Introduction
Sepsis, a severe systemic inflammatory response
syndrome (1), results in multiple
organ dysfunction, shock and even death (2-4).
The high incidence of sepsis in elderly populations results in high
mortality and morbidity rates worldwide (5,6).
Although great efforts have been made to improve the outcome for
the diagnosis and treatment of sepsis, targets for earlier
treatment require further investigation (7,8).
MicroRNAs (miRNAs), a class of endogenous
non-encoding small molecules of 20-22 nucleotides in length, can
regulate the target gene expression by interacting with the
3'-untranslated region (3'-UTR) of target mRNAs (9,10).
miRNAs are involved in the regulation of numerous cell functions,
including cell growth, differentiation, proliferation and apoptosis
(11). Studies have generated
promising treatments for complex human diseases by restraining
translational, destroying targets and silencing genes (12,13).
Moreover, inflammation is a potential sepsis biomarker, and a
number of studies have demonstrated that miRNAs serve as important
regulators for the inflammatory response by preventing NF-κ
activation (14). Multiple miRNAs
have been confirmed to be dysregulated in sepsis, including
miRNA-133a, miRNA-146a, miRNA-297 and miRNA-122, which provide
vital references for the diagnosis and treatment of sepsis
(15-19).
Previous studies have indicated that miRNA (miR)-146a prevents NF-κ
activation and inflammation by targeting tumor necrosis factor
(TNF) receptor-associated factor 6 and interleukin (IL)-1
receptor-associated kinase (20,21).
NF-κ, a transcription factor, has been reported to regulate the
inflammatory response and be activated by several different
stimulants, including lipopolysaccharide (LPS). Additionally, NF-κ
participates in the inflammation process by regulating the
expression of inflammatory cytokines and mediator genes (22). Notably, miR-15a-5p is considered a
tumor suppressor and serves important roles in a number of cancer
types including endometrial cancer (23), human hepatocellular carcinoma
(24) and non-small cell lung cancer
(25). However, its participation in
the progression of sepsis remains unclear.
A previous study has demonstrated that the
macrophage-mediated inflammatory response is involved in severe
inflammation and the immune suppression of sepsis (20). During sepsis, macrophages mediate the
inflammatory response and are heavily involved in the serious
inflammation and immune suppression during sepsis. LPS is widely
used in both in vitro and in vivo models focusing on
inflammation (26). LPS stimulation
causes various intracellular activities in macrophages, including
the phosphorylation and activation of p65. Phosphorylated (p)-p65
is subsequently transferred into the nucleus where it regulates the
expression of genes associated with inflammation (27). TNF-α induced protein 3-interacting
protein (TNIP) 2 is a negative regulator of NF-κ signaling.
Overexpression of TNIP2 has been reported to suppress NF-κ
activation (28). However, whether
and how miR-15a-5p functions in the inflammatory process in sepsis
in vivo and in vitro remains unknown.
In the present study, increased levels of miR-15a-5p
and pro-inflammatory cytokines in RAW264.7 macrophages stimulated
with LPS were observed. It was subsequently confirmed that TNIP2 is
a direct target of miR-15a-5p. Furthermore, miR-15a-5p inhibitor
was found to protect against LPS-induced pro-inflammatory cytokine
secretions by inhibiting NF-κ signal activation in macrophages. In
an in vivo model, miR-15a-5p inhibitor exhibited
anti-inflammatory and organ-protective effects. In particular, the
effects of miR-15a-5p inhibitor were significantly eliminated by
TNIP2-small interfering RNA (siRNA) treatment.
In summary, the present study provided novel
evidence that miR-15a-5p is a vital regulator and is involved in
the inflammatory process in sepsis. miR-15a-5p may regulate
inflammatory responses by inhibiting TNIP2, while activating the
NF-κ pathway.
Materials and methods
Animals.
Healthy adult male C57BL/6 mice (age, 6-8 weeks;
weight, 20-22 g) were purchased from the Experimental Animal Center
of the Fourth Military Medical University. A total of 50 C57BL/6
mice were used in the present investigation. All animals were
housed under standard conditions at room temperature (22-24˚C) and
humidity (60-65%) on a 12-h light/dark cycle with ad libitum
supply of food and water. This study was performed in accordance to
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (29) and was
approved by the Committee of Experimental Animals of the Affiliated
Hospital of Medical School of Ningbo University. The mice were
acclimated to the environment for 1 week prior to experiments.
Animal model.
C57BL/6 mice were intraperitoneally injected with
LPS (10 mg/kg; Sigma-Aldrich; Merck KGaA) to induce sepsis. For
treatment, the inhibitor control (80 mg/kg/day;
5'-CAGUACUUUUGUGUAGUACAA-3'; Shanghai GenePharma Co., Ltd.),
miR-15a-5p inhibitor (80 mg/kg/day; Guangzhou RiboBio Co., Ltd.) or
miR-15a-5p inhibitor (80 mg/kg/day; 5'-CACUGGUACAAGGGUUGGGAGA-3';
Shanghai GenePharma Co., Ltd.) + TNIP2-siRNA (80 mg/kg/day) were
injected by caudal vein for 3 consecutive days as previously
described (30), followed by LPS (10
mg/kg) injection at 24 h following the last administration. The
mice were anaesthetized with sodium pentobarbital (30 mg/kg;
intraperitoneal injection) and sacrificed by cervical dislocation.
The blood was subsequently collected for further analysis at 24 h
after LPS injection. The duration of the experiment was 4 days in
total, and the health and behavior of all mice were monitored every
2 days. No mice died during the experiment. The experiment was
terminated when the mice lost >15% of their body weight, and
throughout the experiment attempts were made to alleviate the pain
of the mice.
ELISA and biochemical marker
detection.
At 24 h after LPS injection, blood was collected
from each mouse. The blood was centrifuged for 15 min at 1,600 x g
at 4˚C and the serum was collected for biochemical analysis. The
levels of cytokines, including IL-1β (cat. no. PI301), IL-6 (cat.
no. PI326) and TNF-α (cat. no. PT512), were examined using ELISA
(Beyotime Institute of Biotechnology). The signal produced from
each well was detected at a wavelength of 450 nm. Alanine
aminotransferase (ALT), aspartate aminotransferase (AST), creatine
(Cr) and blood urea nitrogen (BUN; all kits supplied by Nanjing
Jiancheng Bioengineering Institute) were detected in the serum to
evaluate organ function, according to the manufacturer's
protocols.
Cell culture and LPS treatment.
The mouse macrophage cell line RAW264.7 (cat. no.
(ATCC® TIB-71™; American Type Culture Collection) and 293 cells
(cat. no. (ATCC® CRL-1573; American Type Culture
Collection) were grown in DMEM (Gibco; Thermo Fisher Scientific,
Inc.), including 10% FBS (Thermo Fisher Scientific, Inc.) and 1%
penicillin and streptomycin (Beyotime Institute of Biotechnology).
The cells were maintained at 37˚C with 5% CO2. In the
present study, a concentration of 1 µg/ml LPS was used to stimulate
RAW264.7 cells for 4 h, and cells were harvested for further
experiments.
Cell transfection and reagents.
