1R knockdown
also enhanced the inhibitory effects of miR-4458 on cell
proliferation in HDECs (P<0.01) when compared with miR-4458
mimics transfection alone. Furthermore, flow cytometry analysis
revealed that IGF1R knockdown induced cell cycle G0/G1 phase arrest
(Fig. 4C and D; P<0.001) and apoptosis (Fig. 4E and F; P<0.001) when compared with si-NC
transfection. Similarly, the miR-4458-induced cell cycle G0/G1
phase arrest (Fig. 4C and D, P<0.01) and apoptosis (Fig. 4E and F; P<0.001) was significantly enhanced
when IGF1R was knocked down when compared with miR-4458 mimics
transfection alone. Taken together, these results indicated that
IGF1R may be a downstream target of miR-4458 in HDECs.
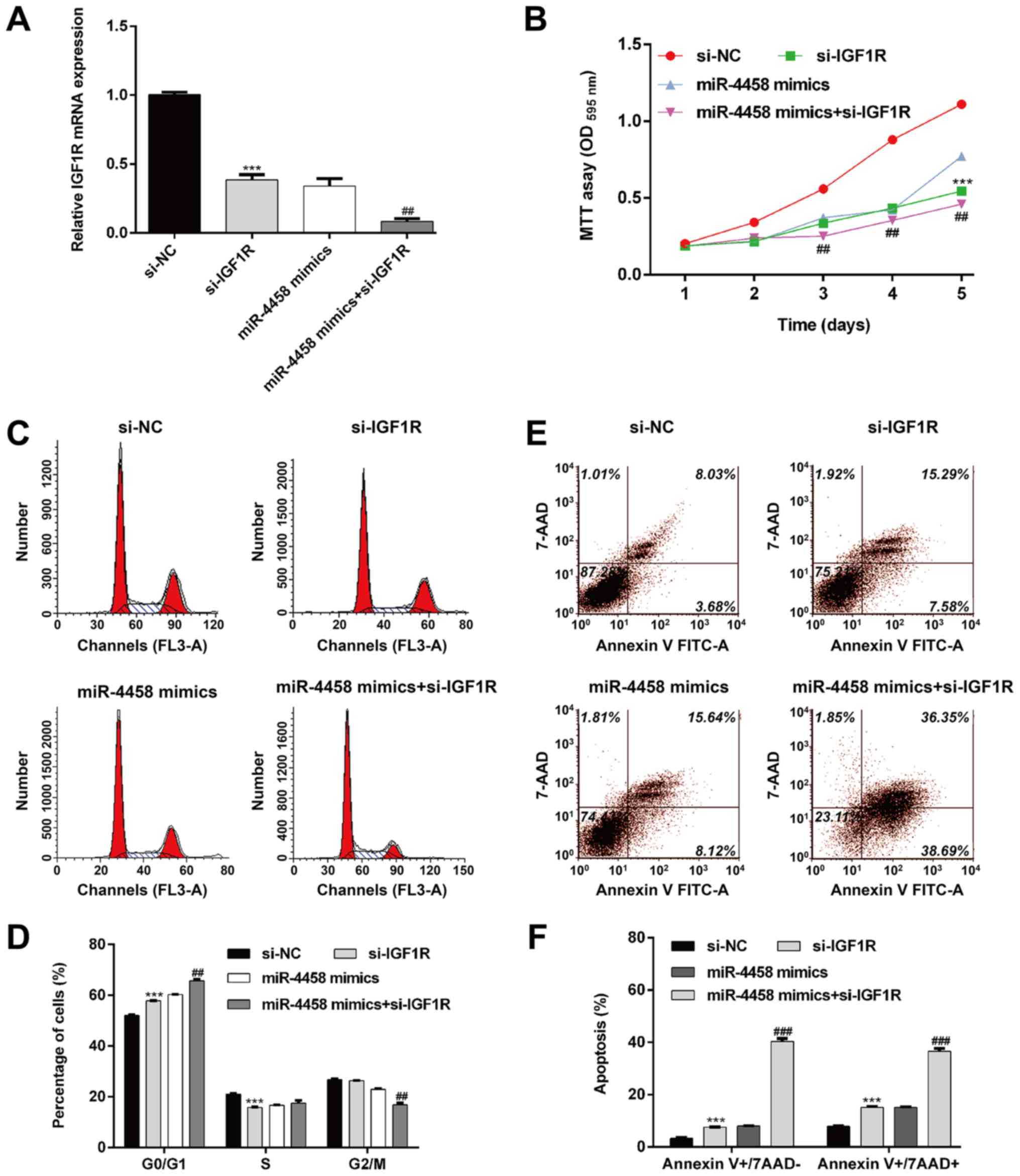 | Figure 4IGF1R regulates miR-4458-mediated
cell proliferation, cell cycle progression and apoptosis. HDECs
were transfected with si-IGF1R, miR-4458 mimics or miR-4458 mimics
+ si-IGF1R plasmid. (A) Reverse transcription-quantitative
polymerase chain reaction was performed to determine the expression
of miR-4458. ***P<0.001 vs.
si-NC; ##P<0.01 vs. miR-4458 mimics; (B) Cell
proliferation was determined using an MTT assay.
