Introduction
Despite the demonstrable benefit of inhaled
glucocorticoids (GCs) for improving asthma symptoms and lung
function in patients with asthma, referred to as steroid-sensitive
(SS) asthma, it is estimated that ≤5% of asthma cases are
relatively insensitive to steroid therapy (1). Aside from Th17-induced airway
inflammation, it is apparent that activation of monocytes and
macrophages is associated with steroid-resistant (SR) asthma
(2,3). Macrophage-induced airway inflammation
in mice is associated with SR airway inflammation and airway
hyperresponsiveness (AHR) (4).
Mechanistically, monocytes contribute to the amplification of
inflammation in an asthma mouse model, including generation of
cytokines and acceleration of the T helper cell 2 (Th2)-mediated
immune response via their differentiation into dendritic cells and
inflammatory macrophages (5-7).
Clinical studies have also revealed that monocytes from patients
with SR asthma show functional activation compared with those from
patients with SS asthma (8). Thus,
modulation of monocyte activation may be an effective therapeutic
approach for SR asthma.
GCs exert a wide range of anti-inflammatory effects
on monocytes after binding to their glucocorticoid receptors (GR),
which translocate into the nucleus from cytoplasm (9). GRs then bind to glucocorticoid response
elements (GRE) and regulate the transcription of related genes,
including mitogen-activated kinase phosphatase (MKP-1) (10). MKP-1, a phosphatase, selectively
inactivates phosphorylated-p38 mitogen activated kinases (p-p38
MAPK), thus suppressing the production of pro-inflammatory
cytokines (11). It has been
reported that p38 MAPK pathway activation in peripheral blood
mononuclear cells (PBMCs) is significantly increased in patients
with SR asthma compared with patients with SS asthma (8). In addition, previous studies showed
that inhibition of p38 MAPK pathway activation using vitamin D or
p-p38 MAPK inhibitor significantly enhanced dexamethasone
(DEX)-induced inhibition of cellular responsiveness to GCs in
patients with SR asthma (3,12,13).
Therefore, targeting the GRE-based signaling pathway may be a novel
therapeutic strategy for SR asthma.
Interleukin (IL)-35 is an important
anti-inflammatory cytokine formed by IL-12α and Epstein-Barr
virus-induced gene 3 (EBI3), and underpins the pathogenesis of
asthma (14). In a mouse model,
administration of recombinant IL-35 (AdIL-35) reversed
IL-17-dependent AHR and suppressed the severity of airway
inflammation (15,16). Histological analysis shows that
AdIL-35 reduces the number of pro-inflammatory cells and levels of
pro-inflammatory cytokines, including IL-17, IL-4 and IL-5, in
bronchoalveolar lavage fluid (17,18).
Interleukin-35-induced regulatory T cells (iTr35) are known to
suppress the proliferation of Th17 cells and to inhibit the
inflammatory response of immune cells (16,19).
However, it is still unknown whether IL-35 can efficiently enhance
the inhibitory effect of DEX on monocytes in patients with SR
asthma.
Therefore, the present study investigated the
percentage of iTr35 cells and the expression levels of serum IL-35
in peripheral blood from patients with SR or SS asthma. In
addition, the effects of IL-35 on cellular responses to
glucocorticoids in vitro were examined in the monocytes of
these patients. Furthermore, the present study analyzed the effects
of IL-35 on the induction of mitogen-activated protein kinase
phosphatase-1 (MKP-1) expression and the recruitment of GR to the
GRE in monocytes. It was found that decreased IL-35 expression
levels in peripheral blood from patients with SR asthma were
involved in the corticosteroid insensitivity of monocytes,
suggesting potential benefits of IL-35 supplementation in patients
with asthma with DEX.
Materials and methods
Study population
The present study was carried out under ethical
approval from the Ethics Committee of Binhai Hospital (approval no.
2017/05). After detailed explanation, informed consent was provided
by all patients. The sample size was determined using a calculation
for two-samples t-test and it was expected that a difference of 0.2
ng/ml would be detected in serum IL-35 between patients with SR or
SS asthma. To have a study with a power of 1-β=0.90 and a
statistical difference of P<0.05, a sample of 18 patients in
each group was required.
A total of 392 adults with asthma (172 women and 220
men; mean age, 39.1±8.3 years) were enrolled at the respiratory
clinic of Binhai County People's Hospital (Jiangsu, China) between
August 2017 and November 2018. No patients had received oral
glucocorticoids for >1 month before enrollment in the present
study. Patients who had a history of infection, who had received
immunotherapy or who had smoked in the previous month were
excluded.
All 392 asthmatic patients received oral
prednisolone (40 mg/1.73 m2/d; Wockhardt, Ltd.) for 14
days. The clinical response of patients to corticosteroid therapy
was measured with a PC-based spirometer (WinspiroPRO; Medical
International Research). Patients with asthma were defined as SR if
they experienced <10% improvement, or as SS if they experienced
≥10% improvement in baseline forced expiratory volume (FEV) in 1
sec after a 14-day course of oral prednisolone (20). In total, 20 patients were diagnosed
with SR asthma and 372 patients were diagnosed with SS asthma.
According to the SR group, 34 sex and age-matched SS asthma
controls were selected from 372 hormone therapy sensitive patients.
Patient characteristics are presented in Table I.
 | Table IClinical characteristics of patients
with asthma. |
Table I
Clinical characteristics of patients
with asthma.
|
Characteristics | SR asthma
(n=20) | SS asthma
(n=34) |
|---|
| Age, years | 37.4±6.2 | 38.6±6.4 |
| Sex,
male/female | 10/10 | 17/17 |
| BMI,
kg/m2 | 27.4±2.3 | 27.1±2.4 |
| Baseline predicted
FEV1% |
62.5±5.7a | 73.1±6.3 |
| IgE, U/ml |
180.6±55.2a | 129.6±43.2 |
| Change in FEV1%
after prednisone burst |
2.5±0.9a | 25.6±8.2 |
Peripheral blood sample
preparation
A total of 20 ml peripheral venous blood was
collected from each patient. Then, 1 ml was collected into a tube
containing heparin sodium (10 IU/ml; Sigma-Aldrich; Merck KGaA) for
iTr35 cell and intracellular p-p38 MAPK flow cytometric analysis
within 24 h, and another 1 ml was centrifuged at room temperature
for 10 min at 840 x g to prepare serum, which was stored at -80˚C
for later cytokine IL-35 determination. The remaining 18 ml was
used for monocyte isolation and culture.
Flow cytometric detection of iTr35
cells
For iTr35 cell analysis, 100 µl venous blood was
activated with ionomycin (1 µg/ml), monensin (1 µg/ml) and
phorbol-12-myristate-13-acetate (50 ng/ml; all from Sigma-Aldrich;
Merck KGaA) for 5 h at 37˚C. After erythrocytes were lysed in
fluorescence-activated cell sorting lysing solution (BD
Biosciences), the remaining cells were stained with
PerCP-cy5.5-conjugated anti-CD4 antibodies (1:10; cat. no. 4291864;
ebioscience; Thermo Fisher Scientific, Inc.) for 30 min at room
temperature. Next, cells were fixed and permeabilized using the BD
cytofix/cytoperm kit according to the manufacturer's protocol (BD
Biosciences), before staining with phycoerythrin-conjugated
anti-IL-27 EBI3 (1:10; cat. no 4329546), eFluor 660 labeled
anti-IL-12P35 (1:10; cat. no 4323861), and FITC-conjugated
anti-forkhead box P3 (Foxp3) antibodies (1:10; cat. no E15373-103)
(all from ebioscience; Thermo Fisher Scientific, Inc.) for 20 min
at room temperature. Then, cells were analyzed by a flow cytometer
(Becton, Dickinson and Company).
iTr35 cell were identified as follows: i) Forward
and side scattering was used to gate lymphocytes; ii) CD4-positive
and Foxp3-negative cells were detected in gated lymphocytes; and
iii) IL-12P35 and IL-27EBI3 double positive cells were detected in
CD4+FoxP3- lymphocytes. The iTr35 cell
percentage was measured as the percentage of
CD4+FoxP3-IL-12P35+IL-27EBI3+
cells among the CD4+ T cells.
