Introduction
Glioblastoma is a heterogeneous group of tumor types
that arise in neuroepithelial tissue, and account for 45.6% of
primary malignant brain tumors in the central nervous system
(1,2). Due to diffuse brain infiltration of
glioblastomas and the potential risk of nervous system impairments
from surgical resection, complete surgical resection is usually not
feasible. Despite substantial advances in surgical strategies,
radiotherapy and chemotherapy, the median survival rates for
patients with high-grade glioblastoma is only 12-15 months
(3,4). Due to these poor clinical outcomes,
there is an urgent need for further glioblastoma research.
Epidemiological data indicates that the incidence of
glioblastoma is higher in males compared with females, particularly
in premenopausal females (5). This
suggests that elevated levels of estrogen may serve a function in
reducing the risk of glioblastoma. Endogenous estrogens primarily
consist of 17β-estradiol (E2), estrone (E1) and estriol (E3). E2 is
produced by the ovaries and is also a neurosteroid hormone
synthesized by nerve cells (6-8). In
the 1970s, Naftolin et al (9)
demonstrated that E2 is derived from the activity of aromatase
enzymes in the brain. The androgen hormone testosterone is
catalyzed by aromatase, which aromatizes the A-ring of testosterone
to form E2(6). Cells of the central
nervous system and glial cells are thought to be targets of E2. The
biological effects of E2 are primarily mediated by estrogen
receptors (ER) α and ERβ. Batistatou et al (10,11)
reported that the expression of ERβ was detectable in 56
glioblastoma samples and paired adjacent normal brain tissues. In
addition, patients with high-grade glioblastoma exhibited lower
expression levels of ERβ than low-grade glioblastoma patients, as
determined by using immunohistochemical analysis (10,11).
These results suggest that ERβ may serve a crucial function in the
progression of glioblastoma and may be an important biomarker of
patient prognosis.
The blood-brain barrier prevents the delivery of the
vast majority of candidate anti-glioblastoma compounds. Icaritin
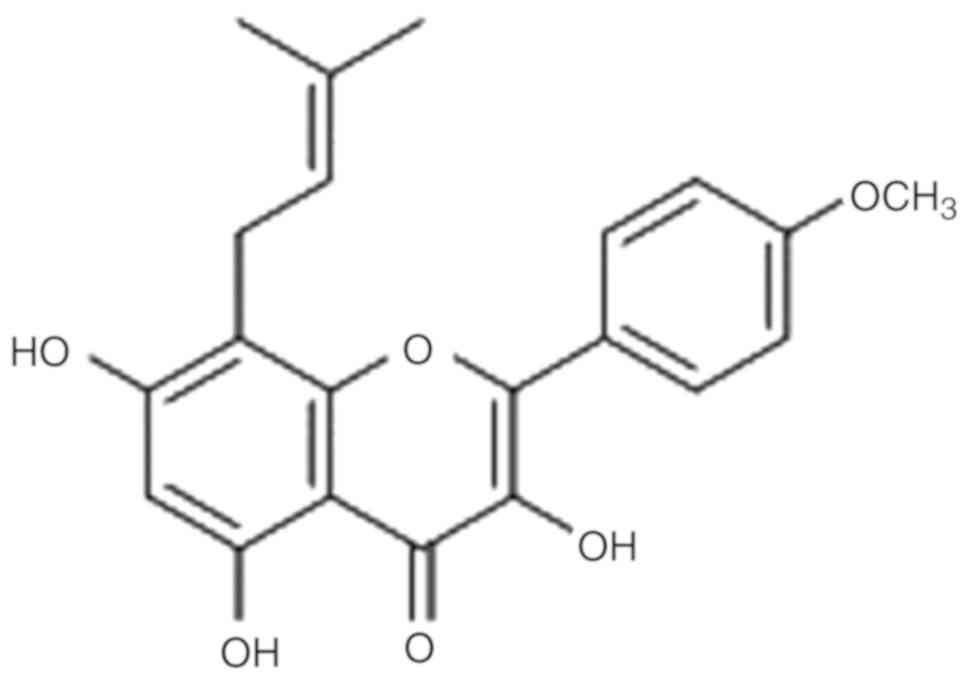
(C21H20O6) has a small molecular
weight (368.38) and is a hydrolytic product of Epimedium
brevicornu Maxim; a member of the berberine family (12,13). Of
particular note, icaritin is able to cross the blood-brain barrier
and may therefore present a useful therapeutic option for patients
with glioblastoma (14). It was
previously reported that icaritin may exert anti-myeloma effects in
nude mice (15). In addition, one
previous study demonstrated that icaritin may regulate the
proliferation, invasion and apoptosis of glioblastoma cells
(13). However, studies
investigating the mechanisms underlying the anti-glioblastoma
effects of icaritin via ERβ are limited.
As a traditional Chinese medicine, icaritin is
thought to exert estrogenic effects and has been used to treat
impotence and infertility (16).
Icaritin (Fig. 1) is a hydrolyzed
product of icariin, which is also an important metabolite of
Epimedium in the human body (13). Therefore, studies investigating the
effect of icaritin on the ER signaling pathway and its ability to
mediate the inhibition of glioblastoma cell migration are required.
Phosphatase and tensin homolog (PTEN) is a lipid phosphatase enzyme
that catalyzes the phosphorylation of phosphoinositide 3-kinase
(PI3K) and is a major negative regulator of protein kinase B (Akt)
signaling. Loss of PTEN function is associated with the development
of numerous malignant tumor types. ERβ reduces phosphorylated
(p)-Akt levels by promoting the expression of PTEN, which mediates
its anti-tumor effects (17).
The aim of the present study was to investigate the
anti-glioblastoma effects of icaritin and the underlying mechanisms
mediating these effects, with a particular relevance to ERβ. To the
best of the authors' knowledge, the present study is the first to
demonstrate that icaritin exerts anti-glioblastoma effects via the
modulation of ERβ. This suggests that ERβ modulators, including
icaritin, may be a useful therapeutic strategy for the treatment of
patients with malignant glioblastoma.
Materials and methods
Cell culture and drug treatment
Human U87-MG glioblastoma cells glioblastoma of
unknown origin (cat. no. HTB-14) and rat C6 glioblastoma cells (cat
no. CCL-107) were obtained from the American Type Culture
Collection, and U87-MG cells were authenticated with short tandem
repeat profiling. The two cell lines were maintained in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Lonsera,
Canelones, Uruguay) and 1% 100 U/ml penicillin/streptomycin
(Hyclone; GE Healthcare Life Sciences, Little Chalfont, UK) and
cultured in an incubator with a humidified atmosphere at 37˚C and
5% CO2.
Cell viability assay
The effect of icaritin on cell viability was
determined using an MTT assay. U87-MG and C6 cells at a density of
2x104 cells/ml growing in logarithmic phase were seeded
in 96-well plates and treated with icaritin at different doses
(6.25, 12.5, 25, 50 and 100 µM) at 37˚C for 24, 48 and 72 h. MTT
(10 µl; 0.5 mg/ml) was added to the medium of each well and the
cells were subsequently incubated at 37˚C for 4 h. The medium was
then removed and 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added to each well. The optical
density was read using an ELx800 microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA) at 490 nm. The effect of E2,
ERβ-specific antagonist ICI 182,780 (Shanghai Amquar Biological
Technology Co., Ltd.) at 37˚C for 24 h and icaritin on cell
viability was assessed using a cell counting Kit-8 (CCK-8) assay.
CCK-8 (10 µl) was added to each well and cells were subsequently
incubated at 37˚C for 3 h. The optical density was then measured at
450 nm. For MTT and CCK-8 assays, a total of five replicates were
prepared for each treatment.
Hoechst 33342 fluorescence
staining
U87-MG and C6 cells (5x104 cells/ml) were
stained with Hoechst 33342 (5 µg/ml) at 37˚C for 20 min in the dark
and photographed under a DP80 fluorescence microscope at x100
magnification (Olympus Corporation) with a 340-nm excitation
filter. Cell shrinkage and condensed nuclei were used to
characterize apoptotic cells. The proportion of apoptotic cells in
the total population were quantified in three randomly selected
fields of view under a microscope.
