Introduction
Rheumatoid arthritis (RA) is an autoimmune disease
that is mainly mediated by cytokines and is characterized by the
abnormal proliferation of synovial cells, the massive infiltration
of inflammatory cells and the progressive destruction of joints
(1). T helper cells (Th)
are divided into Th1, Th2 and Th17
subgroups according to the different expression profiles of
secretory cytokines (2). It has
become clear in the last few years that T cell-derived cytokines
expressed preferentially by Th1 cells contribute to
joint destruction and inflammatory response in RA, whereas
Th2 cell-associated cytokines may be protective
(2,3). However, recent research on
Th17 cells and regulatory T cells (Treg) has
demonstrated that an imbalance between
Th1/Th2/Th17/Treg cells is
important to the pathogenesis of RA (4-6). Although the origin
of RA is unclear, it has been determined that lymphocytes
accumulate around the terminal blood vessels of the subsynovial
layer prior to the inflammatory response (7). The synovium is composed of two layers,
namely the synovial lining layer (lining cells) and the lower
synovial lining layer (or supporting layer) (8). Another major feature of RA is the
abnormal proliferation of synovial cells in the lining layer and
the expression of transformed cells, which erode the surrounding
bone and cartilage (7-9).
Increasing evidence has confirmed that the activation state of RA
synovial fibroblasts (RASFs) is critically dependent on TLR
expression, which in turn is known to serve an essential role in T
cell differentiation and function (10-12).
TLR2 and TLR4 have been revealed to serve important functions in
pathogenesis of RA and they has been determined that the expression
of TLR-2 and TLR-4 is increased and regulated by proinflammatory
cytokines that are present in the synovial compartment (10). Activation of these RASF-expressed
TLRs exacerbates inflammatory Th1 and Th17
cell expansion in cell-cell contact-dependent and inflammatory
cytokine-dependent pathways, inducing the increased accumulation of
interferon (IFN)-γ and interleukin (IL)-17(11). Targeting TLRs may therefore modulate
inflammation in RA and provide novel therapeutic strategies for
overcoming this persistent disease (12).
Xixin is isolated from the dried roots and rhizomes
of Asarum heterotropoides f. mandshuricum
(Maximowicz) Kitagawa, A. sieboldii Miq. var.
seoulense Nakai and A. sieboldii Miq.
(Aristolochiaceae), and is a commonly used herbal medicine in China
(13). Xixin is used to treat colds,
fever, chills, headaches, acute toothaches, sinusitis, coughs and
RA in traditional Chinese medicine (13). Asarinin (molecular weight, 354.35) is
one of the main active chemical components isolated from Xixin
(13-16).
Many pharmacological effects have been identified in asarinin,
including anti-inflammatory effects, antipyretic properties and
immune inhibition (13-16).
A previous study indicated that asarinin
significantly inhibited the macroscopic score and cartilage
destruction of collagen-induced arthritis (17). However, little is known about the
effect of asarinin on RA synovial fibroblasts. The present study
aimed to determine the pharmacological profile of asarinin and its
potential effect on RA (18).
The present study used RA synovial fibroblasts to
examine the effect of asarinin and the possible roles of
Th17, Th1 and TLRs in the pathogenesis of
arthritis.
Materials and methods
Reagents
Recombinant Human tumor necrosis factor (TNF)-α and
IL-1β were obtained from PeproTech China. Fetal bovine serum and
high-glucose DMEM were purchased from HyClone; GE Healthcare Life
Sciences. Trypsin-EDTA solution and the Trypan Blue Staining Cell
Viability Assay kit were purchased from Beyotime Institute of
Biotechnology. The RNA PCR kit (EX TAQ R-PCR Version 2.1) was
obtained from Takara Biotechnology Co., Ltd., and primers and
probes were purchased from Sangon Biotech Co., Ltd.
TRIzol® reagent was purchased from Invitrogen. Asarinin
was purchased from Chengdu Must Bio-Technology Co., Ltd., and ELISA
kits for TNF-α (cat. no. 555212), IL-6 (cat. no. 555220) and IFN-γ
(cat. no. 555142) from BD Biosciences. The ELISA kit for IL-17A
(cat. no. D1700) was obtained from R&D Systems, Inc. Primary
antibodies for TNF-α (cat. no. TA808184), IL-17A (cat. no.
TA337063), IL-6 (cat. no. TA500067) and IFN-γ (cat. no. TA353236)
were from OriGene Technologies, Inc. Peptidoglycan (PGN) and
lipopolysaccharide (LPS) were purchased from Sigma-Aldrich.
Preparation of the asarinin-medicated
serum
Asarinin and olive oil were mixed in a ratio of 5:1
to form a suspension for the asarinin-medicated serum. The
asarinin-medicated serum was generated according to previous
studies (19,20). A total of 20 female Sprague-Dawley
(age range, 6-8 weeks; weight 200-250 g) were supplied by the
Experimental Animals Department of the Second Affiliated Hospital
of Harbin Medical University. Animals were housed in an
airconditioned room (temperature, 22±2˚C; humidity, 55±5%) with a
12 h light-dark cycle and free access to a standard diet with free
access to tap water. Sprague-Dawley (SD) rats were randomly divided
into an asarinin group and a blank serum group. Asarinin (30 mg/kg)
was administered orally to SD rats twice daily for 5 days. Rats in
the blank serum group received saline twice daily for 5 days. The
same dose was administered orally to the blank serum group and the
asarinin group. Then, 1 h after the last administration, rats were
anesthetized via intraperitoneal injection of pentobarbital and
blood samples (10 ml) were collected from the abdominal aorta for
subsequent experiments. The Ethics Committee for Experimental
Animals of Harbin Medical University reviewed and approved the
current study and all animals were treated according to the
guidelines of the animal ethics committee.
Rat plasma sample preparation for
high-performance liquid chromatography (HPLC) analysis
Rat medicated sera and blank sera were subjected to
HPLC analysis using a Thermo Fisher Ultimate 3000 UHPL (Thermo
Fisher Scientific, Inc.) (21,22).
Chromatographic conditions: The C18 column used was supplied by
Thermo Fisher Scientific, Inc. (4.6x150 mm; 5 µm). The mobile phase
consisted of acetonitrile-water (50:50) and the running time was 32
min. Additionally, the column temperature was 30˚C and the flow
rate 1 ml/min. The detection wavelengths were set at 287 nm with a
sample size of 10 µl. Asarinin serum (1 ml) was obtained and
dissolved by ultrasonography with methanol added to a final volume
of 5 ml. Sera were filtered through 0.45 microporous membranes for
analysis.