Isolation and treatment of
monocytes
PBMCs were isolated by two-step gradient
centrifugation (21). PBMCs were
obtained by Ficoll-Hypaque density gradient centrifugation (at room
temperature for 20 min at 800 x g) and resuspended in Percoll
solution 1 (ρ=1.123 g/ml). Then, the cells were overlaid with
Percoll solution 2 (ρ=1.064 g/ml) and Percoll solution 3 (ρ=1.032
g/ml; all, Beijing Solarbio Science & Technology Co., Ltd.) at
room temperature for 5 min. Monocytes were obtained from the
RPMI/Percoll interface after centrifugation at 1,000 x g for 90 min
at 20˚C. The purity of monocytes was 80-85% as measured by flow
cytometric analysis of the CD14+ cells as previously
described (6). Isolated monocytes
were resuspended in RPMI 1640 (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS (Sigma-Aldrich; Merck KGaA) for in
vitro culture.
To examine the cellular responses to IL-35
treatment, monocytes were seeded at a density of 5x105
cells/ml in 24-well plates (Corning, Inc.) and treated with RPMI
1,640 containing 20 ng/ml rIL-35 (PeproTech, Inc.) for 4 h at 37˚C.
This was followed by cell stimulation by 10 nM DEX (Sigma-Aldrich;
Merck KGaA), with or without 1 ng/ml LPS (Sigma-Aldrich; Merck
KGaA) for 3 h at 37˚C. Cells were collected for MKP-1 mRNA reverse
transcription-quantitative PCR (RT-qPCR) or chromatin
immunoprecipitation (ChIP) assay. The expression level of p-p38
MAPK in CD14+ cells was analyzed by flow cytometric
analysis.
Corticosteroid sensitivity assay
Monocytes were seeded at a density of
1x104 cells/ml in 96-well plates (Corning, Inc.) and
pre-incubated with different concentrations of DEX
(10-11-10-6 M) for 1 h prior to stimulation
with 1 ng/ml LPS at 37˚C. After 24 h in culture, the cell
supernatant was collected via centrifugation at a speed of 800 x g
for 5 min at room temperature and stored at -80˚C for IL-6 analysis
using the Bio-plex 200 suspension array Luminex system (Bio-rad
Laboratories, Inc.). Corticosteroid sensitivity was evaluated
according to the half-maximal inhibitory concentration of DEX with
respect to LPS-induced IL-6 maximal production in monocytes
(DEX-IC50). The percentage of maximal inhibition of IL-6
by DEX was presented as Emax. The values for individual
patients were presented as log (DEX-IC50) and compared
among groups.
Cytokine assay
Serum IL-35 and IL-6 concentrations in the culture
supernatant were detected via the Luminex 200 platform based on the
manufacturer's protocol (Bio-rad, Laboratories, Inc.). Each
experiment was performed twice. The minimum detectable levels of
cytokines IL-35 and IL-6 in the assay were 0.15 ng/ml and 1.34
pg/ml, respectively.
p-p38 MAPK detection by flow
cytometric analysis
For intracellular p-p38 MAPK staining, 100 µl
heparin-anticoagulated venous blood was stained with
phycoerythrin-conjugated anti-CD14 monoclonal antibodies for 20 min
at room temperature (1:10; cat. no 1543861; BD Biosciences). Cell
fixation and permeabilization were performed using the BD
cytofix/cytoperm kit according to the manufacturer's protocol (BD
Biosciences). Subsequently, the cells were stained with
FITC-conjugated anti-p-p38 MAPK monoclonal antibodies for 20 min at
room temperature. (1:10; cat. no 127841; BD Biosciences). Then, the
expression levels of p-p38 MAPK in CD14+ cells from
patients were analyzed using a FACScaliber instrument (BD
Biosciences).
MKP-1 mRNA detection by RT-qPCR
analysis
After monocytes were treated with rIL-35 followed by
DEX with or without LPS, cells were collected and total RNA was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). cDNA synthesis was carried out using the
cDNA RT kit (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The temperature protocol was as follows: 10 min at 25˚C, followed
by 50˚C for 20 min. Next, qPCR was carried out using the QuantiTect
SYBR Green PCR kit (Invitrogen; Thermo Fisher Scientific, Inc.).
The thermocycling conditions for PCR were as follows: 95˚C for 5
min; followed by 40 cycles of 95˚C for 15 sec, 55˚C for 25 sec,
72˚C for 30 sec and final extension at 72˚C for 5 min. The
following primer sequences were used: MKP-1 sense,
5'-GTCGGTAGAGAGTCTACGCT-3' and antisense,
5'-TCGTGTGGAACATTCATTC-3'; and β-actin sense,
5'-TGGCACCCAGCACAATGAA-3' and antisense,
5'-CTAAGTCATAGTCCGCCTAGAAGCA-3'. PCR was performed using the ABI
7500 PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Relative MKP-1 mRNA expression levels were calculated by the
2-ΔΔCq method and normalized
against β-actin expression levels (22).
ChIP assay
To examine the effect of IL-35 on GR binding to the
GRE, a GR-binding site in the MKP-1 gene promoter, a ChIP assay was
performed using the ChIP-IT Express kit (Zhonghong Boyuan
Biotechnology Co., Ltd.), according to the manufacturer's
instructions. Monocytes were pre-incubated with 20 ng/ml rIL-35 for
4 h at 37˚C, followed by 10 nM DEX for 3 h at 37˚C. DNA was
precipitated by rabbit polyclonal anti-GR antibodies (1:20; cat. no
172832; Zhonghong Boyuan Biotechnology Co., Ltd.) and measured by
RT-qPCR. The primers used to detect MKP-1 promoter were synthesized
as described previously (23) using
the same protocol as listed above. The quantity of anti-GR
antibody-precipitated DNA was calculated after normalization to
input DNA.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 7.0 software (GraphPad Software, Inc.). Mann-Whitney U test
was used for the skewed data expressed as the median (25-75th
percentile). Normally distributed data are presented as the mean ±
SD. Statistical significance was analyzed by Student's t-test or
one-way ANOVA followed by Tukey's test. Spearman correlation
analysis was used to determine the linear correlation coefficients.
P<0.05 was considered to indicated a statistical significance
difference.
Results
Clinical characteristics of study
population
The clinical characteristics of the 20 patients who
were hormone therapy resistant and the 34 sex and age-matched
patients who were hormone therapy sensitive are presented in
Table I.
