Introduction
Insulin secretion occurs in a pulsatile manner into
the circulation of humans and animals, with fast pulses occurring
every 5-15 min (1,2). Insulin is secreted into the portal vein
and undergoes partial (40-80%) hepatic extraction, before being
diluted into the systemic insulin pool. Peripheral insulin
concentrations oscillate because of the pulsatility of insulin
secretions (3). Pulsatile insulin
concentrations are important to achieve optimal insulin action,
especially the metabolic effects of insulin, such as the
suppression of hepatic glucose output and overall insulin secretion
(4). However, insulin pulsatility is
disrupted in patients with type 2 diabetes mellitus (T2DM)
(5), first-degree relatives who lack
significant metabolic abnormalities (4), and patients with early type 1 diabetes
mellitus (T1DM) (6).
Pulsatile infusion and multiple daily subcutaneous
injections have been reported to have a greater hypoglycemic effect
than continuous delivery (7), and
also to prevent metabolic and microvascular complications in T1DM
(6,8-10).
Whereas the commonly used methods of therapeutic insulin
administration are not pulsatile in nature, several well-known
classes of pharmacological agents that are used to treat patients
with T2DM increase plasma insulin in a pulsatile manner in both
humans and animals, but without significantly modifying insulin
pulse frequency (11,12).
Using an artificial pancreas system to replicate the
physiological insulin pulse patterns could be one approach to
providing effective intravenous insulin infusion in patients with
DM for whom traditional therapy is insufficient (13,14).
Pulsatile intravenous insulin therapy (PIVIT) involves once-weekly
sessions during which pulsatile intravenous insulin and concurrent
oral glucose or quantified amounts of carbohydrate are
administered, according to a standard protocol and under medical
supervision (15).
Respiratory quotient (RQ) is the ratio of the volume
of carbon dioxide produced to the volume of oxygen consumed, and is
an excellent indicator of substrate oxidation (16). Oxygen consumption (VO2)
and carbon dioxide production (VCO2) occur during the
oxidation of carbohydrate, protein and fat, but the associated RQ
values differ according to the substrate being metabolized.; the RQ
values are 0.7 for fat, 0.8 for protein and 1.0 for glucose
(16,17). Low RQ has been frequently observed in
patients with DM (18), because in
diabetes, fat replaces glucose as the main source of energy, and
this increase in fat consumption, alongside that of protein, is a
contributing factor to hypermetabolism.
The purpose of the present study was to assess the
efficacy and safety of PIVIT for the improvement of RQ in Chinese
patients with DM.
Subjects and methods
Ethics statement
The present study was retrospectively registered in
the research registry (www.chictr.org.cn; registration no. ChiCTR1900027510;
November 13th 2019). The study protocol and informed written
consent forms were approved by the research ethics committee of
Peking University First Hospital [approval no. (2012)-Instrument
Registration No. (15)], and the study was carried out in accordance
