Introduction
Vitiligo is a common skin disease which is the most
frequent cause of depigmentation, resulting in prominent and
disfiguring white spots. At present, to the best of the authors'
knowledge, there are no specific drugs for treatment of this kind
of skin disorder (1,2). Vitiligo pathogenesis often involves
dysfunction or absence of melanocytes. A number of previous studies
showed that oxidative stress injury of melanocytes plays an
important role in vitiligo (1-3).
Particulate matter 2.5 (PM2.5) refers to particles
in the atmosphere with an aerodynamic diameter <2.5 µm (4). It is well known to contribute to air
pollution and is closely associated with human health problems
(4,5). Previous epidemiological studies have
demonstrated that PM2.5 was associated with the increasing
prevalence and mortality rates of respiratory and cardiovascular
diseases (6-10).
In addition, a previous study identified that PM2.5 was a crucial
risk factor for skin diseases and skin aging (11). The cytotoxicity of PM2.5 on human
keratinocytes (HaCaT cells), which is related to the inflammatory
response, could be a cause of PM2.5-induced skin injury (12). The main pathways of the PM2.5 toxic
effect are related to oxidative stress injury, the inflammatory
response, skin barrier function impairment and genetic damage
(13-15).
In a previous study, human lung epithelial cells (BEAS-2B) exposed
to PM2.5 caused a high expression of heme oxygenase-1 (HO-1) and
autophagy-related cell necrosis (16). While, hydrogen peroxide could induce
oxidative stress in human melanocytes via nuclear factor erythroid
2-related factor 2 (Nrf2)-driven transcriptional activation of
HO-1, and it could be a possible mechanism for melanocyte
degeneration in vitiligo (17,18).
However, more research into the mechanisms linking PM2.5 and skin
damage is still required. Considering the epidermal oxidative
stress in patients with vitiligo, the present study aimed to
identify the possible association between PM2.5 and vitiligo, which
could provide insight on the underlying mechanisms of vitiligo.
Based on previous investigations on the functions of
PM2.5, the present study aimed to investigate the effects of PM2.5
exposure on human keratinocytes (immortalized human keratinocyte
HaCaT cells) and human melanocytes (immortalized human epidermal
melanocyte PIG1 cells and immortalized vitiligo melanocyte PIG3V
cells) in vitro. Specifically, the effects of PM2.5 exposure
on cell viability and the secretions of stem cell factor (SCF) and
basic fibroblast growth factor (bFGF) in HaCaT cells, and cell
migration, apoptosis and oxidative stress injury in PIG1 cells and
PIG3V cells were investigated. The findings of the present study
suggested that PM2.5 exposure could inhibit the secretions of SCF
and bFGF in keratinocytes, and cause oxidative stress injury and
melanin metabolic disorder in melanocytes. Thus, PM2.5 could be a
new risk factor for vitiligo.
Materials and methods
Collection of PM2.5
PM2.5 was obtained from the mouth of the Yangtze
River at China's central eastern coast in Shanghai using a QJS-100
multi-level flow particulate matter cutter (Jinzhou Licheng
Technology Development Co., Ltd.). The cutter was placed on the
roadside for 48 h at a constant aspiration flow rate (100 l/min).
Then, the PM2.5 fiber filters were transferred to ultrapure water
and subjected to ultrasonic oscillations for 15 min in order to
elude the particulate matter. The sample was vacuum freeze-dried
for 24 h and then, the sample was collected in a 50 ml centrifuge
tube and diluted with PBS. The PM2.5 suspension was autoclaved at
120˚C for 30 min. Finally, the PM2.5 suspension was collected and
stored at 4˚C. According to the literature (19), the main compositions of PM2.5 were
analyzed by gas-mass spectrometry and X-ray fluorescence
spectroscopy.
Cell culture and treatment
The human keratinocyte HaCaT cells (American Type
Culture Collection; ATCC) were cultured in DMEM (containing 1.05 mM
calcium chloride; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
in a humidified atmosphere of 5% CO2 at 37˚C. The
immortalized human epidermal melanocyte PIG1 cells and immortalized
vitiligo melanocyte PIG3V cells (both obtained from ATCC) were
cultured in M154/HMGS medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 0.25 µg/ml amphotericin B, 100 U/ml
penicillin and 10 µg/ml neomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) in a humidified atmosphere of 5% CO2
at 37˚C. For assessment of the effects of PM2.5 exposure on cells,
the cells were incubated with PM2.5 (0-200 µg/ml) for 24 h.
Short tandem repeat (STR)
profiling
Authenticity of the human keratinocyte HaCaT cells
was verified by performing STR analysis. DNA was prepared and
suspended in double-distilled water, and then analyzed by STR
profiling. The selected markers included the sex chromosome locus,
amelogenin (Xp22.10-22.3 and Y) and eight core STR loci (CSF1PO;
5q33.3-34, D16S539; 16q24-qter, D13S317; 13q22-q31, D7S820; 7q,
D5S818; 5q21-q31, TH01; 11p15.5, TPOX; 2p23-2pter, and vWA;
12p12-pter). The STR profiling result was compared with the STR
profiles for HaCaT cell in the ATCC STR profiling database
(https://www.atcc.org/en/STR_Database.aspx), and the
results showed that STR profiles of HaCaT cells were identical to
the STR profiles in the ATCC database. STR profiles of HaCaT cells
were as follows: Amelogenin; XX, CSFIPO; 9 11, D16S539; 9 12,
D13S317; 10 12, D7S820; 9 11, D5S818; 12 12, THOI; 9.3, TPOX; 11
12, and vWA; 16 17.
Cell viability assay
Cell viability was measured using a Cell Counting
Kit-8 (CCK-8; Guangzhou Yiyuan Biological Technology Co., Ltd.)
according to the manufacturer's instruction. Cells were plated in a
96-well plate (at a cell density of 2.0x104 cells/ml),
and exposed to PM2.5 (0-200 µg/ml) for 24 h. Zinc protoporphyrin IX
(ZnPP; HO-1 inhibitor) and hemin chloride (Hemin; HO-1 agonist)
were used to pre-incubate human melanocytes for 2 h at 37˚C prior
to PM2.5 exposure (200 µg/ml). The cells were then incubated with
10 µl CCK-8 reagent for 2 h, and then the absorbance was measured
at 450 nm wavelength using a microplate reader (MRP-2100;
Syntron).
Determination of SCF and bFGF
levels
The secretions of SCF and bFGF in HaCaT cells were
measured using specific SCF (cat. no. F02540) and bFGF (cat. no.
F00760) ELISA kits, respectively, according to the manufacturer's
instructions (Shanghai Westang Biotechnology Co., Ltd.). After the
indicated treatment, the absorbance was measured at 450 nm
wavelength using a microplate reader (MRP-2100; Syntron). The
levels of SCF and bFGF were quantified according to the standard
curve.
