Introduction
The mevalonate kinase (MVK) gene has 10 coding exons
and one non-coding exon at chromosome 12q24. The MVK protein is
encoded by two transcripts of MVK and is expressed in various
tissues, such as the human skin (1).
MVK catalyzes the phosphorylation of mevalonic acid into
5-phosphomevalonate, and functions downstream of
3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA) in the
mevalonate pathway. The mevalonate pathway provides crucial
molecules to cells, and may be an important metabolic pathway in
human skin (2). Cholesterol, which
is essential in the function of skin barrier, derives from
mevalonate pathway (3) and MVK plays
an important role in the synthesis of isoprenoid and cholesterol.
Furthermore, the mevalonate pathway was reported to regulate the
gene expression of keratin (4). MVK
mutations were previously identified in disseminated superficial
actinic porokeratosis by exome sequencing (5). Moreover, MVK plays an important role in
regulating calcium-induced keratinocyte differentiation and
protecting keratinocytes from apoptosis induced by type A
ultraviolet radiation in cultured primary keratinocytes (5).
Keratin 1 (KRT1) and involucrin (IVL) are markers of
the differentiation of keratinocytes in the spinous layer and
granular layer of skin, respectively. Apoptosis and dysregulation
of keratinocyte differentiation was reported to be associated with
the pathogenesis of porokeratosis (6). Prenylation (farnesylation and
geranylgeranylation), also known as lipidation, facilitates the
attachment of molecules to cell membranes; hydrophobic molecules
are added to a chemical compound or protein during prenylation. The
inhibition of prenylation by HMG-CoA reductase inhibitors
fluvastatin or compactin was shown to decrease the
bradykinin-stimulated generation of inositol 1,4,5-triphosphate in
human keratinocytes (7). However,
the role of MVK in the differentiation, apoptosis and prenylation
of human keratinocytes requires further investigation.
Short-chain isoprenoids farnesyl pyrophosphate (FPP)
and geranylgeranyl pyrophosphate (GGPP) are the intermediate
products of the mevalonate pathway; these attach to small G
proteins covalently, and serve as molecular switches in many
biochemical pathways (8). Small G
proteins, such as lamin A, HRAS, KRAS, NRAS, Rho E, Rho B, Rho A,
Ras-related C3 botulinum toxin substrate 1 (RAC1) and cell division
control protein 42 homolog (CDC42), independently hydrolyze
guanosine triphosphate (GTP) in cytosol. However, it is unclear
whether FPP and GGPP could rescue the downregulation of MVK
expression and whether MVK expression affects the processing of
small G proteins.
Thus, the present study aimed to investigate the
role of interference and overexpression of MVK in the expression of
keratin 1 and involucrin, apoptosis, protein prenylation, and the
processing of small G proteins.
Materials and methods
Cell culture
HaCaT cells (cat. no. C01-BH; Shanghai
Novobioscience, Co., Ltd.) were cultured in Dulbecco's modified
Eagle's medium (DMEM) with GlutaMax™, supplemented with 10% fetal
bovine serum (FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). Cells were seeded at
1x105 cells/ml, and the medium was changed every other
day up to 90% confluency.
Construction of recombinant plasmids
and lentiviral packaging
According to gene information of MVK (NM_000431.3),
three-pairs of oligos encoding the target sequence of MVK (Table I) and CDS domain sequences were
designed, synthesized and inserted into interference vector
PDS019_PL-shRNA and overexpression vector
PDS159_pL6.3-CMV-GFPa1-IRES-MCS (Shanghai Novobioscience, Co.,
Ltd.), respectively. Interference lentiviral vectors
(CL926-1-pL-shRNA-HOMO-MVK-348, CL926-2-PL-shRNA-HOMO-MVK-520, and
CL926-3-pL-shRNA-HOMO-MVK-1283) and overexpression lentiviral
vector (CL927-pL6.3-CMV-GFPa1-IRES-homo-MVK) were produced. The
negative control for interference was non-targeting sequence
(5'-GAAACGATATGGGCTGAATAC-3'), which was inserted into the vector
pL-shRNA (Novobio). The negative control for overexpression was an
empty vector pL6.3-CMV-GFPa1-IRES-MCS (Novobio). Correct sequences
were confirmed by Sanger sequencing. ViraPower™ Lentiviral
Packaging Mix (9 µg; Invitrogen; Thermo Fisher Scientific, Inc.)
and the recombinant lentiviral vectors (3 µg; Invitrogen; Thermo
Fisher Scientific, Inc.) were added into Opti-Minimum Essential
Medium (MEM; Gibco; Thermo Fisher Scientific, Inc.) and mixed,
respectively. Lipofectamine® 2000 (36 µl; Invitrogen; Thermo Fisher
Scientific, Inc.) was mixed with Opti-MEM (1.5 ml), and incubated
at room temperature for 5 min. The plasmid solution and diluted
Lipofectamine® 2000 were subsequently mixed, and incubated at room
temperature for 5 min. The mixture was added into a culture dish
with 293T cells (3x106) and cells were cultured for 48
h. Cell supernatant was collected, centrifuged at 1,500 x g for 10
min at room temperature and filtered. The virus solution was then
condensed by centrifuging at 50,000 x g for 2 h at 4˚C, and
re-suspended in DMEM. Viruses carrying MVK interference (LV542-1,
LV542-2 and LV542-3, respectively) and overexpression vectors were
derived, respectively. Human keratinocytes HaCaT were transfected
with the viruses (multiplicity of transfection, 10) for 48 h, and
reverse transcription-quantitative PCR was utilized to detect
efficiency of interference and overexpression, respectively.
 | Table IOligo sequences for interference. |
Table I
Oligo sequences for interference.