with the principles of the Declaration of Helsinki. All patients
gave their informed written consent to participate in the
study.
Study population
The inclusion criteria were a diagnosis of DM that
was made according to the 1999 World Health Organization diagnostic
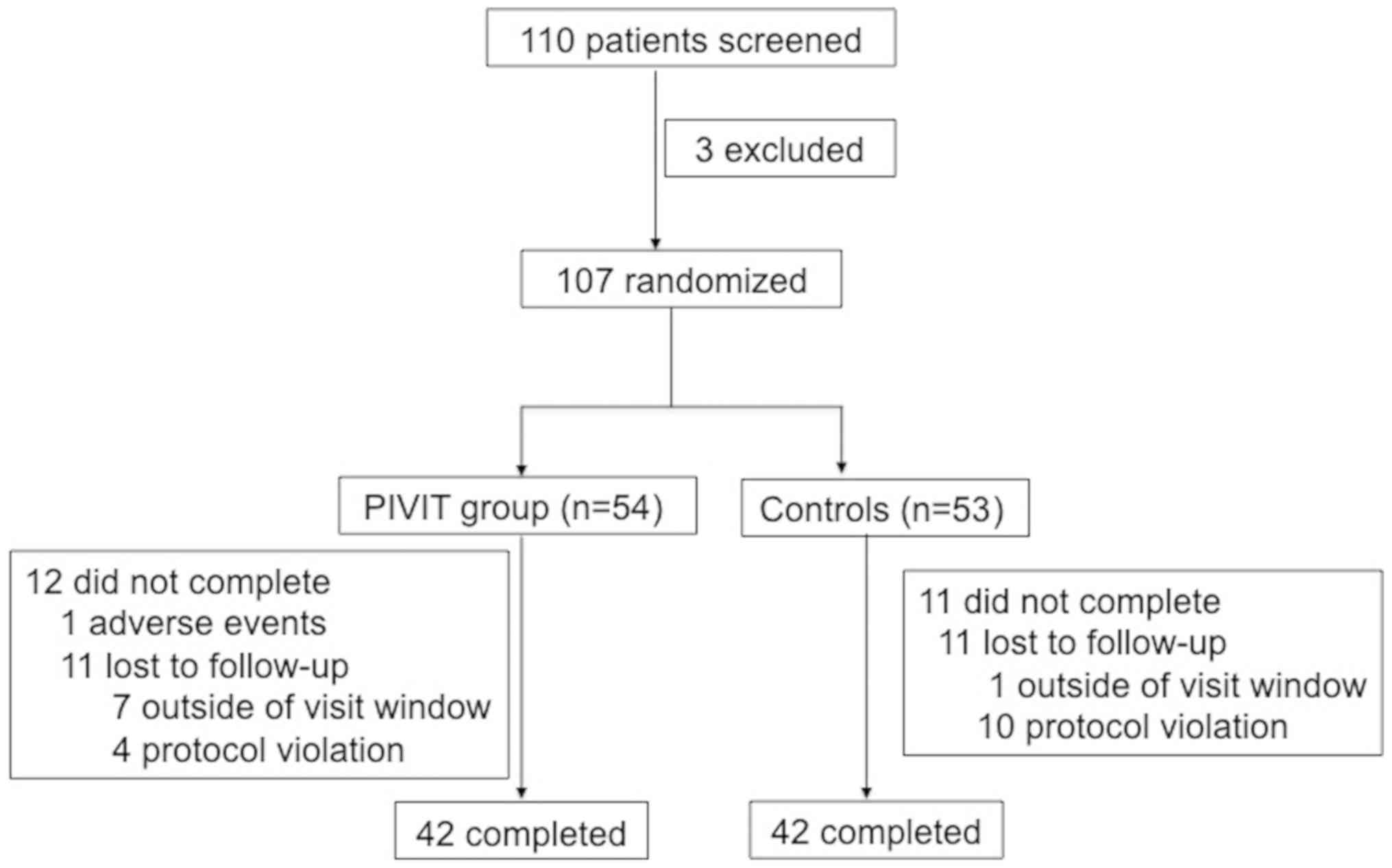
criteria, and a measured RQ of <0.8. A total of 110 patients
with DM were recruited from July 2012 to September 2013 from Peking
University First Hospital and Peking University Shougang Hospital,
and were enrolled in the present study (Fig. 1). Specific anti-diabetic therapies,
including the use of any oral anti-diabetic agents and insulin,
were recorded in a questionnaire.
The exclusion criteria were as follows: The presence
of hypokalemia; pregnancy or intended pregnancy; or a history of
unstable cardiac disease (including new or worsening signs or
symptoms of coronary heart disease within 3 months of study entry).
Additional exclusion criteria were any of the following within 6
months of study entry: Acute coronary syndrome, stroke or ischemic
event; coronary artery intervention or New York Heart Association
Class II-IV congestive heart failure; significant renal impairment
(creatinine clearance rate <50 ml/min); high (>2X the upper
limit of normal) plasma alanine aminotransferase or aspartate
aminotransferase activities; or high plasma triglycerides (>600
mg/dl).
The key withdrawal criteria were intolerance of the
study drugs, an inability to continue adherence to the protocol and
unwillingness to continue in the study.
Randomization
Randomization codes were generated using SAS version
9.10 (SAS Institute, Inc.) for the eligible patients. The
researchers randomly assigned cards and ensured that they were
sealed inside envelopes with sequence numbers that were the same as
the card numbers. Patients were randomly assigned (1:1) to each of
the two treatment groups.
Procedures
The total duration of the study was 12 weeks. A
total of 53 patients formed the control group. These patients
continued their standard anti-diabetic therapy, and had their RQ
evaluated every 4 weeks, while 54 patients formed the treatment
group (the PIVIT group) and underwent weekly PIVIT in addition to
their standard anti-diabetic therapy.
RQ, the ratio of the mean CO2 produced to
the mean O2 consumed/minute, was automatically
calculated using a VacuMed® (VacuMed YD 17590;
Vacumetrics, Inc.). Patients fasted for at least 8 h overnight,
after which indirect calorimetry was performed the following
morning. The humidity of the testing room was maintained at 45-60%
and the temperature at 24-26˚C.
The initial PIVIT treatment was carried out three
times a day, at 30 min intervals for 2 days, and was then continued
once weekly, three times a day. The PIVIT protocol was performed as
previously described (6). RQ was
evaluated before and after every treatment, and the fasting blood
of all patients was collected for the measurement of glucose and
lipid levels. Glucose and lipid levels were measured using Beckman
Coulter AU chemistry analyzers obtained from commercially available
kits (Beckman Coulter, Inc.) according to manufacturer's protocol.