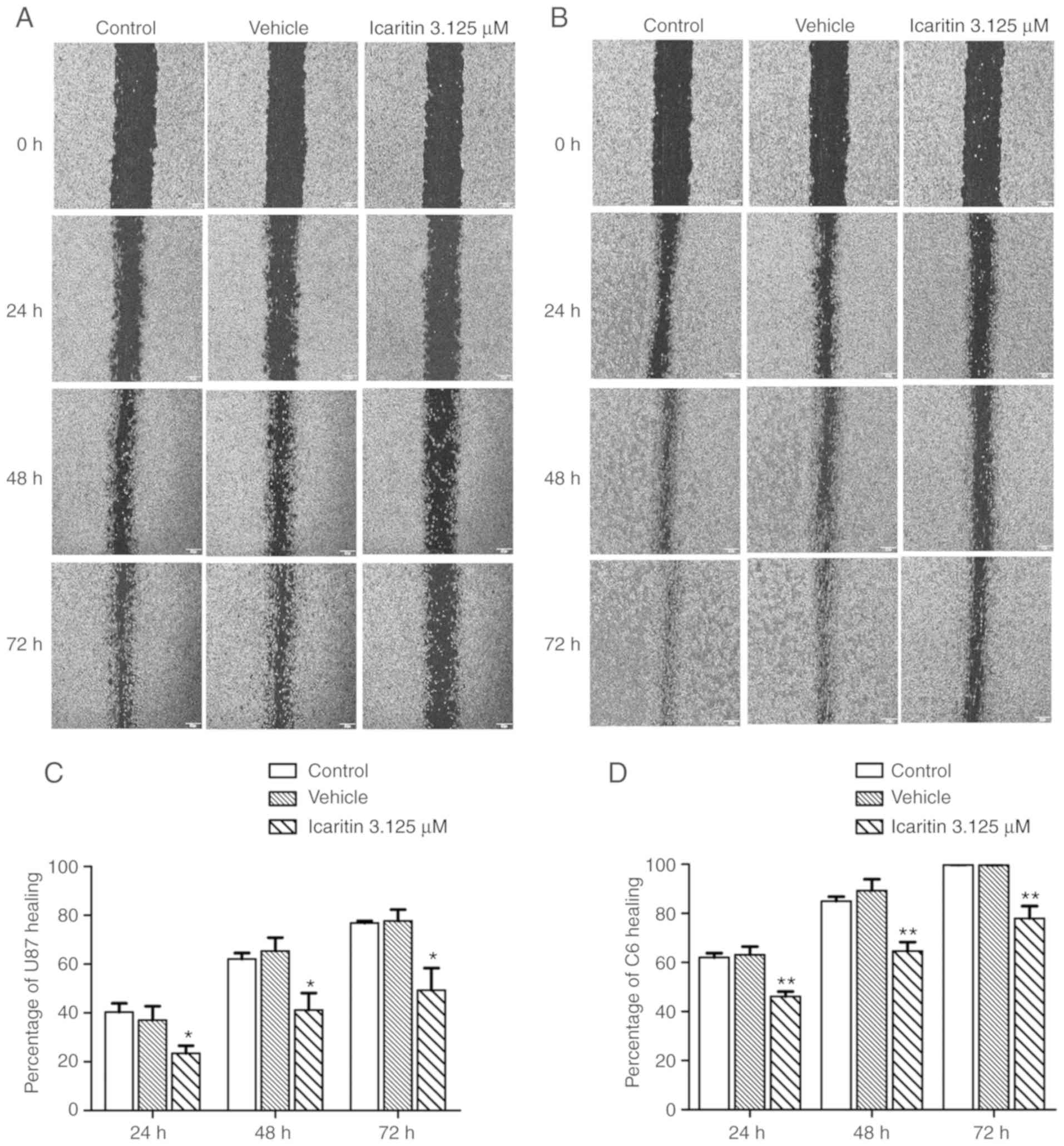
Wound healing assay
U87-MG and C6 cells (5x104 cells/ml) were
seeded in 35-mm plates and cultured until they reached confluence
prior to being treated with 3.125 µM icaritin at 37˚C for 24, 48
and 72 h. In each well, two perpendicular scratch-wounds with an
initial width of 300 µm were generated in the confluent cell
monolayer using a pipette tip. The culture medium was then
refreshed with medium containing the drugs. Phase-contrast images
of the cells were obtained immediately and at 24, 48 and 72 h
following generation of the scratch-wound using a charge-coupled
device camera connected to an Olympus fluorescence microscope (x40
magnification) (Olympus Corporation). The region imaged at the 0 h
time point was marked to enable the same region to be imaged at 24,
48 and 72 h, thus allowing examination of a specific population of
migrating cells. Wound closure was measured using Image-Pro Plus
6.0 (Media Cybernetics, Inc.) and data acquired from four regions
of the scratch-wound on each plate were averaged to obtain the mean
wound-width at a given time.
Immunofluorescence staining
U87-MG and C6 cells were seeded at a density of
2.5x105 cells/well on glass coverslips placed in 6-well
tissue culture plates, and allowed to adhere overnight. Cells were
then treated with 25 µM icaritin at 37˚C for 24 h. The following
day, cells were washed with phosphate buffered saline (PBS) and
fixed in 4% (v/v) paraformaldehyde for 30 min at room temperature.
Fixed cells were subsequently washed with PBS and permeabilized
with 0.3% (v/v) Triton X-100 in PBS for 1 h, prior to being washed
again in PBS. In order to block non-specific binding sites, cells
were incubated with PBS containing 5% (v/v) bovine serum albumin at
room temperature for 10 min, and then incubated with a primary
antibody against ERβ (1:5,000; diluted in 0.1% Triton X-100;
ab3577; Abcam) overnight at 4˚C. The following day, cells were
washed with PBS and incubated with a secondary antibody [1:500;
diluted in mixture of 1% PBS containing 1% BSA and 0.3% Triton
X-100; FITC-labeled goat anti-rabbit IgG (H+L); A0562; Beyotime
Institute of Biotechnology] for 1 h in the dark. Cells were then
gently washed. Finally, the cell nucleus was stained with DAPI (1
µg/ml; 4083; CST Biological Reagents Co., Ltd.) at room temperature
for 10 min and visualized under a DP80 fluorescence microscope
(x400 magnification; Olympus Corporation).
Western blotting
Western blotting was performed as previously
described (18). β-actin protein was
used as a reference protein. U87-MG and C6 cells
(1.25x105 cells/ml) were dissolved in SDS sample buffer
(Beyotime Institute of Biotechnology) following lysis with RIPA
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.).
Protein concentration was determined using a BCA kit (Beijing
Solarbio Science & Technology Co., Ltd.). Equal quantities of
protein (25 µg/lane) were loaded and separated by 10 or 12%
SDS-PAGE (Bio-Rad Laboratories, Inc.) and electroblotted onto PVDF
membranes (Merck KGaA). The membranes were incubated in 5% non-fat
dry milk in tris-buffered saline/Tween buffer (1x; Epizyme, Inc.,
Cambridge, MA, USA) containing 0.1% Tween-20 for 2 h at room
temperature, prior to incubation with the following primary
antibodies. Caspase-3 (9665), caspase-7 (12827), caspase-8 (4790),
caspase-9 (9508), cleaved caspase-3 (9664), cleaved caspase-7
(8438), cleaved caspase-8 (9496), cleaved caspase-9 (7237), poly
(ADP-ribose) polymerase (PARP; 9542), cleaved PARP (5625), AKT
(4691), p-AKT (2965), PTEN (9559), matrix metalloproteinase (MMP-2;
40994), MMP-9 (13667; all 1:1,000; CST Biological Reagents Co.,
Ltd.), ERβ (ab3577), Bcl2 (ab182858) and Bax (ab32503; all 1:1,000;
Abcam) overnight at 4˚C. ECL solution (Merck KGaA) was mixed with
equal volumes of luminol reagent and peroxide solution in a clean
tube. Approximately 0.1 ml of working horseradish peroxidase (HRP)
substrate is required per cm2 membrane area. Blots were
incubated for 5 mins at room temperature. The blots were exposed
using a Tanon-5200 molecular imager (Tanon Science and Technology
Co., Ltd.) following incubation with a HRP-labeled secondary
antibody [(HRP-labeled goat anti-mouse IgG (H+L); A0216; Beyotime
Institute of Biotechnology); (HRP-labeled goat anti-rabbit IgG
(H+L); A0208, Beyotime Institute of Biotechnology) at room
temperature for 2 h. Image-Pro Plus 6.0 (Media Cybernetics, Inc.)