Prior to transfection, cells were changed to
antibiotic-free media for 24 h, then RAW264.7 macrophage cells
(5x104 cells per well) were transfected with 0.2 µM
control-siRNA (cat. no. sc-36869; Santa Cruz Biotechnology, Inc.),
0.2 µM TINP2-siRNA (cat. no. sc-44638; Santa Cruz Biotechnology,
Inc.), 100 nM inhibitor control (Shanghai GenePharma Co., Ltd.;
5'-CAGUACUUUUGUGUAGUACAA-3'), 100 nM miR-15a-5p inhibitor (Shanghai
GenePharma Co., Ltd.; 5'-CACUGGUACAAGGGUUGGGAGA-3') or 100 nM
miR-15a-5p inhibitor + 0.2 µM TNIP2-siRNA using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
efficiency of cell transfection was measured using reverse
transcription quantitative-PCR (RT-qPCR) and western blot assays
with samples collected 48 h after cell transfection. Cells without
any treatment were used as the control.
Detection of IL-1β, IL-6 and TNF-α
level in cell supernatant.
RAW264.7 cells were transfected with inhibitor
control, miR-15a-5p inhibitor, or miR-15a-5p inhibitor+TNIP2-siRNA
for 48 h. Then the cells were subjected to 1 µg/ml LPS for 4 h.
Subsequently, the expression levels of IL-1β, IL-6 and TNF-α in the
cell supernatant were also detected using ELISA kits, according to
the manufacturer's protocols.
Dual-luciferase reporter assay.
Target prediction database (TargetScan 7.2;
http://www.targetscan.org/vert_72/)
was used to examine the association between miR-15a-5p and TNIP2.
The results indicated that TNIP2 was a potential target of
miR-15a-5p. The 3'-UTR of TNIP2, containing wild-type (WT) or
mutant (MUT) target sites for miR-15a-5p, was subsequently
amplified and inserted into the pGL3-Control Vector (Promega
Corporation) to form the reporter vector TNIP2-WT or TNIP2-MUT,
respectively. For the luciferase reporter assay, the luciferase
reporter vectors and 50 nM mimic control (Shanghai GenePharma Co.,
Ltd.; sense, 5'-UUCUCCGAACGUGUCACGUTT-3'; and antisense,
5'-ACGUGACACGUUCGGAGAATT-3') or 50 nM miR-15a-5p mimic (Shanghai
GenePharma Co., Ltd.; sense, 5'-UCUCCCAACCCUUGUACCAGUG-3'; and
antisense, 5'-CUGGUACAAGGGUUGGGAGAUU-3') were co-transfected into
293 cells (5x104 cells per well) using Lipofectamine
2000 for 48 h according to the manufacturer's protocol.
Dual-Luciferase Reporter assay system (Promega Corporation) was
used to measure the luciferase activity and results were normalized
to that of Renilla luciferase, according to the
manufacturer's protocol. Each experiment was repeated in
triplicate.
RT-qPCR.
Total RNA was extracted from serum samples and
RAW264.7 macrophages using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
In order to detect mRNA expression levels, PrimeScript™ RT reagent
kit (Takara Biotechnology Co., Ltd.) was used for cDNA synthesis.
The reaction conditions were as follows: Initial annealing at 25˚C
for 5 min, followed by extension at 42˚C for 60 min and termination
at 80˚C for 2 min. The expression levels of miR-15a-5p and TNIP2
were measured using Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with the SYBR Green PCR kit (Takara
Biotechnology Co., Ltd.), according to the manufacturer's
protocols. GAPDH or U6 was used as an internal control. Primer
sequences (all provided by Sangon Biotech Co., Ltd.) are as
following: U6 forward, 5'GCTTCGGCAGCACATATACTAAAAT3' and reverse,
5'CGCTTCACGAATTTGCGTGTCAT3'; GAPDH forward,
5'CTTTGGTATCGTGGAAGGACTC3' and reverse, 5'GTAGAGGCAGGGATGATGTTCT3';
miR-15a-5p forward, 5'GGGTAGCAGCACATAATGGTTTGTG3' and reverse,
5'CAGTGCGTGTCGTGGAGT3'; TNIP2 forward, 5'-CTAAAGAGGCGGCAGGTCCCTC-3'
and reverse, 5'-CAAGATGACCTTCCAGTGAC-3'. The following
thermocycling conditions were used: 35 cycles of initial
denaturation at 95˚C for 15 sec, annealing at 60˚C for 1 min,
extension at 72˚C for 1 min; and a final extension step at 72˚C for
10 min. The relative expression of genes was determined using the
2-ΔΔCq method (31).
Western blot assay.
Total protein was obtained from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Protein
concentration was measured using a bicinchoninic acid protein assay
kit (Thermo Fisher Scientific, Inc.). Equal amounts of protein (40
µg) were separated by 10% SDS-PAGE (Bio-Rad Laboratories, Inc.) and
transferred onto PVDF membranes (EMD Millipore). Membranes were
subsequently blocked with 5% fat-free skim milk in PBS-Tween
(PBST), containing 0.05% Tween-20, for 1 h at room temperature, and
incubated with the following primary antibodies at 4˚C overnight:
TNIP2 (1:1,000; cat. no. ab205925; Abcam), p-p65 (1:1,000; cat. no.
ab86299; Abcam), p65 (1:1,000; cat. no. ab16502; Abcam) and β-actin
(1:2,000; cat. no. ab8227; Abcam). The membranes were washed in
triplicate with PBST and then incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG H&L (1:2,000; cat.
no. Ab7090; Abcam) at room temperature for 1 h. The protein bands
were visualized using the ECL detection reagent (Beyotime Institute
of Biotechnology). The intensity of each band was quantified using
Image Lab™ Software (version 5.2.1; Bio-Rad Laboratories
Inc.).
Statistical analysis
SPSS 21.0 statistical software (IBM Corp.) was used
to analyze data. All experiments were performed at least three
times. Measurement data were presented as the mean ± standard
deviation. Differences between multiple groups were tested using
ANOVA followed by Student-Newman-Keuls tests. Differences between
two groups were analyzed by Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
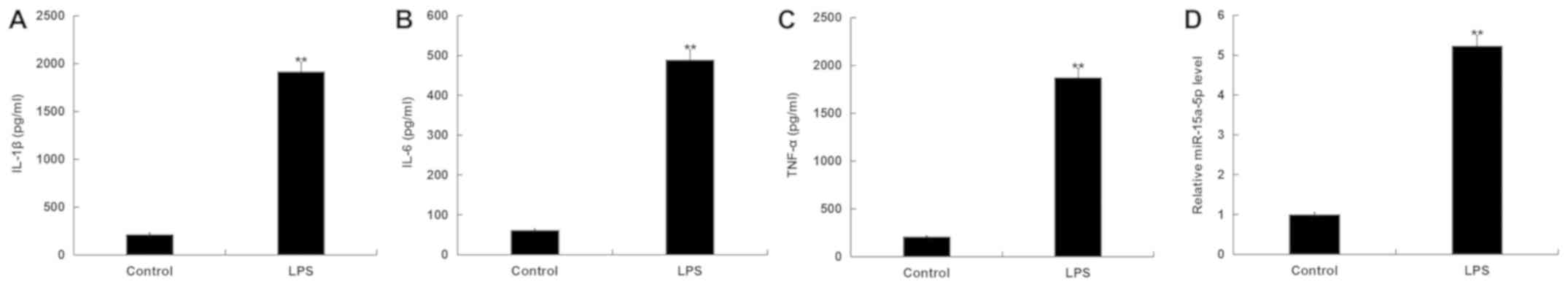
miR-15a-5p and inflammatory factors'
expression levels are significantly increased in RAW264.7
macrophages after LPS treatment.