A standard questionnaire was completed, which included general
information, family history, medical history and details of the
medication being used. During the evaluation, height, body mass,
blood pressure and pulse were measured. Blood and urine were
collected at the baseline stage and after 12 weeks to determine the
levels of glucose and glycated hemoglobin (HbA1c), as well as the
lipid profile and urinary protein excretion.
The procedures undertaken for the PIVIT group were
as follows: i) Capillary blood glucose was measured using an
Accu-CHEK® Performa Blood Glucose Meter (Roche Diagnostics). If the
blood glucose was <8 mmol/l, oral glucose (50% glucose
injection, 20 ml, 10 g; Otsuka Pharmaceutical Co., Ltd.) was
administered to increase the blood concentration. ii) An ambulatory
infusion pump (10 pulses/h; Microdose®; Bionica Corp.) was used. A
volume of 1 ml insulin (Novolin R®; 3 ml/300 IU; Novo Nordisk A/S)
and 9 ml normal saline (0.9% saline; Baxter International, Inc.)
were mixed in a 10-ml syringe, and the syringe was loaded into the
syringe driver on the left of the pump. iii) The infusion pump was
then connected to a hand or forearm vein. RQ was evaluated before
connecting the pump to a vein. iv) The capillary blood glucose of
patients was then monitored every 30 min by nurses. If blood
glucose levels were <8 mmol/l, patients were administered oral
50% glucose therapy and their blood glucose concentration was
measured every 15 min until it reached 8 mmol/l. v) Resting
VO2 and VCO2 were measured after the
treatment. vi) The patients left the hospital when they had
completed the treatment and their blood glucose was >8
mmol/l.
Assessment of efficacy
After 12 weeks of treatment, an RQ ≥0.9 was
considered to represent clinical efficacy (6), while an RQ <0.9 was considered to
represent inefficacy.
Safety assessment
Adverse events (AEs), serious AEs and adverse
reactions were recorded during the study period. A serious AE was
defined as an event that resulted in death; required inpatient
hospitalization or the prolongation of existing hospitalization;
resulted in persistent or significant disability/incapacity.
Statistical analysis
All statistical analyses were performed using the
SPSS statistical package (version 13.0; SPSS, Inc.). Quantitative
data are presented as the mean ± SD, or the median with minimum,
maximum and quartile values. Qualitative data are described as a
number and percentage. Paired t-tests were used to analyze changes
in clinical parameters from the baseline values. Comparisons of
clinical and laboratory parameters between the PIVIT and control
groups were performed using paired t-tests or the Wilcoxon rank sum
test, as appropriate. The paired-sample t-test was used to compare
data before and after treatment. Comparisons of the safety of each
therapy were performed using the χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline clinical characteristics
The baseline clinical characteristics of the
subjects are presented in Table I.
Compared with treatment group, the control group had a higher
proportion of alcohol drinkers and had higher blood glucose
concentrations. The age, sex, body mass index, baseline RQ,
diabetic complications, blood pressure, respiration rate, heart
rate, HbA1c, and anti-diabetic treatments did not differ between
the two groups.
 | Table IBaseline characteristics of the
treatment and control groups. |
Table I
Baseline characteristics of the
treatment and control groups.
| Characteristics | T, n=54 | C, n=53 | P-value |
|---|
| Sex, male/female | 32/23 | 37/16 |
0.208a |
| Age, years | 58.52±11.40 | 56.14±10.49 |
0.264b |
| Height, m | 1.69±0.09 | 1.69±0.09 |
0.996b |
| Body mass, kg | 72.6±11.79 | 71.95±12.51 |
0.770b |
| BMI,
kg/m2 | 25.39±3.02 | 25.17±3.45 |
0.718b |
| VO2,
l/min | 0.24±0.10 | 0.24±0.11 |
0.704b |
| VCO2,
l/min | 0.17±0.07 | 0.17±0.08 |
0.907b |
| RQ | 0.70±0.11 | 0.73±0.06 |
0.210b |
| Smoker, yes/no | 19/36 | 22/31 |
0.456a |
| Alcohol drinker,
yes/no | 6/49 | 14/39 |
0.038a |
| Diabetic
complications, yes/no | 24/31 | 25/28 |
0.712a |
| Oral hypoglycemic
agents (%) | 24 (44.4) | 21 (39.6) |
0.613a |
| Insulin treatment
(%) | 13 (24.1) | 7 (13.2) |
0.478a |
| Combination of OAD
and insulin (%) | 17 (31.5) | 25 (47.2) |
0.203a |
| SBP, mmHg | 133.69±17.37 | 131.08±13.71 |
0.658b |
| DBP, mmHg | 77.26±9.67 | 80.28±8.42 |
0.265b |
| RR,
breaths/minute | 17.85±1.15 | 17.66±1.53 |
0.913b |
| HR,
beats/minute | 74.78±6.98 | 73.75±8.60 |
0.376b |
| Glucose levels,
mmol/l | 9.79±3.18 | 8.28±3.22 |
0.017b |
| HbA1c, % | 8.02±1.62 | 7.84±1.92 |
0.596b |
Assessment of PIVIT treatment
efficacy
Patients in the treatment group showed a significant
increase in the RQ after the weekly PIVIT treatment. At the end of
the 12-week study period (13 visits), the mean RQ of the PIVIT
group had increased from 0.70 to 0.90, but there was no change in
the control group (Table II). The
RQ before treatment at each visit was also compared between the
treatment and control groups. The RQ at the first visit did not
differ between the two groups (P=0.273), but during the 6, 10 and
13th visits, the RQ before the daily treatment was significantly
higher in the treatment than in the control group (P<0.05)
(Table II).