| Oligo | Sequences (5' to
3') |
|---|
| MVK-348-F |
CACCGCTTACCCAACATTGGTATCACGAATGATACCAATGTTGGGTAAGC |
| MVK-348-R |
AAAAGCTTACCCAACATTGGTATCATTCGTGATACCAATGTTGGGTAAGC |
| MVK-520-F |
CACCGCTGGCCTTTCTTTACTTATACGAATATAAGTAAAGAAAGGCCAGC |
| MVK-520-R |
AAAAGCTGGCCTTTCTTTACTTATATTCGTATAAGTAAAGAAAGGCCAGC |
| MVK-1283-F |
CACCGGCTTTGACTGCTTGGAAACCCGAAGGTTTCCAAGCAGTCAAAGCC |
| MVK-1283-R |
AAAAGGCTTTGACTGCTTGGAAACCTTCGGGTTTCCAAGCAGTCAAAGCC |
| Non-targeting
sequence |
GAAACGATATGGGCTGAATAC |
RT-qPCR
The expression of MVK, KRT1 and IVL in HaCat cells
was detected by RT-qPCR. HaCat human keratinocytes were divided
into 5 groups: i) negative control; ii) MVK interference; iii) MVK
interference+FPP (1 µM); iv) MVK interference+GGPP (1 µM); v) MVK
overexpression. Prior to detecting the expression of KRT1 and IVL
by RT-qPCR, HaCat cells were treated with CaCl2 (0.5 mM)
for 48 h. Total RNA was extracted from cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. SuperScript™ First-Strand Synthesis System
(cat. no. 11904018; Invitrogen; Thermo Fisher Scientific, Inc.) was
used for RT by applying the following temperature protocol: 65˚C
for 5 min; 42˚C for 60 min and 70˚C for 15 min. Each reaction
mixture contained 0.5 µl random primers (0.2 µg/µl) and 1 µl
SuperScript III reverse transcriptase (200 U/µl; Invitrogen; Thermo
Fisher Scientific, Inc.). The specific primers used are listed in
Table II. PCR was performed using a
SYBR RT-qPCR mix kit (cat. no. 4309155; Invitrogen; Thermo Fisher
Scientific, Inc.). The PCR conditions were as follows: Initial
denaturation at 95˚C for 2 min, followed by 40 cycles of
denaturation at 95˚C for 10 sec, annealing at 60˚C for 30 sec and
elongation at 70˚C for 45 sec. PCR was performed using a CFX96
Touch™ Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.).
Gene expression was determined and normalized to β-actin. The
2-ΔΔCq method was utilized to determine the relative
gene expression (9).
 | Table IIPrimers for reverse
transcription-quantitative PCR. |
Table II
Primers for reverse
transcription-quantitative PCR.
| Primers | Sequences (5' to
3') |
|---|
| MVK-F |
TTCCCAGGAGCCATGTTGTC |
| MVK-R |
CTTGCTCTGAGGTGGGTGTT |
| KRT1-F |
TGGACAACAACCGCAGTCTC |
| KRT1-R |
CTCTGGTACAAGGACTCGGC |
| IVL-F |
TATTTCGGGTCCGCTAGGTG |
| IVL-R |
TGAGGTTGGGATTGGGGTCA |
| Actin-F |
AGGGAAATCGTGCGTGAC |
| Actin-R |
CGCTCATTGCCGATAGTG |
Western blot analysis
Protein expression of MVK, keratin 1, involucrin,
lamin A, HRAS, KRAS, NRAS, Rho E, Rho B, Rho A, RAC1 and CDC42 in
HaCat cells was detected by western blot analysis. Prior to
detecting the expression of KRT1 and IVL by western blotting, HaCat
cells were treated with CaCl2 (0.5 mM) for 48 h. HaCat
cells were lysed in lysis buffer (cat. no. P0013; Beyotime
Institute of Biotechnology) at 4˚C with inhibitors of phosphatase
and protease (cat. no. P1045; Beyotime Institute of Biotechnology).
The lysis mixture was centrifuged at 4˚C at 10,000 x g for 10 min,
and the supernatant containing cellular proteins was utilized in
following experiments. The protein concentration was measured using
Bicinchoninic Acid Assay kit (Beyotime Institute of Biotechnology).
Proteins were separated by SDS-PAGE on a 10% gel (40 µg per lane;
at 120 V). The separated proteins were then transferred to
polyvinylidene fluoride membranes (at 100 V for 120 min). The
membranes were blocked at room temperature with 5% non-fat milk for
1 h, and incubated with primary antibodies against MVK (1:1,000;
cat. no. 12228-1-AP; ProteinTech Group, Inc.), cytokeratin 1
(1:1,000; cat. no. 16848-1-AP; ProteinTech Group, Inc.), involucrin
(1:1,000; cat. no. 55328-1-AP; ProteinTech Group, Inc.), lamin A
(1:1,000; cat. no. 10298-1-AP; ProteinTech Group, Inc.), HRAS
(1:1,000; cat. no. 18295-1-AP; ProteinTech Group, Inc.), Rho E
(1:1,000; cat. no. D223036; Sangon Biotech Co., Ltd.), Rho B
(1:1,000; cat. no. 14326-1-AP; ProteinTech Group, Inc.), Rho A
(1:1,000; cat. no. 10749-1-AP; ProteinTech Group, Inc.), RAC1
(1:1,000; cat. no. 24072-1-AP; ProteinTech Group, Inc.) and CDC42
(1:1,000; cat. no. 10155-1-AP; ProteinTech Group, Inc.) at 4˚C
overnight, respectively. Membranes were washed with Tris-buffered
saline containing Tween-20 and incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:2,000;
cat. no. D110058; Sangon Biotech Co., Ltd.) at room temperature for
1 h. Membranes were incubated in enhanced chemiluminescence
solution (cat. no. P0018A; Beyotime Institute of Biotechnology).
Images were captured on film (cat. no. FF057, Beyotime Institute of
Biotechnology) in the dark. Experiments were repeated three times.
Blot images were quantified in greyscale using the Image-Pro Plus
software (version 6.0; Media Cybernetics, Inc.).
Detection of cell apoptosis by flow
cytometry
HaCat cells were radiated with UVA for 5 min, and
incubated overnight. The apoptosis of HaCat cells was detected with
the Annexin V-PE/7-Aminoactinomycin D (7-AAD) apoptosis assay kit
(cat. no. 40310ES20; Shanghai Yeasen Biotechnology Co., Ltd.) using
flow cytometry. The cells from each group were washed twice with
PBS and incubated with trypsin at 37˚C for 1 min. Following
digestion, the cell suspension was centrifuged at 400 x g at room
temperature for 5 min. The cell pellet was resuspended with PBS and
the centrifugation and resuspension steps were repeated twice. The
cells were blocked with 2% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) for 30 min at room temperature. 7-AAD (5 µl) and
Annexin V-PE (1 µl) reagents were added to 100 µl cell suspension,
followed by incubation at room temperature for 10 min. Cells were
centrifuged at 400 x g at room temperature for 5 min and
re-suspended with PBS three times. Cell fluorescence was detected
by flow cytometry. Data were acquired on an LSRII flow cytometer
(BD Biosciences) and analyzed with FlowJo software (version
10.2.64; FlowJo LLC). Annexin V+ cells were calculated
in each experimental group. Apoptotic rate was defined as
percentage of Annexin V+ cells in each group.
Experiments were performed for a total of three times.
Detection of protein prenylation by
flow cytometry
The protein prenylation levels of HaCat cells were
detected using flow cytometry. The cells from each group were
washed with PBS twice and incubated with trypsin at 37˚C for 1 min.