Patients, tissue specimens and ethics
statement
Synovial tissue specimens used for the culture of OA
synovial fibroblasts (OASFs; n=3) and RASFs (n=4) were obtained
from the knees of patients following joint replacement surgery,
which was a procedure performed in patients who had severe,
long-term disease and who had received numerous therapies over many
years. All patients fulfilled the American College of Rheumatology
2010 criteria for RA and the 1995 criteria for osteoarthritis (OA)
and provided their written consent to participate in the current
study (23,24). Synovial tissue specimens from 4
patients with RA [2 females (mean age, 67.9±4.8 years) and 2 males
(age, 70.1±3.5 years)] and 3 patients with OA [3 females (age,
68.2±5.3 years)]. Patients were recruited from September 2017 to
September 2018.The study protocols, consent forms and consent
procedure were approved by the Institutional Medical Ethics Review
Board of the Second Affiliated Hospital of Harbin Medical
University.
Synovial fibroblast (SF) isolation,
culture and treatment
Human SFs were isolated from synovial tissue
obtained at the time of knee replacement surgery from 4 patients
with RA and 3 patients with OA. Synoviocytes were trypsinized and
suspended in DMEM containing 10-20% fetal bovine serum, inoculated
in a culture flask and cultured in a cell incubator at 37˚C and 5%
CO2. The following day, the medium was changed, and the
unattached cells were discarded. The cells that remained adherent
were considered to be synovial cells. The medium was subsequently
changed once every 3-4 days and the culture solution was discarded
once the synovial cells reached 80-90% confluence in the flask.
Cells were then rinsed twice with PBS. Digestion was terminated
when the majority of cells changed shape from diamond to round and
were bright in appearance. Cells were generally passaged at a ratio
of 1:2 or 1:3. After 3 generations, it was revealed that 95% of
cells were fibroid synovial cells, which were used in the present
study. OASFs and RASFs were pretreated with IL-1β (2 ng/ml) and
TNF-α (10 ng/ml). OASFs (5x106 cells) and RASFs
(5x106 cells) per well were stimulated with medicated
serum of asarinin, toll-like receptor (TLR)2 ligand PGN (10 ng/ml)
and TLR4 ligand LPS (100 ng/ml).
Cell viability determination
OASFs (5x106 cells) and RASFs
(5x106 cells) were seeded into 96-well plates and
treated with medicated serum of asarinin at a series of
concentrations (5, 10, 15, 20, 25 or 30%) for 6 h or medicated
serum of asarinin (15%) for 6, 12, 18 or 24 h. Asarinin at a dosage
of 15% was selected as cell death did not affect subsequent
experiments. Cell suspensions in PBS (10 µl) were mixed with trypan
blue (40 µl) for 5 min at room temperature and the number of
stained (dead) and unstained (alive) cells were counted using a
hemocytometer (25,26).
ELISA
OASFs (5x106 cells) and RASFs
(5x106 cells) per well were stimulated with medicated
serum of asarinin (15%) for 6 h, after which the culture
supernatants were collected and stored at -20˚C for subsequent
ELISA. Levels of TNF-α, IFN-γ, IL-17A and IL-6 in cell culture
supernatants were measured using ELISA kits, in accordance with the
manufacturer's protocol.
RNA preparation and reverse
transcription-quantitative (RT-q) PCR
OASFs (5x106 cells) and RASFs
(5x106 cells) per well were stimulated with medicated
serum of asarinin (15%) for 6 h and the cells were harvested and
stored at -80˚C until analysis. Total mRNA was isolated from
synovial fibroblasts using TRIzol® and cDNA was
synthesized from 1 µg total RNA using oligo-dT primers and AMV
reverse transcriptase according to the manufacturer's protocol. The
conditions of reverse transcription were as follows: 30˚C for 10
min, 42˚C for 30 min, 99˚C for 5 min and 5˚C for 5 min. Samples
were stored at -20˚C until further use. The following primer
sequences were used for RT-qPCR. β-actin forward,
5'-AGCGGTTCCGATGCCCT-3' and reverse, 5'-AGAGGTCTTTACGGATGTCAACG-3';
IFN-γ forward, 5'-TGAACGCTACACACTGCATCTTGG and reverse,
5'-CGACTCCTTTTCCGCTTCCTG G-3'; IL-17A forward,
5'-AGTGAAGGCAGCAGCGATCAT-3' and reverse, 5'-CGCCAAGGGAGTTAAAG-3';
TNF-α forward, 5'-TCTCATCAGTTCTATGGCCC-3' and reverse,
5'-GGGAGTAGACAAGGTACAAC-3'; IL-6 forward,
5'-TCCAGTTGCCTTCTTGGGAC-3' and reverse,
5'-GTGTAATTAAGCCTCCGACTTG-3'; TLR2 forward,
5'-ACCAAGTGAAGGTACCTGTGGGGC-3' and reverse,
5'-GCACCAGAGCCTGGAGGTTCAC-3'; TLR4 forward,
5'-CCCCGACAACCTCCCCTTCTCA-3' and reverse,
5'-TCCAGAAAAGGCTCCCAGGGCT-3'.
RT-qPCR was performed at a final concentration of
0.15 µl primer and 0.25 µl of Ex Taq HS polymerase (Takara
Biotechnology Co., Ltd.) in standard PCR buffer. Transcripts were
quantified using EX TAQ R-PCR. PCR was performed with a reaction
mix comprising 0.5 µl forward and reverse primers, 2 µl cDNA and 1
µl TaqMan probe template to a total volume of 25 µl. PCR was
initiated for 2 min at 95˚C, continued with 40 cycles of 10 sec at
95˚C, 40 sec at 60˚C and final extension for 5 min at 72˚C. The
fold changes in the expression of each gene were calculated using
the 2-ΔΔCq method (27), with the housekeeping gene β-actin
mRNA as an internal control.
Western blot analysis
RASFs (5x106 cells) and OASFs
(5x106 cells) were pretreated with IL-1β (2 ng/ml) and
TNF-α (10 ng/ml), and incubated with medicated serum of asarinin at
37˚C for 6 h. The cells were harvested and stored at -80˚C until
analysis. Ice-cold lysis buffer (40 ml) was used to lyse cells.