 | Table IIRQ values before and after weekly
treatment using PIVIT, compared with control subjects. |
Table II
RQ values before and after weekly
treatment using PIVIT, compared with control subjects.
| | T | C | | |
|---|
| Visit no. | n | RQ before
treatment | RQ after
treatment | n | RQa |
P-valueb,c |
P-valueb,d |
|---|
| 1 | 53 | 0.70±0.11 | 0.86±0.08 | 53 | 0.73±0.06 | 0.273 | <0.0001 |
| 6 | 50 | 0.79±0.11 | 0.89±0.08 | 52 | 0.74±0.07 | 0.016 | <0.0001 |
| 10 | 47 | 0.81±0.11 | 0.89±0.09 | 52 | 0.75±0.09 | 0.007 | <0.0001 |
| 13 | 47 | 0.81±0.09 | 0.90±0.08 | 52 | 0.72±0.06 | <0.0001 | <0.0001 |
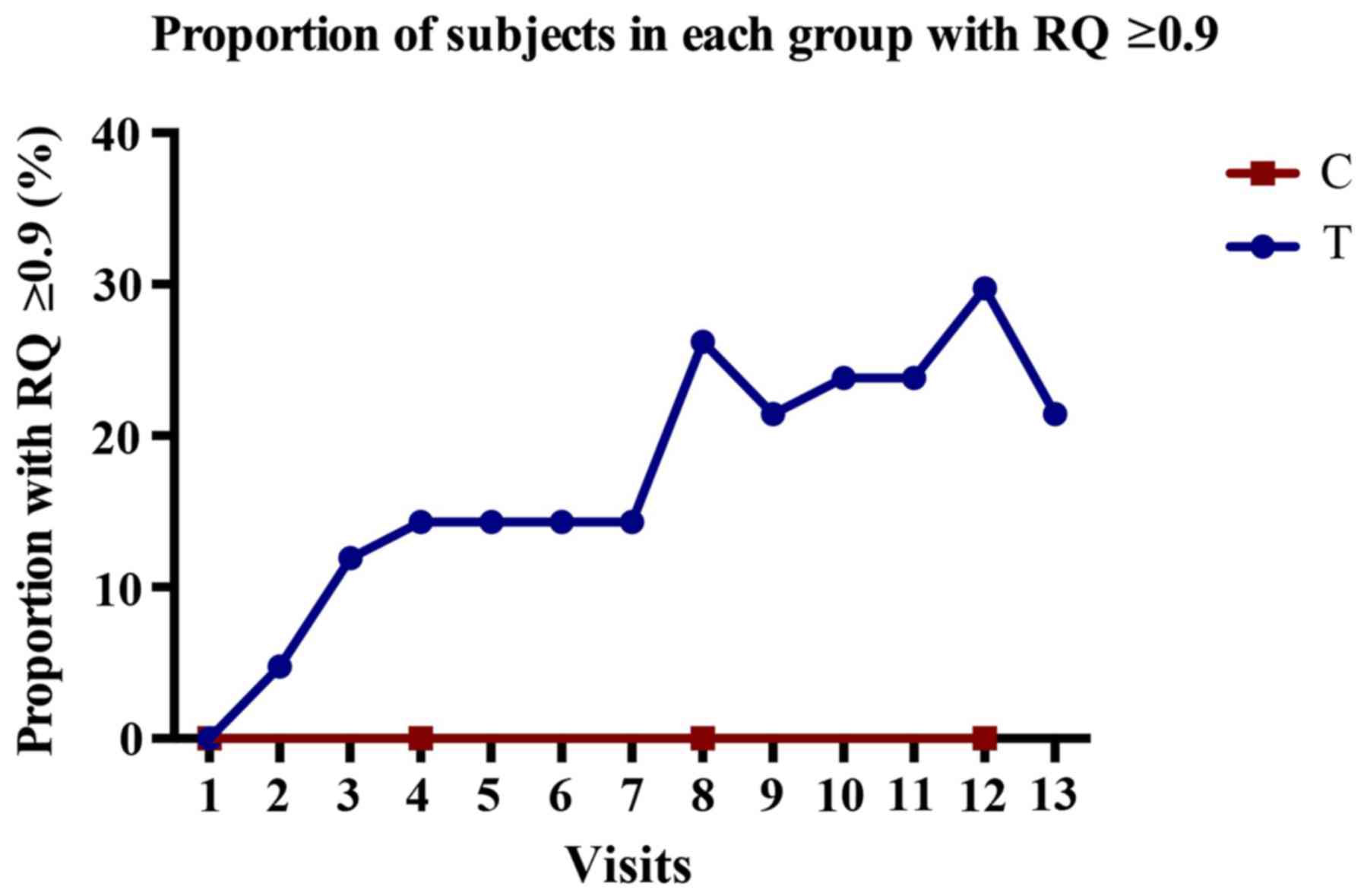
After PIVIT treatment, 26 patients in the treatment
group had an RQ ≥0.9, and the clinical efficacy rate was therefore
61.9% (26/42). By contrast, none of the patients in the control
group had an RQ ≥0.9. The proportion of subjects with RQ ≥0.9
before treatment at each visit is shown in Fig. 2. In the treatment group, the
percentage of subjects with RQ ≥0.9 gradually increased over the
study period, peaking at the 12th visit (29.7%), but slightly
decreasing at the 13th visit (to 21.4%). However, there was no
improvement in the control group.