Following digestion, the cell suspension was centrifuged at 400 x g
at room temperature for 5 min. The cell pellet was resuspended with
PBS and the centrifugation and resuspension steps were repeated
twice. The cells were blocked at room temperature with 2% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) overnight.
Anti-farnesylation antibody (1:1,000; cat. no. ab199481; Abcam) was
added to 100 µl cell suspension, followed by incubation at room
temperature for 2 h. Cells were then incubated with iFluor goat
anti-rabbit IgG (H+L) (1:2,000; cat. no. 16803; AAT Bioquest Inc.)
at room temperature for 1 h. Cells were centrifuged at 400 x g at
room temperature for 5 min and resuspended with PBS three times.
Cell fluorescence was then detected by flow cytometry. Data were
acquired on an LSRII flow cytometer (BD Biosciences,) and analyzed
with FlowJo software. Cells with positive staining were calculated
in each experimental group. Experiments were performed for a total
of three times.
Statistical analysis
Statistical data were analyzed by GraphPad Prism
version 5.0 software (GraphPad Software, Inc.). The results are
presented as mean ± standard deviation (SD). Differences among ≥3
groups were compared by one-way analysis of variance followed by
the Bonferroni post-hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
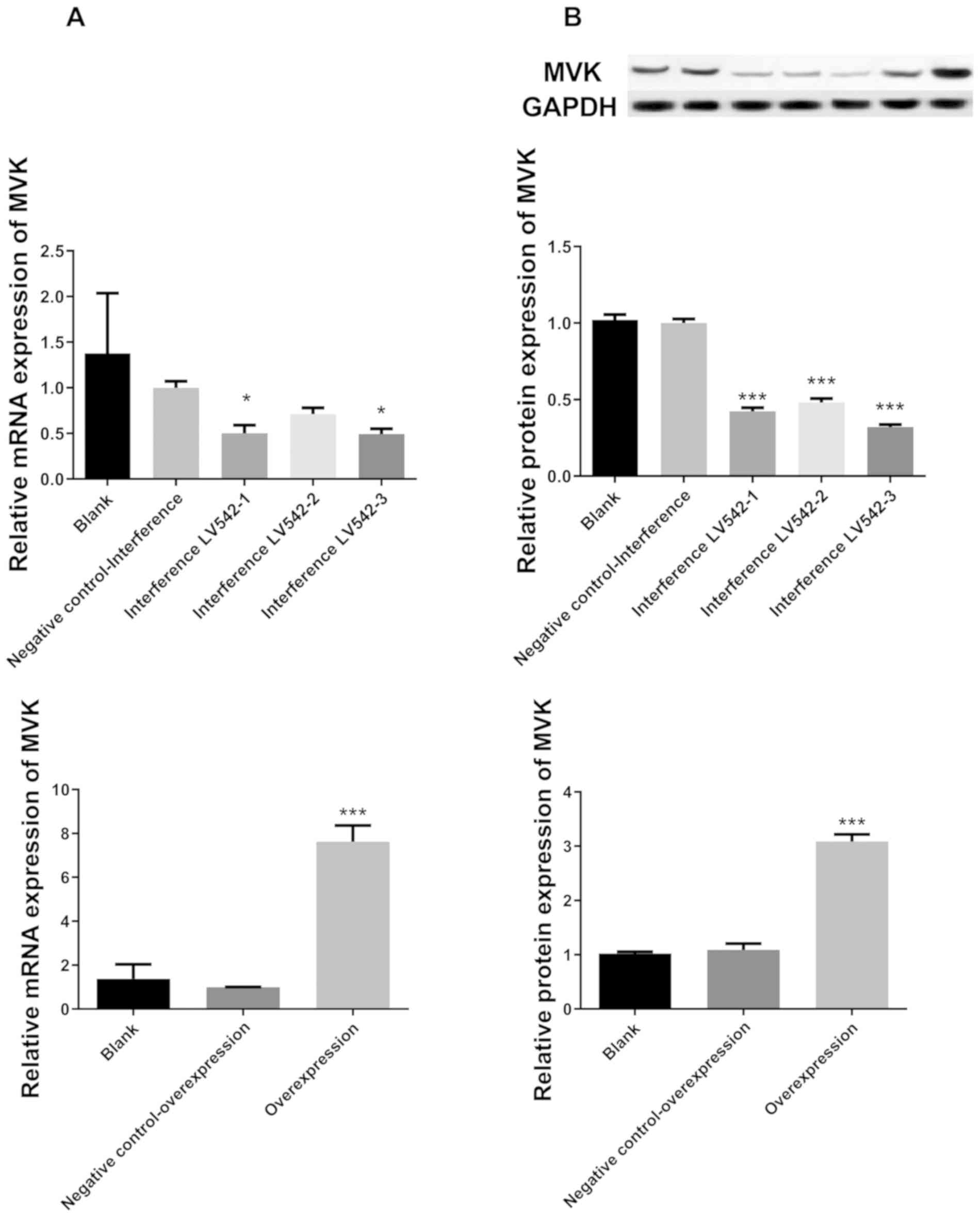
Confirmation of interference and
overexpression of MVK in HaCat cells
Following transfection, the mRNA expression of MVK
in HaCat cells was significantly decreased in LV542-1 (MVK-348) and
LV542-3 (MVK-1283) groups compared with the negative control group
(P<0.05; Fig. 1). In addition,
the mRNA expression of MVK in HaCat cells was markedly increased in
the overexpression group compared with the negative control group
(P<0.001; Fig. 1A). Although
there was no statistical significance in the relative mRNA
expression of MVK between LV542-1 and LV542-3 groups, the mean
value was lower in LV542-3 group (0.5, LV542-1 group; and 0.49,
LV542-3 group). Thus, LV542-3 was used for interference of MVK in
subsequent experiments. Moreover, the protein expression of MVK was
markedly decreased in the interference groups, and increased in the
overexpression group, compared with the negative control groups
(P<0.001 for all groups; Fig.
1B). These research findings indicate that the interference and
overexpression in HaCat cells were successfully induced.