Protein concentration was measured using the BCA method. Samples
(10 µl) were separated using SDS-PAGE in a 10% polyacrylamide gel
and transferred electronically to a PVDF membranes. The membranes
were then blocked with Tris-buffered saline containing 0.1%
Tween-20 and 5% nonfat milk at 37˚C for 1 h. Samples were then
incubated with the following primary antibodies at 4˚C overnight:
IFN-γ, IL-6, TNF-α and IL-17A (1:500). After three washes with PBST
(0.5% Tween 20 in PBS), membranes were incubated with horseradish
peroxidase-conjugated IgG (cat. no. PV6002; OriGene Technologies,
Inc.) secondary antibodies for 1 h at 37˚C. Samples were visualized
via chemiluminescence using an ECL Plus Detection kit (Beyotime
Institute of Biotechnology). The protein expression levels were
then determined by analyzing the signals captured on the PVDF
membranes using a ChampGel 5500 (Beijing Sage Creation Science Co,
Ltd.).
Statistical analysis
Samples were run in triplicate and the data were
presented as the mean ± standard deviation. Statistically
significant differences were analyzed via one-way analysis of
variance followed by and SNK test using SPSS software 13.0 for
Windows (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
HPLC of medicated serum of
asarinin
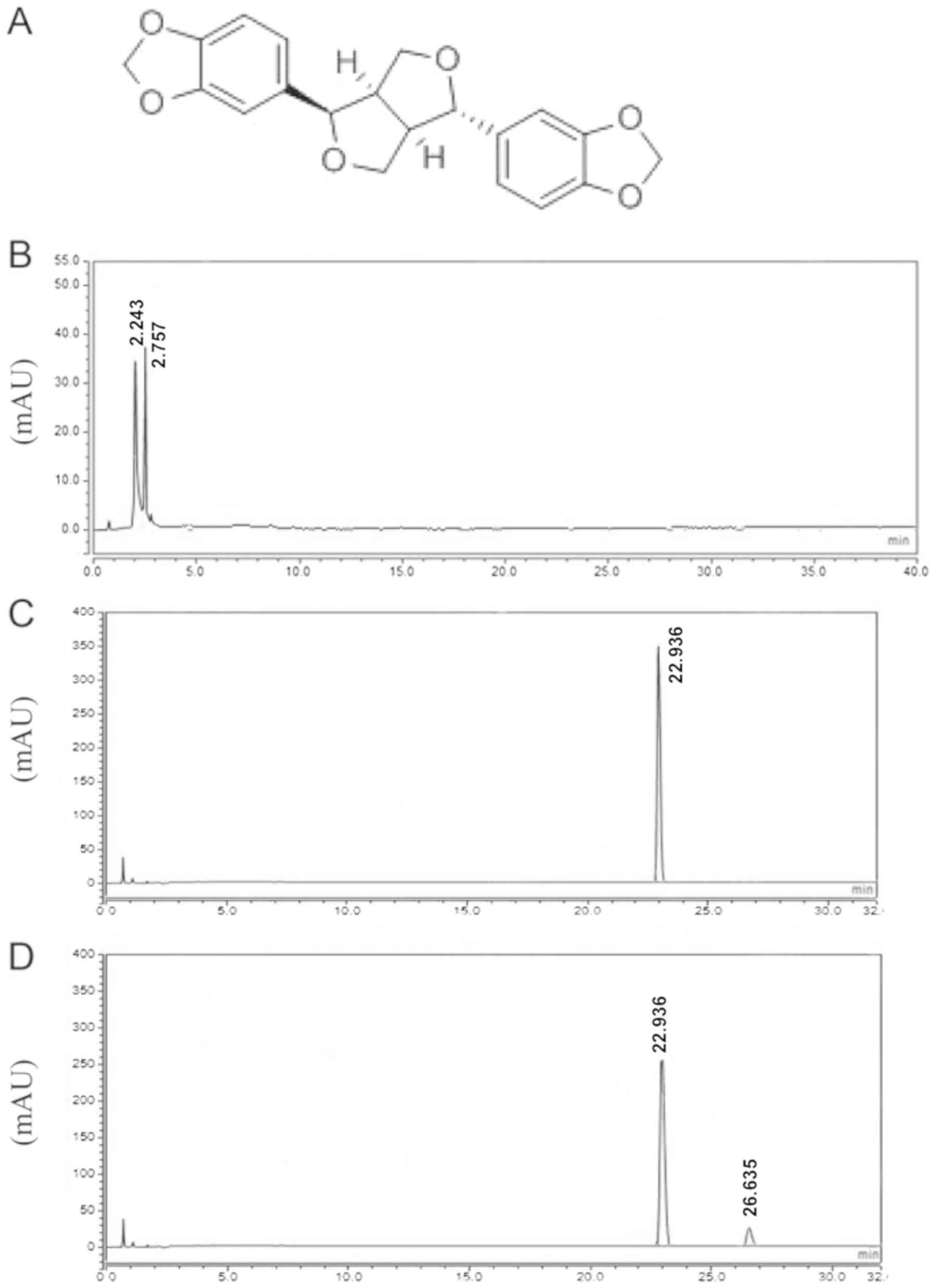
The chemical structures of asarinin are provided in
Fig. 1A. No peak for asarinin was
detected in the blank serum group (Fig.
1B) but was present for the control asarinin (Fig. 1C) and in rat medicated serum
(Fig. 1D). The extra peak (~26.635
min) may represent a secondary metabolite of asarinin (Fig. 1D).