Patients with SR asthma show lower
IL-35 concentration and iTr35 cell proportion
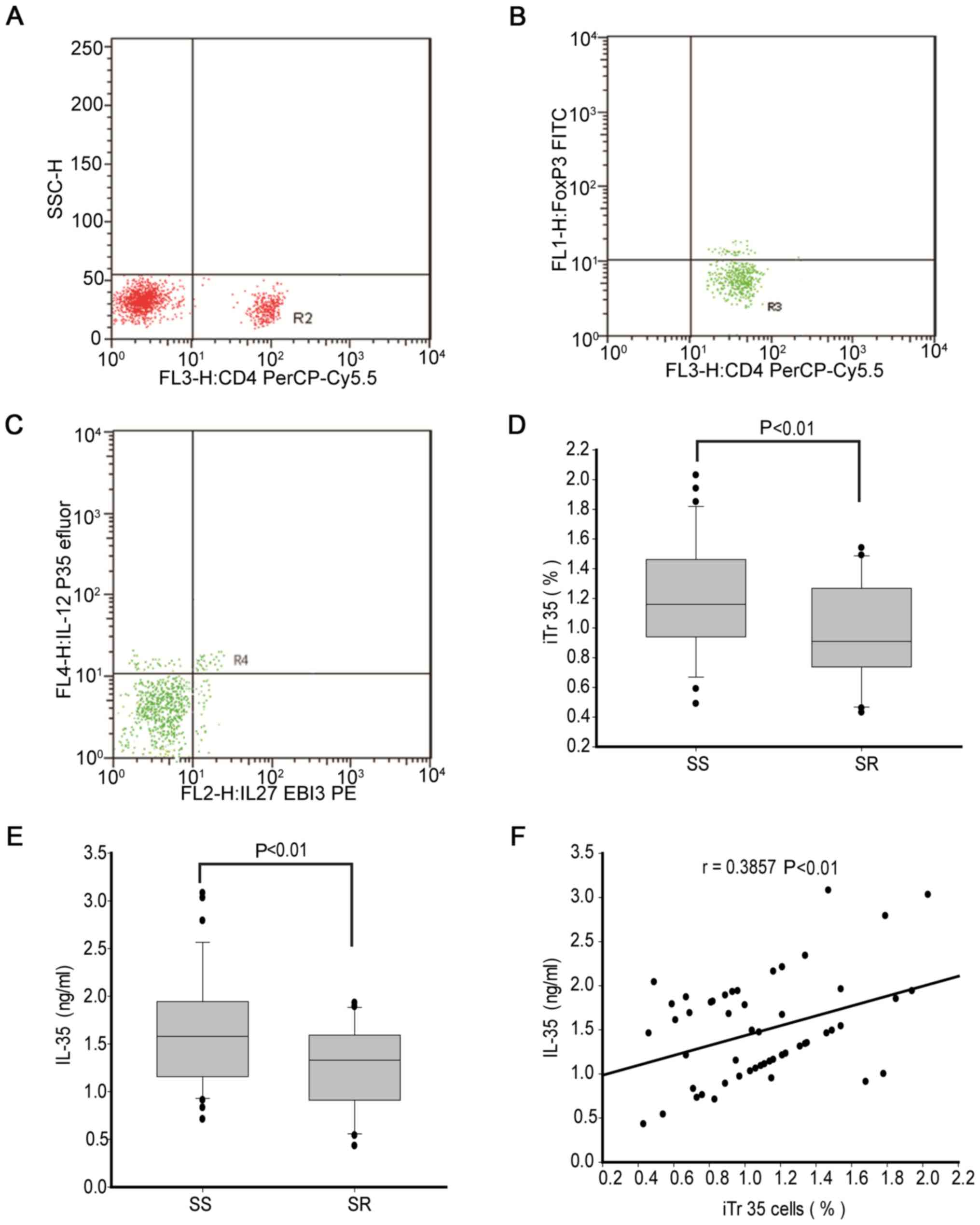
By gating for CD4+T lymphocytes (Fig. 1A), CD4+FoxP3- T
cells were classified (Fig. 1B) and
then the percentage of iTr35 cells was analyzed in the peripheral
blood (Fig. 1C). The percentage of
iTr35 cells was significantly lower in patients with SR asthma
(median, 0.83%; range, 0.73-1.24%) compared with patients with SS
asthma (median, 1.18%; range, 0.95-1.44%; Fig. 1D).
In addition, the present study measured the
expression levels of IL-35 in serum. The concentration of IL-35 was
significantly lower in patients with SR asthma (median, 1.32 ng/ml;
range, 0.91-1.59 ng/ml) compared with patients with SS asthma
(median, 1.63 ng/ml; range, 1.17-1.93 ng/ml; Fig. 1E). Correlation analysis results
demonstrated a weak positive correlation between the percentage of
iTr35 cells (r=0.386; P<0.01; Fig.
1F) and IL-35 concentration in all patients with asthma.
IL-35 displays a positive correlation
with corticosteroid sensitivity in monocytes from patients with
asthma
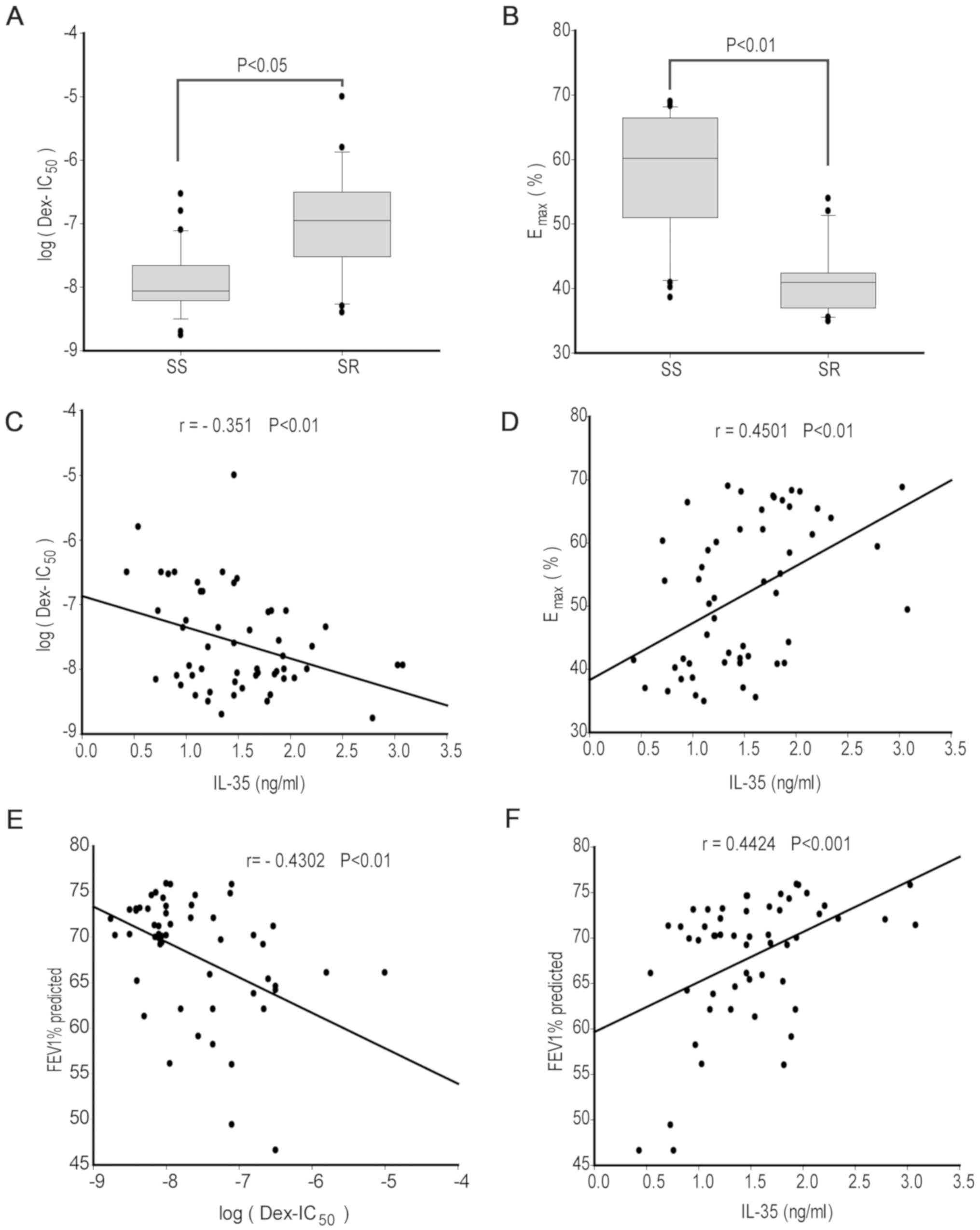
Monocytes from 34 patients with SS asthma and 20
patients with SR asthma were treated with different concentrations
of DEX followed by LPS stimulation for 24 h. A higher log
(DEX-IC50) was found for patients with SR asthma
(median, -7.1; range, -6.5- -7.5) compared with patients with SS
asthma (median, -8.1; range, -7.7- -8.2; P<0.05; Fig. 2A). In addition, the Emax
value was significantly lower in patients with SR asthma (median,
40.6%; range, 37.2-41.8%) compared with patients with SS asthma
(median, 60.3%; range, 51.3-67.1%; P<0.01; Fig. 2B). Therefore, the present results
suggested that monocytes in patients with SR asthma were less
sensitive to steroid treatment compared with those from patients
with SS asthma.