After RAW264.7 macrophages were stimulated with LPS
for 4 h, the expression levels of miR-15a-5p and inflammatory
factors were detected by RT-qPCR and ELISAs, respectively. IL-1β,
IL-6 and TNF-α levels were detected to evaluate the inflammatory
response. The results indicated that the expression levels of
IL-1β, IL-6 and TNF-α were significantly increased in RAW264.7
macrophages after treatment with LPS compared with the control
group (Fig. 1A-C). Additionally, the
data showed that the expression level of miR-15a-5p was increased
with LPS stimulation (Fig. 1D).
TNIP2 is a target gene of
miR-15a-5p.
In order to investigate the molecular mechanism of
action for miR-15a-5p, the present study predicted the potential
targets of miR-15a-5p using a bioinformatics prediction tool
(TargetScan). The binding sites between miR-15a-5p and TNIP2 were
observed and are presented in Fig.
2A. A dual-luciferase reporter assay was subsequently used to
further verify that there is a specific regulatory connection
between miR-15a-5p and TNIP2. The results showed that increased
expression levels of miR-15a-5p decreased the luciferase activity
of WT 3'-UTR segment of TNIP2 compared with mimic control group,
however no significant differences were observed in the luciferase
activity of TNIP2-MUT (Fig. 2B).
Together, these results suggested that TNIP2 was a direct target of
miR-15a-5p.
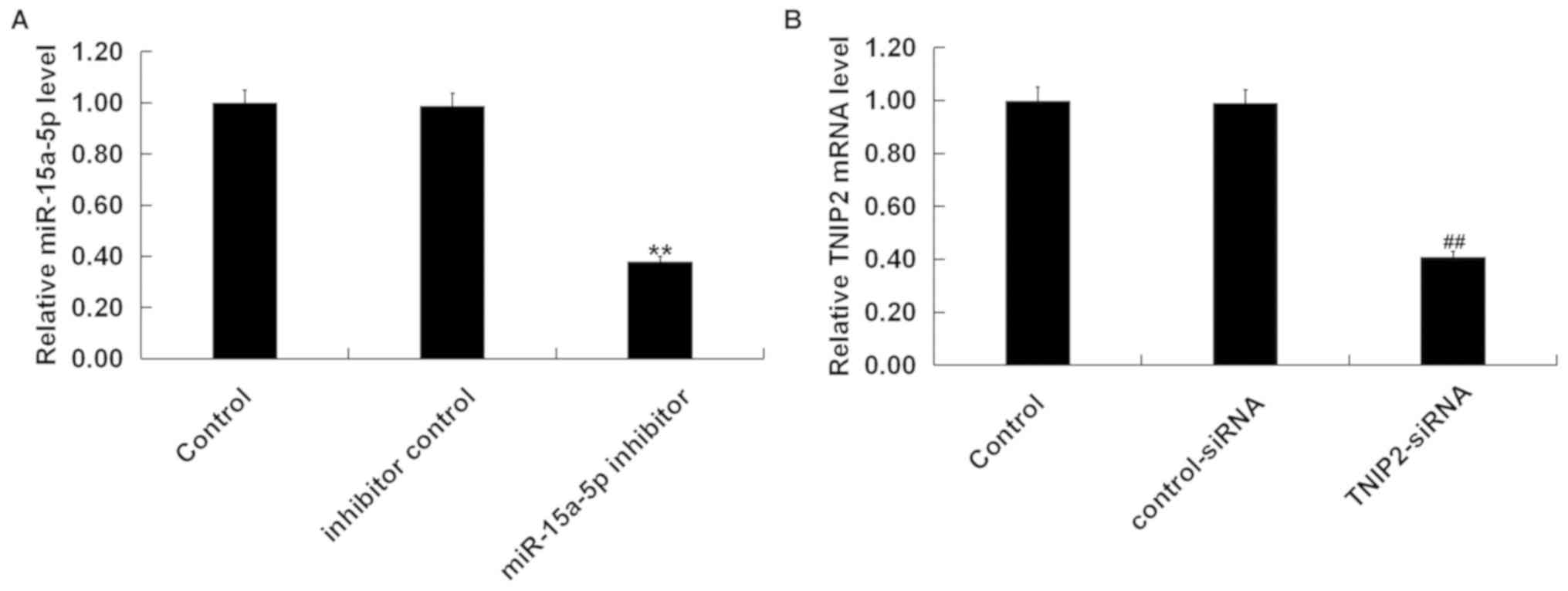
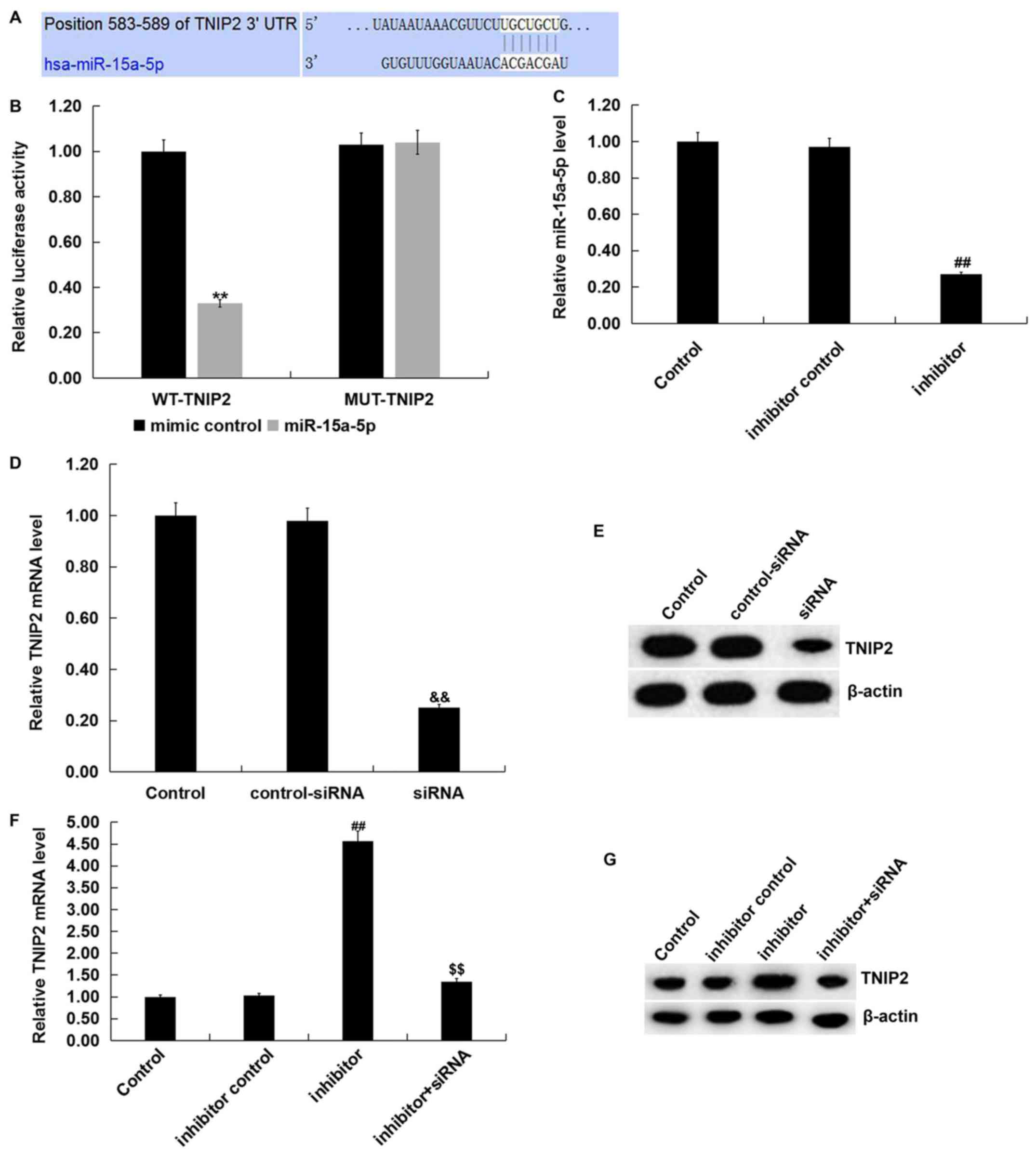 | Figure 2TNIP2 is a direct target of
miR-15a-5p. (A) The binding sites between miR-15a-5p and TNIP2
3'-UTR. (B) Dual-luciferase reporter assays were performed to
measure the luciferase activities.