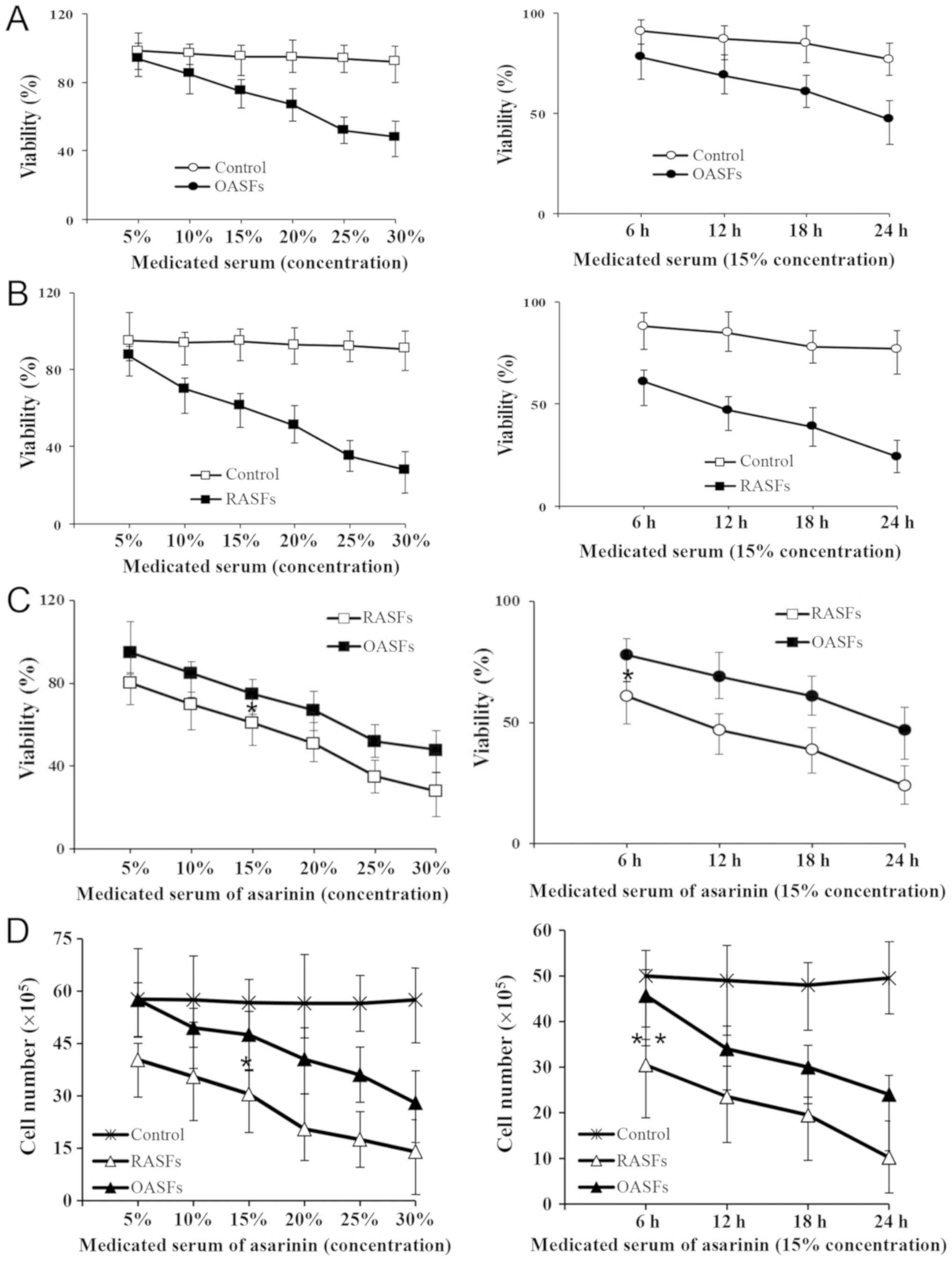
Medicated serum of asarinin inhibits
human RASF proliferation
To examine the effect of medicated serum of asarinin
on the proliferation of RASFs, trypan blue staining was performed
to detect the viability of RASFs. The results demonstrated that
medicated serum of asarinin decreased the viability of RASFs and
OASFs in a dose- and time-dependent manner (Fig. 2A-C). A significant decrease was
observed in the cell number of RASFs compared with that of OASFs
(Fig. 2D).
Regulation of inflammatory cytokines
on rheumatoid synoviocytes by medicated serum of asarinin
RA and OA samples were collected from the discarded
tissue of patients following knee joint replacement surgery. Third
generation rheumatoid synoviocytes and osteoarthritic synoviocytes
were used in the current study. The results revealed that third
generation rheumatoid synoviocytes and osteoarthritic synoviocytes
exhibited fibroblast-like synovial cell morphology (Fig. 3A). TNF-α, IFN-γ, IL-6 and IL-17A are
important inflammatory mediators in RA (2). RT-qPCR analysis for IL-17A, TNF-α,
IFN-γ and IL-6 expression revealed a decrease following asarinin
treatment compared with control and treated cells (Fig. 3B). Western blot analyses for TNF-α,
IFN-γ, IL-6 and IL-17A expression also revealed a decrease compared
with the controls (Fig. 3C). The
effect of asarinin on IL-17A, TNF-α, IFN-γ and IL-6 in RASFs was
also measured by ELISA, revealing similar results (Fig. 3D).