Correlation analysis results identified that the
expression levels of IL-35 showed a weak negative correlation with
log (DEX-IC50; r=-0.351; P<0.01; Fig. 2C) and a moderate positive correlation
with Emax value (r=0.4501; P<0.01; Fig. 2D) in all patients with asthma. In
addition, the FEV1% predicted had a moderate negative correlation
with log (DEX-IC50; r=-0.4302; P<0.01; Fig. 2E) and a moderately positive
correlation with IL-35 (r=0.4424; P<0.001; Fig. 2F). Collectively, the present results
indicated that the decreased IL-35 expression levels may be
involved in the corticosteroid insensitivity of monocytes from all
patients with asthma.
IL-35 shows a negative correlation
with p-p38 MAPK in CD14+ monocytes of patients with
asthma
The present study analyzed the mean fluorescence
intensity (MFI) of p-p38 MAPK (p-p38 MFI) in monocytes by flow
cytometry (Fig. 3A). Compared to the
level in patients with SS asthma (median, 2.7; range, 1.8-4.0),
p-p38 MAPK was significantly increased in CD14+
monocytes of patients with SR asthma (median, 12.7; range,
10.4-15.1; P<0.01; Fig. 3B).
Furthermore, the concentration of IL-35 showed a weak negative
correlation with p-p38 MAPK in all patients with asthma (r=0.352;
P<0.01; Fig. 3C). In addition,
there was a strong negative correlation between p-p38 MAPK and
FEV1% (r=-0.718; P<0.001; Fig.
3D). Moreover, p38 phosphorylation was found to be moderately
positively correlated with log(DEX-IC50) (r=0.594;
P<0.001; Fig. 3E) and moderately
negatively correlated with the Emax value in patients
(r=-0.631; P<0.001; Fig. 3F).
Therefore, the present results suggested that IL-35 may be involved
in p38 phosphorylation in monocytes.
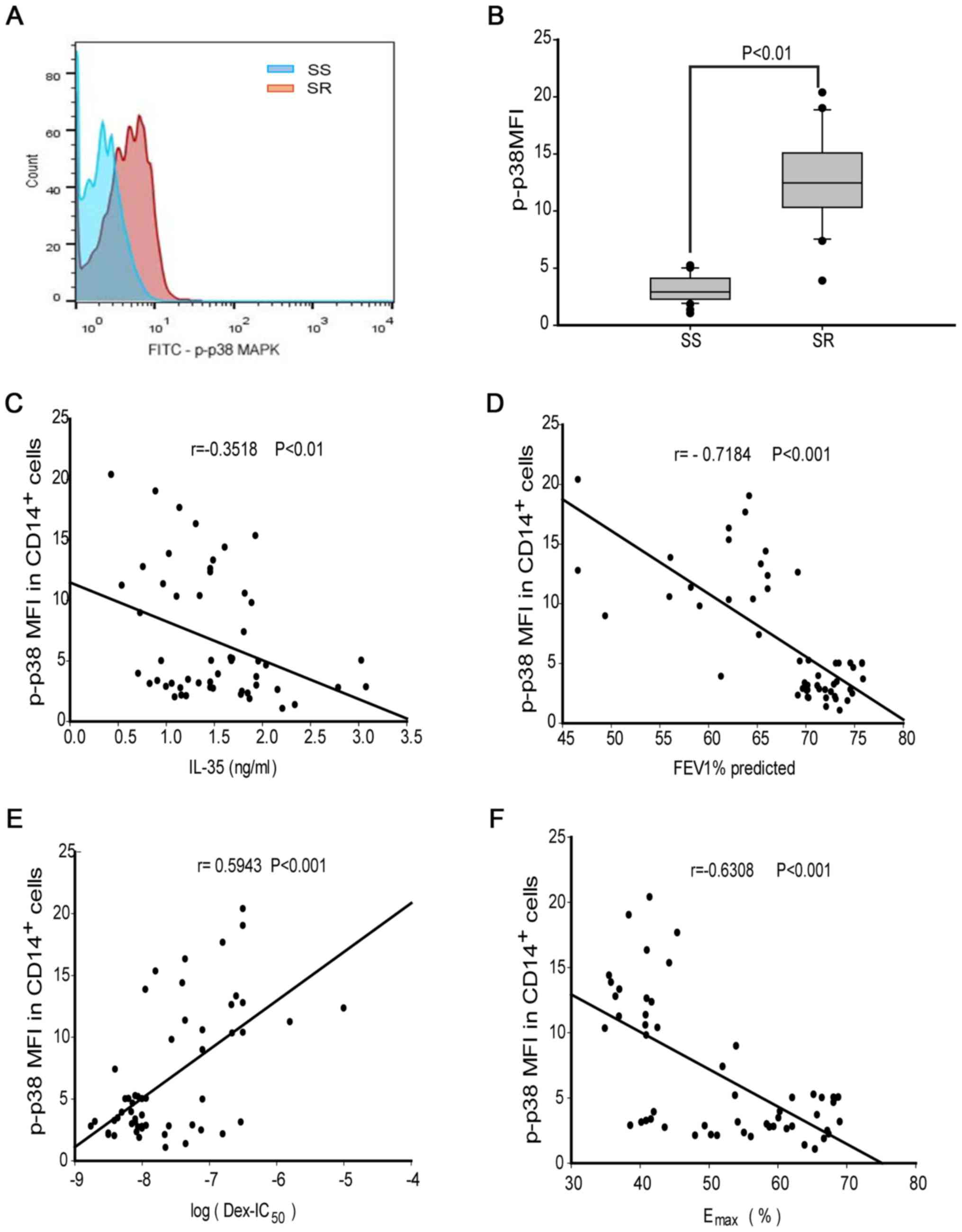 | Figure 3MAPK phosphorylation in
CD14+ monocytes from patients with SR or SS asthma. (A)
Plots of p-p38 MAPK MFI in gated CD14+ monocytes. (B)
p-p38 MAPK MFI in CD14+ monocytes from patients with SR
or SS asthma. Correlations between p-p38 MAPK MFI and (C) IL-35
level, (D) predicted FEV1%, (E) log(DEX-IC50) and (F)
Emax were analyzed by Spearman correlation test.