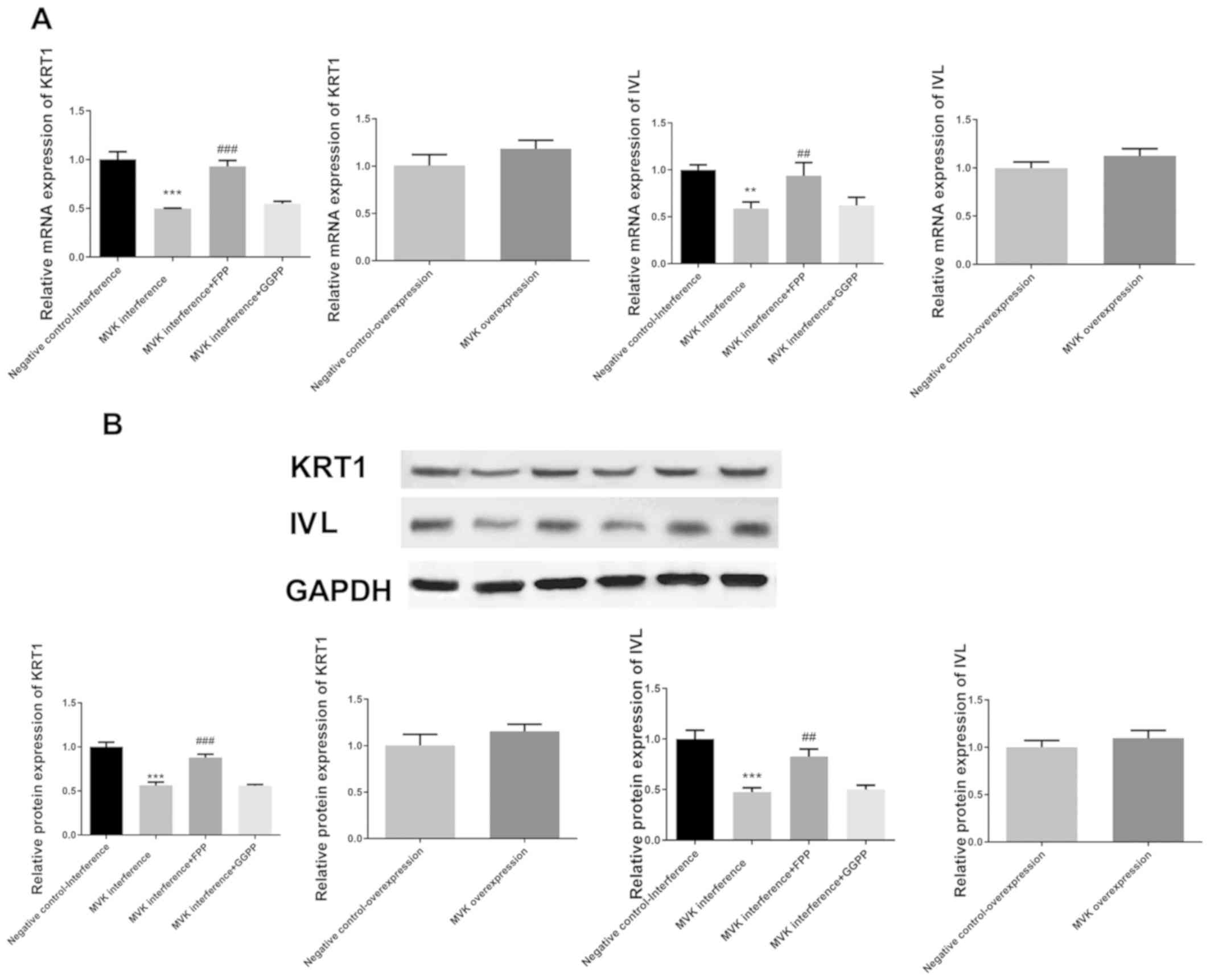
Decreased expression of keratin 1 and
involucrin following MVK interference was markedly attenuated by
FPP
Compared with the negative control group, the mRNA
and protein expression of keratin 1 and involucrin were
significantly decreased following interference of MVK expression
(P<0.01; Fig. 2). The decrease in
the expression of keratin 1 and involucrin following MVK
interference was markedly attenuated by FPP (P<0.01; Fig. 2). GGPP was not shown to restore the
expression of keratin 1 and involucrin following MVK interference.
On the other hand, the overexpression of MVK did not alter the
expression of keratin 1 and involucrin.
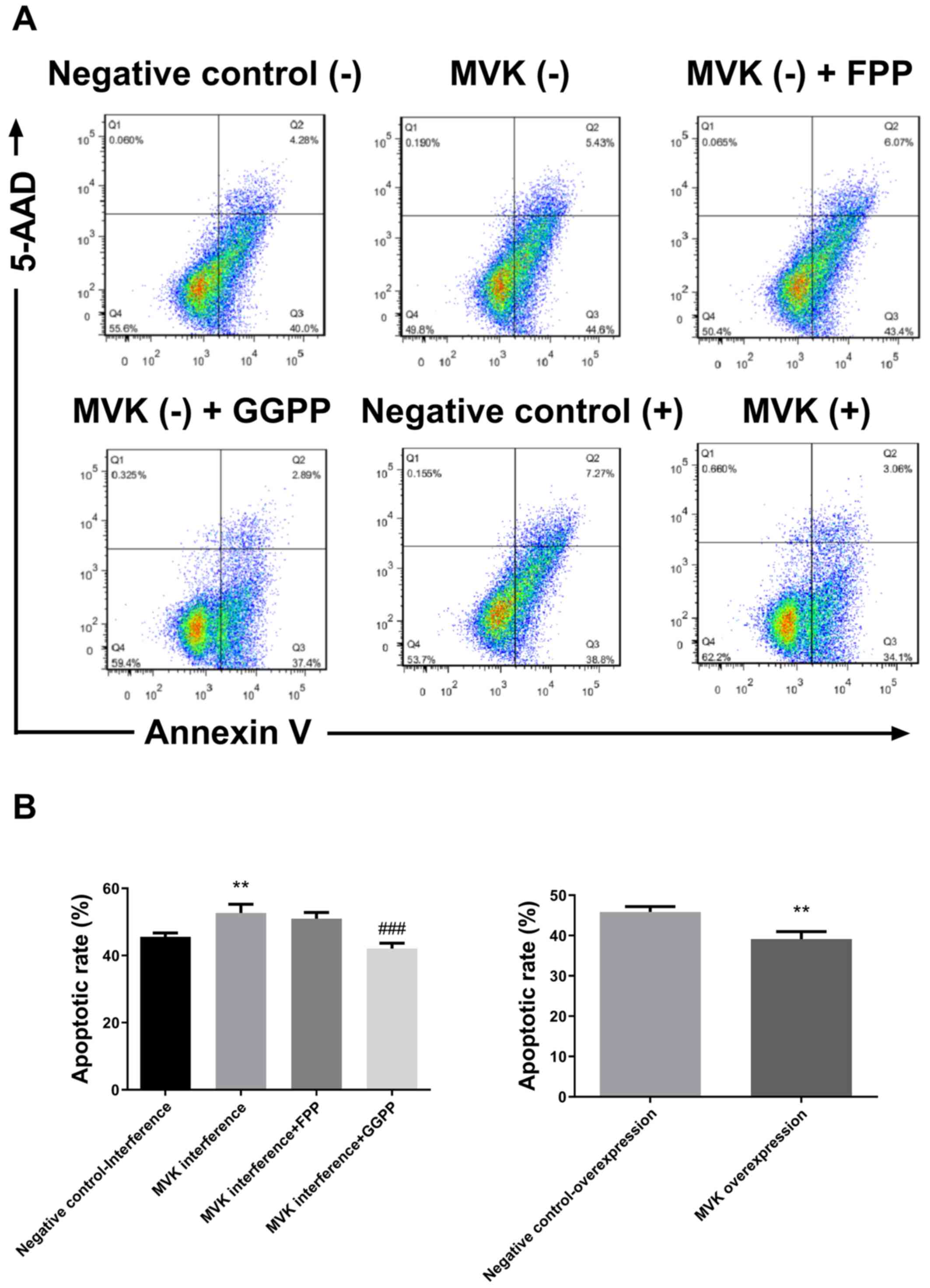
Increase in apoptotic rate following
MVK interference was significantly attenuated by GGPP
The apoptotic rate following MVK interference was
markedly increased compared with the negative control group
(P<0.01; Fig. 3). The increase in
apoptotic rate following MVK interference was significantly
attenuated by GGPP (P<0.001; Fig.