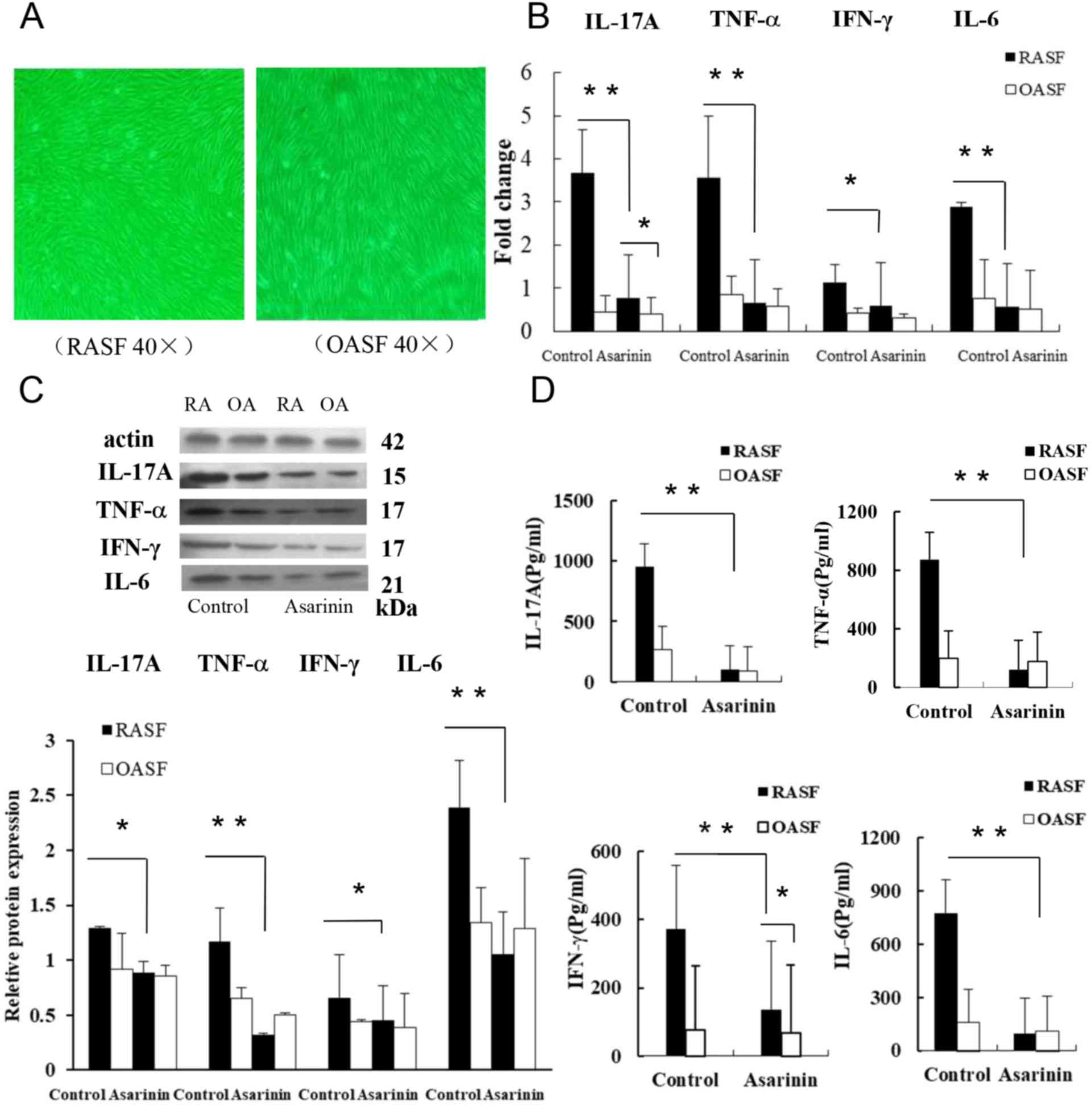 | Figure 3Effect of asarinin on cytokine
expression of rheumatoid synoviocytes. (A) Left: Fibroblast-like
synovial cell morphology of third generation osteoarthritic
synoviocytes. Right: third generation of the fibroblast-like
synovial cell morphology of rheumatoid synoviocytes (magnification,
x40). (B) Changes in the expression of IL-17A, TNF-α, IFN-γ and
IL-6 were determined by performing reverse
transcription-quantitative PCR. (C) Protein expression of cytokines
in rheumatoid synoviocytes. Upper panel: Expression of IL-17A,
TNF-α, IFN-γ and IL-6 was analyzed using western blotting with
β-actin as a loading control. Lower panel: Density histogram data
from three separate western blot analyses (mean ± standard
deviation), which represent the relative expression of IL-17A,
TNF-α, IFN-γ and IL-6. (D) Quantitative analyses of IL-17A, TNF-α,
IFN-γ and IL-6 using ELISA. *P<0.05 and
**P<0.01 vs. control cells. IL, interleukin;
TNF, tumor necrosis factor; IFN, interferon; RASF, rheumatoid
arthritis synovial fibroblast; OASF, osteoarthritis synovial
fibroblast. |
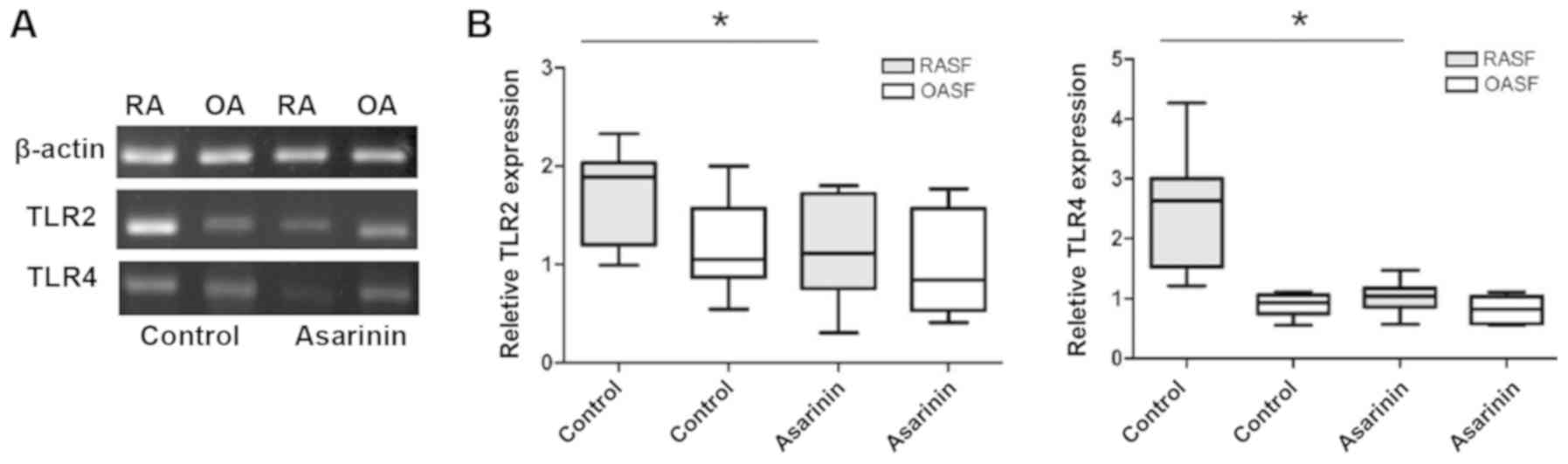
Inhibition of TLR2 and TLR4 on
rheumatoid synoviocytes by medicated serum of asarinin
RT-qPCR analysis for TLR2 and TLR4 expression
revealed a decrease following asarinin treatment (Fig. 4).
Discussion
Xixin is a commonly used herbal medicine in China
and other Asian countries. Asarinin is one of the main active
chemical components isolated from Xixin (13). Despite a previous study revealing
that asarinin significantly inhibited the macroscopic score and
cartilage destruction of collagen-induced arthritis (17), little is known about the effect of
asarinin on RA synovial fibroblasts. The present study therefore
aimed to assess the effects of asarinin on RASFs.