Assessment of clinical characteristics
after PIVIT treatment
The clinical measurement values before and after
PIVIT were measured in the treatment group. HbA1c was improved
during the study period in subjects in both groups in the present
study (PIVIT: 8.02±1.62 vs. 7.76±1.23 mmol/l, P=0.024; and Control:
7.84±1.92 vs. 7.36±1.30 mmol/l, P=0.003). However, there were no
significant differences between the two groups after the study
period (P=0.507; data not shown). Systolic blood pressure improved
(P<0.05), and blood glucose also showed a non-significant
improvement (Table III).
 | Table IIIClinical characteristics of the
subjects before and after treatment. |
Table III
Clinical characteristics of the
subjects before and after treatment.
| | PIVIT group | Control group |
|---|
|
Characteristics | Baseline | Week 12 |
P-valuea | Baseline | Week 12 |
P-valuea |
|---|
| SBP, mmHg | 133.69±17.37 | 128.00±16.77 | 0.028 | 131.08±13.71 | 131.65±14.94 | 0.82 |
| DBP, mmHg | 77.26±9.67 | 75.55±9.54 | 0.302 | 80.28±8.42 | 79.12±8.66 | 0.28 |
| RR,
breaths/minute | 17.85±1.15 | 17.81±1.66 | 0.926 | 17.66±1.53 | 17.88±1.49 | 0.85 |
| HR,
beats/minute | 74.78±6.98 | 74.15±7.62 | 0.327 | 73.75±8.6 | 75.58±9.87 | 0.104 |
| Glucose levels,
mmol/l | 9.79±3.18 | 9.37±3.69 | 0.324 | 8.28±3.22 | 8.18±2.83 | 0.32 |
| HbA1c, % | 8.02±1.62 | 7.76±1.23 | 0.024 | 7.84±1.92 | 7.36±1.3 | 0.035 |
Assessment of the safety of PIVIT
The percentage of subjects reporting AEs was 31.5%
(17/54) in the treatment group and 9.43% (5/53) in the control
group (P=0.0053). No serious AEs occurred in either group. The AEs
in the treatment group included 12 cases of gastrointestinal
reactions, comprising mainly nausea, vomiting, diarrhea and
anorexia, all of which were closely related to taking 10-50 g of
glucose to maintain a blood glucose of >8 mmol/l during the
treatment, and were relieved by eating or symptomatic treatment.
The other adverse reactions are listed in Table IV. Hypoglycemia and gastrointestinal
reactions were the most common. Hypoglycemia in the treatment group
mostly occurred after treatment or during the evening of the
treatment day, whereas hypoglycemia in the control group did not
tend to occur at a specific time, and could be rapidly alleviated
by eating or consuming oral glucose.
 | Table IVAEs in the two groups. |
Table IV
AEs in the two groups.
| AEs | T, n=54 | C, n=53 |
P-valuea |
|---|
| Total AEs (%) | 17/54 (31.5) | 5/53 (9.43) | 0.0053 |
|
Gastrointestinal
reactions | 12 | 0 | - |
|
Hypoglycemia | 7 | 2 | - |
|
Fracture | 0 | 1 | - |
|
Fever | 1 | 0 | - |
|
Fatigue | 2 | 1 | - |
|
Chest
pain | 0 | 1 | - |
|
Orthostatic
hypotension | 1 | 0 | - |
Discussion
The purpose of the present clinical study was to
evaluate the safety and efficacy of PIVIT for the improvement of RQ
in patients with T1DM and T2DM, using a repeatable standardized
metabolic assay. A total of 110 subjects were enrolled in the
present study, of whom 54 were enrolled in the treatment group and
53 in the control group. There were no significant differences in
the demographics of the subjects and the baseline data for the two
groups were broadly comparable.