Determination of melanin contents
Melanin contents in PIG1 or PIG3V cells were
detected with a NaOH method, as previously described (20). The cells were plated in a 6-well
plate (at a cell density of 2x104 cells/ml), and exposed
to PM2.5 (0-200 µg/ml) for 24 h. Then, the cells were washed with
PBS three times and separated by 0.25% trypsin digestion. Cells
were dissolved in 100 µl NaOH (1 M) for 1 h at 80˚C, then,
centrifuged (16,000 x g) for 20 min at room temperature. The
supernatants were transferred to a 96-well plate, and the
absorbance was measured at 405 nm wavelength using a microplate
reader (MRP-2100; Syntron).
Tyrosinase activity assay
Tyrosinase activity in PIG1 or PIG3V cells was
measured with a DOPA oxidase method, as previously described
(21). The cells were plated in a
6-well plate (at a cell density of 2x104 cells/ml) and
exposed to PM2.5 (0-200 µg/ml) for 24 h. Then cells were washed
with PBS three times, lysed with 1% Triton X-100 and centrifuged
(16,000 x g) at room temperature for 15 min. The supernatants were
transferred to a 96-well plate and 100 µl 1% L-DOPA was added for 1
h at 37˚C. The absorbance was measured at 490 nm wavelength using a
microplate reader (MRP-2100; Syntron).
Cell apoptosis
Cell apoptosis was assessed by Annexin
V-FITC/Propidium Iodide (PI) double staining using a cell apoptosis
detection kit (Nanjing KeyGen Biotech Co., Ltd.) as previously
described (16). After the indicated
treatment, PIG1 and PIG3V cells were collected by centrifugation
(500 x g for 5 min at 4˚C). Then, the cells were washed with
ice-cold PBS three times and suspended in binding buffer. Finally,
the cells were incubated with annexin V-FITC and PI for 15 min at
room temperature in the dark, and the apoptotic cells were counted
using a flow cytometer (BD Biosciences). The cell apoptotic ratio
was subsequently analyzed using WinMDI v2.9 software (The Scripps
Research Institute).
Cell migration
Cell migration was evaluated by a transwell assay,
as previously described (20). PIG1
or PIG3V cells were exposed to PM2.5 (0, 25, 50, 100 and 200 µg/ml)
for 24 h. Cells (2x105 cells/ml; 100 µl serum-free cell
suspension) were added into the upper chamber of the transwell
insert (Corning, Inc.) and 600 µl M154/HMGS medium (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 20% FBS (Gibco;
Thermo Fisher Scientific, Inc.) was added into the lower chamber.
After incubation for 24 h, the transwell insert was removed from
the plate. The unmigrated cells in the upper chamber were carefully
removed using a cotton-tipped applicator. The migrated cells were
fixed with 70% ethanol for 10 min at room temperature and stained
with 0.2% crystal violet for 10 min at room temperature. The excess
crystal violet was discarded, then the cells were washed and
counted in five different fields of view under an inverted light
microscope (magnification, x400; Olympus Corporation) to get an
average cell migration rate.
Determination of malondialdehyde
(MDA), superoxide dismutase (SOD) and glutathione peroxidase
(GSH-Px) levels
MDA contents, SOD and GSH-Px levels were measured
using specific MDA (cat. no. 10009055), SOD (cat. no. 706002) and
GSH-Px (cat. no. 703102) assay kits, respectively, according to the
manufacturer's instructions (Cayman Chemical). After the indicated
treatment, the absorbance was measured at 490 nm wavelength using a
microplate reader (MRP-2100; Syntron) and quantified according to
the standard curve. MDA content is presented as nmol/mg protein,
SOD activity is presented as U/mg protein and GSH-Px activity is
presented as U/g protein.
Total and nuclear protein
isolation
Total protein was isolated using a RIPA kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instruction. After treatment, the cells were washed
with ice-cold PBS three times and lysed with RIPA lysis buffer for
40 min. Then total protein was collected by centrifugation (12,000
x g) for 12 min at 4˚C and stored at -80˚C for the following
experiments. Nuclear protein was isolated using a Scientific NE-PER
nuclear extraction kit (Thermo Fisher Scientific, Inc.), according
to the manufacturer's instruction. After treatment, the cells were
washed with ice-cold PBS three times and incubated with NE-PER
Nuclear lysis buffer for 10 min. Then, the nuclear protein was
collected by centrifugation (15,000 x g) for 10 min at 4˚C and
stored at -80˚C for the following experiments.
Western blot analysis
A western blotting assay was performed to analyze
the related protein expression, as previously described (16). Protein concentration was determined
by a BCA assay (Beyotime Institute of Biotechnology). In total, 60
µg total protein or nuclear protein of each sample was denatured
for 5 min at 95˚C and separated by SDS-PAGE on 8-12% gels. After
the separated protein was transferred onto PVDF membranes (EMD
Millipore) and blocked with 5% skim milk for 120 min at room
temperature, the PVDF membranes were incubated with primary
antibodies according to manufacturer's instruction at 4˚C
overnight. After rewarming, the PVDF membranes were washed with PBS
three times for 5 min, and incubated with horse-radish peroxidase
(HRP) conjugated Rabbit Anti-Mouse IgG H&R (1:1,000; cat. no.
ab6728; Abcam) or Goat Anti-Rabbit IgG H&R HRP conjugated
(1:1,000; cat. no. ab6721; Abcam) for 1 h at room temperature.
Blots of target protein were developed with enhanced
chemiluminescence substrates (Guangzhou Ladder Biotech. Co. Ltd.)
and visualized with exposure to X-ray film (Kodak). Integrated
optical density of target bands was accurately determined using the
ImageJ 1.48u analysis system (National Institutes of Health). In
the present study, antibodies against cytochrome C (1:200; cat. no.
ab90529) and CoxIV (1:500; cat. no. ab153709) were purchased from
Abcam, antibodies against β-actin (1:1,000; cat. no. 4967), cleaved
caspase 3 (1:1,000; cat. no. 9661) and caspase 3 (1:1,000; cat. no.
9662) were purchased from Cell Signaling Technology, Inc., and
antibodies against Nrf2 (1:1,000; cat. no. sc-365949), H3 (1:1,000;
cat. no. sc-376769) and HO-1 (1:1,000; cat. no. sc-136960) were
purchased from Santa Cruz Biotechnology, Inc.