RA is an autoimmune disease characterized by chronic
inflammation that leads to joint destruction (28). Although the pathogenesis of RA,
mediated by T cells, is not completely clear, certain
Th1-type cytokines, including IFN-γ, TNF-α and IL-1,
Th2-type cytokines, including IL-4 and IL-10,
Th17 cytokines, including IL-17 and IL-22, and Treg
cells interact to activate synovial macrophages, fibroblasts and
osteoclasts to affect chronic inflammation and joint destruction
(29-32).
RASFs are leading cells in joint erosion and contribute actively to
inflammation.
Synovial cells are the most important cells in the
synovial layer. The primary target of RA immune activity is located
in the synovium. There are two types of cells in the synovial
layer: Type A (macrophage-like synovial cells) and type B
(fibroblast-like synoviocytes; FLSs) (33). Synovial cells, particularly FLSs of
the synovial layer, participate in the function of motor joints,
producing joint lubrication fluids and maintaining the compliance
and integrity of the synovial fluid, cartilage nutrition and the
synovial layer (33,34). Rheumatoid arthritis synovial
fibroblasts are leading cells in joint erosion and contribute
actively to inflammation, and enhance cell proliferation and
invasiveness under the action of various cytokines and growth
factors (28). During the disease
progression of RA, the fibroid synovial cells function as passive
responders and active invaders after transformation (35). Therefore, the present study used
third generation rheumatoid synoviocytes and osteoarthritic
synoviocytes. Since certain compounds of asarinin may change at
room temperature after being dissolved in solvents, the current
study used medicated serum of asarinin on cells. The results
demonstrated that medicated serum of asarinin decreased the
viability of RASFs and OASFs in a time-dependent manner within 24
h. Furthermore, when the concentration and duration of
drug-containing serum were significantly increased, the number of
RASFs was significantly reduced and the number of OASFs was
correspondingly reduced. However, the reduction of the latter was
less compared with that of the former. When OASFs and RASFs were
treated with blank serum, no significant decrease in cell viability
was observed. FLSs of RA exhibit tumor-like growth characteristics,
and the excessive abnormal proliferation and apoptosis of these
cells serve an important role in synovial inflammation and bone
destruction in RA (36). The effect
of asarinin on FLS may be associated with the immunosuppressive
effect of asarinin. However, whether the medicated serum of
asarinin causes RASF necrosis or apoptosis requires
elucidation.
To clarify the mechanism of action of asarinin on
RA, OASFs were used as control cells to detect the cytokines
associated with RA pathogenesis. The results demonstrated that
asarinin downregulated the expression of TNF-α, IFN-γ, IL-6 and
IL-17A in RA synovial cells. The expression of Th2
cytokines (IL-4 and IL-5) were too low to be detected (data not
shown). TNF-α, IFN-γ, IL-6 and IL-17A were also expressed in OA
synovial cells, but TNF-α, IFN-γ, IL-6 and IL-17A expression was
low and no significant differences were observed before and after
asarinin treatment. IFN-γ, IL-6, TNF-α and IL-17A are inflammatory
cytokines and IFN-γ and TNF-α are Th1-type cytokines
(6). IL-6 is also the main
inflammatory factor in RA joint inflammation, but its pathogenic
effect is different from that of IL-1 and TNF-α, which mainly
induces the production of immunoglobulins and the formation of
acute phase proteins (37-40).
Although Th2 cells secrete IL-6, IL-6 still serves a
role as an inflammatory cytokine. Additionally, Th17
cells secrete IL-17, which is generally considered an important
inflammatory mediator that modulates local infiltration and tissue
injury of inflammatory cells by inducing the expression of other
inflammatory cytokines, such as IL-6 and TNF-α, and chemokines,
such as monocyte chemotactic protein 1 and macrophage inflammatory
protein-2 (1,6). Mice deficient in IL-17 exhibit reduced
severity of arthritis, and mice with increased IL-17 levels exhibit
exacerbated levels of the disease (41,42).
Inflammatory cytokines are selectively recruited to the synovial
cavity in RA and various relevant cytokines in the synovial tissue
were detected in the present study (13,43).
IL-1 and TNF-α stimulate the proliferation of FLS to secrete
cytokines and prostaglandins, inducing FLS proliferation (44). FLS produce large numbers of cytokines
(including IL-1, TNF-α and IL-6) in spontaneous and stimulating
conditions (45-47).
The cytokine production profiles by synovial cells in patients with
RA and OA are similar regarding the types of cytokines produced
(44,48). However, the quantity of inflammatory
cytokines secreted in patients with RA is significantly higher
compared with that in patients with OA, indicating that synovial
cells may be excessively activated in patients with RA (49). Streptococcal cell wall induced
arthritis is markedly reduced in
TLR2-/- mice and TLR4 deficiency
results in impaired osteoclast formation as well as a reduction in
Th-17 inducing cytokines such as IL-1, IL-6 and IL-23 in the same
animals (50). TLR4 and TLR2 serve
crucial roles in the production of inflammatory cytokines and TLR
activation exacerbates RASF-mediated inflammatory Th1
and Th17 responses (11).
In conclusion, the inhibitory effect of asarinin drug serum on
human RASFs may be achieved by inhibiting Th1 and
Th17 cytokines via the suppression of TLR2 and TLR4.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fund of
Heilongjiang Science and Technical Office (grant no. QC2011C059),
the Harbin Science and Technology Bureau of Heilongjiang Province
(grant no. 2012RFQXS020), the Postdoctoral Foundation of China and
Heilongjiang Province (grant nos. 2013M531081, LBH-Z11004 and
LBHQ15138) and the Excellent Innovative Talents Support Program of
Heilongjiang University of Traditional Chinese Medicine (Excellent
Young Academic Leader; grant no. 2018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QD, YL and JL conceived and designed the current
study, and wrote and revised the manuscript. QD, MW and YZL
conducted the experiments and analyzed and interpreted the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were performed in accordance
with the guidelines for the Care and Use of Laboratory Animals and
were approved by the Institutional Animal Care and Use Committee of
Harbin Medical University. The study protocols, consent forms and
consent procedure were approved by the Institutional Medical Ethics
Review Board of the Second Affiliated Hospital of Harbin Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dai Q, Li Y, Zhang F, Yu H and Wang X:
Therapeutic effect of low-dose IL-18 combined with IL-10 on
collagen-induced arthritis by down-regulation of inflammatory and
Th1 responses and induction of Th2 responses. Rheumatol Int.