Statistical significance was analyzed by Mann-Whitney U test. DEX,
dexamethasone; DEX-IC50, half-maximal inhibitory
concentration of DEX; Emax, percentage inhibition at
maximal concentration; IL, interleukin; FEV1, forced expiratory
volume in 1 sec; SR, steroid-resistant; SS, steroid-sensitive;
p-p38 MAPK, phosphorylated P38 mitogen activated kinase; MFI, mean
fluorescence intensity. |
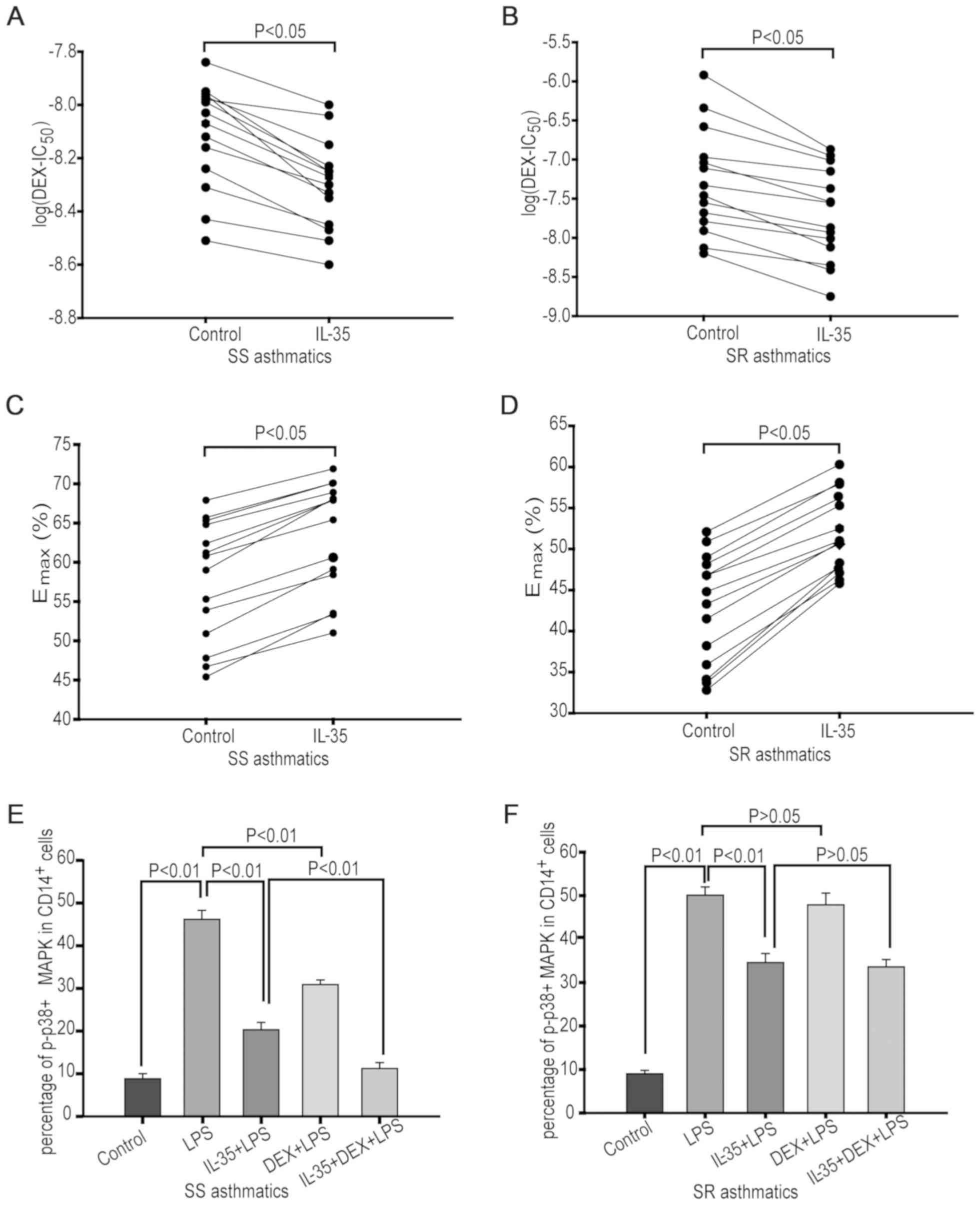
IL-35 enhances corticosteroid
responses and inhibits p-p38 MAPK activation in monocytes
To examine the effects of IL-35 on corticosteroid
responses, monocytes were treated with 20 ng/ml IL-35 and various
concentrations of DEX for 4 h followed by 1 ng/ml LPS stimulation
for 24 h. The value of log(DEX-IC50) was decreased
(P<0.05; Fig. 4A and B), and Emax values were
increased (P<0.05; Fig. 4C and
D) by pretreatment with IL-35 in
monocytes from both patients with SS and SR asthma. Thus, IL-35 may
improve corticosteroid sensitivity in monocytes from both patient
groups.
The present study also assessed the effects of IL-35
on suppression of p-p38 MAPK activation. Monocytes were treated
with 20 ng/ml IL-35 for 4 h followed by 10 nM DEX, with or without
subsequent treatment with 1 ng/ml of LPS for 24 h. It was found
that LPS significantly enhanced the percentage of
p-p38+CD14+ cells in monocytes from patients
with SS (P<0.01) and SR asthma (P<0.01; n=14; Fig. 4E and F). However, DEX treatment significantly
reversed the LPS-induced p-p38 MAPK activation in monocytes from
patients with SS asthma (P<0.01; n=14), but no significant
suppression was observed in monocytes from patients with SR asthma
(P=0.423; n=14). Furthermore, it was demonstrated that IL-35
pretreatment significantly suppressed LPS-induced p-p38 MAPK
activation in monocytes from patients with SS (P<0.01, n=14) and
SR asthma (P<0.01; n=14). In addition, the inhibition achieved
with IL-35 + DEX treatment was significantly higher compared with
the inhibitory effect caused by DEX or IL-35 alone in monocytes of
patients with SS asthma (P<0.01; n=14). However, IL-35 + DEX
treatment did not further increase the inhibition caused by IL-35
alone in monocytes from patients with SR asthma (P=0.442;
n=14).
IL-35 enhances DEX-induced MKP-1
expression in monocytes
A previous study showed that DEX can suppress
LPS-induced p38 MAPK activation in monocytes via MKP-1(13). To investigate the mRNA expression of
MKP-1 in response to IL-35 or IL-35 + DEX treatment, monocytes were
pretreated with IL-35 for 4 h followed by DEX treatment for 3 h.
Then, cells were collected to examine the mRNA expression of MKP-1
by RT-qPCR.
Compared to media control treatment, DEX
pretreatment significantly increased the expression of MKP-1 mRNA
in monocytes from both patients with SS (P<0.01; n=14) and SR
asthma (P<0.01; n=14). Furthermore, IL-35 treatment alone did
not significantly increase the MKP-1 mRNA expression level in
monocytes from both patients with SS (P=0.576; n=14) and SR asthma
(P=0.881; n=14). However, IL-35 co-treatment did significantly
increase the DEX-induced upregulation of MKP-1 mRNA expression
levels in monocytes from both patients with SS (P<0.01; n=14)
and SR asthma (P<0.01, n=14; Fig.
5A). However, it was found that the mRNA expression level of
MKP-1 was significantly higher in monocytes from patients with SS
asthma compared with those with SR asthma (P<0.01; n=14;
Fig. 5A).