***P<0.001 vs. si-NC;
##P<0.01 vs. miR-4458 mimics. (C) Cell cycle
distribution was determined using flow cytometry and propidium
iodide staining and was quantified in (D).
***P<0.001 vs. si-NC;
##P<0.01 vs. miR-4458 mimics. (E) Apoptosis was
determined using flow cytometry with Annexin V-FITC/7-AAD double
staining and is quantified in (F).
***P<0.001 vs. si-NC;
###P<0.001 vs. miR-4458 mimics. IGF1R, insulin-like
growth factor 1 receptor; miR, microRNA; HDEC, hemangioma derived
epithelial cells; si, small interfering; PI, propidium iodide;
7-AAD, 7-Aminoactinomyosin D; FITC, fluorescein isothiocyanate; NC,
negative control. |
Discussion
The present study demonstrated that the increased
expression of miR-4458 decreased proliferation and induced G0/G1
cell cycle arrest and apoptosis in HDECs, whereas transfection of
miR-4458 mimics had the opposite effect. It was additionally
revealed that miR-4458 mediated the repression of its target gene,
IGF1R. In particular, IGF1R knockdown was demonstrated to imitate
the function of endogenous miR-4458 overexpression.
Numerous miRNAs have been demonstrated to serve
important roles in the development and maintenance of normal
cellular functions and the dysregulated expression of miRNAs is
associated with the initiation and progression of a number of
different types of malignancy (28).
Previous research has revealed that miR-4458 was involved in the
regulation of glycolysis and lactate production in colon cancer
cells by targeting hexokinase 2(15). The suppression of cell proliferation
via miR-4458 overexpression was demonstrated in HCC cells,
indicating a novel tumor suppressor function of miR-4458 in HCC
(14). Overexpression of miR-4458
arrested non-small-cell lung cancer A549 and H460 cells at G0/G1
stage and suppressed cell proliferation by inhibiting cyclin
D1(29). However, to the best of our
knowledge, no studies have determined the effect of miR-4458 on the
behavior of cellular biology in HA. In the present study, miR-4458
mimics decreased the proliferation of HDECs, induced G0/G1 phase
arrest and induced apoptosis. These results demonstrated that
miR-4458 exhibited an anti-proliferative and pro-apoptotic effect
on HDECs.
It has been hypothesized that each miRNA has the
potential to regulate a very large number (10-100) of genes by
binding to specific elements in the 3'UTR of their target mRNAs
(30). In the present study, IGF1R
regulation by miR-4458 was demonstrated in vitro in HDECs
with the use of luciferase reporter assays.
IGF1R is amplified in various types of tumors and
mediates tumor transformation and malignant cell apoptosis
(31). It has been demonstrated that
the diminished activation of the IGF1R signaling pathway when IGF1R
is downregulated, or in the presence of IGF1R-specific inhibitor
picropodophyllin, significantly reduced the proliferation of
cultured mouse spermatogonial stem cells by arresting cells at G2/M
(32). Additionally, there is a
crosstalk between the IGF1R signaling pathway and other signaling
components involved in cell growth and survival (33). IGF-1R inhibitor treatment has
demonstrated a notable anti-proliferative and pro-apoptotic effect,
as well as G0/G1 cell cycle arrest in MDA-MB-231 cells (34). Another study revealed that the
inactivation of the IGF1/IGF1R-PI3K/Akt pathway when treated with
formononetin was associated with G0/G1 phase arrest in breast
cancer cells (35). Mechanistically,
PI3K/Akt and the Raf/MEK/ERK signaling pathways are implicated in
IGF1R-mediated cell cycle distribution and protect against
apoptosis in hematopoietic cells (36). Additionally, a number of growth
factors and receptors, including vascular endothelial growth factor
and epidermal growth factor receptor can interfere with IGF1R
signaling and are considered as the key functional elements in
cellular viability, cell cycle progression and apoptosis (31,37). In
the present study, IGF1R expression knockdown resulted in a
decrease in HDEC proliferation by preventing cell cycle
progression. Apoptosis analysis revealed that miR-4458 exhibited a
pro-apoptotic effect in HDECs, at least in part through the direct
suppression of IGF1R. However, further studies are required to
determine the underlying molecular mechanisms by which IGF1R exerts
its effects in HA.
In summary, the results of the present study
demonstrated that miR-4458 decreased proliferation and induced
apoptosis in HDECs by targeting IGF1R. The tumor suppressive
effects of miR-4458 in HA may serve as a basis for the exploration
of new potential therapeutic strategies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the author on reasonable request.