29:615–622. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guggino G, Giardina AR, Raimondo S,
Giardina G, Sireci G, Dieli F, Peralta M, Alessandro R, Triolo G
and Ciccia F: Targeting IL-6 signalling in early rheumatoid
arthritis is followed by Th1 and Th17 suppression and Th2
expansion. Clin Exp Rheumato. l32:77–81. 2014.PubMed/NCBI
|
|
3
|
Gonzalo-Gil E, Criado G, Santiago B, Dotor
J, Pablos JL and Galindo M: Transforming growth factor (TGF)-β
signalling is increased in rheumatoid synovium but TGF-β blockade
does not modify experimental arthritis. Clin Exp Immunol.
174:245–255. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thepmalee C, Panya A, Junking M,
Chieochansin T and Yenchitsomanus PT: Inhibition of IL-10 and TGF-β
receptors on dendritic cells enhances activation of effector
T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother.
14:1423–1431. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dai Q, Li J, Yun Y and Wang J: Toll-like
receptor 4-myeloid differentiation primary response gene 88 pathway
is involved in the shikonin treatment of CIA by regulating
Treg/Th17 expression. Evid Based Complement Alternat Med.
2018(2428546)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dai Q, Li Y, Yu H and Wang X: Suppression
of Th1 and Th17 responses and induction of Treg responses by
IL-18-expressing plasmid gene combined with IL-4 on
collagen-induced arthritis. Biomed Res Int.
2018(5164715)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Müller-Ladner U, Ospelt C, Gay S, Distler
O and Pap T: Cells of the synovium in rheumatoid arthritis.
Synovial fibroblasts. Arthritis Res Ther. 9(223)2007.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Schönfeld C, Pap T, Neumann E and
Müller-Ladner U: Fibroblasts as pathogenic cells in rheumatic
inflammation. Z Rheumatol. 74:33–38. 2015.(In German). PubMed/NCBI View Article : Google Scholar
|
|
9
|
Davis LS, Cush JJ, Schulze-Koops H and
Lipsky PE: Rheumatoid synovial CD4+ T cells exhibit a reduced
capacity to differentiate into IL-4-producing T-helper-2 effector
cells. Arthritis Res. 3:54–64. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Radstake TR, Roelofs MF, Jenniskens YM,
Oppers-Walgreen B, van Riel PL, Barrera P, Joosten LA and van den
Berg WB: Expression of toll-like receptors 2 and 4 in rheumatoid
synovial tissue and regulation by proinflammatory cytokines
interleukin-12 and interleukin-18 via interferon-gamma. Arthritis
Rheum. 50:3856–3865. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu F, Li Y, Zheng L, Shi L, Liu H, Zhang
X, Zhu H, Tang S, Zhu L, Xu L, et al: Toll-like receptors expressed
by synovial fibroblasts perpetuate Th1 and Th17 cell responses in
rheumatoid arthritis. PLoS One. 9(e100266)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Elshabrawy HA, Essani AE, Szekanecz Z, Fox
DA and Shahrara S: TLRs, future potential therapeutic targets for
RA. Autoimmun Rev. 16:103–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jing Y, Zhang YF, Shang MY, Liu GX, Li YL,
Wang X and Cai SQ: Chemical constituents from the roots and
rhizomes of asarum heterotropoides var. mandshuricum and the in
vitro anti-inflammatory activity. Molecules. 22(125)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gu J, Zhang L, Wang Z, Chen Y, Zhang G,
Zhang D, Wang X, Bai X, Li X and Lili Z: The effect of asarinin on
toll-like pathway in rats after cardiac allograft implantation.
Transplant Proc. 47:545–548. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park HJ, Lee KS, Zhao TT, Lee KE and Lee
MK: Effects of asarinin on dopamine biosynthesis and
6-hydroxydopamine-induced cytotoxicity in PC12 cells. Arch Pharm
Res. 40:631–639. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang LL, Lu SF, Zhang S, Nie HG, Guan ZZ
and Yang BF: Effect of asarinin on the acute heart transplantation
rejection and the expression of adhesion molecule. Zhongguo Zhong
Yao Za Zhi. 31:494–497. 2006.(In Chinese). PubMed/NCBI
|
|
17
|
Dai Q, Wang M, Li Y and Li J: Amelioration
of CIA by Asarinin is associated to a downregulation of TLR9/NF-κB
and regulation of Th1/Th2/Treg expression. Biol Pharm Bull.
42:1172–1178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xiong Y, Jing Y, Shang M, Li C, Ye J, Wang
X and Cai S: Anti-inflammatory and anti-nociceptive effects in mice
of water and ethanol extracts of roots and rhizomes of Asarum
heterotropoides var. mandshuricum. Zhongguo Zhong Yao Za Zhi.
34:2252–2257. 2009.(In Chinese). PubMed/NCBI
|
|
19
|
He Q, Liu Q, Chen Y, Meng J and Zou L:
Long-zhi decoction medicated serum promotes angiogenesis in human
umbilical vein endothelial cells based on autophagy. Evid Based
Complement Alternat Med. 2018(6857398)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu X, Lu H, Deng YL, Wan Q and Yie SM:
Effect of rat medicated serum containing Zuo Gui Wan and/or You Gui
Wan on the differentiation of stem cells derived from human first
trimester umbilical cord into oocyte-like cells in vitro. Evid
Based Complement Alternat Med. 2015(825805)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aaltonen K, Niemelä T, Sankari S and
Tulamo RM: Determination of the unsaturated disaccharides of
hyaluronic acid in equine synovial fluid by high-performance liquid
chromatography and fluorescence detection. Acta Vet Scand.
57(12)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Di Y, Zhao M, Nie Y, Wang F and Lv J: A
high-performance liquid chromatography: Chemiluminescence method
for potential determination of vardenafil in dietary supplement. J
Autom Methods Manag Chem. 2011(982186)2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 rheumatoid arthritis classification criteria:
An American college of rheumatology/european league against
rheumatism collaborative initiative. Arthritis Rheum. 62:2569–2581.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Klein K, Aeschlimann A, Jordan S, Gay R,
Gay S and Sprott H: ATP induced brain-derived neurotrophic factor
expression and release from osteoarthritis synovial fibroblasts is
mediated by purinergic receptor P2X4. PLoS One.