was used to calculate densitometry.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least 3 replicates. SPSS 17 statistical software (SPSS, Inc.,
Chicago, IL, USA) was used to analyze the data, and statistical
analysis was performed using one-way ANOVAs, with Fisher's least
significant difference post-hoc tests used to determine the
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
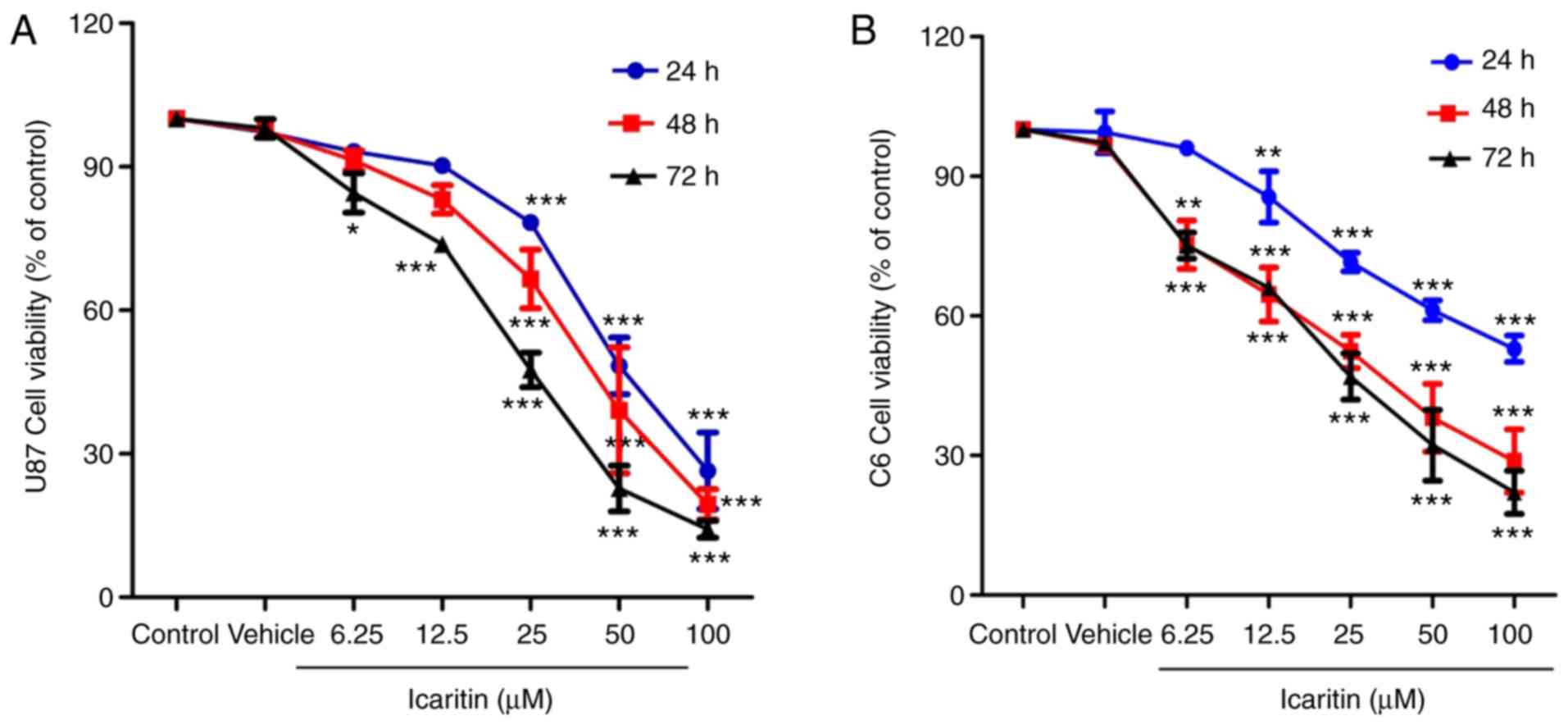
Icaritin suppresses the growth of
U87-MG and C6 cells
As presented in Fig.
2A and B, U87-MG and C6 cells
were incubated with different concentrations of icaritin for 24, 48
and 72 h. The results indicated that icaritin suppressed the
viability of U87-MG and C6 cells at different time points and
concentrations. Notably, compared with 0 µM icaritin, U87-MG and C6
cells treated with 100 µM icaritin for 72 h showed the greatest
inhibition of cell viability (P<0.001). Viability suppression of
U87-MG and C6 cells was time-dependent upon treatment with 12.5 µM
icaritin, and dose-dependent upon treatment with 6.25, 12.5, 25, 50
and 100 µM icaritin.
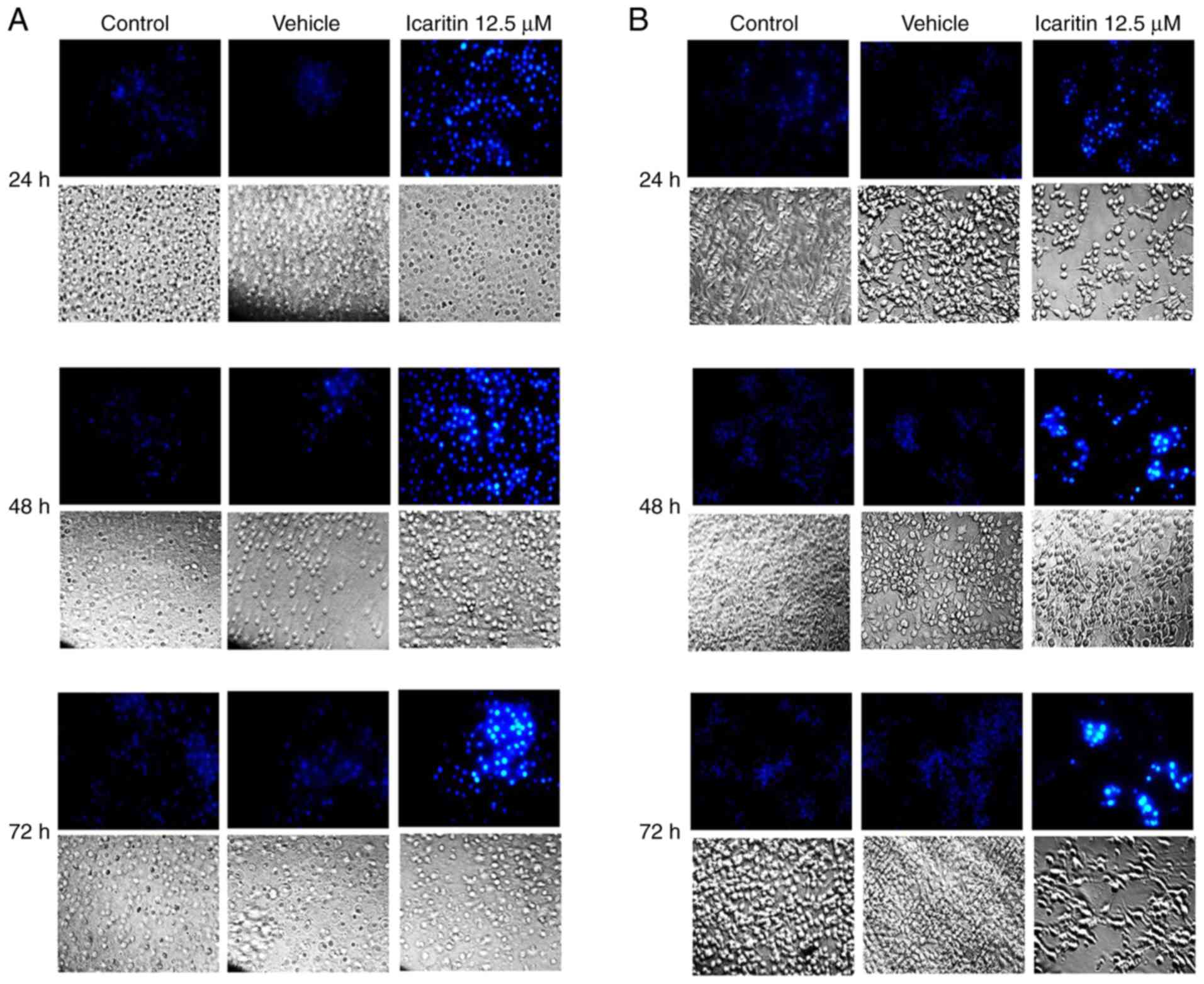
Icaritin induces apoptosis of U87-MG
and C6 cells
U87-MG and C6 cells were treated with 12.5 µM
icaritin for 24, 48 and 72 h prior to Hoechst 33342 fluorescence
staining being performed. As demonstrated in Fig. 3A and B, a greater fluorescence intensity was
observed in the 12.5 µM icaritin group when compared with the
control group. Fluorescence intensity increased in a treatment
time-dependent manner.