Authors' contributions
XL designed the study. MW, YT and GH performed the
experiments. CY and KY analyzed the data and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spence-Shishido AA, Good WV, Baselga E and
Frieden IJ: Hemangiomas and the eye. Clin Dermatol. 33:170–182.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Phillips JD, Zhang H, Wei T and Richter
GT: Expression of β-adrenergic receptor subtypes in proliferative,
involuted, and propranolol-responsive infantile hemangiomas. JAMA
Facial Plast Surg. 19:102–107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Janmohamed SR, Madern GC, de Laat PC and
Oranje AP: Educational paper: Pathogenesis of infantile
haemangioma, an update 2014 (part I). Eur J Pediatr. 174:97–103.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nakayama H, Huang L, Kelly RP, Oudenaarden
CR, Dagher A, Hofmann NA, Moses MA, Bischoff J and Klagsbrun M:
Infantile hemangioma-derived stem cells and endothelial cells are
inhibited by class 3 semaphorins. Biochem Biophys Res Commun.
464:126–132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Itinteang T, Davis PF and Tan ST:
Infantile hemangiomas exhibit neural crest and pericyte markers.
Ann Plast Surg. 74(383)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv Biol Regul. 57:1–9.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Koshizuka K, Nohata N, Hanazawa T, Kikkawa
N, Arai T, Okato A, Fukumoto I, Katada K, Okamoto Y and Seki N:
Deep sequencing-based microRNA expression signatures in head and
neck squamous cell carcinoma: Dual strands of pre-miR-150 as
antitumor miRNAs. Oncotarget. 8:30288–30304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Biswas A, Pan X, Meyer M, Khanna S, Roy S,
Pearson G, Kirschner R, Witman P, Faith EF, Sen CK and Gordillo GM:
Urinary excretion of MicroRNA-126 is a biomarker for hemangioma
proliferation. Plast Reconstr Surg. 139(1277e-1284e)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fei Z, Qiu M, Qi X, Dai Y, Wang S, Quan Z,
Liu Y and Ou J: MicroRNA-424 suppresses the proliferation of
hemangioma-derived endothelial cells by targeting VEGFR-2. Mol Med
Rep. 18:4065–4071. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang C, Huang J, Ma P and Yu G:
microRNA-143 acts as a suppressor of hemangioma growth by targeting
Bcl-2. Gene. 628:211–217. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mong EF, Akat KM, Canfield J, Lockhart J,
VanWye J, Matar A, Tsibris JCM, Wu JK, Tuschl T and Totary-Jain H:
Modulation of LIN28B/Let-7 signaling by propranolol contributes to
infantile hemangioma involution. Arterioscler Thromb Vasc Biol.
38:1321–1332. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu CH, Lv DS, Li M, Sun G, Zhang XF and
Bai Y: MicroRNA-4458 suppresses the proliferation of human lung
cancer cells in vitro by directly targeting Lin28B. Acta Pharmacol
Sin. 38:1297–1304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tang D, Sun B, Yu H, Yang Z and Zhu L:
Tumor-suppressing effect of miR-4458 on human hepatocellular
carcinoma. Cell Physiol Biochem. 35:1797–1807. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qin Y, Cheng C, Lu H and Wang Y: miR-4458
suppresses glycolysis and lactate production by directly targeting
hexokinase2 in colon cancer cells. Biochem Biophys Res Commun.
469:37–43. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cai W, Sakaguchi M, Kleinridders A,
Gonzalez-Del Pino G, Dreyfuss JM, O'Neill BT, Ramirez AK, Pan H,
Winnay JN, Boucher J, et al: Domain-dependent effects of insulin
and IGF-1 receptors on signalling and gene expression. Nat Commun.