3), but not by FPP. The overexpression of MVK significantly
decreased the apoptotic rate of HaCat cells (P<0.01; Fig. 3).
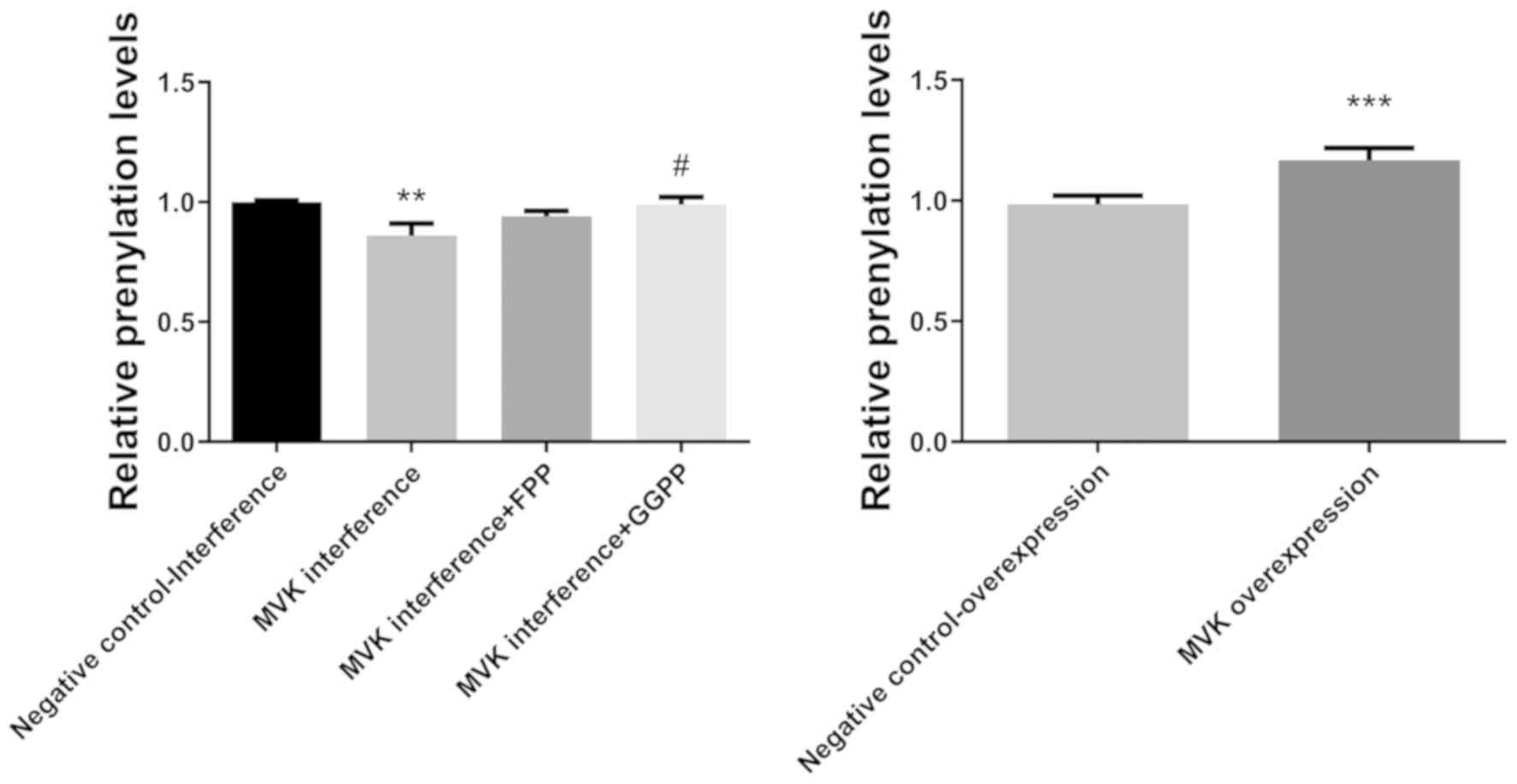
Decrease in protein prenylation levels
following MVK interference was markedly attenuated by GGPP
The protein prenylation levels following MVK
interference was notably decreased compared with the negative
control group (P<0.01; Fig. 4).
The decrease in prenylation levels following MVK interference was
notably attenuated by GGPP (P<0.05; Fig. 4), but not by FPP. The overexpression
of MVK significantly increased the prenylation levels of HaCat
cells (P<0.001; Fig. 4).
FPP or GGPP reversed MVK
interference-induced decrease in geranylgeranylation levels of
small G proteins
To examine the geranylgeranylation levels of small G
proteins in HaCat cells following interference of MVK, the presence
of processed (geranylated) and unprocessed (not geranylated) forms
of proteins were evaluated (10).
Geranylated lamin A, HRAS, KRAS, NRAS, Rho E, Rho B, Rho A, RAC1
and cdc42 decreased in HaCat cells following the interference of
MVK (Fig. 5). Furthermore, FPP
reversed MVK interference-induced decrease in geranylgeranylation
levels of Lamin A, HRAS and Rho E. In addition, GGPP reversed MVK
interference-induced decrease in geranylgeranylation levels of Rho
A, RAC1 and cdc42. Both FPP and GGPP reversed MVK
interference-induced decrease in geranylgeranylation levels of
KRAS, NRAS and Rho B (Fig. 5).