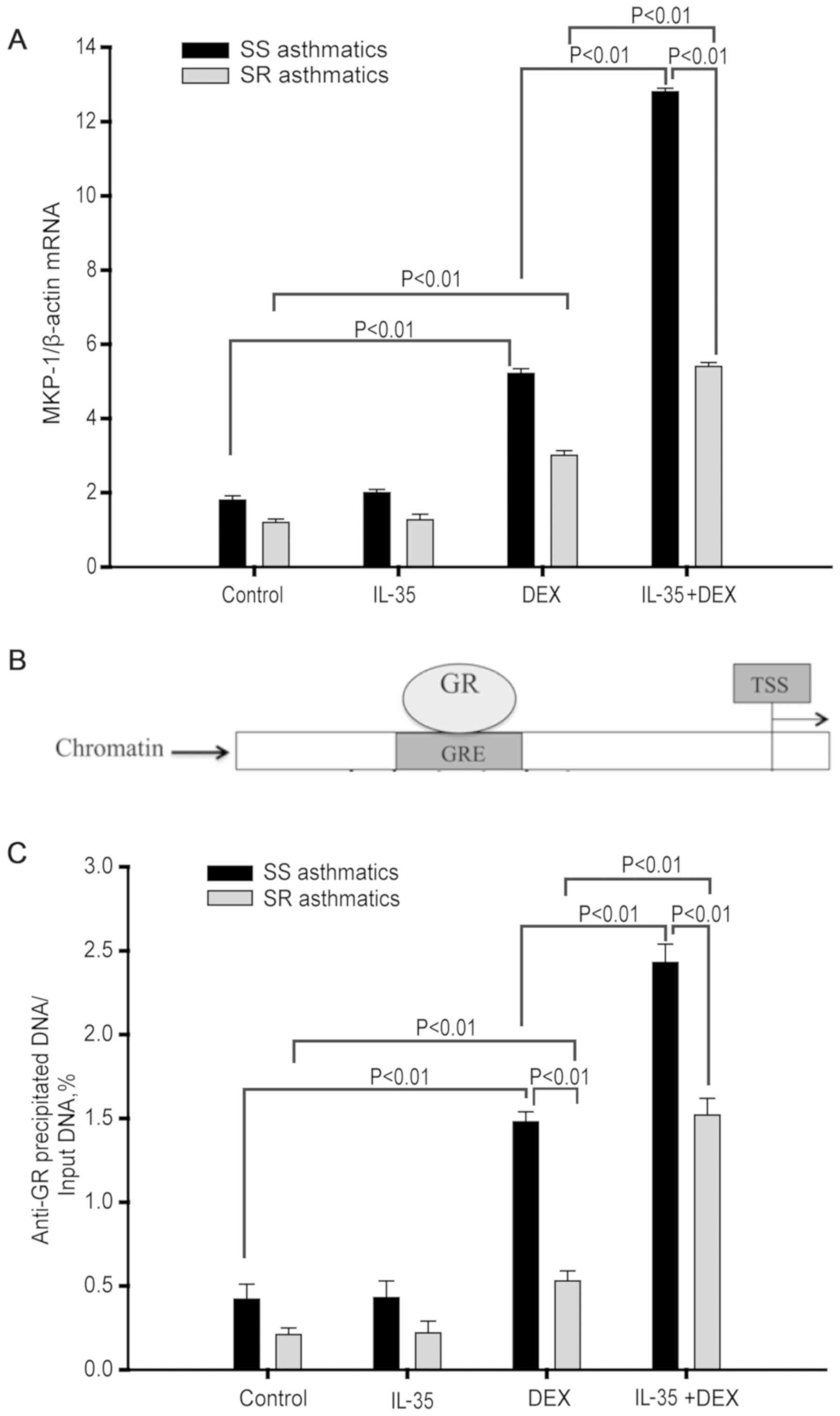 | Figure 5IL-35 enhances DEX-induced MKP-1
expression and recruitment of GR to the GRE in monocytes. (A) MKP-1
mRNA expression level was analyzed by reserve
transcription-quantitative PCR. SR, n=12; SS, n=12. (B) Schematic
of GR binding to GRE upstream of the TSS of the human MKP-1 gene.
(C) Amount of GR bound to GRE as measured by chromatin
immunoprecipitation analysis. SR, n=6; SS, n=6. Data are presented
as the mean ± SD. TSS, transcriptional start site; SR,
steroid-resistant; SS, steroid-sensitive; IL, interleukin; DEX,
dexamethasone; GR, glucocorticoid receptor; GRE, glucocorticoid
response element; MKP-1, mitogen-activated protein kinase
phosphatase-1. |
IL-35 enhances GR binding to the GRE
in the MKP-1 promoter
GRE, a GR-binding site in the MKP-1 gene promoter,
is located in 4.6 kb upstream of the MKP-1 gene transcriptional
start site (Fig. 5B) (23). The present results suggested that
IL-35 + DEX differentially upregulated MKP-1 expression levels in
monocytes from patients with SS and SR asthma. ChIP assays were
performed to further investigate whether this effect was due to
differential GR binding to the GRE.
The ChIP assay results found that DEX significantly
enhanced GR binding to GRE compared with the media control
treatment in monocytes from both patients with SS (P<0.01; n=12)
and SR asthma (P<0.01; n=12; Fig.
5C). However, this GR binding to GRE induced by DEX treatment
was significantly higher in monocytes from patients with SS asthma
compared with those with SR asthma (P<0.01; n=6; Fig. 5C). Moreover, IL-35 treatment did not
enhance GR binding to the GRE in monocytes from either patients
with SS (P=0.749; n=6) or SR asthma (P=0.776; n=6; Fig. 5C). Furthermore, IL-35 pre-treatment
significantly enhanced the DEX-induced increase in GR binding to
GRE in monocytes from both patients with SS (P<0.01; n=6) and SR
asthma (P<0.01; n=6; Fig. 5C).
However, it was found that in response to IL-35 + DEX, the amount
of GR bound to GRE was significantly increased in monocytes from
patients with SS asthma compared with those with SR asthma
(P<0.01; n=6; Fig. 5C).
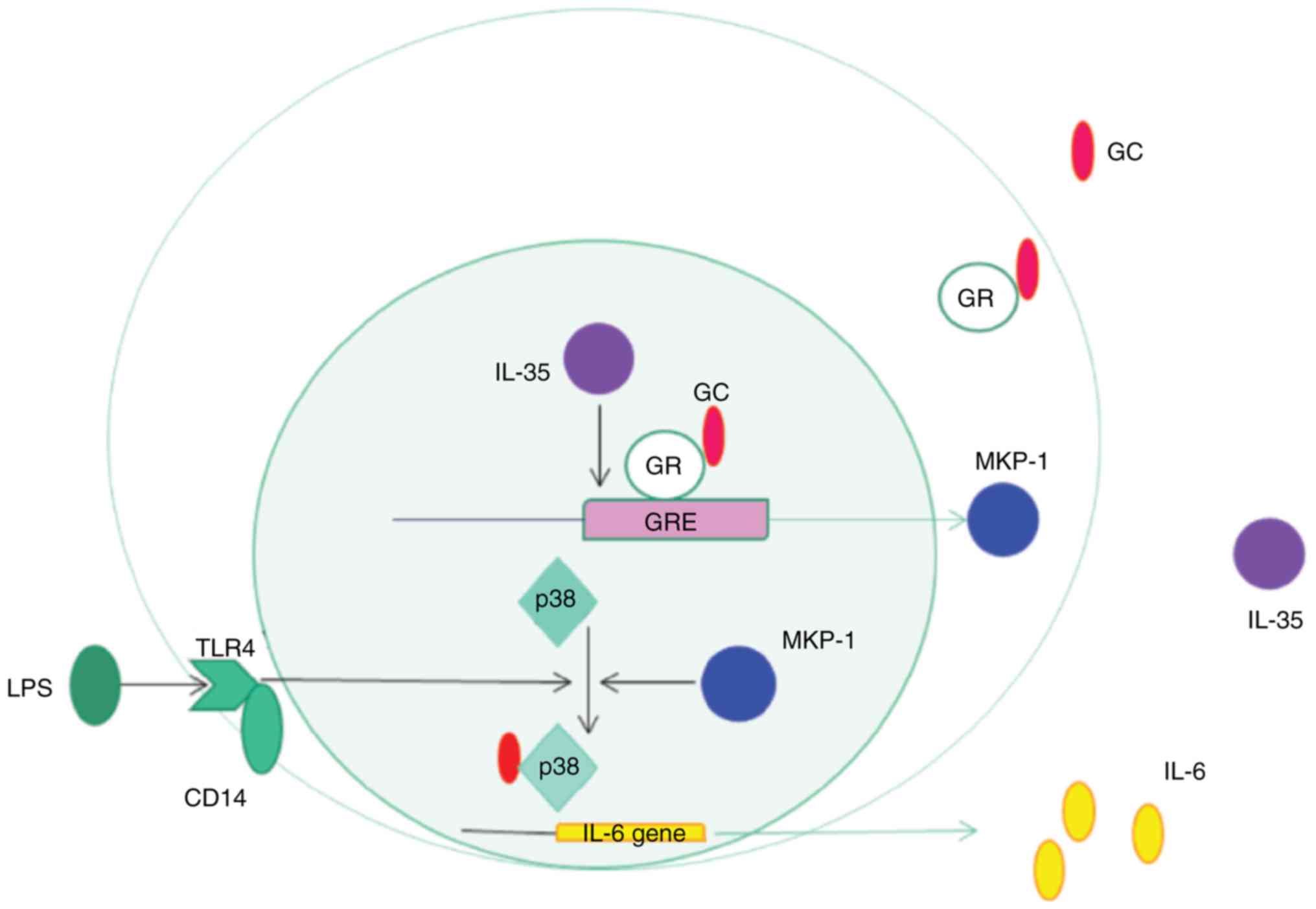
Collectively, the present results suggested that there may be a
regulatory signaling pathway by which IL-35 enhanced the binding of
GR to the GRE and induced MKP-1 expression, thus suppressing
LPS-induced p-p38 MAPK activation and enhancing corticosteroid
sensitivity in monocytes (Fig.