8(14892)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cannarella R, Mattina T, Condorelli RA,
Mongioì LM, Pandini G, La Vignera S and Calogero AE: Chromosome 15
structural abnormalities: Effect on IGF1R gene expression and
function. Endocr Connect. 6:528–539. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Taliaferro-Smith LT, Oberlick E, Liu T,
McGlothen T, Alcaide T, Tobin R, Donnelly S, Commander R, Kline E,
Nagaraju GP, et al: FAK activation is required for IGF1R-mediated
regulation of EMT, migration, and invasion in mesenchymal triple
negative breast cancer cells. Oncotarget. 6:4757–4772.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ball MW, Bezerra SM, Chaux A, Faraj SF,
Gonzalez-Roibon N, Munari E, Sharma R, Bivalacqua TJ, Netto GJ and
Burnett AL: Overexpression of insulin-like growth factor-1 receptor
is associated with penile cancer progression. Urology. 92:51–56.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qian Y, Teng Y, Li Y, Lin X, Guan M, Li Y,
Cao X and Gao Y: miR-143-3p suppresses the progression of nasal
squamous cell carcinoma by targeting Bcl-2 and IGF1R. Biochem
Biophys Res Commun. 518:492–499. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zaanan A, Calmel C, Henriques J, Svrcek M,
Blons H, Desbois-Mouthon C, Merabtene F, Goumard C, Parc Y, Gayet
B, et al: High IGF1R protein expression correlates with
disease-free survival of patients with stage III colon cancer. Cell
Oncol (Dordr). Dec 10. 2019.(Epub ahead of print). doi:
10.1007/s13402-019-00484-6. PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang XH, Wu HY, Gao J, Wang XH, Gao TH and
Zhang SF: IGF1R facilitates epithelial-mesenchymal transition and
cancer stem cell properties in neuroblastoma via the STAT3/AKT
axis. Cancer Manag Res. 11:5459–5472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ou JM, Lian WS, Qiu MK, Dai YX, Dong Q,
Shen J, Dong P, Wang XF, Liu YB, Quan ZW and Fei ZW: Knockdown of
IGF2R suppresses proliferation and induces apoptosis in hemangioma
cells in vitro and in vivo. Int J Oncol. 45:1241–1249.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu ZQ, Fu WQ, Zhao S and Zhao X:
Regulation of insulin-like growth factor 1 receptor signaling by
microRNA-4458 in the development of lumbar disc degeneration. Am J
Transl Res. 8:2309–2316. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fu X, Zhai S and Yuan J: Interleukin-6
(IL-6) triggers the malignancy of hemangioma cells via activation
of HIF-1α/VEGFA signals. Eur J Pharmacol. 41:82–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qiu MK, Wang SQ, Pan C, Wang Y, Quan ZW,
Liu YB and Ou JM: ROCK inhibition as a potential therapeutic target
involved in apoptosis in hemangioma. Oncol Rep. 37:2987–2993.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bandres E, Bitarte N, Arias F, Agorreta J,
Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ,
et al: microRNA-451 regulates macrophage migration inhibitory
factor production and proliferation of gastrointestinal cancer
cells. Clin Cancer Res. 15:2281–2290. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bao L, Wang L, Wei G, Wang Y, Wuyun G and
Bo A: Role of microRNA-4458 in patients with non-small-cell lung
cancer. Oncol Lett. 12:3958–3966. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hoffman Y, Bublik DR, Ugalde AP, Elkon R,
Biniashvili T, Agami R, Oren M and Pilpel Y: 3'UTR shortening
potentiates MicroRNA-based repression of pro-differentiation genes
in proliferating human cells. PLoS Genet.
12(e1005879)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zakraoui O, Marcinkiewicz C, Aloui Z,
Othman H, Grépin R, Haoues M, Essafi M, Srairi-Abid N, Gasmi A,
Karoui H, et al: Lebein, a snake venom disintegrin, suppresses
human colon cancer cells proliferation and tumor-induced
angiogenesis through cell cycle arrest, apoptosis induction and
inhibition of VEGF expression. Mol Carcinog. 56:18–37.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Wang S, Wang X, Wu Y and Han C: IGF-1R
signaling is essential for the proliferation of cultured mouse
spermatogonial stem cells by promoting the G2/M progression of the
cell cycle. Stem Cells Dev. 24:471–483. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang M, Liu J, Li M, Zhang S, Lu Y, Liang
Y, Zhao K and Li Y: Insulin-like growth factor 1/insulin-like
growth factor 1 receptor signaling protects against cell apoptosis
through the PI3K/AKT pathway in glioblastoma cells. Exp Ther Med.
16:1477–1482. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ayub A, Yip WK and Seow HF: Dual
treatments targeting IGF-1R, PI3K, mTORC or MEK synergize to
inhibit cell growth, induce apoptosis, and arrest cell cycle at G1
phase in MDA-MB-231 cell line. Biomed Pharmacother. 75:40–50.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li T, Zhao X, Mo Z, Huang W, Yan H, Ling Z
and Ye Y: Formononetin promotes cell cycle arrest via
downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer
cells. Cell Physiol Biochem. 34:1351–1358. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen J, Duan Y, Zhang X, Ye Y, Ge B and
Chen J: Genistein induces apoptosis by the inactivation of the
IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells.
Food Funct. 6:995–1000. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dai Z, Wang L, Wang X, Zhao B, Zhao W,
Bhardwaj SS, Ye J, Yin Z, Zhang J and Zhao S: Oxymatrine induces
cell cycle arrest and apoptosis and suppresses the invasion of
human glioblastoma cells through the EGFR/PI3K/Akt/mTOR signaling
pathway and STAT3. Oncol Rep. 40:867–876. 2018.PubMed/NCBI View Article : Google Scholar
|