Statistical analysis
Data from six independent experiments were presented
as the mean value ± standard deviation. Statistical significance
was performed by one-way ANOVA followed by the Bonferroni multiple
comparison test using SPSS 21.0 software (SPSS, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
PM2.5 exposure inhibits cell
viability, and downregulates the production of bFGF and SCF in
human keratinocytes
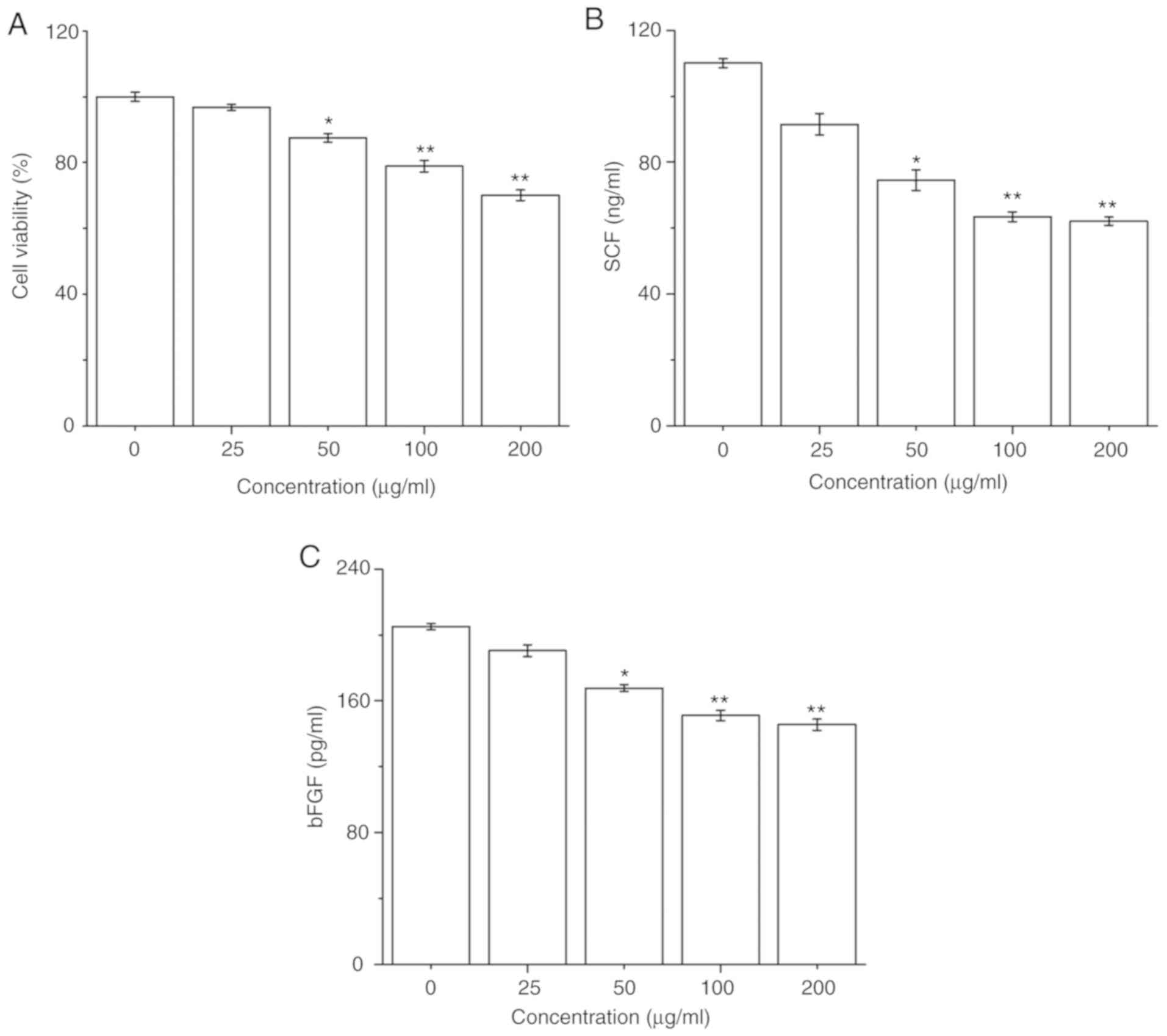
The effect of PM2.5 on HaCaT cell survival was
firstly determined; HaCaT cells were exposed to PM2.5 (0-200 µg/ml)
for 24 h, and then cell viability was detected by a CCK-8 assay.
PM2.5 exposure significantly decreased HaCaT cell viability in a
dose-dependent manner from 50 µg/ml PM2.5 (Fig. 1A). Furthermore, the secretions of SCF
and bFGF in HaCaT cells after PM2.5 exposure for 24 h were
evaluated. PM2.5 exposure significantly inhibited the secretions of
SCF and bFGF in HaCaT cells in a dose-dependent manner from 50
µg/ml PM2.5 (Fig. 1B and C).
PM2.5 exposure attenuates the
melanization process in human melanocytes
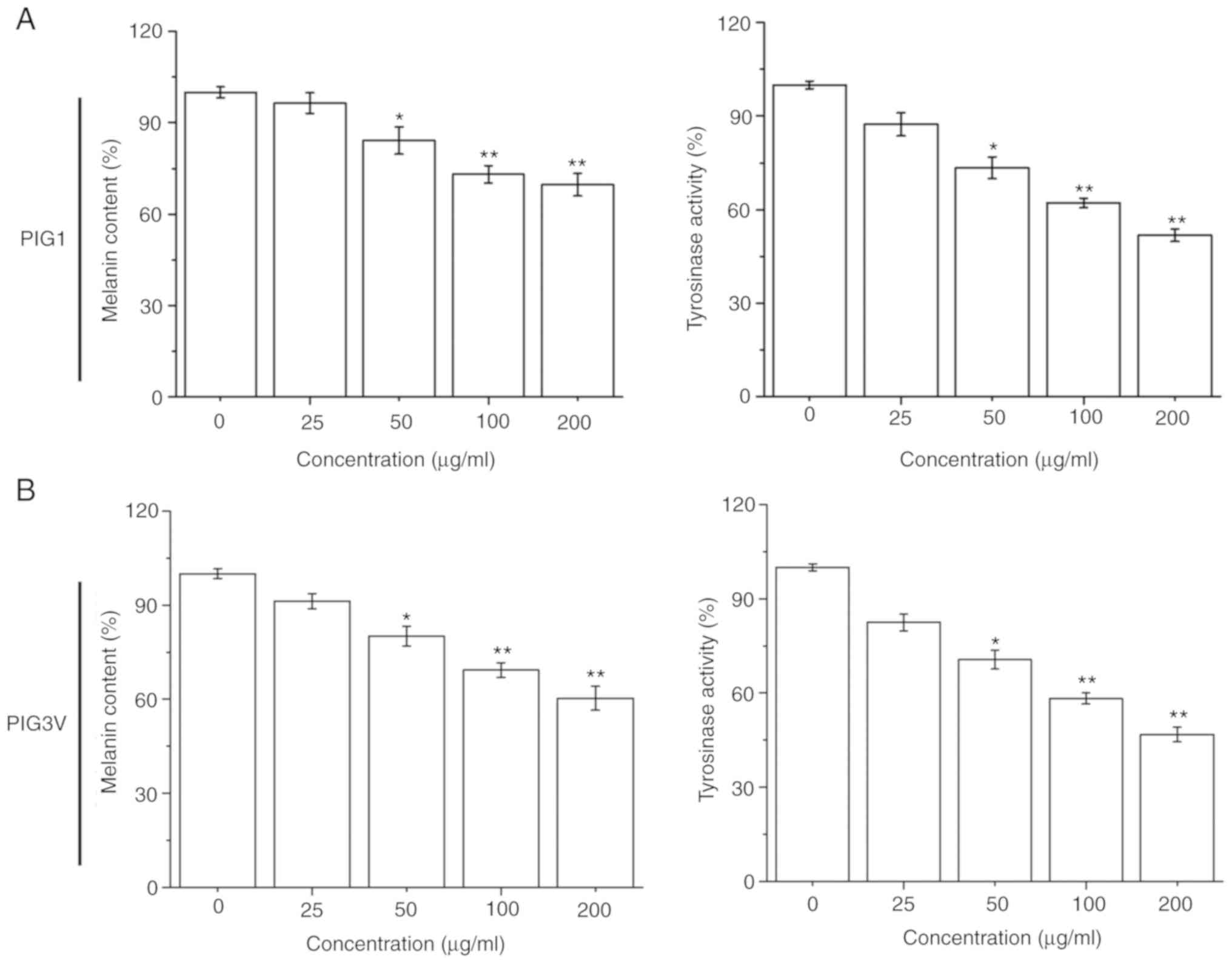
The effectiveness of the melanization process was
estimated by measuring the melanin contents and cellular tyrosinase
activity in melanocytes after PM2.5 (0-200 µg/ml) exposure for 24
h. PM2.5 exposure significantly inhibited the melanin contents and
tyrosinase activity in both PIG1 and PIG3V cells in a
dose-dependent manner from 50 µg/ml PM2.5 (Fig. 2). These data demonstrated that PM2.5
had a negative effect on the melanization process in human
melanocytes.