RQ is the ratio of CO2 produced to
O2 consumed and is helpful in determining the metabolic
substrate that is being predominantly catabolized to produce energy
(17,19). In resting, post-absorptive
non-diabetic individuals, energy requirements are met primarily by
fat oxidation, which is reflected in an RQ
(VCO2/VO2) of 0.7-0.8. After glucose
administration, CO2 production (and consequently RQ)
increases (to 0.9-1.0), indicating that glucose has become the
primary source of energy (20). In
theory, RQ may have a value between 0.7 (reflecting 100% fat
oxidation) and 1.0 (reflecting 100% carbohydrate oxidation).
However, healthy adults typically have an RQ of ~0.90 and patients
with DM patients typically have an RQ of 0.7-0.8 (21,22). An
impairment in glycogen storage and insulin resistance results in
earlier preferential use of fats and proteins as fuel sources. This
leads to greater free fatty acid and ketone body production, and a
reduction in the RQ. Furthermore, the capacity of patients with DM
to oxidize and store exogenous carbohydrate is markedly impaired
(23,24), possibly as a result of hyperglycemia
(25). Instead, patients with
diabetes typically utilize lipid oxidation as their main source of
energy (21,26).
In the present study, the changes in RQ during
treatment and over the study period were measured and used to
evaluate the clinical efficacy of PIVIT. The treatment group
underwent PIVIT over 12 weeks, while the subjects in the control
group only underwent periodic RQ measurements. RQ gradually
increased to >0.8 in the treatment group during the early part
of the study period, and the mean RQ was >0.9 after the 7, 8, 9,
12 and 13th visits. Therefore, PIVIT had a beneficial effect on RQ,
especially in the early phase of treatment, although there was a
slight deterioration at the final visit. This yielded an efficacy
rate was significantly higher than that achieved by the regimen
used by the control group, indicating that each PIVIT had a
beneficial effect on the RQ.
In previous studies investigating the effects of a
combination of weekly outpatient PIVIT and daily subcutaneous
insulin treatment, blood pressure was found to improve
significantly (27), the progression
of diabetic nephropathy slowed (8,10) and
diabetic autonomic neuropathy was reversed (9). In the present study, a decrease in
systolic blood pressure after treatment was observed. However, the
exact mechanism of action behind how PIVIT reduces blood pressure
remains to be determined. Because an increase in vascular smooth
muscle tone is a hallmark of the hypertensive state in diabetic
patients (28), and insulin may play
a role in the regulation of vascular smooth muscle (29), it is possible that this therapy
partially normalizes vascular reactivity, lowering blood pressure.
HbA1c was improved during the study period in subjects in both
groups in the present study. This effect of PIVIT may be explained
by an increase in the suppression of gluconeogenesis and a
consequent reduction in hepatic glucose production (30). However, it may also be that
physicians adjusted the therapeutic regimens more frequently, or
the patients improved their self-monitoring. An effect of PIVIT on
blood glucose, the frequency of complications and blood pressure
may require longer periods of study to become apparent.
The incidence of AEs was significantly higher in the
PIVIT group than in the control group. The AEs were mild and did
not affect the course of treatment. Gastrointestinal reactions
included nausea, vomiting, diarrhea, acid reflux, anorexia and a
burning sensation in the stomach. All of these occurred in subjects
in the treatment group when they were treated with oral high-dose
glucose to maintain their blood glucose ≥8 mmol/l during PIVIT.
Oral high-dose glucose commonly causes gastrointestinal reactions.
Previous studies using the 75 g oral glucose tolerance test
(glucose concentration, 25-28.5%) show a 51.2-66.0% incidence of
gastrointestinal reactions (31,32).
Future studies will aim to explore ways to replace high-dose
glucose, such as with juice or food.
Hypoglycemia generally occurred after treatment or
during the evening of the day of treatment in the PIVIT group. By
contrast, hypoglycemia in the control group did not occur at a
fixed time and could be rapidly alleviated by eating or consuming
oral glucose. Subjects in the treatment group also experienced
postural hypotension and cramps in their lower limbs, which were
relieved after a break from PIVIT treatment. Hypoglycemia may have
been more prevalent in the PIVIT group because the subjects
received an intravenous injection of insulin, meaning that they
needed to ingest glucose rapidly to avoid this AE. In the early
stages of the present study, the lack of timely glucose
supplementation at the end of each intervention may have led to
this hypoglycemia. The incidence of hypoglycemia was significantly
lower in the middle and late phases of the study.
In the present study, PIVIT significantly improved
the RQ and blood pressure of diabetic patients. Although the blood
glucose concentrations of patients were also slightly improved,
this improvement was also demonstrated in the control group,
suggesting that the improvement in RQ may be independent of blood
glucose concentrations. Previous studies suggest that long-term use
of PIVIT delays the progression of diabetic complications (6,9),
especially involving the microvascular circulation (8,10). For
future studies, the effects of a longer duration of PIVIT treatment
on the incidence of the chronic complications of diabetes should be
evaluated.