**P<0.01 vs. mimic control. (C) Inhibitor
control or miR-15a-5p inhibitor was transfected into RAW264.7
macrophages for 48 h to detect the mRNA levels of miR-15a-5p.
Control-siRNA or TNIP2-siRNA was transfected into RAW264.7
macrophages for 48 h to detect the TNIP2 (D) mRNA and (E) protein
levels. Following transfection with miR-15a-5p inhibitor, inhibitor
control, or miR-15a-5p inhibitor + TNIP2-siRNA, the (F) mRNA and
(G) protein expression level of TNIP2 in RAW264.7 macrophages was
measured. ##P<0.01 vs. inhibitor control;
&&P<0.01 vs. control-siRNA;
$$P<0.01 vs. inhibitor. miR, microRNA; MUT, mutant;
LPS, lipopolysaccharide; siRNA, small interfering RNA; TNIP, tumor
necrosis factor-α induced protein 3-interacting protein; WT,
wild-type; UTR, untranslated region. |
Furthermore, the present study examined whether
miR-15a-5p could regulate the expression of TNIP2 in RAW264.7
macrophages. The inhibitor control, miR-15a-5p inhibitor,
control-siRNA, TNIP2-siRNA or miR-15a-5p inhibitor + TNIP2-siRNA
were transfected into RAW264.7 macrophages for 48 h. As indicated
in Fig. 2C, it was observed that
compared with the inhibitor control group, the expression levels of
miR-15a-5p were significantly suppressed in RAW264.7 macrophages
transfected with the miR-15a-5p inhibitor. Meanwhile, compared with
the control-siRNA group reduced mRNA and protein expression levels
of TNIP2 were detected when RAW264.7 macrophages were transfection
with TNIP2-siRNA (Fig. 2D and
E). Additionally, RT-qPCR and
western blot analyses demonstrated that the mRNA and protein
expression levels of TNIP2 were significantly higher in RAW264.7
macrophages after miR-15a-5p inhibitor transfection compared with
the inhibitor control group (Fig. 2F
and G). This increase was reversed
by TNIP2-siRNA co-transfection. These data indicated that TNIP2 was
a direct target of miR-15a-5p. Furthermore, a negative association
between miR-15a-5p and TNIP2 was shown, which may be related to the
progression of sepsis.
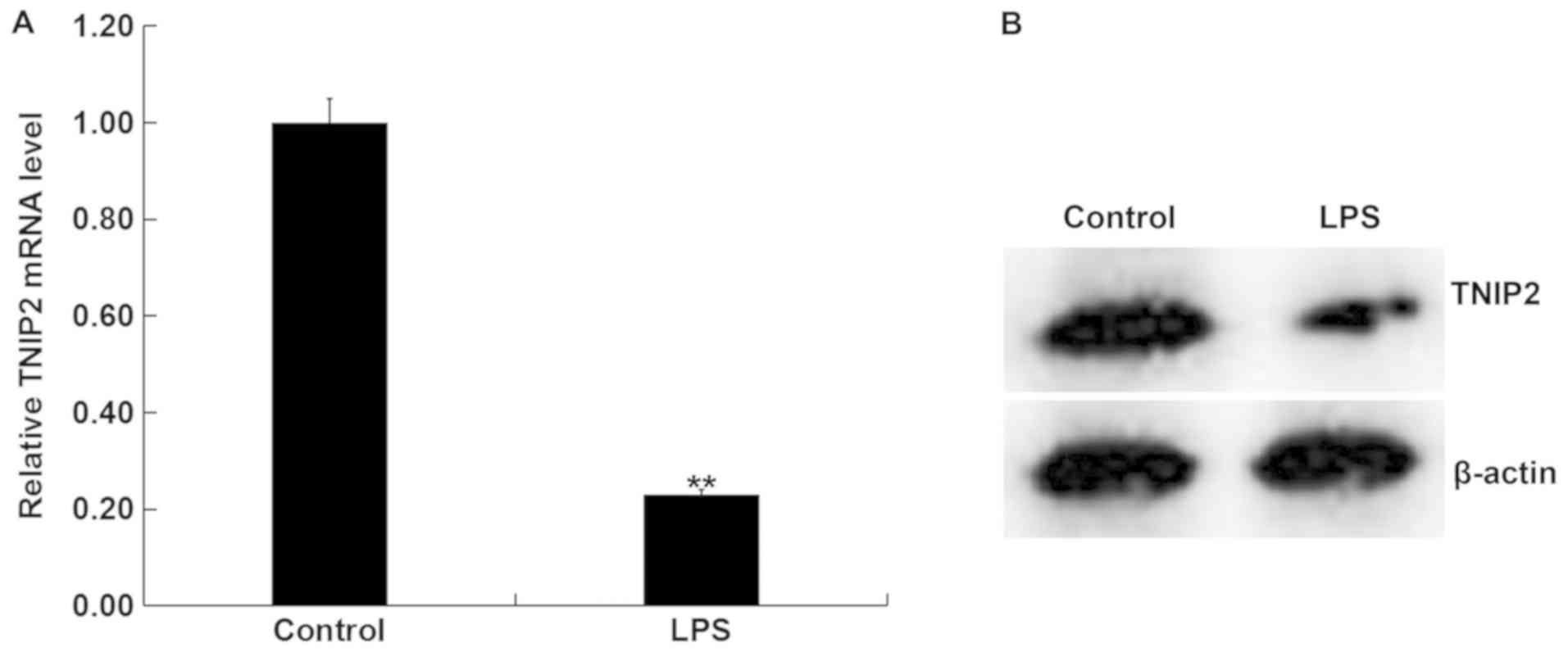
Expression of TNIP2 is reduced in
LPS-activated RAW264.7 macrophages.