7(e36693)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He Y, Zhu Q, Chen M, Huang Q, Wang W, Li
Q, Huang Y and Di W: The changing 50% inhibitory concentration
(IC50) of cisplatin: A pilot study on the artifacts of the MTT
assay and the precise measurement of density-dependent
chemoresistance in ovarian cancer. Oncotarget. 25:70803–70821.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rai Y, Pathak R, Kumari N, Sah DK, Pandey
S, Kalra N, Soni R, Dwarakanath BS and Bhatt AN: Mitochondrial
biogenesis and metabolic hyperactivation limits the application of
MTT assay in the estimation of radiation induced growth inhibition.
Sci Rep. 8(1531)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Dleta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Neumann E, Lefèvre S, Zimmermann B, Gay S
and Müller-Ladner U: Rheumatoid arthritis progression mediated by
activated synovial fibroblasts. Trends Mol Med. 16:458–468.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dai Q, Fang J and Zhang FS: Dual role of
shikonin in early and late stages of collagen type Ⅱ arthritis. Mol
Biol Rep. 36:1597–1604. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kinoshita M, Kuranaga N, Matsumoto A, Ono
S, Shinomiya N, Hiraide H and Seki S: Multiple interleukin-18
injections promote both mouse Th1 and Th2 responses after sublethal
Escherichia coli infection. Clin Exp Immunol. 143:41–49.
2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: An effector CD4 T cell lineage with
regulatory T cell ties. Immunity. 24:677–688. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Veenbergen S, Smeets RL, Bennink MB, Arntz
OJ, Joosten LA, van den Berg WB and vandeLoo FA: The natural
soluble form of IL-18 receptor β exacerbates collagen-induced
arthritis via modulation of T-cell immune responses. Ann Rheum Dis.
69:276–283. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Croft AP, Naylor AJ, Marshall JL, Hardie
DL, Zimmermann B, Turner J, Desanti G, Adams H, Yemm AI,
Müller-Ladner U, et al: Rheumatoid synovial fibroblasts
differentiate into distinct subsets in the presence of cytokines
and cartilage. Arthritis Res Ther. 18(270)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bustamante MF, Garcia-Carbonell R,
Whisenant KD and Guma M: Fibroblast-like synoviocyte metabolism in
the pathogenesis of rheumatoid arthritis. Arthritis Res Ther.
19(110)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lefèvre S, Knedla A, Tennie C, Kampmann A,
Wunrau C, Dinser R, Korb A, Schnäker EM, Tarner IH, Robbins PD, et
al: Synovial fibroblasts spread rheumatoid arthritis to unaffected
joints. Nat Med. 15:1414–1420. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Alzabin S, Abraham SM, Taher TE,
Palfreeman A, Hull D, McNamee K, Jawad A, Pathan E, Kinderlerer A,
Taylor PC, et al: Incomplete response of inflammatory arthritis to
TNF-α blockade is associated with the Th17 pathway. Ann Rheum Dis.
71:1741–1748. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bevington SL, Cauchy P, Withers DR, Lane
PJ and Cockerill PN: T cell receptor and cytokine signaling can
function at different stages to establish and maintain
transcriptional memory and enable T helper cell differentiation.
Front Immunol. 8(204)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Macallan DC, Borghans JA and Asquith B:
Human T cell memory: A dynamic view. Vaccines (Basel).
4(E5)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yanes RE, Gustafson CE, Weyand CM and
Goronzy JJ: Lymphocyte generation and population homeostasis
throughout life. Semin Hemato. 54:33–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nakae S, Nambu A, Sudo K and Iwakura Y:
Suppression of immune induction of collagen-induced arthritis in
IL-17-deficient mice. J Immunol. 171:6173–6177. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lubberts E, van den Bersselaar L,
Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK,
Joosten LA and van den Berg WB: IL-17 promotes bone erosion in
murine collagen-induced arthritis through loss of the receptor
activator of NF-κB ligand/osteoprotegerin balance. J Immunol.
170:2655–2662. 2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mellado M, Martínez-Muñoz L, Cascio G,
Cascio G, Lucas P, Pablos JL and Rodríguez-Frade JM: T cell
migration in rheumatoid arthritis. Front Immunol.
27(384)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Millán O and Brunet M: Cytokine-based
immune monitoring. Clin Biochem. 49:338–346. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sun J, Yan P, Chen Y, Chen Y, Yang J, Xu
G, Mao H and Qiu Y: MicroRNA-26b inhibits cell proliferation and
cytokine secretion in human RASF cells via the Wnt/GSK-3β/β-catenin
pathway. Diagn Pathol. 10(72)2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shikhagaie MM, Germar K, Bal SM, Ros XR
and Spits H: Innate lymphoid cells in autoimmunity: Emerging
regulators in rheumatic diseases. Nat Rev Rheumatol. 13:164–173.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ahmad SF, Zoheir KM, Abdel-Hamied HE,
Ashour AE, Bakheet SA, Attia SM and Abd-Allah AR: Amelioration of
autoimmune arthritis by naringin through modulation of T regulatory
cells and Th1/Th2 cytokines. Cell Immunol. 287:112–120.
2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lubberts E: Th17 cytokines and arthritis.
Semin Immunopathol. 32:43–53. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
de Lange-Brokaar BJ, Ioan-Facsinay A, van
Osch GJ, Zuurmond AM, Schoones J, Toes RE, Huizinga TW and
Kloppenburg M: Synovial inflammation, immune cells and their
cytokines in osteoarthritis: A review. Osteoarthritis Cartilage.
20:1484–1499. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Firestein GS: Invasive fibroblast-like
synoviocytes in rheumatoid arthritis. Arthritis Rheum.
39:1781–1790. 1996.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Abdollahi-Roodsaz S, Joosten LA, Helsen
MM, Walgreen B, van Lent PL, van den Bersselaar LA, Koenders MI and
van den Berg WB: Shift from toll-like receptor 2 (TLR-2) toward
TLR-4 dependency in the erosive stage of chronic streptococcal cell
wall arthritis coincident with TLR-4-mediated interleukin-17
production. Arthritis Rheum. 58:3753–3764. 2008.PubMed/NCBI View Article : Google Scholar
|