PM2.5 exposure induces apoptosis in
human melanocytes
PIG1 or PIG3V cells were exposed to PM2.5 (0-200
µg/ml) for 24 h, then the cell viability was detected by a CCK-8
assay. PM2.5 exposure significantly decreased cell viability in
both PIG1 (Fig. 3A) and PIG3V
(Fig. 3B) cells in a dose-dependent
manner from 50 µg/ml PM2.5. PIG1 or PIG3V cells were exposed to
PM2.5 (0-200 µg/ml) for 24 h, and the extent of apoptosis was
quantified by AnnexinV-FITC/PI double staining by flow cytometry.
Apoptosis was significantly increased in both PIG1 (Fig. 3C) and PIG3V (Fig. 3D) cells after PM2.5 exposure. The
present results demonstrated that PM2.5 was able to facilitate cell
apoptosis in human melanocytes.
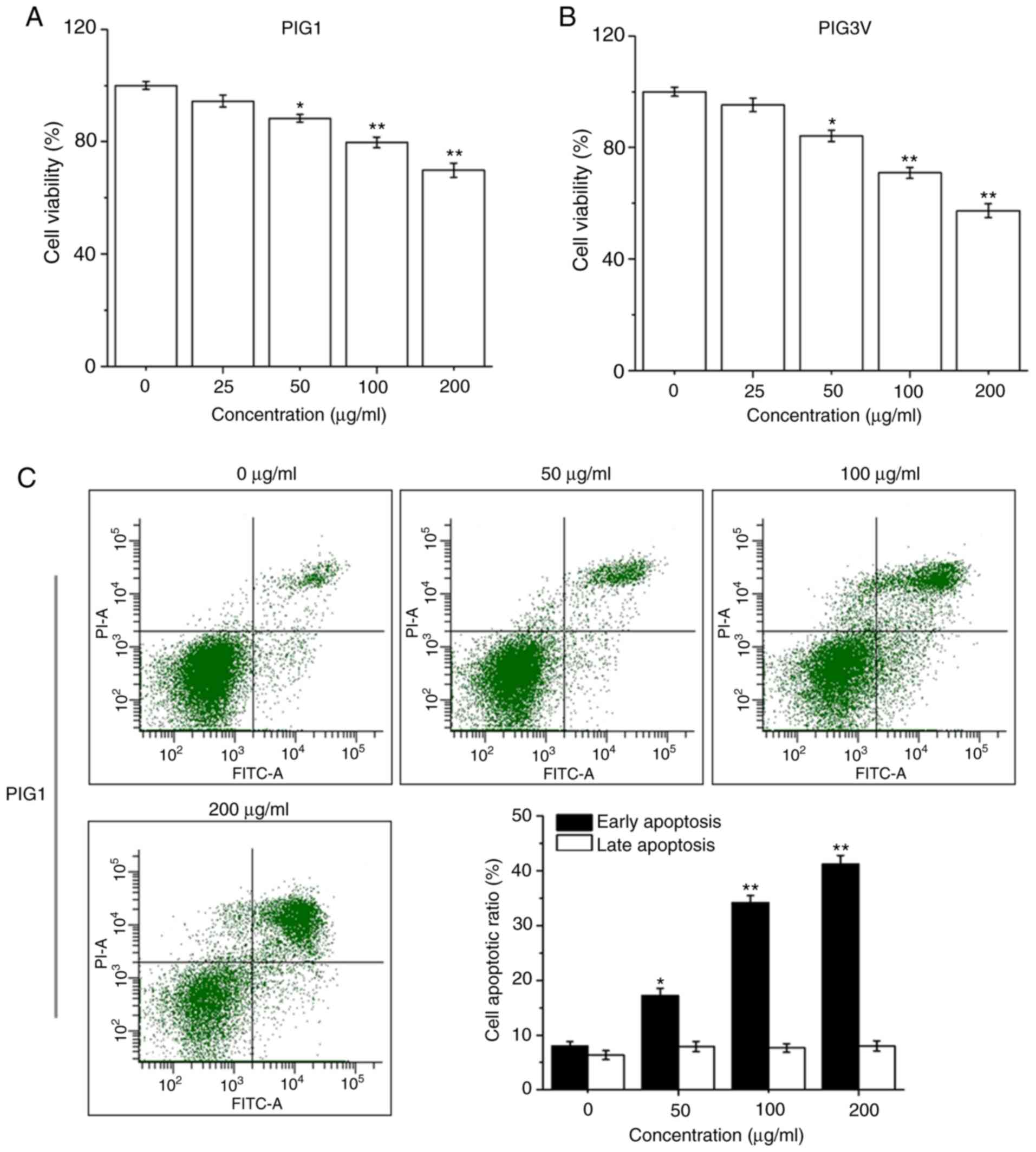 | Figure 3PM2.5 exposure induces cell apoptosis
in human melanocytes. PIG1 or PIG3V cells were exposed to (0-200
µg/ml) PM2.5 for 24 h. Cell viability was measured by Cell Counting
Kit-8 assay, cell apoptosis was evaluated by AnnexinⅤ-FITC/PI
double staining, and the expressions of apoptosis related-proteins
were determined by western blotting. PM2.5 exposure decreased (A)
PIG1 and (B) PIG3V cell viability in a dose-dependent manner. PM2.5
exposure increased cell apoptosis in (C) PIG1 and (D) PIG3V cells.