The current study did have limitations. There were
no inclusion criteria regarding diabetes duration, medication or
blood glucose concentration. Furthermore, no previous studies
appeared to identify the effects of diabetes or the degree of
glycemic control. However, during the screening period, not all
diabetic patients demonstrated a decrease in their RQ values, which
may have been because of the duration of their diabetes or better
blood glucose control. Patients with a longer disease course and
poor blood glucose control should be selected for further studies.
Furthermore, patients had to visit a clinic once a week, which may
have led to sub-optimal compliance. Additional studies are required
to explore effective ways of improving patient compliance.
In conclusion, PIVIT is an effective and tolerated
means of improving RQ in Chinese T1DM and T2DM patients.
Acknowledgements
Not applicable.
Funding
Beijing Fuji Sunshine Technology Co., Ltd. supported
this work.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NG interpreted the patient data and was a major
contributor to the writing of the manuscript. AD, LG and WW
interpreted the patient data. CX and PH performed the intravenous
insulin treatment and RQ measurements. SZ and CY analyzed the
patient data. JZ and XG designed the treatment. XG interpreted the
data and reviewed the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol and informed consent forms were
approved by the Research Ethics Committee of Peking University
First Hospital [approval no. (2012)-Instrument Registration No.
(15)], and the study was carried out in accordance with the
principles of the Declaration of Helsinki. All patients gave their
informed written consent to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Satin LS, Butler PC, Ha J and Sherman AS:
Pulsatile insulin secretion, impaired glucose tolerance and type 2
diabetes. Mol Aspects Med. 42:61–77. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lang DA, Matthews DR, Burnett M, Ward GM
and Turner RC: Pulsatile, synchronous basal insulin and glucagon
secretion in man. Diabetes. 31:22–26. 1982.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pørksen N: The in vivo regulation of
pulsatile insulin secretion. Diabetologia. 45:3–20. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pørksen N, Nyholm B, Veldhuis JD, Butler
PC and Schmitz O: In humans at least 75% of insulin secretion
arises from punctuated insulin secretory bursts. Am J Physiol.
273:E908–E914. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Scheen AJ, Sturis J, Polonsky KS and Van
Cauter E: Alterations in the ultradian oscillations of insulin
secretion and plasma glucose in aging. Diabetologia. 39:564–572.
1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aoki TT, Benbarka MM, Okimura MC,
Arcangeli MA, Walter RM Jr, Wilson LD, Truong MP, Barber AR and
Kumagai LF: Long-term intermittent intravenous insulin therapy and
type 1 diabetes mellitus. Lancet. 342:515–518. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matthews DR, Naylor BA, Jones RG, Ward GM
and Turner RC: Pulsatile insulin has greater hypoglycemic effect
than continuous delivery. Diabetes. 32:617–621. 1983.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Weinrauch LA, Burger AJ, Aepfelbacher F,
Lee AT, Gleason RE and D'Elia JA: A pilot study to test the effect
of pulsatile insulin infusion on cardiovascular mechanisms that
might contribute to attenuation of renal compromise in type 1
diabetes mellitus patients with proteinuria. Metabolism.
56:1453–1457. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weinrauch LA, Bayliss G, Gleason RE, Lee
AT and D'Elia JA: Utilization of an abbreviated diabetes impact
management scale to assess change in subjective disability during a
trial of pulsatile insulin delivery demonstrates benefit.
Metabolism. 58:488–491. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dailey GE, Boden GH, Creech RH, Johnson
DG, Gleason RE, Kennedy FP, Weinrauch LA, Weir M and D'Elia J88A:
Effects of pulsatile intravenous insulin therapy on the progression
of diabetic nephropathy. Metabolism. 49:1491–1495. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pørksen NK, Munn SR, Steers JL, Schmitz O,
Veldhuis JD and Butler PC: Mechanisms of sulfonylurea's stimulation
of insulin secretion in vivo: Selective amplification of insulin
secretory burst mass. Diabetes. 45:1792–1797. 1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Juhl CB, Hollingdal M, Pørksen N, Prange
A, Lönnqvist F and Schmitz O: Influence of rosiglitazone treatment
on beta-cell function in type 2 diabetes: Evidence of an increased
ability of glucose to entrain high-frequency insulin pulsatility. J
Clin Endocrinol Metab. 88:3794–3800. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Juhl CB, Gjedsted J, Nielsen MF and
Schmitz O: Increased action of pulsatile compared to non-pulsatile
insulin delivery during a meal-like glucose exposure simulated by
computerized infusion in healthy humans. Metabolism. 61:1177–1181.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Skjaervold NK, Ostling D, Hjelme DR,
Spigset O, Lyng O and Aadahl P: Blood glucose control using a novel
continuous blood glucose monitor and repetitive intravenous insulin
boluses: Exploiting natural insulin pulsatility as a principle for
a future artificial pancreas. Int J Endocrinol.
