Introduction
Aristolochic acid (AA) is present in
Aristolochia plants and used in several types of traditional
Chinese medicine. In total, 99 types of AA exist, with AAI and AAII
being particularly nephrotoxic (1,2). AA was
originally used as a weight-loss supplement or for the treatment of
snakebite, diarrhea and gynecological conditions (3). However, long-term use of agents
containing AAI can lead to aristolochic acid nephropathy (AAN) or
urothelial carcinoma (2,4). AAN is an interstitial nephritis
characterized by transient necrosis in its early stages and
interstitial inflammation, fibrosis and tubular atrophy during its
chronic phase (5). Effective
therapies are needed to help improve the conditions of patients
with AAN.
The mechanism of AAN initially involve apoptosis of
proximal tubular epithelial cells (PTEC), followed by renal
interstitial fibrosis. In addition, AAI is metabolized into
intermediates that form covalent adducts with DNA, which leads to
the blockage of DNA replication, inducing cell cycle arrest in the
G2 phrase or cell apoptosis (6,7). AAI
itself can cause apoptosis by activating the p53 signaling pathway,
with AAI being especially pro-apoptotic (6). Renal interstitial fibrosis also
accelerates the development of AAN, as indicated by increased
expression of bone morphogenetic protein-7(8), transforming growth factor-β (9) and reduced levels of matrix
metalloproteinase-9(10).
The mTOR signaling pathway serves a critical role in
tissue fibrosis due to its ability to catalyze cell proliferation
and protein synthesis, which are also associated with cell cycle
progression (11,12). Rapamycin functions as an inhibitor of
the mTOR signaling pathway by binding with FK506-binding protein 12
and mTOR complex 1(13). This causes
inhibition of the bioactivities of mTOR, including regulation of
proliferation, differentiation, growth and transference of cells
(14-16).
The present study was designed to investigate
whether rapamycin has therapeutic effects on chronic aristolochic
acid nephropathy (CAAN). For the in vitro experiments, the
HK-2 cell line was used to examine the influence of rapamycin on
cell cycle arrest and apoptosis. Murine in vivo experiments
were also conducted, whereby analysis of urea nitrogen and
creatinine, and hematoxylin and eosin (H&E) staining of renal
tissue determined whether the damage to kidney function structure
induced by AAI could be reversed by rapamycin treatment. It was
hypothesized that any ameliorative effects would be facilitated
through the mTOR signaling pathway, which were observed as
reduction in the expression of genes associated with cell
apoptosis, proliferation and fibrosis.
Materials and methods
In vitro experiments Cell culture
Rapamycin was purchased from MedChemExpress. HK-2
cells were purchased from Kunming Cell Bank, Chinese Academy of
Sciences and cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) in a 5% CO2 incubator at 37˚C. Cells used for
in vitro experiments were divided into three groups at
1x105 cells/well: Untreated group (control group), cells
dosed with 10 µg/ml AAI (AAI group) and AAI cells treated with
rapamycin (RMS; AAI + RMS). The dosage used was based on a previous
study (17,18), which used 50 and 100 nM rapamycin for
24 h. Since no difference in morphology was found in cells treated
with 50 and 100 nM AAI, 50 nM was used for subsequent experiments.
Cells in the control group were grown in DMEM, while the AAI + RMS
group was pre-treated with 50 and 100 nm rapamycin for 24 h. Cells
in the AAI and treatment groups were dosed with 10 µg/ml AAI (Sigma
Aldrich; Merck KGaA) for 24 h to induce injury. Cell morphological
changes were observed under an light microscope (S800T-950HK; Leica
Microsystems GmbH) after 48 h.
Flow cytometry.
A cell cycle staining kit [Multi Sciences (Lianke)
Biotech Co., Ltd.] was used to analyze the cell cycle. A total of
2x106 cells were washed with 3 ml of 1X PBS.
Subsequently, 10 µl permeabilization solution and 1 ml DNA staining
solution were incubated with the cells at room temperature for 30
min. The cells were then detected by flow cytometry. An Annexin
V-FITC/propidium iodide (PI) apoptosis kit [Multi Sciences (Lianke)
Biotech Co., Ltd.] was used to detect cell apoptosis. A total of
2x106 cells were suspended in 500 µl of binding buffer.
Each sample was then incubated with 5 µl Annexin V-FITC and 10 µl
PI at room temperature in the dark for 5 min. Apoptotic cells were
subsequently analyzed using a flow cytometer coupled with the
FlowJo software (version 7.6; FlowJo LLC).
In vivo experiments Mice and
treatment
A total of 60 8-week-old specific pathogen free
grade C57BL/6 male mice weighing 25-30 g were purchased from the
Experimental Animal Center of Wenzhou Medical University [SCXK
(Zhe) 2015-0001, Wenzhou, China]. Animal procedures were based on
international guidelines and approved by The Wenzhou Medical
University Animal Policy and Welfare Committee (approval no. wydw
2014-0066). All mice were housed in controlled conditions, at
24±1˚C and 50±1% relative humidity, a 12-h light/dark cycle and had
free access to food and water. Mice were randomly divided into
three different groups: Control group (n=20), CAAN group (n=20) and
treatment group (n=20). A CAAN mouse model was constructed through
reiterative intraperitoneal injection of AAI (dissolved in PBS
supplemented with 0.15% DMSO) at a dose of 5 mg/kg/every two days
for 6 consecutive weeks. Simultaneously, rapamycin (Selleck
Chemicals) was administered to the treatment group by
intraperitoneal injection at a dose of 1 mg/kg/day for 6
consecutive weeks. The normal control group was injected with the
same volume of normal saline over the same 6-week period. Blood and
kidney tissues were collected for analysis.
Tissue sampling.