6).
Discussion
The present study provided several novel findings.
First, the percentage of iTr35 cells and the level of IL-35 in the
peripheral blood of patients with SR asthma were significantly
lower compared with those in SS asthma. Second, IL-35 enhanced the
corticosteroid sensitivity and inhibition of LPS-induced p-p38 MAPK
in monocytes treated with DEX from both patients groups. Moreover,
it was found that IL-35 may enhance effect of DEX by increasing the
MKP-1 mRNA expression level and GR binding to GRE.
The present results, indicating a significant
decrease in the percentage of iTr35 cells and expression level of
IL-35 in peripheral blood from patients with SR, are consistent
with previous studies showing that decreased IL-35 protein and mRNA
expression levels in peripheral blood are significantly related to
disease severity, as evidenced by the predicted FEV1% (24,25).
Furthermore, SR asthma is associated with Th1 and Th17-mediated
responses, and monocytic or neutrophilic-mediated airway
inflammation, most of which are involved in severe asthma, with
lower levels of anti-inflammatory cytokines and regulatory immune
cells compared with SS asthma (26).
In addition, the present study identified a positive correlation
between the iTr35 cell percentage and IL-35 expression level.
Therefore, the present results suggested that reductions in IL-35
and iTr35 cells may be involved in the pathogenesis of asthma.
It was found that the expression level of serum
IL-35 was negatively correlated with corticosteroid insensitivity
in monocytes. In in vitro cell culture, it was demonstrated
that IL-35 may improve corticosteroid sensitivity in monocytes from
patients with asthma. In addition, IL-35 increased the
Emax of corticosteroids, suggesting that a smaller dose
of corticosteroids may be required for asthma treatment. Similarly,
it has been demonstrated that vitamin D treatment enhances
corticosteroid sensitivity in monocytes from patients with asthma
(27). Vitamin D and IL-35 are known
for their anti-inflammatory effects in the healthy population and
patients with asthma (17,24,27). The
present results suggested that anti-inflammatory substances and
cytokines may act synergistically with corticosteroid to inhibit
the activation of monocytes.
The present study investigated the role of IL-35 in
p38 MAPK phosphorylation, which regulates the production of
pro-inflammatory cytokines, such as IL-6, IL-8 and tumor necrosis
factor-α (28). Activated p38 MAPK
in alveolar macrophages of patients with asthma show a positive
correlation with the disease severity (12). Activated p38 MAPK in peripheral blood
monocytes can be detected by flow cytometric analysis, western
blotting or immunohistochemical analysis (8,27). In
the present study, flow cytometric detection was chosen due to the
small amount of protein in the specimen. It was found that IL-35
was negatively correlated with p38 phosphorylation in peripheral
blood CD14+ monocytes of patients with asthma. In
addition, IL-35 pretreatment inhibited LPS-induced p38
phosphorylation in monocytes from both patients with SS and SR
asthma. Similarly, using a mouse model of LPS-induced acute
inflammation, a previous study demonstrated that IL-35 inhibits
endothelial cell activation by suppressing the MAPK-AP1 pathway and
attenuating the secretion of proinflammatory cytokines (29).
Glucocorticoid receptor-mediated MKP-1 signaling is
a key process in the regulation of steroid resistance and
sensitivity (30). The MKP-1 gene
promoter region contains binding sites for several transcription
factors, such as activator protein 1 (AP-1), AP-2, vitamin D
receptor element and GRE (31). In
the present study, IL-35 + DEX induced a significantly higher MKP-1
mRNA expression level in patients with SS asthma compared with
those with SR asthma, which is consistent with the present p-p38
MAPK data. The ChIP assay results also indicated that IL-35
enhanced binding of GR to GRE in monocytes from patients with SS
asthma compared with those with SR asthma, indicating higher MKP-1
gene transcriptional activation in patients with SS asthma.
The present study has limitations that should be
considered. First, iTr35 cells can only be detected after
activation and permeabilization, and thus they could not be sorted
for investigation of their function. Second, the present results
suggested that IL-35 alone significantly decreased p-p38 MAPK
expression in monocytes stimulated by LPS, but did not increase
MKP-1 protein expression and GR binding to GRE. Several previous
studies have reported that multiple signaling pathways regulate
p-p38 MAPK activation at the epigenetic, transcriptional and
post-transcriptional levels (32,33).
Therefore, IL-35 may inhibit p-p38 MAPK activation via a variety of
pathways, thus further research is required. Moreover, the present
results indicated that the levels of IL-35 and iTr35 cells in
peripheral blood of patients with SR asthma are at a low basal
expression, suggesting that modulation of IL-35 and iTr35 levels
may be an effective therapeutic approach, such as antigen-specific
immunotherapy and intestinal probiotic immunity (34).
In summary, the present results suggested that the
percentage of iTr35 cells and the expression level of IL-35 in the
peripheral blood of patients with SR asthma were significantly
lower compared with those with SS asthma. In addition, it was found
that IL-35 had a positive correlation with corticosteroid
sensitivity in monocytes from patients with asthma, suggesting
potential benefits of IL-35 supplementation with corticosteroids
for asthma treatment. Mechanistically, it was demonstrated that
IL-35 enhanced the binding of GR to the GRE and induced MKP-1
expression, resulting in suppression of LPS-induced p-p38 MAPK
activation and enhanced corticosteroid sensitivity in monocytes
from both patient groups.
Acknowledgements
Not applicable.
Funding
The current study was supported by The Medical and
Health Technology Development Program in Yancheng City, China
(grant no. YK2016068).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LQ designed the current study and drafted the
manuscript. DX and FX recruited patients and interpreted clinical
data. LQ, ML, XW, GL collected, analyzed and interpreted the data.
GL designed the clinical study and critically revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was carried out under ethical
approval from the Ethics Committee of Binhai Hospital (approval no.
2017/05). After detailed explanation, informed consent was provided
by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
References
|
1
|
Zervas E, Samitas K, Papaioannou AI,
Bakakos P, Loukides S and Gaga M: An algorithmic approach for the
treatment of severe uncontrolled asthma. ERJ Open Res.