In order to further verify the difference in
expression levels of TNIP2 between the LPS-activated RAW264.7
macrophages and untreated RAW264.7 macrophages, RAW264.7
macrophages were treated with LPS for 4 h. It was indicated that
TNIP2 mRNA levels were significantly decreased in the LPS treatment
group compared with the control group (Fig. 3A). Consistently, the protein
expression of TNIP2 appeared reduced in RAW264.7 macrophages upon
LPS activation (Fig. 3B). These data
suggested that LPS may inhibit the expression of TNIP2 in RAW264.7
macrophages.
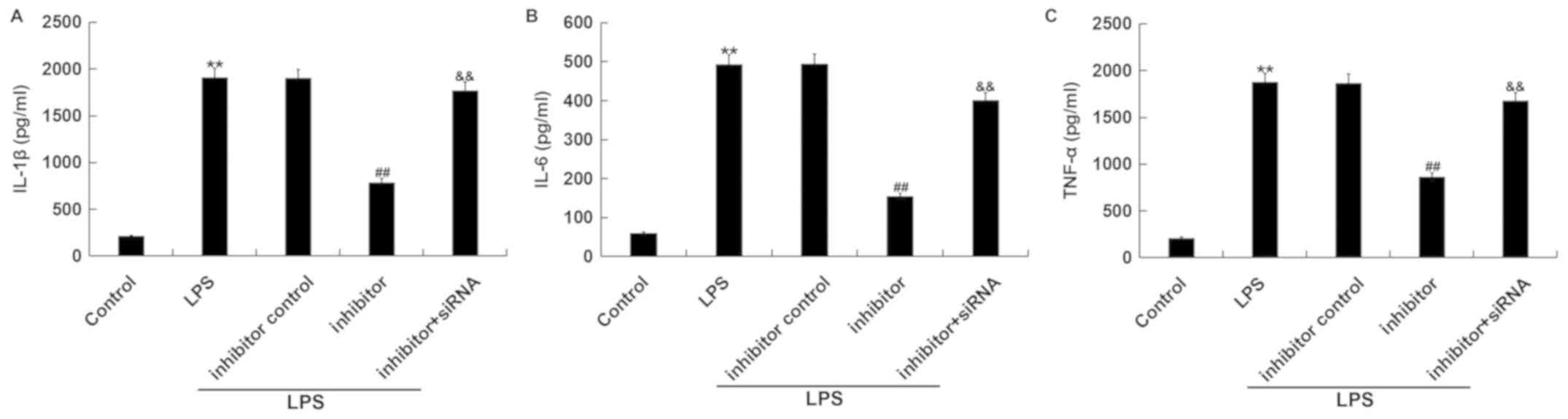
miR-15a-5p inhibitor suppresses
LPS-induced inflammatory factors' expression in RAW264.7
macrophages by TNIP2 regulation.
The expression levels of IL-1β, IL-6 and TNF-α
following transfection and LPS treatment were detected. Results
from the ELISAs showed that LPS triggered an increased secretion of
IL-1β, IL-6 and TNF-α from RAW264.7 macrophages compared with the
control group. Additionally, inhibition of miR-15a-5p suppressed
the secretion of IL-1β, IL-6 and TNF-α in RAW264.7 macrophages
which were stimulated by LPS, compared with the LPS + inhibitor
control treatment group. Meanwhile, the effects were reversed by
TNIP2-siRNA, and the protein expression levels of IL-1β, IL-6 and
TNF-α were increased in the TNIP2-siRNA co-transfection group
compared with the inhibitor group (Fig.
4A-C).
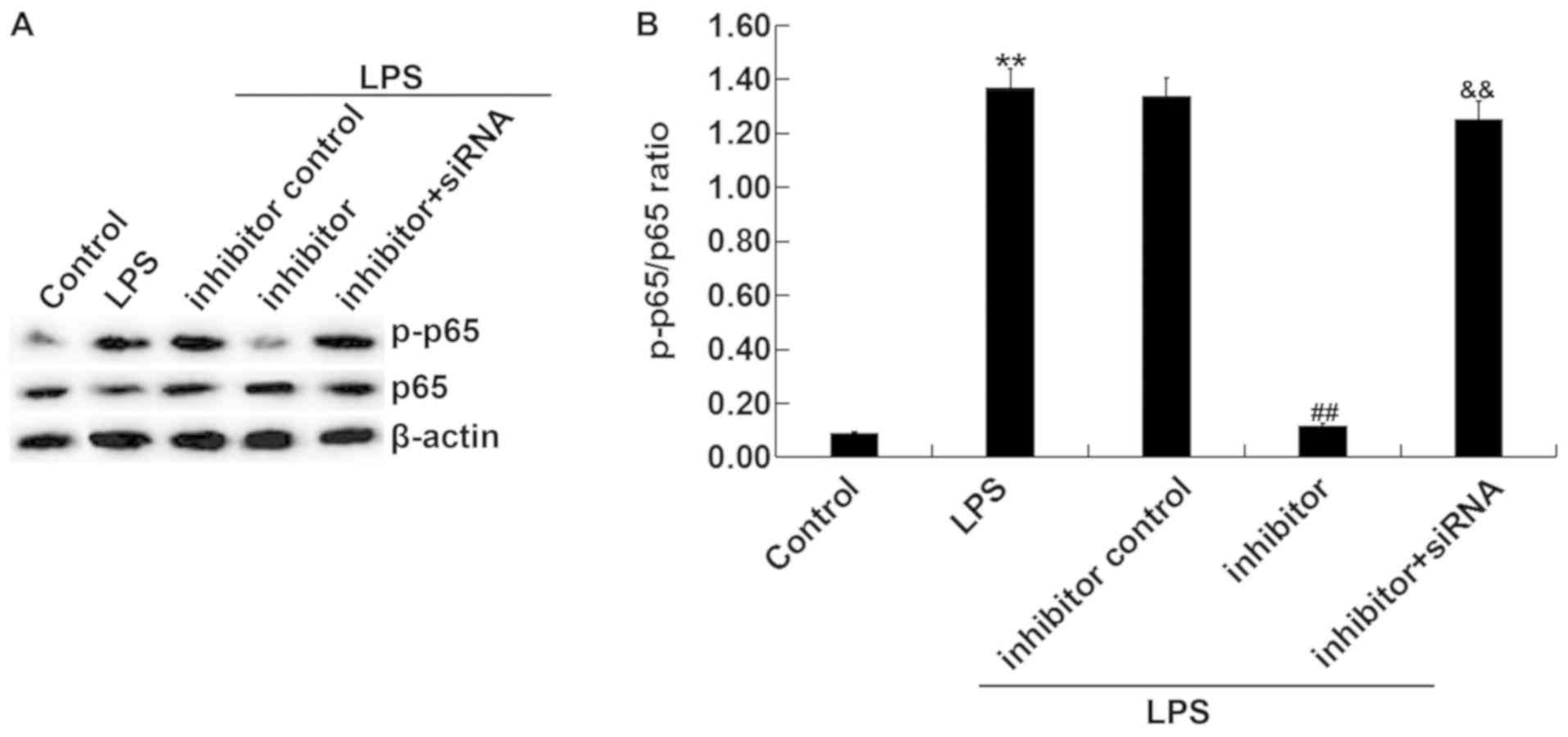
miR-15a-5p inhibitor significantly
affects the activity of NF-κ in LPS-induced RAW264.7 macrophages
via TNIP2 regulation.