All mice were exposed to isoflurane sustained at 5%
for ~2 min in a ventilator. Mice were then sacrificed by vertebral
dislocation. Blood was collected from the orbital veins for
quantification of creatinine and urea nitrogen. Both kidneys were
obtained from each mouse and subsequently divided into two
sections. One section was cut into small cubes and frozen at -80˚C
for later protein analysis, and the remaining section was used for
histopathological staining. Tissue for staining was first fixed in
4% PFA for ≥72 h at room temperature, then embedded in paraffin,
dehydrated and cut into 5 µm-thick slices.
Histopathology and
immunohistochemistry.
Following deparaffinization and rehydration, the
tissue slices were stained with H&E for general
histopathological observation and assessment of parameters
including tubular atrophy and glomerular structure. Subsequently,
the presence of cell apoptosis, proliferation and fibrosis markers
was assessed by immunohistochemical (IHC) staining. All paraffin
tissue sections were dewaxed in xylene and rehydrated using a
descending alcohol gradient. The sections were then processed with
3% hydrogen peroxide to block endogenous peroxidase, citrate buffer
to retrieve antigen and 5% goat serum (Beyotime Institute of
Biotechnology) at 37˚C in an oven for 1 h to eliminate nonspecific
binding sites. Samples were incubated with the following primary
antibodies: Anti-Ki67 (1:200; cat. no. ab15580; Abcam),
anti-cleaved caspase-3 (1:100; cat. no. ab2302; Abcam) and
anti-α-smooth muscle actin (α-SMA; cat. no. ab32575; 1:200) at 4˚C
overnight. The next day, the sections were incubated with
horseradish peroxidase (HRP)-labeled Goat Anti-Rabbit IgG secondary
antibodies (1:50; cat. no. A0208; Beyotime Institute of
Biotechnology) at 37˚C for 1 h and visualized using