Data are presented as the mean ± SD. n=6. *P<0.05,
**P<0.01 vs. respective 0 µg/ml PM2.5. PM2.5 exposure
induces cell apoptosis in human melanocytes. PIG1 or PIG3V cells
were exposed to (0-200 µg/ml) PM2.5 for 24 h. Cell viability was
measured by Cell Counting Kit-8 assay, cell apoptosis was evaluated
by AnnexinⅤ-FITC/PI double staining, and the expressions of
apoptosis related-proteins were determined by western blotting.
PM2.5 exposure regulated the expressions of apoptosis-related
molecules in (E) PIG1 and (F) PIG3V cells. The changes of
cytochrome C and the activation of caspase 3 were detected by
western blotting. Data are presented as the mean ± SD. n=6.
*P<0.05, **P<0.01 vs. respective 0
µg/ml PM2.5. PM2.5, particulate matter 2.5; c-cas3, cleaved
caspase3; cas3, caspase3; c-cyto C, cytosolic cytochrome C; m-cyto
C, mitochondria cytochrome C; PI, propidium iodide. |
The mitochondrial apoptotic pathway is the major
pathway for cell apoptosis activated by various cytotoxic
substances (22). Therefore, the
present study examined the changes of cytochrome C and the
activation of caspase 3 in the model used in the present study.
After PIG1 or PIG3V cells were exposed to PM2.5 (0, 50, 100 and 200
µg/ml) for 24 h, a western blotting assay was used to evaluate the
changes of cytochrome C and the activation of caspase 3. PM2.5
exposure promoted the protein expressions of cleaved caspase 3 and
cytosolic cytochrome C, while the expression of mitochondria
cytochrome C was decreased in both PIG1 (Fig. 3E) and PIG3V (Fig. 3F) cells. These data suggested that
the mitochondrial apoptotic pathway may be involved in
PM2.5-elicited apoptosis in human melanocytes.
PM2.5 inhibits human melanocyte
migration
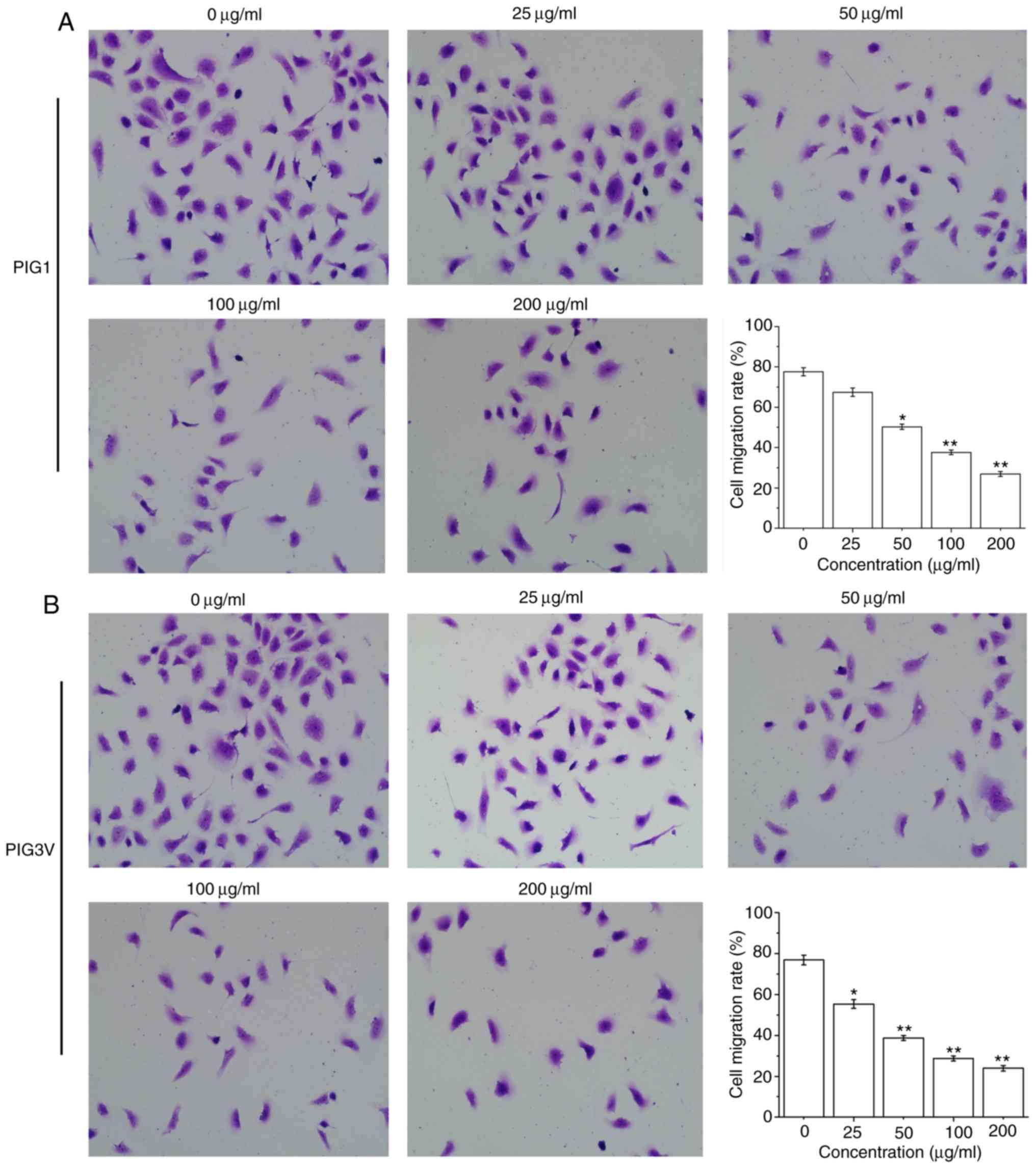
To examine the effect of PM2.5 on the biological
characteristics of human melanocytes, the present study further
examined its effect on PIG1 and PIG3V cells migration by a
transwell assay. The cell migration was significantly decreased
after PIG1 cells were exposed to ≥50 µg/ml PM2.5 for 24 h (Fig. 4A) and PIG3V cells were exposed to ≥25
µg/ml PM2.5 for 24 h (Fig. 4B).
These data demonstrated that PM2.5 inhibited cell migration in
human melanocytes.
PM2.5 induces the oxidative stress
response in human melanocytes
Oxidative stress plays an important role in cell
injury. MDA, SOD and GSH-Px represent the status of oxidative
stress (23). The present data
showed that PM2.5 exposure suppressed the activities of SOD and
GSH-Px, while enhancing MDA contents in both PIG1 (Fig. 5A) and PIG3V (Fig. 5B) cells in a concentration-dependent
manner. These data suggested that the oxidative stress response may
be responsible for PM2.5-elicited apoptosis in human
melanocytes.