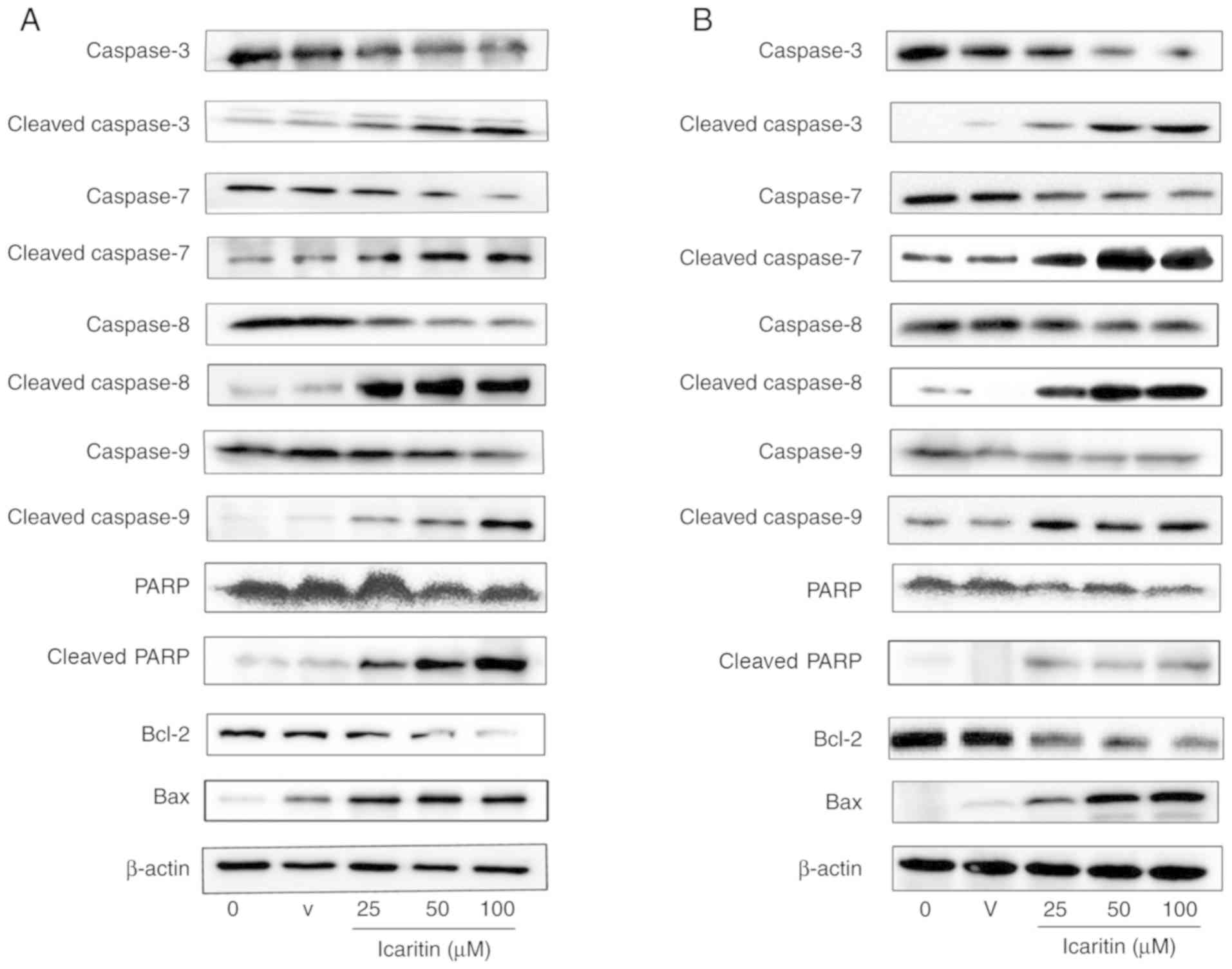
The protein expression of caspase enzymes and BCL2
apoptosis regulator (Bcl-2) in U87-MG and C6 cells treated with
icaritin (25, 50 and 100 µM) for 48 h was then examined (Fig. 4A and B). Compared with the control group,
icaritin treatment appeared to upregulate the protein expression of
the cleaved isoforms of caspase-8, caspase-9, caspase-7, caspase-3,
PARP and Bax. In addition, icaritin appeared to downregulate the
protein expression of caspase-8, caspase-9, caspase-7, caspase-3,
PARP and Bcl-2. The expression of these proteins were positively
associated with the concentration of icaritin.
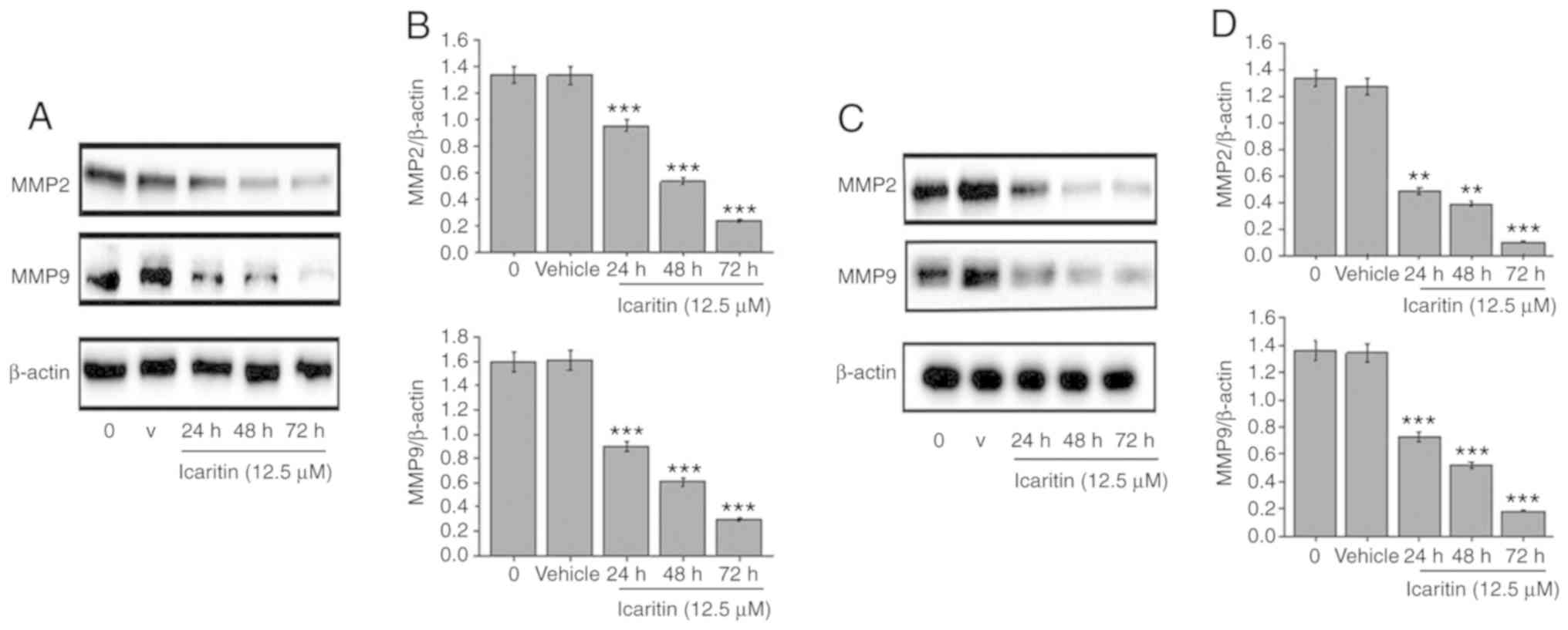
Icaritin suppresses the invasion and
migration of U87-MG and C6 glioblastoma cells
U87-MG and C6 cells were treated with 12.5 µM
icaritin for 24, 48 and 72 h (Fig.