The effect of miR-15a-5p inhibitor on LPS-induced
NF-κ activation was subsequently examined. After transfecting
RAW264.7 macrophages with miR-15a-5p inhibitor or miR-15a-5p
inhibitor + TNIP2-siRNA for 48 h, LPS was used to stimulate the
cells for 4 h. As indicated in Fig.
5, the protein expression levels of p-p65 were enhanced in the
LPS treatment group compared with the control group (Fig. 5A and B). Compared with the LPS + inhibitor
control treatment group, miR-15a-5p inhibitor significantly
inhibited the expression of p-p65 in LPS-treated cells, whereas
TNIP2 downregulation reversed these effects. These data suggested
that miRNA-15a-5p was involved in inflammatory progression by
regulating the activation of the NF-κ pathway and by targeting
TNIP2 in vitro.
Depletion of TNIP2 eliminates the
effects of miR-15a-5p inhibitor in LPS-induced septic mice.
To assess whether miRNA-15a-5p was involved in the
inflammatory progression in septic mice, a mouse model of sepsis
was established using LPS followed by treatment with miR-15a-5p
inhibitor or miR-15a-5p inhibitor + TNIP2-siRNA. It was confirmed
that compared with the inhibitor control injection group,
miR-15a-5p inhibitor significantly decreased the level of
miR-15a-5p in the serum of mice (Fig.
6A). TNIP2-siRNA significantly decreased the mRNA level of
TNIP2 in the serum of mice compared to the control-siRNA group
(Fig. 6B). ELISAs were carried out
to detect the levels of IL-1β, IL-6 and TNF-α in the serum of mice
from the various treatment groups (LPS; LPS + inhibitor control;
LPS + inhibitor; LPS + inhibitor + siRNA). As indicated in Fig. 7A-C, miR-15a-5p inhibitor
significantly decreased the secretion of inflammatory factors,
including IL-1β, IL-6 and TNF-α, in the serum of LPS-treated mice.
Additionally, compared with the LPS treatment alone group, the
levels of kidney and liver injury markers, including Cr, BUN, ALT
and AST, in the serum of LPS-treated mice were reduced in the
miR-15a-5p inhibitor-administered group (Fig. 7D-G). The aforementioned effects of
miR-15a-5p inhibitor on LPS-treated mice were eliminated by the
TNIP2-siRNA combination therapy. These results indicated that
miRNA-15a-5p promoted the inflammatory response by negatively
regulating TNIP2 expression in septic mice.
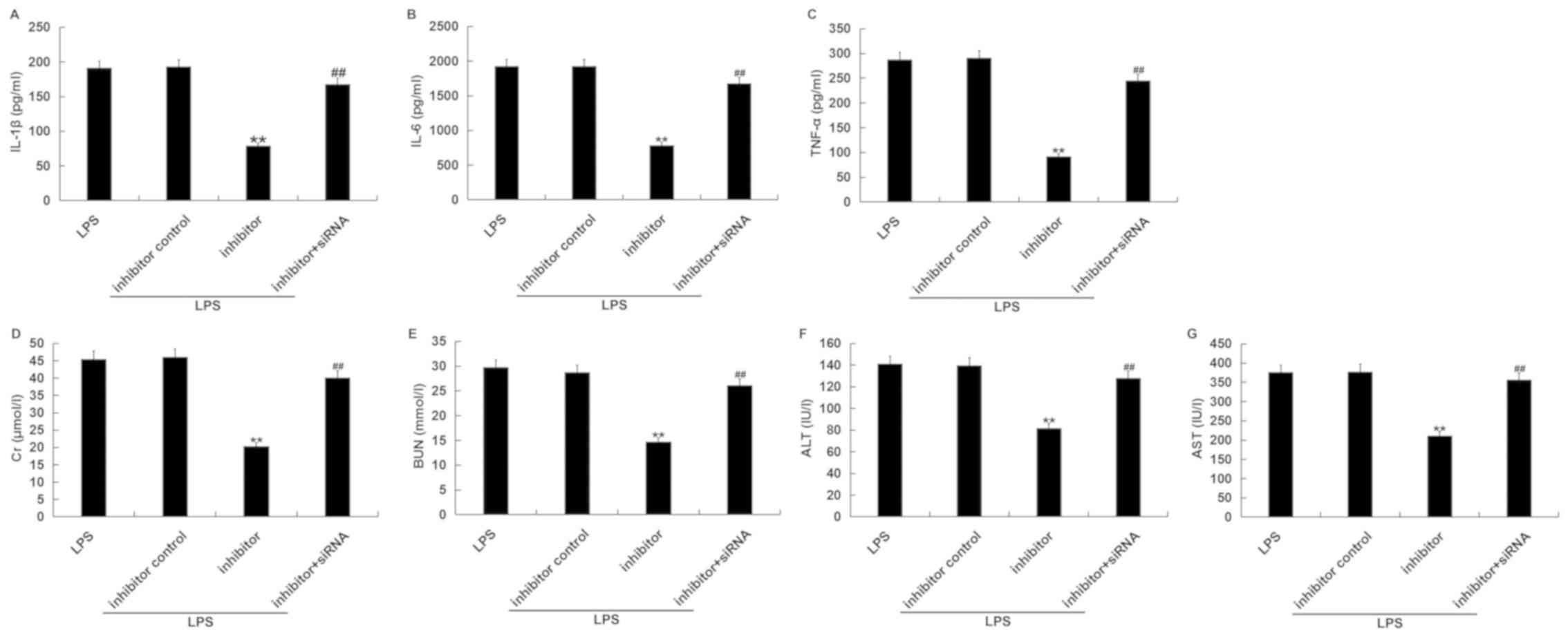 | Figure 7TNIP2-siRNA eliminates the effects of
miR-15a-5p inhibitor in LPS-induced septic mice. The serum (A)
IL-1β, (B) IL-6 and (C) TNF-α levels were evaluated using ELISAs.
The serum (D) Cr, (E) BUN, (F) ALT and (G) AST levels were
evaluated in the various treatment groups.