Moreover, the overexpression of MVK increased the
geranylgeranylation levels of Lamin A, HRAS, KRAS, NRAS, Rho E, Rho
B, Rho A, RAC1 and cdc42 (Fig.
5).
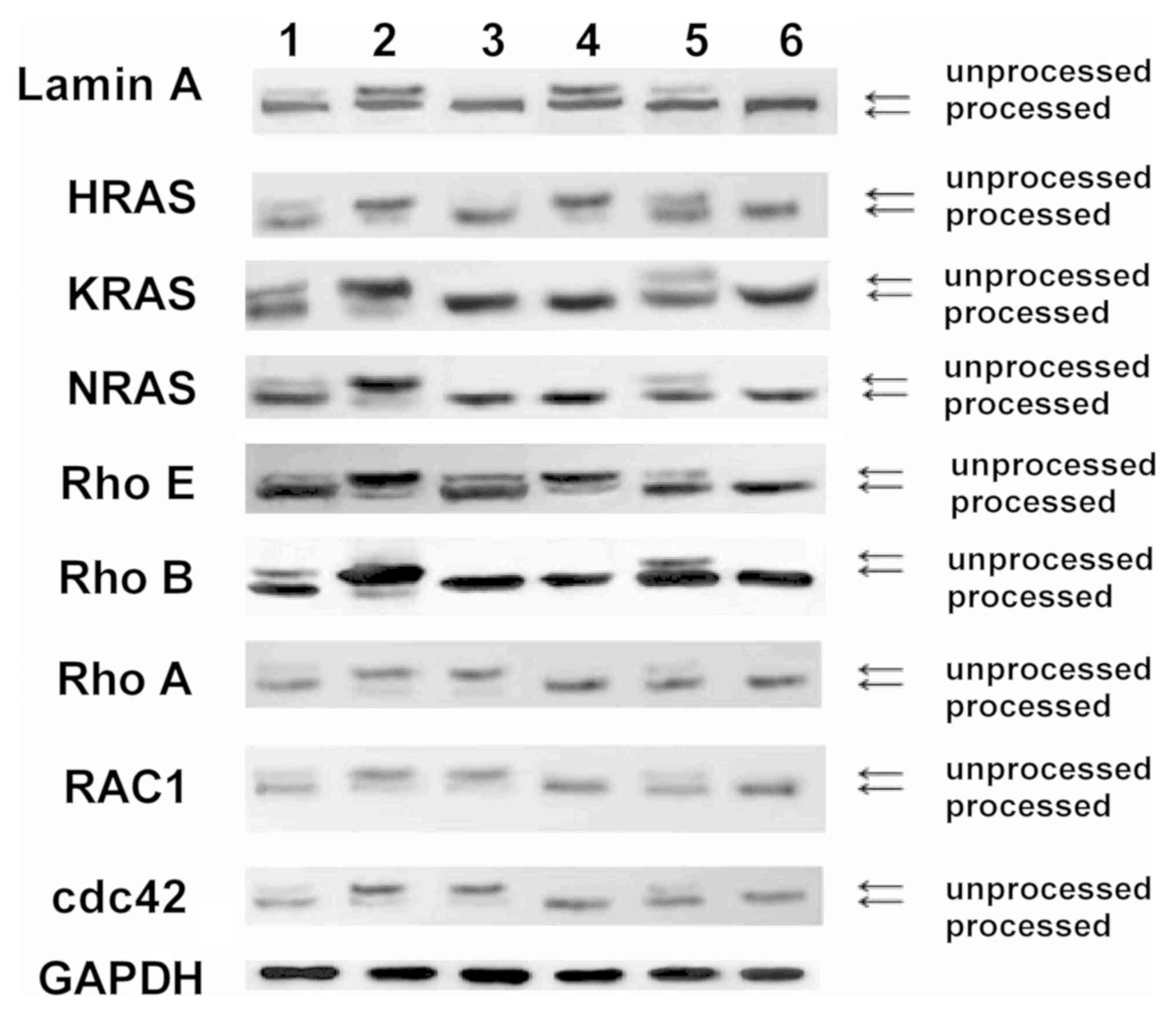 | Figure 5Geranylgeranylation levels of small G
proteins following the interference and overexpression of MVK. The
geranylgeranylated lamin A, HRAS, KRAS, NRAS, Rho E, Rho B, Rho A,
RAC1 and cdc42 decreased in HaCat cells following interference of
MVK. FPP reversed MVK interference-induced decrease in
geranylgeranylation levels of lamin A, HRAS and Rho E. In addition,
GGPP reversed MVK interference-induced decrease in
geranylgeranylation levels of Rho A, RAC1 and cdc42. Both FPP and
GGPP reversed MVK interference-induced decrease in
geranylgeranylation levels of KRAS, NRAS and Rho B. 1, negative
control-interference; 2, MVK interference; 3, MVK interference+FPP,
4, MVK interference+GGPP; 5, negative control-overexpression; 6,
MVK overexpression. MVK, mevalonate kinase; GGPP, geranylgeranyl
pyrophosphate; FPP, farnesyl pyrophosphate. |
Discussion
The present study demonstrated the decreased
expression of keratin 1 and involucrin, following MVK interference,
to be notably attenuated by FPP. In addition, the increase in
apoptosis and decrease in protein prenylation following MVK
interference was significantly attenuated by GGPP. The
overexpression of MVK significantly decreased the apoptotic rate
and increased prenylation levels. The decrease in
geranylgeranylation levels of Lamin A, HRAS, KRAS, NRAS, Rho E, Rho
B, Rho A, RAC1 and cdc42 was attenuated by FPP or GGPP.
The decrease in expression of keratin 1 and
involucrin following MVK interference was notably attenuated by
FPP. The modulation of keratin 1 and involucrin expression was
shown to involved in the response of human keratinocytes to
ultraviolet radiation (11). Keratin
and involucrin were expressed in skin diseases such as discoid
lupus erythematosus and lichen planus (12). High-cell-density phorbol ester and
retinoic acid upregulated involucrin in autocrine cultures of human
epidermal keratinocytes (13).
Keratin and involucrin were expressed in keratoacanthoma, which
might aid in diagnosis (14). The
knockdown of protein kinase D1 in normal human epidermal
keratinocytes also increased the mRNA expression of involucrin
(15). In addition, FPP was shown to
be the skin metabolite that regulated epidermal responses to
inflammation, oxidative stress and migration. The cytoprotective
transcriptional factor Nrf2 and its target genes were induced by
increased levels of FPP. FPP also functioned as a ligand for
glucocorticoid receptor (GR), which is a major regulator of
epidermal homeostasis. Comparative microarray analyses demonstrated
significant but incomplete overlap between glucocorticoid and FPP
regulated genes (16). These
findings suggest that FPP might have wider transcriptional impact.