5). Compared with the control group, the expression levels of
extracellular MMP-2 and MMP-9 decreased significantly in a
time-dependent manner (P<0.01). In addition, U87-MG and C6 cells
were treated with 3.125 µM icaritin for 24, 48 and 72 h, and the
number of migrating cells were observed under an inverted
microscope (Fig. 6). Compared with
the control group, the number of migrated cells significantly
decreased in a time-dependent manner (P<0.05).
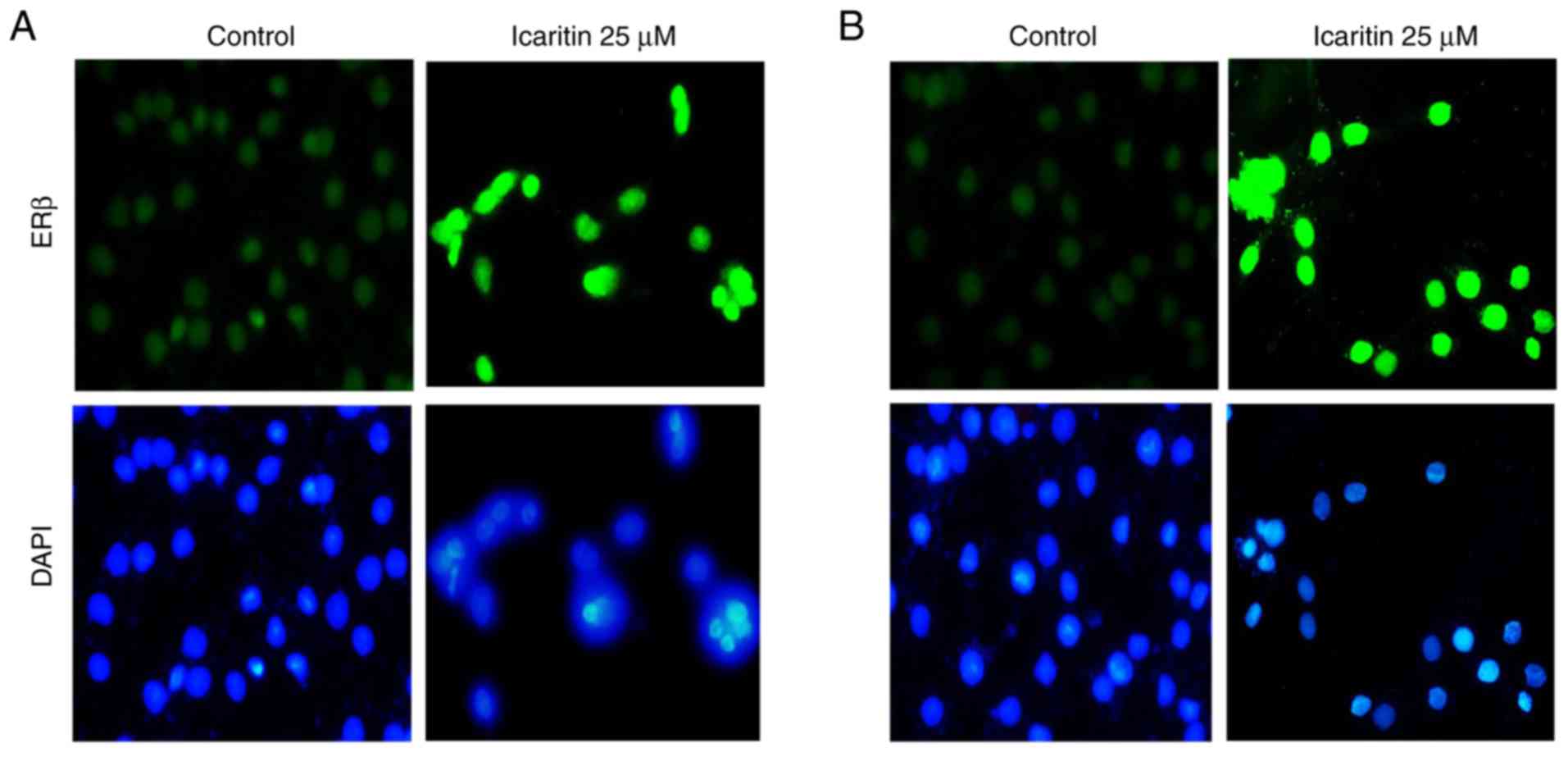
Icaritin upregulates ERβ protein
expression in U87-MG and C6 glioblastoma cells
Results from immunofluorescent staining suggested
that the expression of ERβ in U87-MG and C6 cells increased
substantially following treatment with 25 µM icaritin when compared
with the control group (Fig. 7).
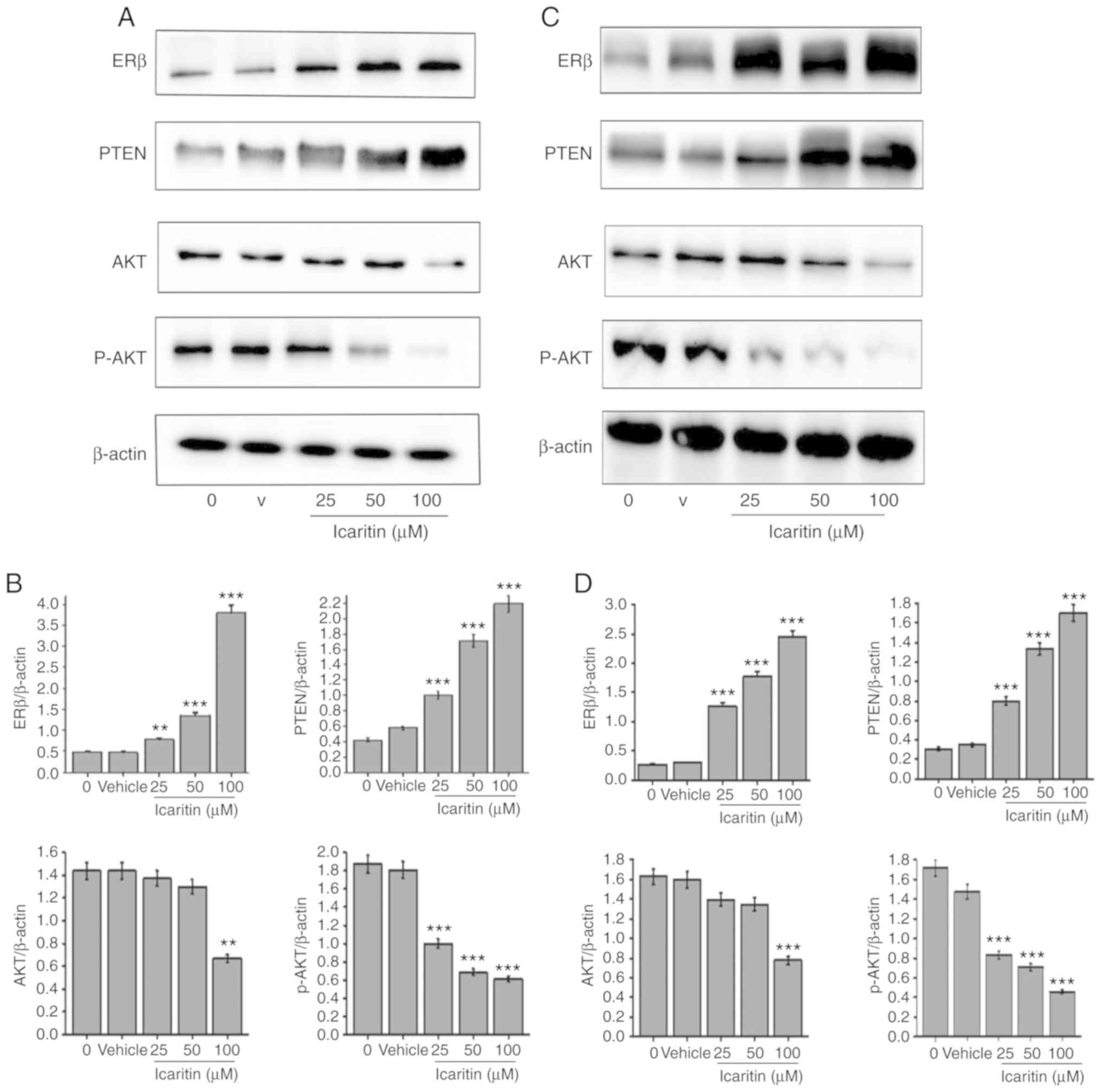
Icaritin affects the protein
expression levels of ERβ, PTEN, Akt and p-Akt
Compared with the 0 µM icaritin group, the protein
expression levels of ERβ and PTEN in U87-MG and C6 cells
significantly increased following treatment with increasing
concentrations of icaritin (25, 50 and 100 µM; P<0.01) for 48 h.