diaminobenzidine (brown staining; Beyotime Institute of
Biotechnology). The intensity and area of positive staining was
determined by measuring the integrated optical density (IOD)/area
value using the Image-Pro Plus 6.0 image analysis software (Media
Cybernetics, Inc.).
Western blot analysis.
The specific method used for western blotting was
described in a previous study (19).
The kidney tissues were first homogenized with RIPA buffer
supplemented with phosphatase inhibitors (Beyotime Institute of
Biotechnology) extract the total protein. Protein concentration was
determined with a bicinchoninic acid Protein Assay kit (Beyotime
Institute of Biotechnology). The following primary antibodies were
used at 4˚C overnight on a shaking table: Proliferating cell
nuclear antigen (PCNA; 1:1,000; cat. no. ab92552; Abcam), Bax
(1:1,000; cat. no. ab32503; Abcam), Bcl-2 (1:1,000; cat. no.
ab182858; Abcam), epithelial cell interstitial transformation (EMT;
1:1,000; cat. no. ab32039; Abcam)-related protein, E-cadherin
(1:1,000; cat. no. ab76055; Abcam) and m-TOR pathway proteins
[phosphorylated (p)-mTOR (1:1,000; cat. no. 5536; Cell Signaling
Technology, Inc.), mTOR (1:1,000; cat. no. 2983; Cell Signaling
Technology, Inc.), p-AKT (1:1,000; cat. no. 4060; Cell Signaling
Technology, Inc.) and AKT (1:1,000; cat. no. 4685; Cell Signaling
Technology, Inc.)] with GAPDH antibody (1:2,000; cat. no. BS72410;
Bioworld Technology, Inc.) used as an internal reference. The next
day, the membranes were incubated with HRP-conjugated goat
anti-Rabbit IgG (1:5,000; cat. no. BS13278; Bioworld Technology,
Inc.) or goat anti-Mouse IgG (1:5,000; cat. no. BS12471; Bioworld
Technology, Inc.) secondary antibodies at room temperature for 1-2
h. Protein bands were visualized using SuperSignal™ West Femto
Maximum Sensitivity Substrate (Thermo Fisher Scientific, Inc.) and