2013(245152)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aoki TT, Grecu EO, Arcangeli MA, Benbarka
MM, Prescott P and Ahn JH: Chronic intermittent intravenous insulin
therapy: A new frontier in diabetes therapy. Diabetes Technol Ther.
3:111–123. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livesey G and Elia M: Estimation of energy
expenditure, net carbohydrate utilization, and net fat oxidation
and synthesis by indirect calorimetry: Evaluation of errors with
special reference to the detailed composition of fuels. Am J Clin
Nutr. 47:608–628. 1988.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Haugen HA, Chan LN and Li F: Indirect
calorimetry: A practical guide for clinicians. Nutr Clin Pract.
22:377–388. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao J, Wang ZY, Li J, Yu HW and Meng QH:
Influence of diabetes mellitus on energy metabolism in patients
with alcoholic liver cirrhosis. Eur J Gastroenterol Hepatol.
32:110–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Graf S, Karsegard VL, Viatte V, Heidegger
CP, Fleury Y, Pichard C and Genton L: Evaluation of three indirect
calorimetry devices in mechanically ventilated patients: Which
device compares best with the Deltatrac II®? A
prospective observational study. Clin Nutr. 34:60–65.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Körner A, Eklöf AC, Celsi G and Aperia A:
Increased renal metabolism in diabetes. Mechanism and functional
implications. Diabetes. 43:629–633. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gonzalez C, Fagour C, Maury E, Cherifi B,
Salandini S, Pierreisnard A, Masquefa-Giraud P, Gin H and Rigalleau
V: Early changes in respiratory quotient and resting energy
expenditure predict later weight changes in patients treated for
poorly controlled type 2 diabetes. Diabetes Metab. 40:299–304.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Perseghin G, Lattuada G, De Cobelli F,
Esposito A, Costantino F, Canu T, Scifo P, De Taddeo F, Maffi P,
Secchi A, et al: Reduced intrahepatic fat content is associated
with increased whole-body lipid oxidation in patients with type 1
diabetes. Diabetologia. 48:2615–2621. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Meyer HU, Curchod B, Maeder E, Pahud P,
Jequier E and Felber JP: Modifications of glucose storage and
oxidation in nonobese diabetics, measured by continuous indirect
calorimetry. Diabetes. 29:752–756. 1980.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Thalacker-Mercer AE, Ingram KH, Guo F,
Ilkayeva O, Newgard CB and Garvey WT: BMI, RQ, diabetes, and sex
affect the relationships between amino acids and clamp measures of
insulin action in humans. Diabetes. 63:791–800. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Buscemi S, Donatelli M, Grosso G, Vasto S,
Galvano F, Costa F, Rosafio G and Verga S: Resting energy
expenditure in type 2 diabetic patients and the effect of insulin
bolus. Diabetes Res Clin Pract. 106:605–610. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mirbolooki MR, Taylor GE, Knutzen VK,
Scharp DW, Willcourt R and Lakey JR: Pulsatile intravenous insulin
therapy: The best practice to reverse diabetes complications? Med
Hypotheses. 73:363–369. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aoki TT, Grecu EO, Prendergast JJ,
Arcangeli MA and Meisenheimer R: Effect of chronic intermittent
intravenous insulin therapy on antihypertensive medication
requirements in IDDM subjects with hypertension and nephropathy.
Diabetes Care. 18:1260–1265. 1995.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nieves-Cintrón M, Syed AU, Nystoriak MA
and Navedo MF: Regulation of voltage-gated potassium channels in
vascular smooth muscle during hypertension and metabolic disorders.
Microcirculation. 25:2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pietruczuk P, Jain A, Simo-Cheyou ER,
Anand-Srivastava MB and Srivastava AK: Protein kinase B/AKT
mediates insulin-like growth factor 1-induced phosphorylation and
nuclear export of histone deacetylase 5 via NADPH oxidase 4
activation in vascular smooth muscle cells. J Cell Physiol.
234:17337–17350. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bratusch-Marrain PR, Komjati M and
Waldhäusl WK: Efficacy of pulsatile versus continuous insulin
administration on hepatic glucose production and glucose
utilization in type I diabetic humans. Diabetes. 35:922–926.
1986.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang H, Wang X, Meng Z, Zhang R, Zhou Y
and Zhao Y: Analysis of influence factors of adverse reactions to
oral glucose tolerance test in healthy people. J Nur. 17:23–25.
2010.
|
|
32
|
Fan L, Wang G, Tao X, Hong B, Li H and
Huang Y: Study on the influencing factors of adverse reactions in
1110 oral glucose tolerance tests. Chin Nur J. 29:387–390.
1994.
|