By contrast, the protein expression levels of p-Akt were
significantly decreased (P<0.01; Fig.
8). The protein expression levels of total Akt in U87-MG and C6
cells treated with 50 µM of icaritin were decreased, although this
did not reach statistical significance. However, compared with the
0 µM icaritin group, the protein expression levels of total Akt in
U87-MG and C6 cells treated with 100 µM icaritin were significantly
decreased (P<0.01).
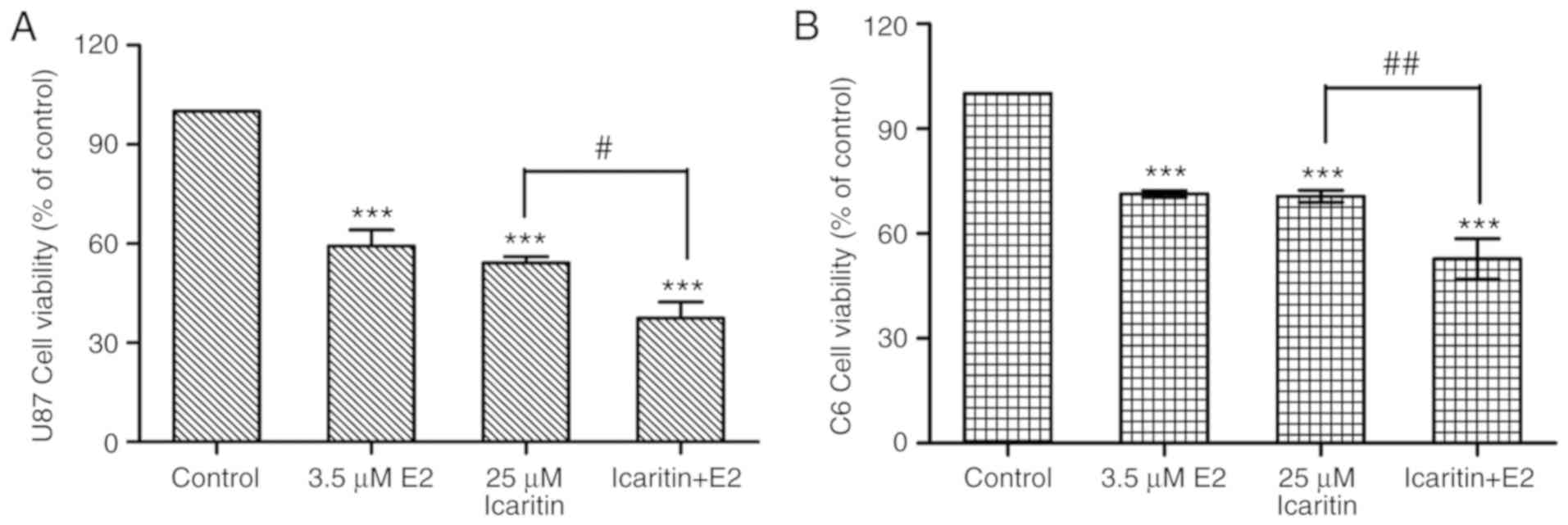
Combined treatment with E2 and
icaritin suppresses the viability of U87-MG and C6 cells
A total of 3.5 µM E2 exerted significant
anti-glioblastoma activity compared with the control group
(P<0.001). Furthermore, the combined treatment of cells with E2
and icaritin exerted significantly higher anti-glioblastoma effects
when compared with the icaritin treatment alone group (P<0.05;
Fig. 9), indicating the involvement
of ER in the icaritin-induced viability inhibition of glioblastoma
cells.
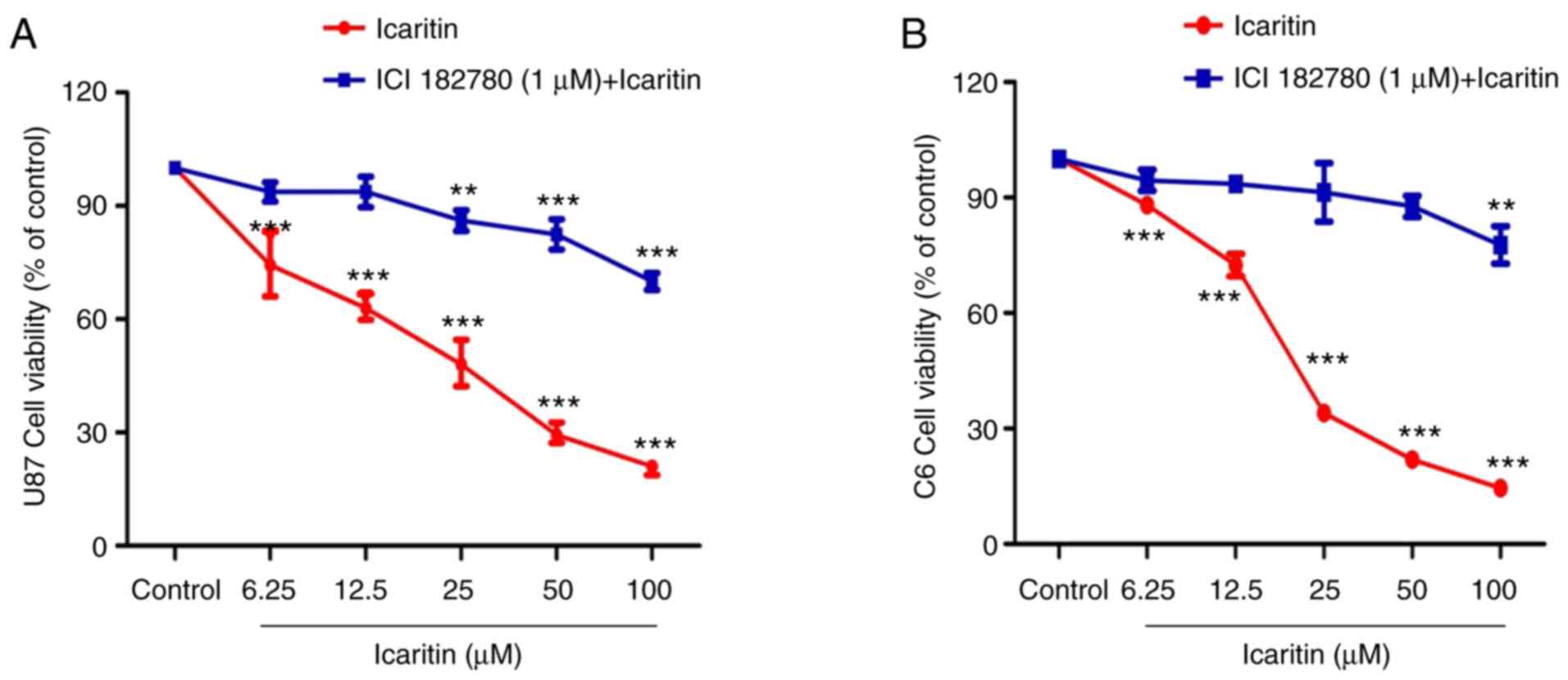
Treatment with the ER antagonist ICI
182,780 attenuates icaritin-mediated inhibition of glioblastoma
cells
U87-MG and C6 cells were treated with 1 µM ICI
182,780 together with 6.25, 12.5, 25, 50 or 100 µM icaritin
(Fig. 10). Compared with the
icaritin treatment alone group, the anti-glioblastoma effects of
icaritin plus ICI 182,780 decreased significantly with increasing
concentrations of icaritin (P<0.01).