the density of the bands were detected by Gel-Pro Analyzer Image
Analysis Software (version 4.0; Meyer Instruments).
Statistical analysis.
Each experiment was performed at least three times.
One-way ANOVA followed by Tukey's post hoc test was applied to
evaluate differences between groups. Data were analyzed using SPSS
16.0 (SPSS, Inc.) and GraphPad Prism 5.0 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
In vitro experiments Morphological
changes of HK-2 cells
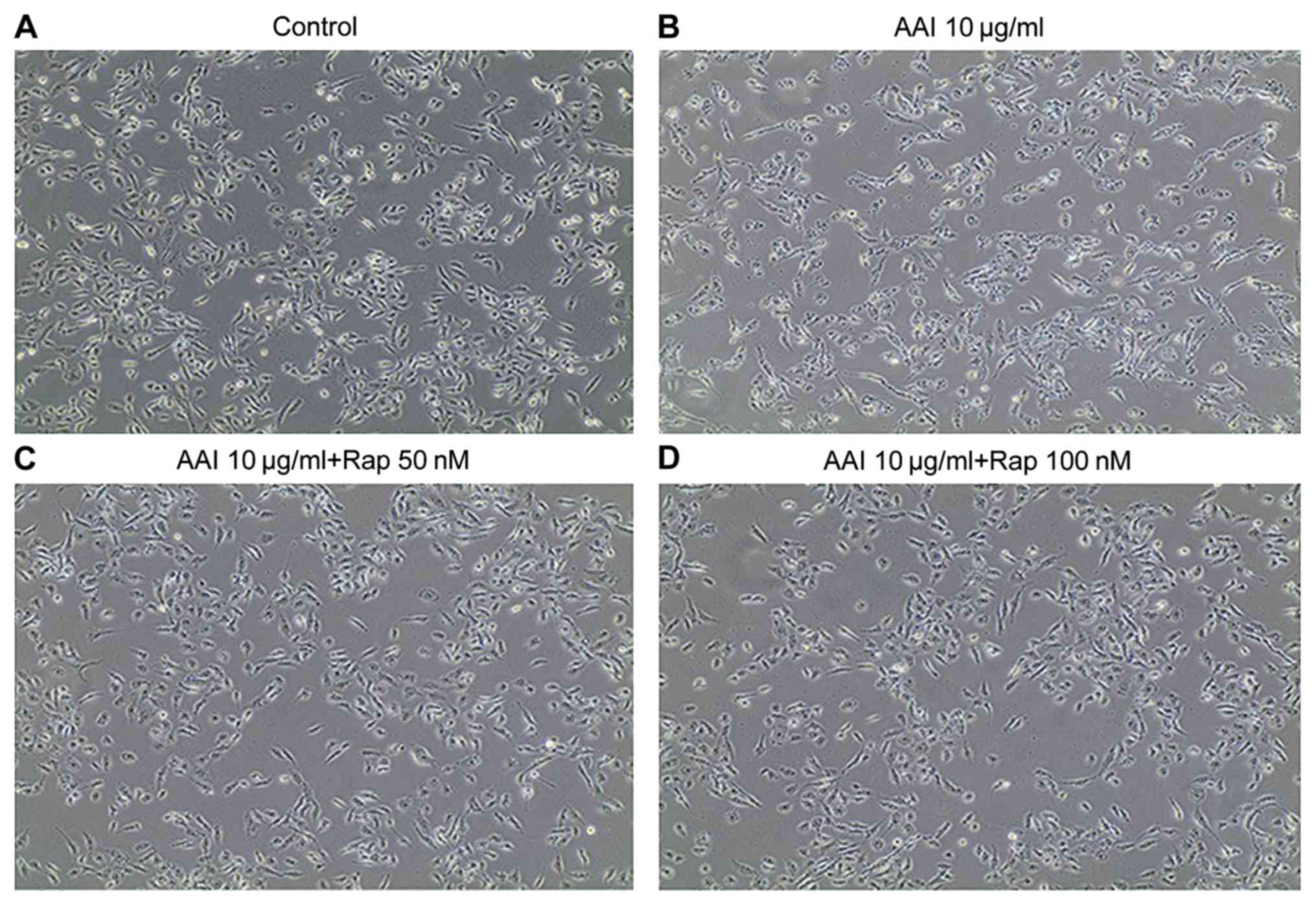
As shown in Fig. 1,
cells in the AAI group (Fig. 1B)
changed from ovate to elongated shapes, whereas cells in the AAI +
RMS group (Fig. 1C) developed smooth
margins. Two doses (50 and 100 nm) were initially used to determine
the optimum concentration of rapamycin. However, little
morphological difference was observed between these differently
dosed groups (Fig. 1C and D). Therefore, 50 nm rapamycin was used for
subsequent experiments.
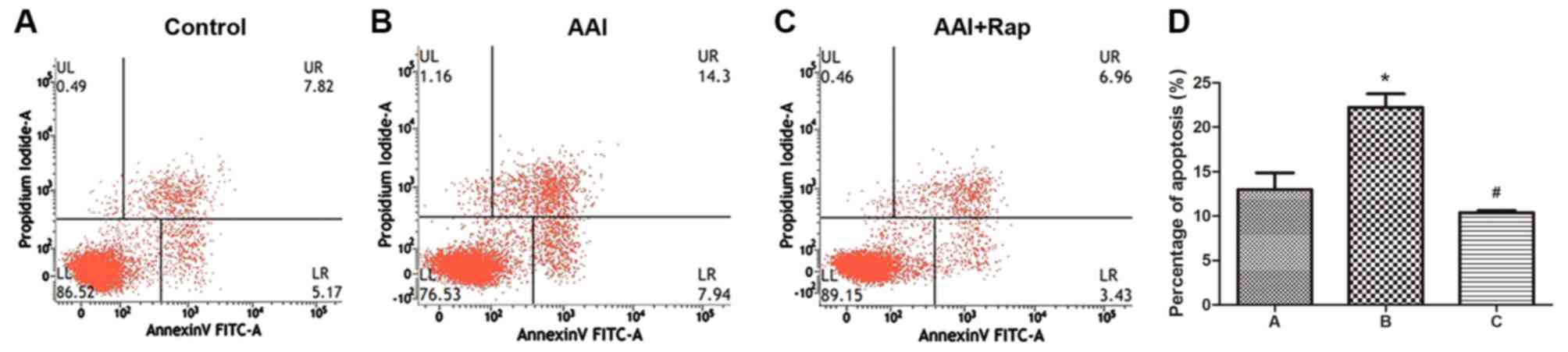
Cell apoptosis and cell cycle alteration results
were as follows. As shown in Fig. 2,
the percentage of apoptotic cells was higher in the AAI group
(22.24±1.53%) and lower in the AAI + RMS group (10.39±0.25%)
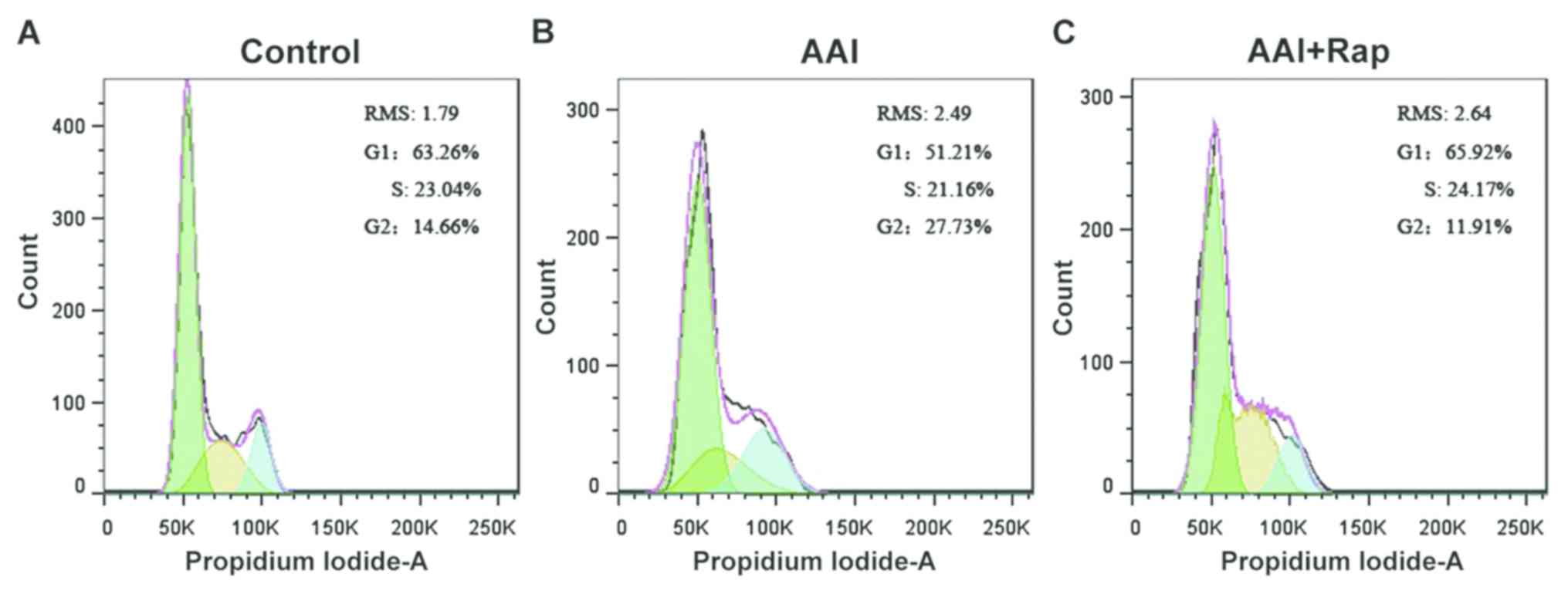
compared with the control group (12.99±1.88%). Cell cycle
alteration was detected based on the amount of chromosomal ploidy
present (Fig. 3). The proportion of
cells in the G1 phase was 51.21% in the AAI group and
65.92% in the AAI + RMS group. The proportion of cells in the
G2/M phase decreased from 27.73% in the AAI group to
11.91% in the AAI + RMS group. The results demonstrated that
rapamycin can decrease apoptosis and prevent G2/M phase
cell cycle arrest.
In vivo experiments Therapeutic
effects of rapamycin
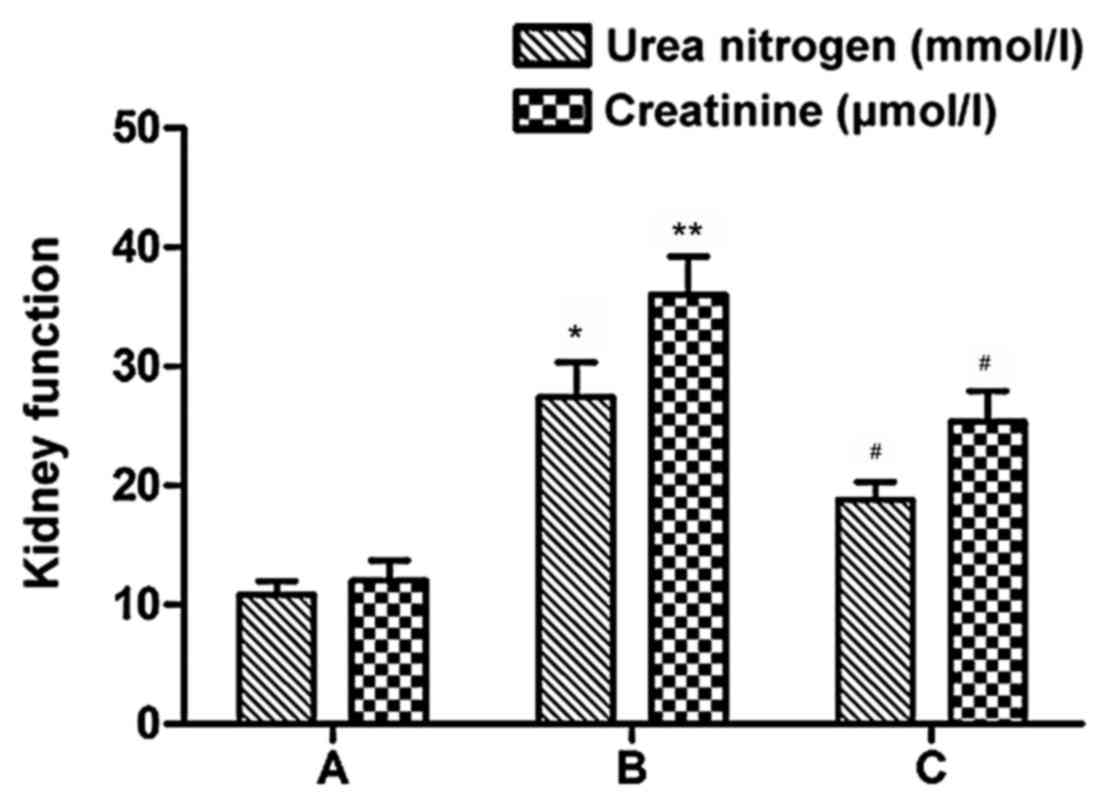