It is likely that FPP has transcriptional impact on the expression
of differentiation markers in keratinocytes, such as keratin 1 and
involucrin. The aforementioned effects of FPP were not seen in
GGPP, which might explain why the expression of keratin 1 and
involucrin following MVK interference was not restored by GGPP.
Studies on the effect of MVK on the differentiation of
keratinocytes are scarce. The present study indicates that MVK is
essential in keratinocyte differentiation.
In addition, the increase in apoptosis following MVK
interference was significantly attenuated by GGPP, whereas the
overexpression of MVK significantly decreased the apoptotic rate of
human keratinocytes. Autophagy impairment, apoptosis, and lack of
prenylated proteins were observed in SH-SY5Y neuronal cell model of
MVK deficiency (17). In addition,
MVK mutation and deficiency were shown to cause a rare autosomal
recessive disease called mevalonic aciduria. Patients with
mevalonic aciduria had recurrent fever episodes with severe
neurologic impairments or death in early childhood. The
neurodegeneration in patients with mevalonic aciduria might be
associated with both the mitochondria-mediated intrinsic apoptosis
pathway (caspase 9 and 3) and pyroptosis (caspase 1) (18). In addition, statins were reported to
induce apoptosis in glioblastoma when the biosynthesis of GGPP was
inhibited and consequently decreased levels of Akt and
phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2)
(19). Therefore, GGPP might inhibit
apoptosis by modulating Akt and ERK. The aforementioned effect of
GGPP was not evident in FPP, which may explain the reason for the
increase in apoptotic rate following MVK interference not being
significantly attenuated by FPP. Further investigation is required
to elucidate the detailed molecular mechanisms.
The present study revealed decreased protein
prenylation level following MVK interference to be significantly
attenuated by GGPP. The overexpression of MVK significantly
increased the prenylation levels in human keratinocytes. Mutations
in MVK were reported to result in temperature-induced defect in the
prenylation of small G proteins in lymphoblast cell lines (20). Furthermore, defective protein
prenylation was shown to be a diagnostic biomarker of MVK
deficiency (21). Moreover,
thienopyrimidine-based bisphosphonate inhibitors of GGPP were shown
to block protein prenylation and subsequently lead to cellular
apoptosis in multiple myeloma cells (22). Hence, the decrease in protein
prenylation might be another explanation for the increase in
apoptosis following MVK interference.
The decrease in geranylgeranylation levels of small
G proteins, such as lamin A, HRAS, Rho E, Rho B, Rho A, RAC1 and
cdc42, was attenuated by FPP or GGPP in keratinocytes. The
geranygeranylation of small G proteins is involved in various
biological processes. HMG-CoA reductase inhibitors were shown to
block the activation of calcium-dependent tyrosine kinase Pyk2 by
geranylgeranylation of small G protein Rap1 in vascular endothelial
cells (23). The inhibition of
geranylgeranylation was also reported to decrease angiotensin
II-mediated free radical production in vascular smooth muscle
cells, and small G protein Rac1 was involved in this process
(24). In addition,
geranylgeranylation determined the Rho migratory function in T
cells (25). Transendothelial
migration and invasion of human breast cancer cells were inhibited
through prevention of geranylgeranylation of Rho (26). Moreover, GGPP-mediated protein
geranylgeranylation was important for establishment of
communication between oocyte and granulose cells, and transition
from primary to secondary follicle in mouse ovary (27). GGPP-dependent plasma membrane
localization of the small G protein RhoA was shown to be required
for RhoA-mediated oncogenic signaling (28). Geranylgeranyl and farnesyl groups
were attached to C termini of eukaryotic cell proteins by protein
geranylgeranyltransferase-I (PGGT-I) and protein
farnesyltransferase (PFT), respectively. Both geranylgeranyl and
farnesyl groups from GGPP and FPP were transferred to their peptide
or protein prenyl acceptor substrates by PGGT-I and PFT (29). It is likely that geranylgeranyl
groups from GGPP and FPP were transferred to prenyl acceptor
substrates of Lamin A, HRAS, Rho E, Rho B, Rho A, RAC1 and cdc42 by
PGGT-I and PFT in keratinocytes. The present study revealed for the
first time that FPP or GGPP attenuated the decrease in
geranylgeranylation of small G proteins in human keratinocytes.
In conclusion, the present study demonstrated that
the decrease in expression of keratin 1 and involucrin following
MVK interference was notably attenuated by FPP. In addition, the
increase in apoptosis and decrease in protein prenylation following
MVK interference was significantly attenuated by GGPP. The decrease
in geranylgeranylation levels of lamin A, HRAS, KRAS, NRAS, Rho E,
Rho B, Rho A, RAC1 and cdc42 was attenuated by FPP or GGPP.
Mutations in MVK may interrupt the biological function of
keratinocytes and cause disseminated superficial actinic
porokeratosis, by affecting protein prenylation in mevalonate
pathway and small G proteins. Although further investigation is
required to shine light on the molecular mechanisms, the present
study might pave the foundation for future therapeutic strategies
for diseases with MVK mutation, such as disseminated superficial
actinic porokeratosis.
Acknowledgements
Not applicable.
Funding
The research was supported by the project of youth
fund of the National Natural Science Foundation of China (grant
nos. 31401071 and 81703144).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
ML and NHZ conceived this study and obtained
financial support. ZLY and QHQ participated in the design of the
study. JBW performed data analyses. LW, YL, WM and ML performed the
cell culture, protein expression studies, gene sequencing, protein
blot analysis and other function studies. ML and WM wrote and
revised the manuscript. All authors approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fu Z, Wang M, Potter D, Miziorko HM and
Kim JJ: The structure of a binary complex between a mammalian
mevalonate kinase and ATP: Insights into the reaction mechanism and
human inherited disease. J Biol Chem. 277:18134–18142.
2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Houten SM, Wanders RJ and Waterham HR:
Biochemical and genetic aspects of mevalonate kinase and its
deficiency. Biochim Biophys Acta. 1529:19–32. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bouwstra JA and Ponec M: The skin barrier
in healthy and diseased state. Biochim Biophys Acta.
1758:2080–2095. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao Y, Gartner U, Smith FJ and McLean WH:
Statins downregulate K6a promoter activity: A possible therapeutic
avenue for pachyonychia congenita. J Invest Dermatol.