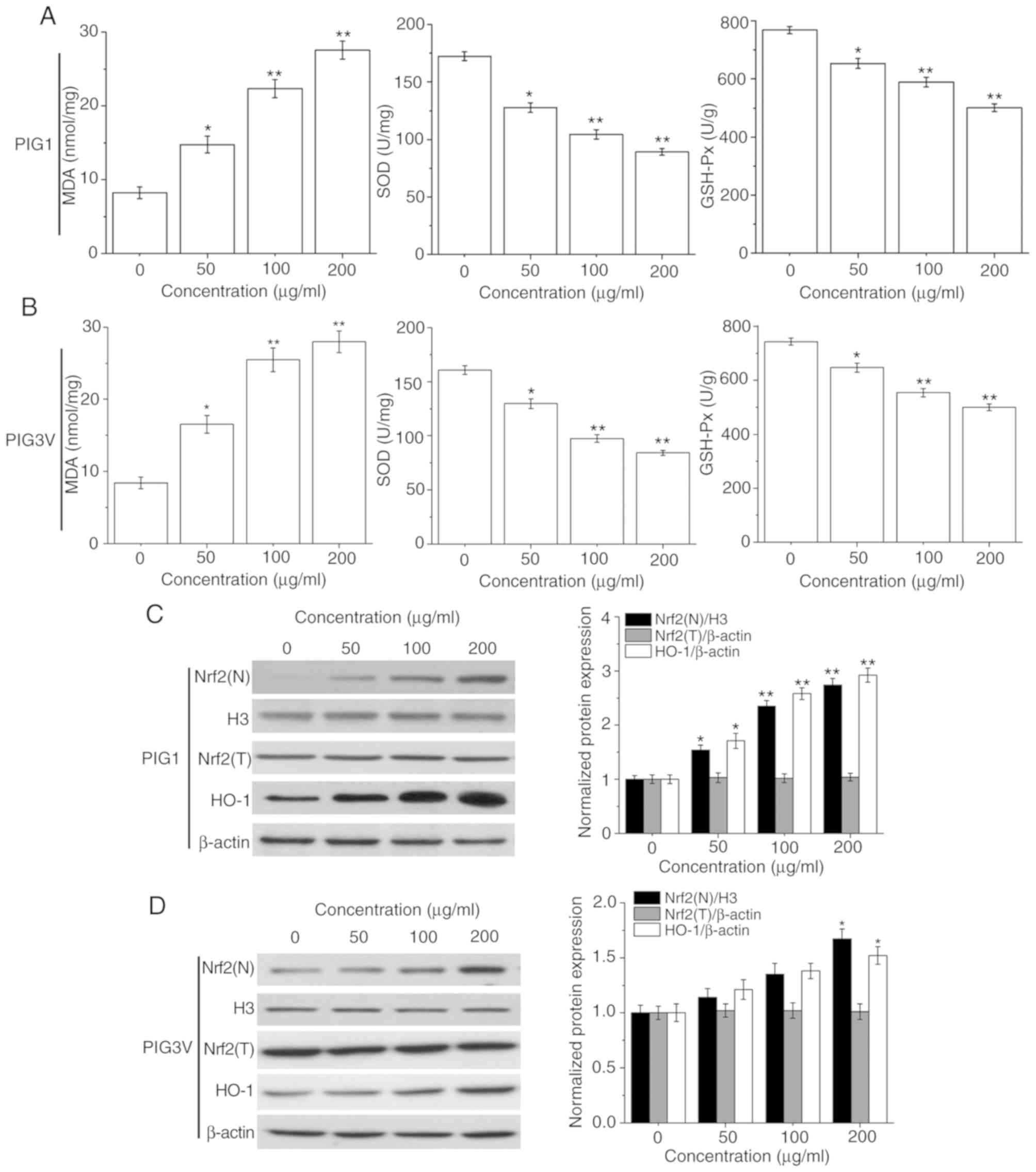 | Figure 5PM2.5 exposure induces the oxidative
stress response and affects the activation of the Nrf2 pathway in
human melanocytes. PIG1 or PIG3V cells were exposed to (0-200
µg/ml) PM2.5 for 24 h, and MDA contents, SOD and GSH-Px levels were
measured using specific respective MDA, SOD and GSH-Px assay kits.
The Nrf2 pathway related-protein expressions were determined by
western blotting. PM2.5 exposure suppressed the activities of SOD
and GSH-Px, while enhancing MDA contents in both (A) PIG1 and (B)
PIG3V cells. PM2.5 exposure activated the Nrf2 pathway in (C) PIG1
cells, while only a high concentration (200 µg/ml) of PM2.5
exposure could significantly promote the expression of Nrf2(N) and
HO-1 in (D) PIG3V cells. Data are presented as the mean ± SD. n=6.
*P<0.05, **P<0.01 vs. respective 0
µg/ml PM2.5. PM2.5, particulate matter 2.5; MDA, malondialdehyde;
GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; Nrf2,
nuclear factor erythroid 2-related factor 2; HO-1, heme
oxygenase-1; N, nuclear; T, total. |
The Nrf2 pathway plays an important role in the
defense against oxidative stress injury (24). Therefore, the protein expressions of
Nrf2 (nuclear), Nrf2 (total) and its downstream molecule HO-1 in
human melanocytes were analyzed to determine its role in
PM2.5-induced oxidative stress injury. As shown in Fig. 5C, after PM2.5 (0, 50, 100 and 200
µg/ml) exposure, the protein expressions of Nrf2 (nuclear) and HO-1
were significantly increased in PIG1 cells. Only 200 µg/ml PM2.5
exposure could lead to a significant upregulation of Nrf2 (nuclear)
and HO-1 expression in PIG3V cells (Fig.
5D).