As shown in Fig. 4,
urea nitrogen and creatinine levels were highest in the CAAN group
(P<0.05), which also indicated that CAAN mice models were
successfully established. However, in the treatment group, these
two indexes declined compared with the CAAN group, especially that
of urea nitrogen (P<0.05). The observations corroborate
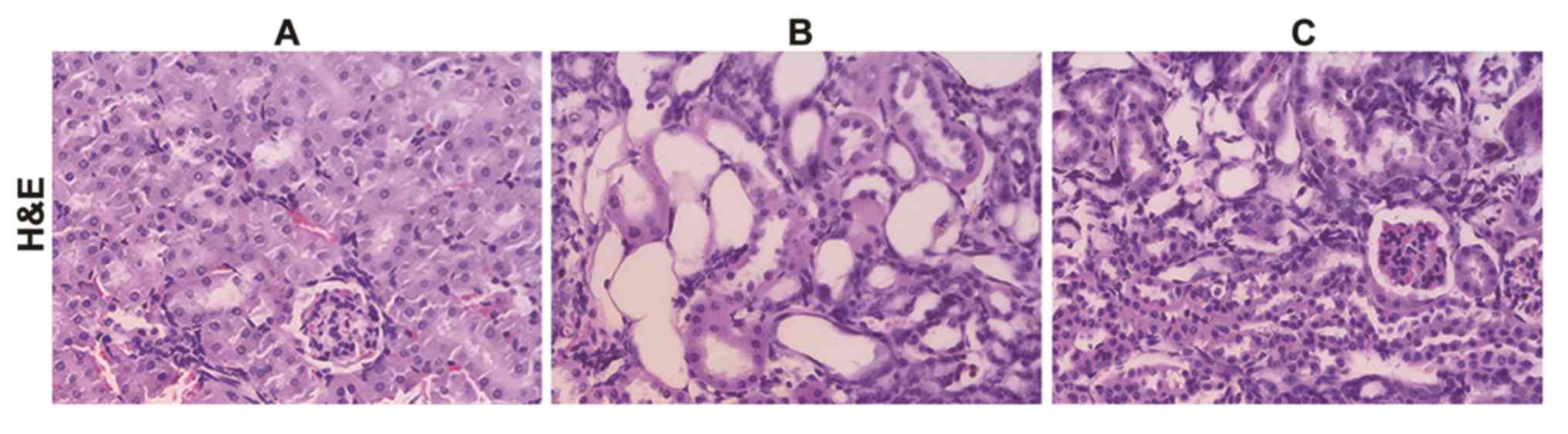
pathological observations. As shown in Fig. 5, compared with the control group, the
structure of kidney tissues in the CAAN group was damaged, with
tubular atrophy and amplified interstitial fibrotic areas. In
addition, glomeruli were barely visible in the whole tissue
section. By contrast, treatment group tissues exhibited reduced
tubular atrophy and larger areas of glomeruli.
Potential therapeutic effects of
rapamycin.
To confirm the therapeutic effects of rapamycin for
alleviating kidney injuries induced by AAI, IHC staining was
performed to determine the expression levels of molecules related
to cell apoptosis, proliferation and tissue fibrosis (Fig. 6A and B). Areas positive for α-SMA, Ki 67 and
cleaved-caspase 3 staining were clearly observed in the CAAN group
(Fig. 6A), and the levels of these
proteins were significantly increased compared with the control
group (Fig. 6B; Ki 67, P<0.05;
cleaved caspase-3, P<0.01; α-SMA, P<0.01). However, following
treatment with rapamycin, the number of positively-stained areas
decreased (Fig. 6B; Ki 67,
P<0.05; cleaved caspase-3, P<0.05; α-SMA, P<0.05).
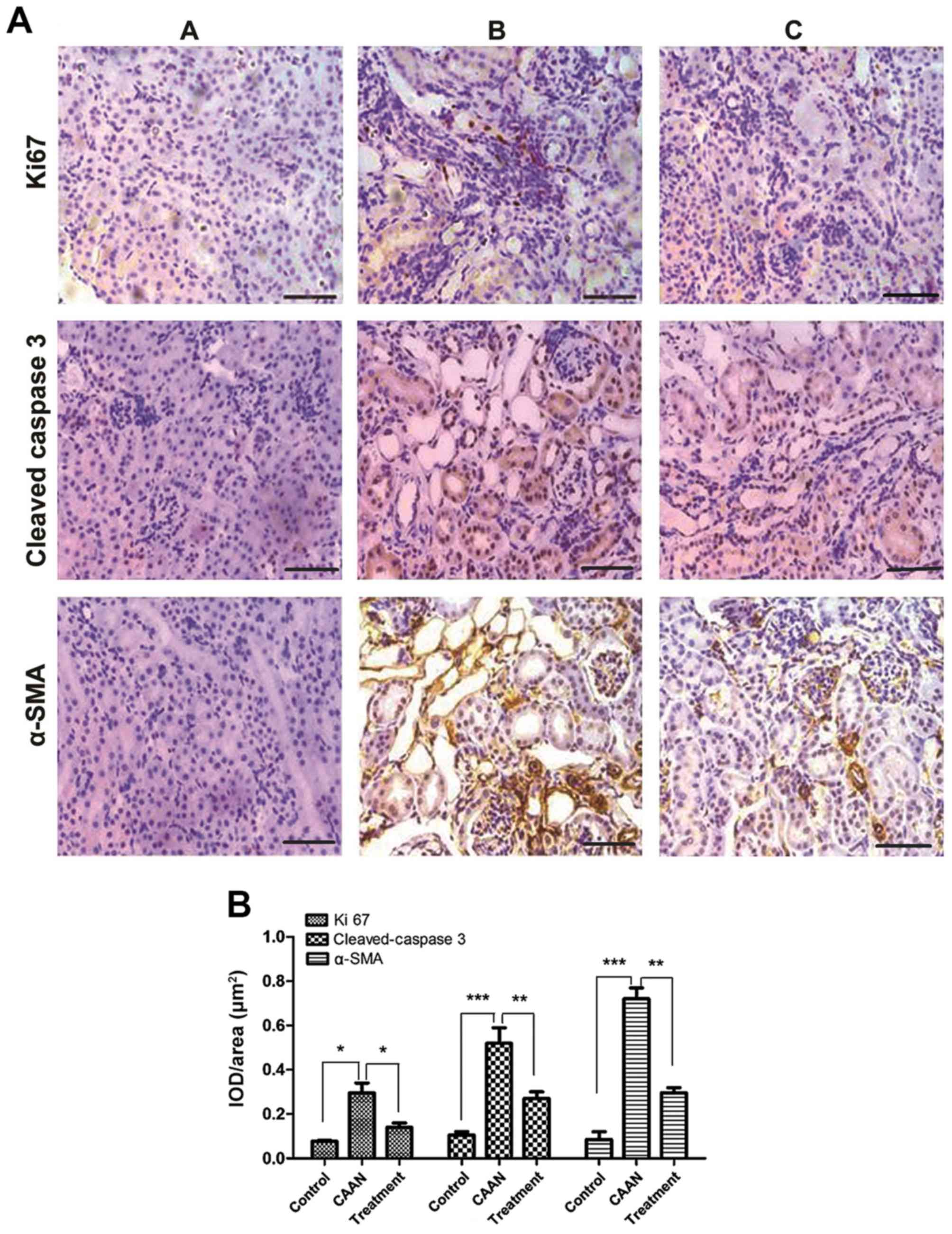 | Figure 6Expression of apoptosis-,
proliferation- and fibrosis-related proteins. (A) Cleaved
caspase-3, Ki67 and α-SMA immunohistochemical staining. Brown color
represents positive staining. (B) Measurement of the IOD/area of
immunohistochemical staining of Ki67, cleaved caspase 3 and α-SMA.