4:00125–2017. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fang SB, Zhang HY, Jiang AY, Fan XL, Lin
YD, Li CL, Wang C, Meng XC and Fu QL: Human iPSC-MSCs prevent
steroid-resistant neutrophilic airway inflammation via modulating
Th17 phenotypes. Stem Cell Res Ther. 9(147)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lea S, Harbron C, Khan N, Booth G,
Armstrong J and Singh D: Corticosteroid insensitive alveolar
macrophages from asthma patients; synergistic interaction with a
p38 mitogen-activated protein kinase (MAPK) inhibitor. Br J Clin
Pharmacol. 79:756–766. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li JJ, Wang W, Baines KJ, Bowden NA,
Hansbro PM, Gibson PG, Kumar RK, Foster PS and Yang M: IL-27/IFN-γ
induce MyD88-dependent steroid-resistant airway hyperresponsiveness
by inhibiting glucocorticoid signaling in macrophages. J Immunol.
185:4401–4409. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baines KJ, Backer V, Gibson PG, Powel H
and Porsbjerg CM: Impaired lung function is associated with
systemic inflammation and macrophage activation. Eur Respir J.
45:557–559. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yuryeva K, Saltykova I, Ogorodova L,
Kirillova N, Kulikov E, Korotkaya E, Iakovleva Y, Feoktistov I,
Sazonov A and Ryzhov S: Expression of adenosine receptors in
monocytes from patients with bronchial asthma. Biochem Biophys Res
Commun. 464:1314–1320. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Plantinga M, Guilliams M, Vanheerswynghels
M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K,
Killeen N, Malissen B, et al: Conventional and monocyte-derived
CD11b(+) dendritic cells initiate and Maintain T helper
2cell-mediated immunity to house dust mite allergen. Immunity.
38:322–335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li LB, Leung DY and Goleva E: Activated
p38 MAPK in peripheral blood monocytes of steroid resistant
asthmatic patients. PLoS One. 10(e0141909)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nixon M, Andrew R and Chapman KE: It takes
two to tango: Dimerisation of glucocorticoid receptor and its
anti-inflammatory functions. Steroids. 78:59–68. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kassel O, Sancono A, Krätzschmar J, Kreft
B, Stassen M and Cato AC: Glucocorticoids inhibit MAP kinase via
increased expression and decreased degradation of MKP-1. EMBO J.
20:7108–7116. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Owens DM and Keyse SM: Differential
regulation of MAP kinase signalling by dual-specificity protein
phosphatases. Oncogene. 26:3203–3213. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bhavsar P, Khorasani N, Hew M, Johnson M
and Chung KF: Effect of p38 MAPK Inhibition on corticosteroid
suppression of cytokine release in severe asthma. Eur Respir J.
35:750–756. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mercado N, Hakim A, Kobayashi Y, Meah S,
Usmani OS, Chung KF, Barnes PJ and Ito K: Restoration of
corticosteroid sensitivity by p38 mitogen activated protein kinase
inhibition in peripheral blood mononuclear cells from severe
asthma. PLoS One. 7(e41582)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma Y, Chen L, Xie G, Zhou Y, Yue C, Yuan
X, Zheng Y, Wang W, Deng L and Shen L: Elevated level of
interleukin-35 in colorectal cancer induces conversion of T cells
into iTr35 by activating STAT1/STAT3. Oncotarget. 7:73003–73015.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Whitehead GS, Wilson RH, Nakano K, Burch
LH, Nakano H and Cook DN: IL-35 production by inducible
costimulator (ICOS)-positive regulatory T cells reverses
established IL-17-dependent allergic airways disease. J Allergy
Clin Immunol. 129:207–215.e1-e5. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao H, Liao XL and Kang Y: Tregs: Where
we are and what comes next? Front Immunol. 8(1578)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Y, Pan X, Peng X, Li S, Zhou Y, Zheng X
and Li M: Adenovirus-mediated interleukin-35 gene transfer
suppresses allergic airway inflammation in a murine model of
asthma. Inflamm Res. 64:767–774. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dong J, Wong CK, Cai Z, Jiao D, Chu M and
Lam CW: Amelioration of allergic airway inflammation in mice by
regulatory IL-35 through dampening inflammatory dendritic cells.
Allergy. 70:921–932. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Collison LW, Chaturvedi V, Henderson AL,
Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ,
Brown SA, et al: IL-35-mediated induction of a potent regulatory T
cell population. Nat Immunol. 11:1093–1101. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Jiang H, Chi X, Zhang X and Wang J:
Increased serum VDBP as a risk predictor for steroid resistance in
asthma patients. Respir Med. 114:111–116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
De Almeida MC, Silva AC, Barral A and
Barral Netto M: A simple method for human peripheral blood monocyte
isolation. Mem Inst Oswaldo Cruz. 95:221–223. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tchen CR, Martins JR, Paktiawal N, Perelli
R, Saklatvala J and Clark AR: Glucocorticoid regulation of mouse
and human dual specificity phosphatase 1 (DUSP1) genes: Unusual
cis-acting elements and unexpected evolutionary divergence. J Biol
Chem. 285:2642–2652. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ma Y, Liu X, Wei Z, Wang X, Xu D, Dai S,
Li Y, Gao M, Ji C, Guo C, et al: The expression of a novel
anti-inflammatory cytokine IL-35 and its possible significance in
childhood asthma. Immunol Lett. 162:11–17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang W, Li P, Chen YF and Yang J: A
potential immunopathogenic role for reduced IL-35 expression in
allergic asthma. J Asthma. 52:763–771. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim RY, Pinkerton JW, Essilfie AT,
Robertson AAB, Baines KJ, Brown AC, Mayall JR, Ali MK, Starkey MR,
Hansbro NG, et al: Role for NLRP3 inflammasome-mediated,
IL-1β-dependent responses in severe, steroid-resistant asthma. Am J
Respir Crit Care Med. 196:283–297. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Y, Leung DYM and Goleva E:
Anti-inflammatory and corticosteroid-enhancing actions of vitamin D
in monocytes of patients with steroid-resistant and those with
steroid-sensitive asthma. J Allergy Clin Immunol. 133:1744–1752.e1.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee HJ, Ko HJ, Song DK and Jung YJ:
Lysophosphatidylcholine promotes phagosome maturation and regulates
inflammatory mediator production through the protein kinase
a-phosphatidylinositol 3 kinase-p38 mitogen-activated protein
kinase signaling pathway during mycobacterium tuberculosis
infection in mouse macrophages. Front Immunol.
9(920)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sha X, Meng S, Li X, Xi H, Maddaloni M,
Pascual DW, Shan H, Jiang X, Wang H and Yang XF: Interleukin-35
inhibits endothelial cell activation by suppressing MAPK-AP-1
pathway. J Biol Chem. 290:19307–19318. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ramamoorthy S and Cidlowski JA: Exploring
the molecular mechanisms of glucocorticoid receptor action from
sensitivity to resistance. Endocr Dev. 24:41–56. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Moosavi SM, Prabhala P and Ammit AJ: Role
and regulation of MKP-1 in airway inflammation. Respir Res.
18(154)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xie SJ, Li JH, Chen HF, Tan YY, Liu SR,
Zhang Y, Xu H, Yang JH, Liu S, Zheng LL, et al: Inhibition of the
JNK/MAPK signaling pathway by myogenesis-associated miRNAs is
required for skeletal muscle development. Cell Death Differ.
25:1581–1597. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang ZK, Dou M, Liu FJ, Jiang P, Ye SL, Ma
L, Cao HJ, Du X, Sun P, Su N, et al: GDF11 induces differentiation
and apoptosis and inhibits migration of C17.2 neural stem cells via
modulating MAPK signaling pathway. PeerJ. 6(e5524)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao ST and Wang CZ: Regulatory T cells
and asthma. J Zhejiang Univ Sci B. 19:663–673. 2018.PubMed/NCBI View Article : Google Scholar
|