**P<0.01 vs. LPS+inhibitor control;
##P<0.01 vs. LPS+inhibitor. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; BUN, blood urea
nitrogen; Cr, creatine; LPS, lipopolysaccharide; miR, microRNA; IL,
interleukin; siRNA, small interfering RNA; TNF, tumor necrosis
factor; TNIP, TNF-α induced protein 3-interacting protein. |
Discussion
Previous studies have reported that sepsis is
associated with an excessive inflammatory response (1,20). A
number of studies have shown that miRNAs participate in the
sepsis-induced inflammatory response by affecting vital signaling
elements. For example, Chen et al (32) found that miR-212-3p depressed
LPS-induced inflammatory responses by targeting high-mobility group
protein B in sepsis. Additionally, Wang et al (33) showed that upregulation of miR-130b
suppressed severe lung inflammation in the sepsis mouse model by
LPS stimulation. However, the underlying mechanisms of action for
miR-15a-5p participation in the progression of sepsis remain
unknown. Based on the aforementioned literature, the aim of the
present study was to elucidate the effect of miR-15a-5p in the
sepsis inflammatory response. The present study provided evidence
that miR-15a-5p inhibitor suppressed the inflammatory response in
both cultured macrophages and in a septic mouse model by repressing
the activation of the NF-κ signaling pathway and by targeting
TNIP2.
RT-qPCR assays and ELISAs were used to detect
miR-15a-5p expression levels as well as inflammatory factors,
including IL-1β, IL-6 and TNF-α, in LPS-induced RAW264.7
macrophages. In accordance with the present study, previous studies
have also reported that IL-1β, IL-6 and TNF-α expression was
enhanced in LPS-treated RAW264.7 macrophages (34,35). The
present results also found that miR-15a-5p was significantly
upregulated in LPS-treated RAW264.7 macrophages. Therefore,
inhibiting miR-15a-5p expression may block the progression of
inflammation and serve an anti-inflammatory role in sepsis.
Additionally, TNIP2 was confirmed as a direct target of miR-15a-5p
through the dual-luciferase reporter assay. Therefore, revealing a
potential mechanism of action for TNIP2 with the inflammatory
response is crucial. In order to confirm the hypothesis,
TNIP2-knockdown was performed using siRNA. The roles of miR-15a-5p
inhibitor and TNIP2-siRNA were investigated in RAW264.7 macrophages
with LPS stimulation. It was observed that miRNA-15a-5p inhibitor
negatively regulated TNIP2 expression in RAW264.7 macrophages and
that the expression levels of TNIP2 in RAW264.7 macrophages with
LPS treatment, significantly decreased. Taking all of the
aforementioned data into consideration, this study suggests that
TNIP2 is a direct target gene of miRNA-15a-5p and a negative
relationship between miR-15a-5p and TNIP2 is indicated. This
relationship may regulate the progression of sepsis.
TNIP2, the binding partner of zinc finger protein
A20 (A20), was first discovered in a yeast two-hybrid screen. TNIP2
was found to regulate NF-κ by binding to A20, a well-known
anti-inflammatory signaling molecule (36). Overexpression of TNIP2 has been
reported to inhibit NF-κ activation and result in cell
proliferation in human cancer types (37). Therefore, the effects of miR-15a-5p
inhibitor or miR-15a-5p inhibitor + TNIP2-siRNA on inflammatory
factor (TNF-α, IL-1β and IL-6) expression levels and the NF-κ
signal pathway in LPS stimulated RAW264.7 macrophages were further
investigated. The results demonstrated that miR-15a-5p inhibitor
alleviated inflammatory responses in macrophages induced by LPS
stimulation, as indicated by decreased levels of IL-1β, IL-6 and
TNF-α. However, there is some disagreement on whether IL-1β is
expressed in RAW264.7 macrophages. For example, Pelegrin et
al (38) reported that RAW264.7
macrophages do not release mature IL-1β. However, a number of other
studies have reported that RAW264.7 macrophages secrete mature
IL-1β, which is consistent with the results of the current study
(39-41).
Furthermore, it was found that the activation of the NF-κ signaling
was inhibited by miR-15a-5p inhibitor in LPS-induced macrophages
compared with the control group, indicated by reduced p-p65
expression and increased TINP2 expression level. However, all of
the observed effects of the miR-15a-5p inhibitor on LPS-stimulated
macrophages were counteracted by TNIP2-siRNA. These data revealed
that miRNA-15a-5p was involved in the inflammatory progression in
macrophages through modulating TNIP2.
In the current study a mouse model was also
established by injecting LPS (10 mg/kg) to induce sepsis. The
results revealed that the levels of inflammatory factors (IL-1β,
IL-6 and TNF-α) and organ damage markers (Cr, BUN, ALT and AST)
were decreased in the miR-15a-5p inhibitor + LPS group compared
with the LPS group, while knockdown of TNIP2 reversed these
effects. These results indicated that miRNA-15a-5p may promote the
development of inflammation by negatively regulating TNIP2
expression in septic mice.
Overall, the present study demonstrated the
anti-inflammatory activity of miRNA-15a-5p inhibitor in both in
vitro and in vivo inflammatory models. To the best of
our knowledge, this study is the first to examine the mechanisms of
action for miRNA-15a-5p in the anti-inflammatory field. The results
of the present study confirmed that miR-15a-5p inhibitor prevented
the activation of the NF-κ signaling pathway by negatively
regulating TNIP2 expression, suppressing the inflammatory response
and therefore providing novel insights into the treatment of
sepsis. However, this study is only a preliminary analysis of the
role of miRNA-15a-5p in sepsis and further investigation of its
role is required. For example, the role of TNIP2 alone in sepsis
should be studied. Additionally, NF-κ activity, as well as the
inflammatory factor levels in RAW264.7 cells with
upregulated/downregulated TNIP2 expression upon LPS treatment
should be investigated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the current study, collected and
analyzed the data, performed statistical analysis, and prepared the
manuscript. ZH collected and analyzed the data, performed
statistical analysis, and prepared the manuscript.
Ethics approval and consent to
participate
The current study was performed in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals and was approved by the Committee of
Experimental Animals of the Affiliated Hospital of Medical School
of Ningbo University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Panagiotou A, Gaiao S and Cruz DN:
Extracorporeal therapies in sepsis. J Intensive Care Med.
28:281–295. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shane AL and Stoll BJ: Neonatal sepsis:
Progress towards improved outcomes. J Infect. 68 (Suppl
1)(S24-S32)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aziz M, Jacob A, Yang WL, Matsuda A and
Wang P: Current trends in inflammatory and immunomodulatory
mediators in sepsis. J Leukoc Biol. 93:329–342. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bosmann M and Ward PA: The inflammatory
response in sepsis. Trends Immunol. 34:129–136. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Burnham JP, Lane MA and Kollef MH: Impact
of sepsis classification and multidrug-resistance status on outcome
among patients treated with appropriate therapy. Crit Care Med.
43:1580–1586. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Carchman EH, Rao J, Loughran PA, Rosengart
MR and Zuckerbraun BS: Heme oxygenase-1-mediated autophagy protects
against hepatocyte cell death and hepatic injury from
infection/sepsis in mice. Hepatology. 53:2053–2062. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hotchkiss RS, Moldawer LL, Opal SM,
Reinhart K, Turnbull IR and Vincent JL: Sepsis and septic shock.
Nat Rev Dis Primers. 2(16045)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Martin GS: Sepsis, severe sepsis and
septic shock: Changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Nakajima TE, Yamada Y, Hamano T, Furuta K,
Matsuda T, Fujita S, Kato K, Hamaguchi T and Shimada Y:
Adipocytokines as new promising markers of colorectal tumors:
Adiponectin for colorectal adenoma, and resistin and visfatin for
colorectal cancer. Cancer Sci. 101:1286–1291. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wichadakul D, Mhuantong W, Jongkaewwattana
A and Ingsriswang S: A computational tool for the design of live
attenuated virus vaccine based on microRNA-mediated gene silencing.