***P<0.001,
**P<0.01, *P<0.05. Scale
bar, 50 µm. Ki67, proliferation marker protein Ki67; α-SMA,
α-smooth muscle actin; CAAN, chronic aristolochic acid nephropathy;
IOD, integrated optical density; A, control group; B, CAAN group;
C, treatment group. |
The western blotting results were in accordance with
microscopic observations (Fig.
7B-D), where the expression of PCNA, Bax and α-SMA were all
elevated in the CAAN group, but reversed by treatment (P<0.01 or
P<0.05). In addition, the expression of anti-apoptotic Bcl-2 and
anti-fibrotic E-cadherin were both elevated in the treatment group
but decreased in the CAAN group compared with the control
group.
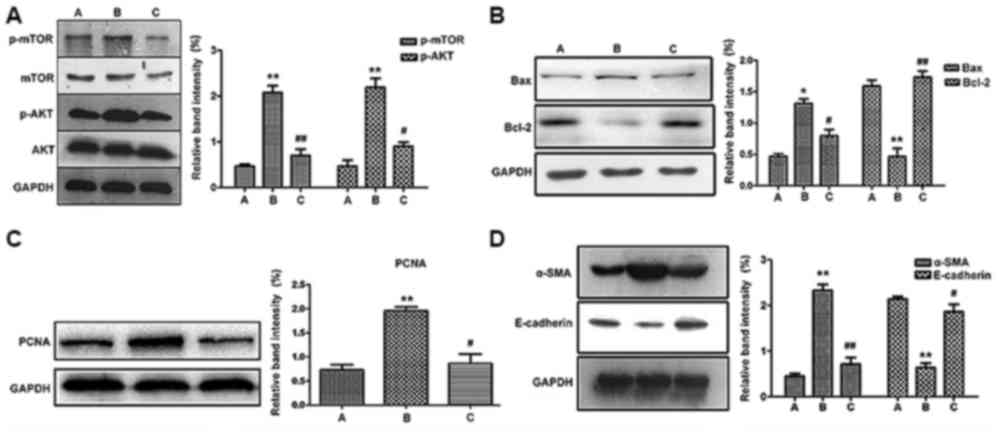 | Figure 7Western blotting results. (A)
Expression levels of key proteins in the mTOR signaling pathway.
The expression of p-AKT and p-mTOR increased in the CAAN group and
decreased in the treatment group. Expression levels of (B) Bax and
Bcl-2, (C) PCNA, (D) α-SMA and E-cadherin. *P<0.05
and **P<0.01 vs. control;
#P<0.05 and ##P<0.01 vs. CAAN. A,
control group; B, CAAN group; C, treatment group; p-AKT,
phospho-AKT; p-mTOR, phospho-mTOR; CAAN, chronic aristolochic acid
nephropathy; PCNA, proliferating cell nuclear antigen; α-SMA,
α-smooth muscle actin. |
As shown in Fig. 7A,
levels of mTOR and AKT phosphorylation were both elevated in the
CAAN group but declined in the treatment group (P<0.01 and
P<0.05). Therefore, rapamycin may block the phosphorylation and
subsequent activation of mTOR signaling, in turn resulting in the
alleviation of cell proliferation, apoptosis and ultimately reduce
tissue fibrosis.
Discussion
AAN is a long-term chronic disease which causes
irreversible structural injury and leaves patients with a poor
quality of life (20,21). However, the progression of AAN cannot
be prevented in most patients (22).
The present research was performed to investigate the ameliorative
effects and mechanism of action of rapamycin on AAN.
The in vitro experiments included in the
current study showed that abnormal cell shape, apoptosis and cell
cycle arrest at the G2/M checkpoint were clearly
observed in the CAAN group. The G2/M checkpoint is a
control point used by cells to protect against injury, as it allows
termination of genotoxicity (23)
and also enables epithelial cells to transdifferentiate (24). Thus, if an organism detects DNA
damage in response to injury, cell cycle progression is inhibited
and DNA-damage repair is activated (25). However, the G2/M
checkpoint is the last defense for DNA repair; if damage cannot be
repaired then, cell death will be induced (26). In the CAAN group, a high proportion
of the cell population in the G2 phase indicated that
extensive DNA damage had occurred and increased the possibility of
cell apoptosis. Furthermore, apoptosis enables cells to change from
an epithelial type to a fibroblast type, while cell cycle arrest
provides cells with the opportunity to transform into fibroblast
types, which are more likely to survive injury (24).