PTEN/Akt is a downstream pathway
modulated by the expression of ERβ
As Fig. 11 presents, compared with icaritin alone,
following the incubation of icaritin with E2 for 24 h in U87-MG and
C6 cells, the protein expression of ERβ and PTEN was increased, and
the protein expression of p-Akt was decreased. When ERβ was
suppressed by ICI 182,780, the downregulation of ERβ and PTEN, as
well as the upregulation of p-Akt, were observed.
Discussion
Icaritin is a flavonoid derived from a traditional
Chinese herb from the Epimedium genus. It has a small
molecular weight, and is able to cross the blood-brain barrier
freely. Previous studies have demonstrated that icaritin possesses
neuroprotective effects and anti-neoplastic functions in colorectal
cancer, oral squamous cell carcinoma and leukemia (19-21).
In addition, it has been reported that icaritin induces apoptosis,
autophagy and cell cycle arrest, and inhibits the invasion and
epithelial-to-mesenchymal transition of human glioblastoma cells
(22-24).
In the present study, the anti-glioblastoma effects of icaritin
were confirmed in a broader therapeutic time window, and a more
detailed dose-effect association was characterized. However, the
detailed mechanisms underlying the anti-glioblastoma effects of
icaritin remain yet to be elucidated.
The incidence of glioblastoma is greater in males
compared with females. In addition, postmenopausal women and those
at a reproductive age have a survival advantage compared with
males, which suggests that estrogen and the ER may serve important
functions in the suppression of glioblastoma (25,26). ERα
and ERβ are the two main isoforms of the ER (11). The expression of ERα in glioblastoma
is low (11), and previous studies
have demonstrated that ERβ functions as a tumor suppressor in
various types of human malignancies, including glioblastoma
(23,27,28). In
addition, it has been reported that icaritin is an ER-regulator
(29) and that ERβ serves a crucial
function in glioblastoma tumorigenesis and prognosis (17). Therefore, the aim of the present
study was to investigate whether icaritin exerts anti-glioblastoma
effects by functioning as a modulator of ERβ. The
immunofluorescence results demonstrated that ERβ protein expression
was observed in the nucleus of glioblastoma cells. The results of
the immunofluorescence staining showed that treatment of
glioblastoma cells with 25 µM icaritin increased the expression of
ERβ. Subsequent western blotting also demonstrated that icaritin
significantly upregulated ERβ expression in glioblastoma cells in a
dose-dependent manner (P<0.01 or P<0.001, compared with the 0
µM icaritin group). These results suggest that icaritin functions
as an agonist of ERβ in glioblastoma.
Estrogen induces PTEN at the post-transcriptional
level. E2 first induces Na+/H+ exchange
regulatory factor 1 (NHERF1) expression by activating the ER. When
competing with neural precursor cell expressed, developmentally
downregulated 4, an ubiquitin E3 ligase, NHERF1 interacts with PTEN
to inhibit its degradation through a ubiquitination-dependent
signaling pathway. This subsequently results in increased PTEN
expression at the protein level (30). A previous study demonstrated that E2
exerts effects on ERβ to increase the transcription of PTEN, and
ERβ binds to the Sp1 region of the PTEN promoter, resulting in
autophagy via inhibition of the PI3K/Akt signaling pathway
(31). The results of phase II and
III clinical trials investigating the ER agonist glycyrrhizin have
demonstrated that it is able to cross the blood-brain barrier and
target glioblastoma cells with good tolerability and low neuronal
toxicity (23). In addition,
glycyrrhizin was observed to enhance sensitivity to temozolomide
due to the activation of multiple ERβ regulatory elements and
downstream target genes, as well as the inhibition of the PI3K/Akt
mechanistic target of rapamycin kinase signaling pathway (32). Therefore, the present study
hypothesized that PTEN/Akt may be a downstream signaling pathway
modulated by the expression of ERβ. In the present study, icaritin
inhibited the protein expression of PTEN, Akt and p-Akt in a
dose-dependent manner. It has been demonstrated that PTEN serves a
critical functions in tumorigenesis and is an important therapeutic
target. Mutations or deletions of the PTEN gene are the main
genetic changes identified in glioblastomas (33). The present study demonstrated that
icaritin activated the ERβ/PTEN/Akt signaling pathway and
investigated whether the anti-glioblastoma effects of icaritin were
dependent on the interaction between icaritin and ERβ. To achieve
this, the effect of E2 on the viability of glioblastoma cells was
initially assessed. A previous study had indicated that E2
activates the c-Jun N-terminal kinase/c-Jun signaling cascade and
inhibits the growth of rat C6 glioblastoma and human T98G
glioblastoma cells, with an half maximal inhibitory concentration
of 3.5 µM (34). The results of the
present study indicated that E2 treatment for 24 h reduced the
viability of C6 and U87-MG cells to 71.33 and 59.28%, respectively.
Treatment with 25 µM icaritin significantly increased the
anti-growth effects of E2(P<0.001, compared with the 0 µM
icaritin group). ICI 182,780, also known as fulvestrant, is a
7α-alkylsulfinyl analogue of E2 and is widely used as a specific
antagonist of intracellular ERs (35). ICI 182,780 binds to ERs in a
dose-dependent manner and inhibits essential receptor dimerization,
which alters the conformation of the ER and prevents it from being
transported to the nucleus (36). In
the present study, ICI 182,780 was demonstrated to exert an
inhibitory effect on the anti-growth activity of icaritin in
glioblastoma cells. This observation supported the notion that the
ER serves an important function in the anti-glioblastoma effects of
icaritin. Therefore, the results indicated that icaritin exerts
anti-glioblastoma effects, at least in part, via modulating
ERβ.