BMC Genomics. 13 (Suppl 7)(S15)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cui XS, Sun SC, Kang YK and Kim NH:
Involvement of microRNA-335-5p in cytoskeleton dynamics in mouse
oocytes. Reprod Fertil Dev. 25:691–699. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Huang J, Sun Z, Yan W, Zhu Y, Lin Y, Chen
J, Shen B and Wang J: Identification of microRNA as sepsis
biomarker based on miRNAs regulatory network analysis. Biomed Res
Int. 2014(594350)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Srivastava M, Khurana P and Sugadev R:
Lung cancer signature biomarkers: Tissue specific semantic
similarity based clustering of digital differential display (DDD)
data. BMC Res Notes. 5(617)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dalamaga M: Nicotinamide
phosphoribosyl-transferase/visfatin: A missing link between
overweight/obesity and postmenopausal breast cancer? Potential
preventive and therapeutic perspectives and challenges. Med
Hypotheses. 79:617–621. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang B, Hasan MK, Alvarado E, Yuan H, Wu H
and Chen WY: NAMPT overexpression in prostate cancer and its
contribution to tumor cell survival and stress response. Oncogene.
30:907–921. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bi TQ, Che XM, Liao XH, Zhang DJ, Long HL,
Li HJ and Zhao W: Overexpression of Nampt in gastric cancer and
chemopotentiating effects of the Nampt inhibitor FK866 in
combination with fluorouracil. Oncol Rep. 26:1251–1257.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Daniel P, Leśniowski B, Mokrowiecka A,
Jasińska A, Pietruczuk M and Małecka-Panas E: Circulating levels of
visfatin, resistin and pro-inflammatory cytokine interleukin-8 in
acute pancreatitis. Pancreatology. 10:477–482. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
van der Poll T, van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vachharajani VT, Liu T, Wang X, Hoth JJ,
Yoza BK and McCall CE: Sirtuins link inflammation and metabolism. J
Immunol Res. 2016(8167273)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Baldwin AJ: The NF-kappa B and I kappa B
proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang ZM, Wan XH, Sang GY, Zhao JD, Zhu QY
and Wang DM: miR-15a-5p suppresses endometrial cancer cell growth
via Wnt/β-catenin signaling pathway by inhibiting WNT3A. Eur Rev
Med Pharmacol Sci. 21:4810–4818. 2017.PubMed/NCBI
|
|
24
|
Long J, Jiang C, Liu B, Fang S and Kuang
M: MicroRNA-15a-5p suppresses cancer proliferation and division in
human hepatocellular carcinoma by targeting BDNF. Tumour Biol.
37:5821–5828. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ergun S, Güney S, Temiz E, Petrovic N and
Gunes S: Significance of miR-15a-5p and CNKSR3 as novel prognostic
biomarkers in non-small cell lung cancer. Anticancer Agents Med
Chem. 18:1695–1701. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee YJ, Choi DY, Choi IS, Kim KH, Kim YH,
Kim HM, Lee K, Cho WG, Jung JK, Han SB, et al: Inhibitory effect of
4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation,
amyloidogenesis and memory impairment via inhibition of nuclear
factor-kappaB in vitro and in vivo models. J Neuroinflammation.
9(35)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hayes JB, Sircy LM, Heusinkveld LE, Ding
W, Leander RN, McClelland EE and Nelson DE: Modulation of
macrophage inflammatory nuclear factor κ (NF-κ) signaling by
intracellular cryptococcus neoformans. J Biol Chem.
291:15614–15627. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Van Huffel S, Delaei F, Heyninck K, De
Valck D and Beyaert R: Identification of a novel A20-binding
inhibitor of nuclear factor-kappa B activation termed ABIN-2. J
Biol Chem. 276:30216–30223. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American Physiological
Society. Physiologist. 39(199): 208–211. 1996.PubMed/NCBI
|
|
30
|
Bai XZ, Zhang JL, Liu Y, Zhang W, Li XQ,
Wang KJ, Cao MY, Zhang JN, Han F, Shi JH and Hu DH: MicroRNA-138
aggravates inflammatory responses of macrophages by targeting SIRT1
and regulating the NF-κ and AKT pathways. Cell Physiol Biochem.
49:489–500. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen W, Ma X, Zhang P, Li Q, Liang X and
Liu J: MiR-212-3p inhibits LPS-induced inflammatory response
through targeting HMGB1 in murine macrophages. Exp Cell Res.
350:318–326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang P, Zhang X, Li F, Yuan K, Li M, Zhang
J, Li B and Liang W: MiR-130b attenuates vascular inflammation via
negatively regulating tumor progression locus 2 (Tpl2) expression.
Int Immunopharmacol. 51:9–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chithra MA, Ijinu TP, Kharkwal H, Sharma
RK, Pushpangadan P and George V: Phenolic rich Cocos nucifera
inflorescence extract ameliorates inflammatory responses in
LPS-stimulated RAW264.7 macrophages and toxin-induced murine
models. Inflammopharmacology. Jul 26. 2019.doi:
10.1007/s10787-019-00620-6 (Epub ahead of print). PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shou J, Kong X, Wang X, Tang Y, Wang C,
Wang M, Zhang L, Liu Y, Fei C, Xue F, et al: Tizoxanide inhibits
inflammation in LPS-Activated RAW264.7 macrophages via the
suppression of NF-κ and MAPK activation. Inflammation.
42:1336–1349. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ma A and Malynn BA: A20: Linking a complex
regulator of ubiquitylation to immunity and human disease. Nat Rev
Immunol. 12:774–785. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Huang L, Verstrepen L, Heyninck K,
Wullaert A, Revets H, De Baetselier P and Beyaert R: ABINs inhibit
EGF receptor-mediated NF-kappaB activation and growth of EGF
receptor-overexpressing tumour cells. Oncogene. 27:6131–6140.
2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pelegrin P, Barroso-Gutierrez C and
Surprenant A: P2X7 receptor differentially couples to distinct
release pathways for IL-1beta in mouse macrophage. J Immunol.
180:7147–7157. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yonezawa Y, Miyashita T, Nejishima H,
Takeda Y, Imai K and Ogawa H: Anti-inflammatory effects of
olive-derived hydroxytyrosol on lipopolysaccharide-induced
inflammation in RAW264.7 cells. J Vet Med Sci. 80:1801–1807.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gao S, Wang Y, Li D, Guo Y, Zhu M, Xu S,
Mao J and Fan G: TanshinoneIIA alleviates inflammatory response and
directs macrophage polarization in lipopolysaccharide-stimulated
RAW264.7 cells. Inflammation. 42:264–275. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu F, Zhang X, Ling P, Liao J, Zhao M,
Mei L, Shao H, Jiang P, Song Z, Chen Q and Wang F: Immunomodulatory
effects of xanthan gum in LPS-stimulated RAW264. .7 macrophages.
Carbohydr Polym. 169:65–74. 2017.PubMed/NCBI View Article : Google Scholar
|