The in vivo experiments showed that rapamycin
was, to a certain extent, able to improve impaired kidney function
and reverse damaged kidney structures such as tubular dilation and
interstitial fibrosis. The expression of p-mTOR and p-AKT both
declined in the treatment group, which indicated that the mTOR
signaling pathway had been successfully blocked by rapamycin.
Furthermore, the expression levels of apoptosis-, cell
proliferation- and tissue fibrosis-related proteins were all
decreased in the treatment group.
The present results show the possible therapeutic
effects of rapamycin on CAAN. Rapamycin has also been used for
other disease research, such as immunoglobulin A nephropathy,
obstructive nephropathy, diabetic nephropathy and kidney neoplasms
(27-29).
These diseases have common pathological features such as cell
apoptosis, proliferation and tissue fibrosis, thus, they may also
be seen as functional targets of rapamycin. Rapamycin is an mTOR
inhibitor and the upstream regulator of mTOR-like serine/threonine
kinase AKT and ERK1/2, which are responsible for extracellular
matrix synthesis and cell proliferation (30,31).
This can account for the decreased levels of cell proliferation and
fibrosis observed in the treatment group.
The apoptosis of tubular epithelial cells is crucial
for nephron destruction (32).
Rapamycin also directly interferes with apoptosis by inhibiting the
mTOR signaling pathway. mTOR plays an important role in
phosphorylating/inactivating Bcl-2 and increasing the expression of
caspase 3 (33,34). Another previous study stated that
rapamycin can interrupt mTOR-triggered transformation of the
nucleus, and block the transcription of p53 and triggering of cell
apoptosis (34). Thus, proapoptotic
proteins such as Bax can be depleted, as was reflected in the
present research, with western blotting and immunohistochemical
staining confirming that Bax and cleaved caspase-3 levels declined
following treatment with rapamycin.
The present study primarily analyzed the
ameliorative effects of rapamycin through cell apoptosis,
proliferation and tissue fibrosis. When cells are injured,
proliferation can be activated to compensate for high levels of
apoptosis (24). However, the newly
proliferated cells can become hypersensitive to injury factors
(35-37),
which can lead to further apoptosis, and eventually result in the
further aggravation of injury, forming a vicious cycle of cell
apoptosis-proliferation. However, treatment with rapamycin can
inhibit the mTOR signaling pathway and thus prevent cell death.
There are some limitations in the present study. In
the in vitro experiments, the dosages of AAI and rapamycin
were determined by referring to published results. Dosages would be
better determined by performing Cell Counting Kit-8 or real time
cell analysis assays. The effects of rapamycin provided by
different suppliers should also be tested. For the in vivo
experiments, only mTOR signaling pathway proteins were examined.
Furthermore, the molecular mechanisms associated with apoptosis,
proliferation and fibrosis need to be studied in more detail. The
rate of cell proliferation and sensitivity to injuries should also
be taken into consideration.
In conclusion, the present findings confirm that
rapamycin can improve CAAN symptoms in vitro and in a mouse
model by blockade of the mTOR signaling pathway, leading to a
decrease in cell apoptosis, proliferation and fibrosis. Therefore,
rapamycin should be investigated as a possible new therapeutic
strategy for the treatment of CAAN.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from The Natural
Science Foundation of Zhejiang Province (grant no. LY14H050006),
The National Natural Science Foundation of China (grant no.
8157080113), The Natural Science Foundation of Shenzhen University
General Hospital (grant no. SUGH2018QD071), and The Natural Science
Foundation of Shenzhen University General Hospital (grant no.
SUGH2018QD013).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MMW performed most of the animal experiments,
including establishment of CAAN mice, H&E and IHC staining. FL
helped to design and guide the study. LLT and FQD performed all
western blot anlaysis and flow cytometry experiments. JJZ, ZS and
BCC were responsible for data analysis. MMW and FL drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
the First Affiliated Hospital of Wenzhou Medical University (grant
no. wydw2014-0066; Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Vanherweghem JL, Depierreux M, Tielemans
C, Abramowicz D, Dratwa M, Jadoul M, Richard C, Vandervelde D,
Verbeelen D, Vanhaelen-Fastre R, et al: Rapidly progressive
interstitial renal fibrosis in young women: Association with
slimming regimen including Chinese herbs. Lancet. 341:387–391.
1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nortier JL, Martinez MC, Schmeiser HH,
Arlt VM, Bieler CA, Petein M, Depierreux MF, De Pauw L, Abramowicz
D, Vereerstraeten P and Vanherweghem JL: Urothelial carcinoma
associated with the use of a Chinese herb (Aristolochia fangchi). N
Engl J Med. 342:1686–1692. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Heinrich M, Chan J, Wanke S, Neinhuis C
and Simmonds MS: Local uses of Aristolochia species and content of
nephrotoxic aristolochic acid 1 and 2-a global assessment based on
bibliographic sources. J Ethnopharmacol. 125:108–144.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hamano Y, Aoki T, Shirai R, Hatano M,
Kimura R, Ogawa M, Yokosuka O and Ueda S: Low-dose darbepoetin
alpha attenuates progression of a mouse model of aristolochic acid
nephropathy through early tubular protection. Nephron Exp Nephrol.
114(e69-e81)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pozdzik AA, Salmon IJ, Husson CP,
Decaestecker C, Rogier E, Bourgeade MF, Deschodt-Lanckman MM,
Vanherweghem JL and Nortier JL: Patterns of interstitial
inflammation during the evolution of renal injury in experimental
aristolochic acid nephropathy. Nephrol Dial Transplant.
23:2480–2491. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou L, Fu P, Huang XR, Liu F, Lai KN and
Lan HY: Activation of p53 promotes renal injury in acute
aristolochic acid nephropathy. J Am Soc Nephrol. 21:31–41.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Arlt VM, Stiborova M and Schmeiser HH:
Aristolochic acid as a probable human cancer hazard in herbal
remedies: A review. Mutagenesis. 17:265–277. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Y, Wang Z, Wang S, Zhao J, Zhang J and
Huang Y: Gremlin-mediated decrease in bone morphogenetic protein
signaling promotes aristolochic acid-induced
epithelial-to-mesenchymal transition (EMT) in HK-2 cells.