HO-1 is responsible for PM2.5-induced
human melanocyte oxidative stress injury
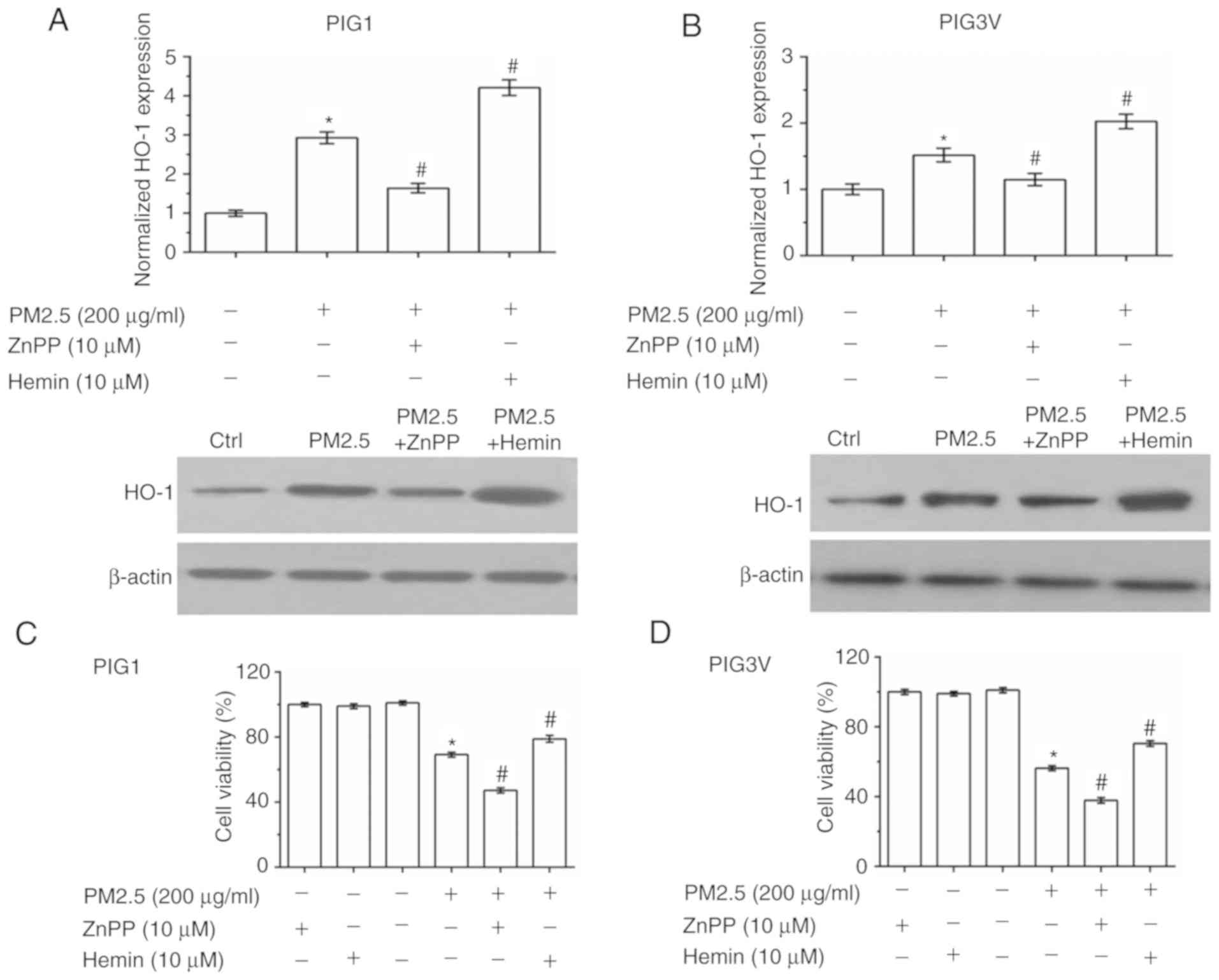
To further verify that HO-1 induction is responsible
for the antioxidant response in human melanocytes, zinc
protoporphyrin Ⅸ (ZnPP, a HO-1 inhibitor) and hemin chloride
(Hemin, a HO-1 agonist) were used to pre-incubate human melanocytes
for 2 h before PM2.5 exposure (Fig.
6A and B). As presented in
Fig. 6A and B, ZnPP pre-incubation inhibited the
expression of HO-1, while Hemin pre-incubation promoted the
expression of HO-1. Cell viability detection data showed that both
ZnPP and Hemin had no toxic effect in human melanocytes. Compared
with the PM2.5 exposure alone, ZnPP pre-incubation exacerbated the
injury induced by PM2.5 exposure, while Hemin pre-incubation
enhanced cell viability in both PIG1 (Fig. 6C) and PIG3V (Fig. 6D) cells. The cell apoptosis detection
data showed that ZnPP pre-incubation exacerbated PM2.5-induced cell
apoptosis, while Hemin pre-incubation partly reversed PM2.5-induced
cell apoptosis in both PIG1 (Fig.
6E) and PIG3V (Fig. 6F)
cells.
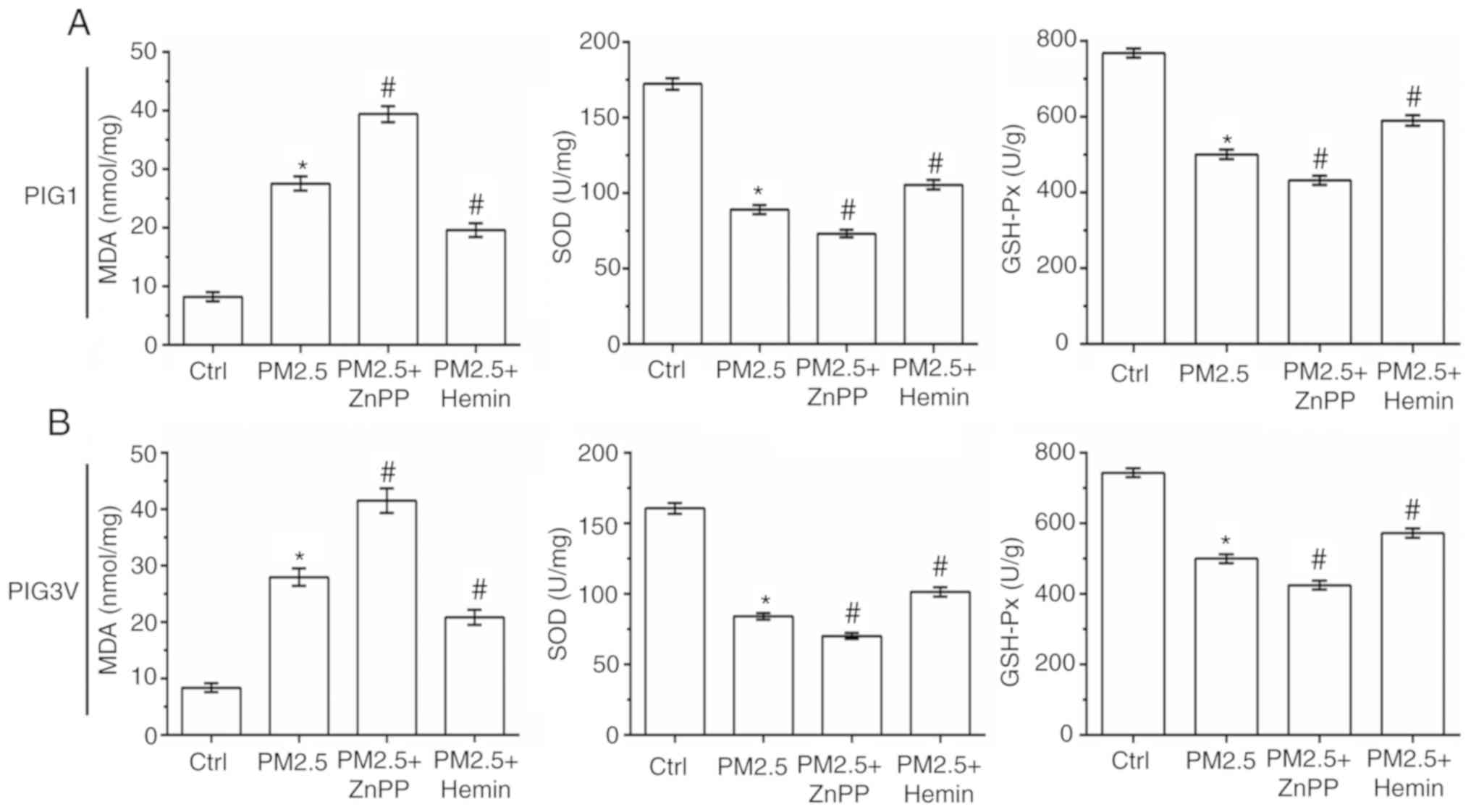
The status of oxidative stress was also measured in
human melanocytes. As shown in Fig.