131:1045–1052. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang SQ, Jiang T, Li M, Zhang X, Ren YQ,
Wei SC, Sun LD, Cheng H, Li Y, Yin XY, et al: Exome sequencing
identifies MVK mutations in disseminated superficial actinic
porokeratosis. Nat Genet. 44:1156–1160. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Shen CS, Tabata K, Matsuki M, Goto T,
Yokochi T and Yamanishi K: Premature apoptosis of keratinocytes and
the dysregulation of keratinization in porokeratosis. Br J
Dermatol. 147:498–502. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alaei P, MacNulty EE and Ryder NS:
Inhibition of protein prenylation down-regulates signalling by
inflammatory mediators in human keratinocytes. Biochem Biophys Res
Commun. 222:133–138. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McTaggart SJ: Isoprenylated proteins. Cell
Mol Life Sci. 63:255–267. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xia Z, Tan MM, Wong WW, Dimitroulakos J,
Minden MD and Penn LZ: Blocking protein geranylgeranylation is
essential for lovastatin-induced apoptosis of human acute myeloid
leukemia cells. Leukemia. 15:1398–1407. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Moravcová M, Libra A, Dvořáková J, Víšková
A, Muthný T, Velebný V and Kubala L: Modulation of keratin 1, 10
and involucrin expression as part of the complex response of the
human keratinocyte cell line HaCaT to ultraviolet radiation.
Interdiscip Toxicol. 6:203–208. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ichikawa E, Watanabe S and Takahashi H:
Keratin and involucrin expression in discoid lupus erythematosus
and lichen planus. Arch Dermatol Res. 289:519–526. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Poumay Y, Herphelin F, Smits P, De Potter
IY and Pittelkow MR: High-cell-density phorbol ester and retinoic
acid upregulate involucrin and downregulate suprabasal keratin 10
in autocrine cultures of human epidermal keratinocytes. Mol Cell
Biol Res Commun. 2:138–144. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ichikawa E, Ohnishi T and Watanabe S:
Expression of keratin and involucrin in keratoacanthoma: An
immunohistochemical aid to diagnosis. J Dermatol Sci. 34:115–117.
2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ivanova P, Atanasova G, Poumay Y and Mitev
V: Knockdown of PKD1 in normal human epidermal keratinocytes
increases mRNA expression of keratin 10 and involucrin: Early
markers of keratinocyte differentiation. Arch Dermatol Res.
300:139–145. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pastar I, Stojadinovic O, Sawaya AP, Stone
RC, Lindley LE, Ojeh N, Vukelic S, Samuels HH and Tomic-Canic M:
Skin metabolite, farnesyl pyrophosphate, regulates epidermal
response to inflammation, oxidative stress, and migration. J Cell
Physiol. 231:2452–2463. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tricarico PM, Romeo A, Gratton R, Crovella
S and Celsi F: Lack of prenylated proteins, autophagy impairment
and apoptosis in SH-SY5Y neuronal cell model of mevalonate kinase
deficiency. Cell Physiol Biochem. 41:1649–1660. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tricarico PM, Marcuzzi A, Piscianz E,
Monasta L, Crovella S and Kleiner G: Mevalonate kinase deficiency
and neuroinflammation: Balance between apoptosis and pyroptosis.
Int J Mol Sci. 14:23274–23288. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yanae M, Tsubaki M, Satou T, Itoh T, Imano
M, Yamazoe Y and Nishida S: Statin-induced apoptosis via the
suppression of ERK1/2 and Akt activation by inhibition of the
geranylgeranyl-pyrophosphate biosynthesis in glioblastoma. J Exp
Clin Cancer Res. 30(74)2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jurczyluk J, Munoz MA, Skinner OP, Chai
RC, Ali N, Palendira U, Quinn JM, Preston A, Tangye SG, Brown AJ,
et al: Mevalonate kinase deficiency leads to decreased prenylation
of Rab GTPases. Immunol Cell Biol. 94:994–999. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Munoz MA, Jurczyluk J, Mehr S, Chai RC,
Arts RJW, Sheu A, McMahon C, Center JR, Singh-Grewal D, Chaitow J,
et al: Defective protein prenylation is a diagnostic biomarker of
mevalonate kinase deficiency. J Allergy Clin Immunol. 140:873–875,
e876. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lacbay CM, Waller DD, Park J, Gómez Palou
M, Vincent F, Huang XF, Ta V, Berghuis AM, Sebag M and Tsantrizos
YS: Unraveling the Prenylation-cancer paradox in multiple myeloma
with novel geranylgeranyl pyrophosphate synthase (GGPPS)
inhibitors. J Med Chem. 61:6904–6917. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Satoh K, Ichihara K, Landon EJ, Inagami T
and Tang H: 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors
block calcium-dependent tyrosine kinase Pyk2 activation by
angiotensin II in vascular endothelial cells. involvement of
geranylgeranylation of small G protein Rap1. J Biol Chem.
276:15761–15767. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wassmann S, Laufs U, Bäumer AT, Müller K,
Konkol C, Sauer H, Böhm M and Nickenig G: Inhibition of
geranylgeranylation reduces angiotensin II-mediated free radical
production in vascular smooth muscle cells: Involvement of
angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol.
59:646–654. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Waiczies S, Bendix I, Prozorovski T,
Ratner M, Nazarenko I, Pfueller CF, Brandt AU, Herz J, Brocke S,
Ullrich O and Zipp F: Geranylgeranylation but not GTP loading
determines rho migratory function in T cells. J Immunol.
179:6024–6032. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kusama T, Mukai M, Tatsuta M, Nakamura H
and Inoue M: Inhibition of transendothelial migration and invasion
of human breast cancer cells by preventing geranylgeranylation of
Rho. Int J Oncol. 29:217–223. 2006.PubMed/NCBI
|
|
27
|
Jiang C, Diao F, Sang YJ, Xu N, Zhu RL,
Wang XX, Chen Z, Tao WW, Yao B, Sun HX, et al: GGPP-mediated
protein geranylgeranylation in oocyte is essential for the
establishment of oocyte-granulosa cell communication and
primary-secondary follicle transition in mouse ovary. PLoS Genet.
13(e1006535)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fuchs D, Berges C, Opelz G, Daniel V and
Naujokat C: HMG-CoA reductase inhibitor simvastatin overcomes
bortezomib-induced apoptosis resistance by disrupting a
geranylgeranyl pyrophosphate-dependent survival pathway. Biochem
Biophys Res Commun. 374:309–314. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yokoyama K, Zimmerman K, Scholten J and
Gelb MH: Differential prenyl pyrophosphate binding to mammalian
protein geranylgeranyltransferase-I and protein farnesyltransferase
and its consequence on the specificity of protein prenylation. J
Biol Chem. 272:3944–3952. 1997.PubMed/NCBI View Article : Google Scholar
|