Toxicology. 297:68–75. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rui HL, Wang YY, Cheng H and Chen YP:
JNK-dependent AP-1 activation is required for aristolochic
acid-induced TGF-β1 synthesis in human renal proximal epithelial
cells. Am J Physiol Renal Physiol. 302(F1569-F1575)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu CJ, Chou YC, Cheng YW, Hsiao CJ, Wang
CH, Wang HY, Sheu JR and Hsiao G: Aristolochic acid downregulates
monocytic matrix metalloproteinase-9 by inhibiting nuclear factor-κ
activation. Chem Biol Interact. 192:209–219. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hartford CM and Ratain MJ: Rapamycin:
Something old, something new, sometimes borrowed and now renewed.
Clin Pharmacol Ther. 82:381–388. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X and Proud CG: mTORC1 signaling:
What we still don't know. J Mol Cell Biol. 3:206–220.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Burnett PE, Barrow RK, Cohen NA, Snyder SH
and Sabatini DM: RAFT1 phosphorylation of the translational
regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA.
95:1432–1437. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brown EJ, Beal PA, Keith CT, Chen J, Shin
TB and Schreiber SL: Control of p70 s6 kinase by kinase activity of
FRAP in vivo. Nature. 377:441–446. 1995.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Li J, Zhang M, Mao Y, Li Y, Zhang X, Peng
X and Yu F: The potential role of aquaporin 1 on aristolochic acid
I induced epithelial mesenchymal transition on HK-2 cells. J Cell
Physiol. 233:4919–4925. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kan WC, Hwang JY, Chuang LY, Guh JY, Ye
YL, Yang YL and Huang JS: Effect of osthole on advanced glycation
end products-induced renal tubular hypertrophy and role of klotho
in its mechanism of action. Phytomedicine. 53:205–212.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou Q, Du J, Hu Z, Walsh K and Wang XH:
Evidence for adipose-muscle cross talk: Opposing regulation of
muscle proteolysis by adiponectin and Fatty acids. Endocrinology.
148:5696–5705. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dickman KG, Sweet DH, Bonala R, Ray T and
Wu A: Physiological and molecular characterization of aristolochic
acid transport by the kidney. J Pharmacol Exp Ther. 338:588–597.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ma DH, Zheng FL, Su Y, Li MX and Guo MH:
Influence and analysis of low-dosage steroid therapy in severe
aristolochic acid nephropathy patients. Nephrology (Carlton).
21:835–840. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luciano RL and Perazella MA: Aristolochic
acid nephropathy: Epidemiology, clinical presentation, and
treatment. Drug Saf. 38:55–64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Stark GR and Taylor WR: Analyzing the G2/M
checkpoint. Methods Mol Biol. 280:51–82. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang L, Besschetnova TY, Brooks CR, Shah
JV and Bonventre JV: Epithelial cell cycle arrest in G2/M mediates
kidney fibrosis after injury. Nat Med. 16:535–543. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Buisson R, Niraj J, Rodrigue A, Ho CK,
Kreuzer J, Foo TK, Hardy EJ, Dellaire G, Haas W, Xia B, et al:
Coupling of homologous recombination and the checkpoint by ATR. Mol
Cell. 65:336–346. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Visconti R, Della Monica R and Grieco D:
Cell cycle checkpoint in cancer: A therapeutically targetable
double-edged sword. J Exp Clin Cancer Res. 35(153)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH
and Tang MJ: Rapamycin attenuates unilateral ureteral
obstruction-induced renal fibrosis. Kidney Int. 69:2029–2036.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tian J, Wang Y, Liu X, Zhou X and Li R:
Rapamycin ameliorates IgA nephropathy via cell cycle-dependent
mechanisms. Exp Biol Med (Maywood). 240:936–945. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Velagapudi C, Bhandari BS, Abboud-Werner
S, Simone S, Abboud HE and Habib SL: The tuberin/mTOR pathway
promotes apoptosis of tubular epithelial cells in diabetes. J Am
Soc Nephrol. 22:262–273. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu DD, Han CC, Wan HF, He F, Xu HY, Wei
SH, Du XH and Xu F: Effects of inhibiting PI3K-Akt-mTOR pathway on
lipid metabolism homeostasis in goose primary hepatocytes. Animal.
10:1319–1327. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zeng R, Xiong Y, Zhu F, Ma Z, Liao W, He
Y, He J, Li W, Yang J, Lu Q, et al: Fenofibrate attenuated
glucose-induced mesangial cells proliferation and extracellular
matrix synthesis via PI3K/AKT and ERK1/2. PLoS One.
8(e76836)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Johnson A and DiPietro LA: Apoptosis and
angiogenesis: An evolving mechanism for fibrosis. FASEB J.
27:3893–3901. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Calastretti A, Bevilacqua A, Ceriani C,
Viganò S, Zancai P, Capaccioli S and Nicolin A: Damaged
microtubules can inactivate BCL-2 by means of the mTOR kinase.
Oncogene. 20:6172–6180. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Shen YL, Sun L, Hu YJ, Liu HJ, Kuang XY,
Niu XL and Huang WY: P53 inhibitor pifithrin-alpha prevents the
renal tubular epithelial cells against injury. Am J Transl Res.
8:4040–4053. 2016.PubMed/NCBI
|
|
36
|
Bonventre JV: Dedifferentiation and
proliferation of surviving epithelial cells in acute renal failure.
J Am Soc Nephrol. 14 (Suppl 1)(S55-S61)2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gniadecki R, Hansen M and Wulf HC: Two
pathways for induction of apoptosis by ultraviolet radiation in
cultured human keratinocytes. J Invest Dermatol. 109:163–169.
1997.PubMed/NCBI View Article : Google Scholar
|