In conclusion, the results of the present study
demonstrated that icaritin may modulate ERβ and the downstream
PTEN/Akt signaling pathway, resulting in subsequent suppression of
glioblastoma cell growth and migration. The results also provide
some evidence to suggest that icaritin may be a potential treatment
for patients with glioblastoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81660031, 81360090
and 81060272) and the Guangxi Natural Science Foundation of China
(grant no. GXNSFBA2014118151).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and JD participated in research design. XL, WZ,
LL conducted experiments. WZ and XD contributed new reagents and
analytic tools. XL, JD, WZ and LL performed data analysis. YZ, JD
and XL wrote or contributed to the writing of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller CR and Perry A: Glioblastoma. Arch
Pathol Lab Med. 131:397–406. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wirsching HG, Galanis E and Weller M:
Glioblastoma. Handb Clin Neurol. 134:381–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Das S and Marsden PA: Angiogenesis in
glioblastoma. N Engl J Med. 369:1561–1563. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Burns DM, D'Ambrogio A, Nottrott S and
Richter JD: CPEB and two poly(A) polymerases control miR-122
stability and p53 mRNA translation. Nature. 473:105–108.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Longstreth WT Jr, Dennis LK, McGuire VM,
Drangsholt MT and Koepsell TD: Epidemiology of intracranial
meningioma. Cancer. 72:639–648. 1993.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Azcoitia I, Arevalo MA and Garcia-Segura
LM: Neural-derived estradiol regulates brain plasticity. J Chem
Neuroanat. 89:53–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Simpson ER: Sources of estrogen and their
importance. J Steroid Biochem Mol Biol. 86:225–230. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ogawa S, Tsukahara S, Choleris E and
Vasudevan N: Estrogenic regulation of social behavior and sexually
dimorphic brain formation. Neurosci Biobehav Rev: Oct 27, 2018
(Epub ahead of print).
|
|
9
|
Naftolin F, Ryan KJ and Petro Z:
Aromatization of androstenedione by the anterior hypothalamus of
adult male and female rats. Endocrinology. 90:295–298.
1972.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Batistatou A, Kyzas PA, Goussia A,
Arkoumani E, Voulgaris S, Polyzoidis K, Agnantis NJ and Stefanou D:
Estrogen receptor beta (ERbeta) protein expression correlates with
BAG-1 and prognosis in brain glial tumours. J Neurooncol. 77:17–23.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Batistatou A, Stefanou D, Goussia A,
Arkoumani E, Papavassiliou AG and Agnantis NJ: Estrogen receptor
beta (ERbeta) is expressed in brain astrocytic tumors and declines
with dedifferentiation of the neoplasm. J Cancer Res Clin Oncol.
130:405–410. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu B, Chen Y, Huang J, Ning Y, Bian Q,
Shan Y, Cai W, Zhang X and Shen Z: Icariin improves cognitive
deficits and activates quiescent neural stem cells in aging rats. J
Ethnopharmacol. 142:746–753. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li Z, Meng X and Jin L: Icaritin induces
apoptotic and autophagic cell death in human glioblastoma cells. Am
J Transl Res. 8:4628–4643. 2016.PubMed/NCBI
|
|
14
|
Dell'Agli M, Galli GV, Dal Cero E, Belluti
F, Matera R, Zironi E, Pagliuca G and Bosisio E: Potent inhibition
of human phosphodiesterase-5 by icariin derivatives. J Nat Prod.
71:1513–1517. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu S, Wang Z, Li Z, Peng H, Luo Y, Deng
M, Li R, Dai C, Xu Y, Liu S and Zhang G: Icaritin suppresses
multiple myeloma, by inhibiting IL-6/JAK2/STAT3. Oncotarget.
6:10460–10472. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma H, He X, Yang Y, Li M, Hao D and Jia Z:
The genus Epimedium: An ethnopharmacological and phytochemical
review. J Ethnopharmacol. 134:519–541. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li W, Winters A, Poteet E, Ryou MG, Lin S,
Hao S, Wu Z, Yuan F, Hatanpaa KJ, Simpkins JW and Yang SH:
Involvement of estrogen receptor beta5 in the progression of
glioma. Brain Res. 1503:97–107. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Akagi T, Murata K, Shishido T and Hanafusa
H: v-Crk activates the phosphoinositide 3-kinase/AKT pathway by
utilizing focal adhesion kinase and H-Ras. Mol Cell Biol.
22:7015–7023. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou C, Gu J, Zhang G, Dong D, Yang Q,
Chen MB and Xu D: AMPK-autophagy inhibition sensitizes
icaritin-induced anti-colorectal cancer cell activity. Oncotarget.
8:14736–14747. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang JG, Lu R, Ye XJ, Zhang J, Tan YQ and
Zhou G: Icaritin Reduces Oral Squamous Cell Carcinoma Progression
via the Inhibition of STAT3 Signaling. Int J Mol Sci.
18(E132)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ma T, Li ZJ, Wang LN, Li Z, Mu XL, Lyu Y
and Xi YM: Effect of Icaritin on K562 cell proliferation and
intracellular reactive oxygen species. Zhongguo Shi Yan Xue Ye Xue
Za Zhi. 24:1003–1007. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Guo Y, Zhang X, Meng J and Wang ZY: An
anticancer agent icaritin induces sustained activation of the
extracellular signal-regulated kinase (ERK) pathway and inhibits
growth of breast cancer cells. Eur J Pharmacol. 658:114–122.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sareddy GR, Nair BC, Gonugunta VK, Zhang
QG, Brenner A, Brann DW, Tekmal RR and Vadlamudi RK: Therapeutic
significance of estrogen receptor beta agonists in gliomas. Mol
Cancer Ther. 11:1174–1182. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu B, Jiang C, Han H, Liu H, Tang M, Liu
L, Ji W, Lu X, Yang X, Zhang Y and Liu Y: Icaritin inhibits the
invasion and epithelial-to-mesenchymal transition of glioblastoma
cells by targeting EMMPRIN via PTEN/AKt/HIF-1α signalling. Clin Exp
Pharmacol Physiol. 42:1296–1307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kabat GC, Etgen AM and Rohan TE: Do
steroid hormones play a role in the etiology of glioma? Cancer
Epidemiol Biomarkers Prev. 19:2421–2427. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
McKinley BP, Michalek AM, Fenstermaker RA
and Plunkett RJ: The impact of age and sex on the incidence of
glial tumors in New York state from 1976 to 1995. J Neurosurg.
93:932–939. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Warner M, Huang B and Gustafsson JA:
Estrogen receptor β as a pharmaceutical target. Trends Pharmacol
Sci. 38:92–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dey P, Barros RP, Warner M, Ström A and
Gustafsson JA: Insight into the mechanisms of action of estrogen
receptor beta in the breast, prostate, colon, and CNS. J Mol
Endocrinol. 51:T61–T74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen MF, Qi L, Li Y, Zu XB, Dai YQ and
Zhang P: Icaritin induces growth inhibition and apoptosis of human
prostatic smooth muscle cells in an estrogen receptor-independent
manner. Amino Acids. 38:1505–1513. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang L, Wang Y, Chen P, Hu J, Xiong Y,
Feng D, Liu H, Zhang H, Yang H and He J: Na(+)/H(+) exchanger
regulatory factor 1 (NHERF1) is required for the
estradiol-dependent increase of phosphatase and tensin homolog
(PTEN) protein expression. Endocrinology. 152:4537–4549.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guido C, Panza S, Santoro M, Avena P,
Panno ML, Perrotta I, Giordano F, Casaburi I, Catalano S, De Amicis
F, et al: Estrogen receptor beta (ERβ) produces autophagy and
necroptosis in human seminoma cell line through the binding of the
Sp1 on the phosphatase and tensin homolog deleted from chromosome
10 (PTEN) promoter gene. Cell Cycle. 11:2911–2921. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Liu X, Wang L, Chen J, Ling Q, Wang H, Li
S, Li L, Yang S, Xia M and Jing L: Estrogen receptor β agonist
enhances temozolomide sensitivity of glioma cells by inhibiting
PI3K/AKT/mTOR pathway. Mol Med Rep. 11:1516–1522. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen P, Zhao D, Li J, Liang X, Li J, Chang
A, Henry VK, Lan Z, Spring DJ, Rao G, et al: Symbiotic
macrophage-glioma cell interactions reveal synthetic lethality in
PTEN-Null glioma. Cancer Cell. 35:868–884, e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Altiok N, Ersoz M and Koyuturk M:
Estradiol induces JNK-dependent apoptosis in glioblastoma cells.
Oncol Lett. 2:1281–1285. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Robertson JF: ICI 182,780
(Fulvestrant)-the first oestrogen receptor down-regulator - current
clinical data. Br J Cancer 85. (Suppl 2):S11–S14. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dauvois S, White R and Parker MG: The
antiestrogen ICI 182780 disrupts estrogen receptor
nucleocytoplasmic shuttling. J Cell Sci. 106:1377–1388.
1993.PubMed/NCBI
|