7, compared with the PM2.5 exposure alone, ZnPP pre-incubation
significantly increased the MDA contents, and decreased the SOD and
GSH-Px activity in both PIG1 and PIG3V cells. Conversely, compared
with the PM2.5 exposure alone Hemin pre-incubation significantly
decreased the MDA contents, and increased the SOD and GSH-Px
activity in both PIG1 and PIG3V cells. All these results suggested
that HO-1 could protect human melanocytes against PM2.5-induced
oxidative stress injury and cell death in human melanocytes.
Discussion
There is an association between vitiligo and
intrinsic defects within melanocytes (2). Intrinsic defects within melanocytes can
activate the cellular oxidative stress response (25). PM2.5 is known for its toxic effects
on skin, cardiovascular and respiration systems (6,9,11). However, to the best of the authors'
knowledge, the effects and mechanisms of PM2.5 on vitiligo are not
fully elucidated. The present study aimed to clarify the effects of
PM2.5 exposure on cell viability, and the secretions of SCF and
bFGF in HaCaT cells, and cell migration, apoptosis and oxidative
stress injury in PIG1 cells and PIG3V cells. The present study
demonstrated that PM2.5 exposure significantly decreased cell
viability of HaCaT cells, PIG1 cells and PIG3V cells in a
dose-dependent manner, and decreased secretions of SCF and bFGF in
HaCaT cells. The present study additionally demonstrated the PM2.5
exposure inhibited cell migration, and promoted apoptosis and
oxidative stress injury in PIG1 cells and PIG3V cells.
The main pathological features of vitiligo are the
destruction or apoptosis of melanocytes (3). Therefore, in the present study, two
types of human melanocytes (PIG1 and PIG3V cells) were selected to
evaluate the effect of PM2.5 exposure on human melanocyte
apoptosis. The present results showed that PM2.5 exposure was able
to promote apoptosis in human melanocytes. Cell apoptosis has two
important pathways: Death receptor-mediated exogenous apoptotic
pathways and mitochondrial/cytochrome C-mediated endogenous
apoptotic pathways (26,27). In the present study, the mechanisms
of PM2.5 exposure on cytochrome C release were examined. The
present results showed that PM2.5 exposure promoted cytochrome C
release into the cytoplasm in both PIG1 and PIG3V cells. It was
hypothesized that the activation of caspase 3 is the central link
of cell apoptosis (28). The present
data further demonstrated that PM2.5 exposure induced caspase 3
activation in PIG1 and PIG3V cells. Collectively, these results
suggested that PM2.5 induced human melanocyte apoptosis through
release of cytochrome C and activation of caspase 3.
Oxidative stress caused by reactive oxygen species
(ROS) is an important part of cell apoptosis (29,30).
Previous studies have demonstrated that oxidative stress injury is
activated in vitiligo (25,31). In addition, previous studies on the
toxic effect of PM2.5 demonstrated that PM2.5 is a major carrier
for some organic chemicals, which can be localized in mitochondria
and reduce SOD generation (11,32). SOD
is a scavenger of endogenous ROS. A reduction of SOD activity not
only results in insufficient clearance of oxygen radicals, but also
induces the synthesis of MDA, resulting in cross-linking between
molecules of the protein and in the molecule, and inducing
apoptosis (33,34). GSH-Px is an important
peroxide-degrading enzyme that protects the structure and function
of cell membranes from peroxide injury (35). In the present study, PM2.5 exposure
was found to suppress the activities of SOD and GSH-Px, while
enhancing MDA contents in both PIG1 cells and PIG3V cells in a
concentration-dependent manner. These data suggested that the
changes of oxidative stress could be responsible for PM2.5-elicited
apoptosis in human melanocytes, which may be responsible for
vitiligo. In addition, the activation of the Nrf2 pathway was
examined, which is an important anti-oxidative stress pathway
(17). The present results
demonstrated that low concentrations (0-100 µg/ml) of PM2.5
exposure could activate the Nrf2 pathway in PIG1 cells, while this
upregulation effect in PIG3V cells was not observed. This may be
due to low concentration of PM2.5-induced ROS generation activating
the self-protection mechanism in normal human melanocytes, while in
vitiligo melanocytes the Nrf2 pathway can be some kind of
dysfunction (17). The Nrf2
translocation could upregulate the expression of antioxidant genes,
such as HO-1(36). Western blot
analysis demonstrated that PM2.5 exposure significantly increased
the protein expression level of HO-1 in human melanocytes, and
pre-incubation of ZnPP exacerbated the oxidative stress response,
while hemin pre-incubation reversed the PM2.5-induced oxidative
stress injury and cell death. These results suggested that HO-1 was
responsible for PM2.5-induced human melanocyte injury.
Melanocyte migration and abnormal melanogenesis are
both important causes of depigmentation in vitiligo (37,38).
Therefore, the effect of PM2.5 exposure on the migration and
melanization processes of human melanocytes was examined in the
present study. The present results showed that PM2.5 exposure
inhibited cell migration, and attenuated the melanization process
in both PIG1 and PIG3V cells. Melanocyte migration and
melanogenesis are controlled by cytokines (such as SCF and bFGF)
secreted by keratinocytes (39,40). The
exosomes secreted by keratinocytes could be involved in melanin
synthesis in melanocytes (31,41). The
present study considered the effect of PM2.5 on cell viability, and
the production of bFGF and SCF in human HaCaT keratinocytes. PM2.5
exposure inhibited the excretion of SCF and bFGF in HaCaT cells in
a dose-dependent manner. The direct contact of keratinocytes and
melanocytes was considered to be important in the melanocyte
metabolism or melanin transfer.
In conclusion, PM2.5 exposure effectively induced
human melanocyte apoptosis through apoptotic-related molecules and
induced oxidative stress injury in human melanocytes. Furthermore,
PM2.5 exposure significantly inhibited melanocyte migration, and
attenuated the activation of tyrosinase and melanogenesis.
Therefore, these findings suggested that PM2.5 could be a risk
factor for vitiligo and may help to better understand the damaging
effects of PM2.5 on the skin.
Acknowledgements
Not applicable.
Funding
The present study was supported by CMA-L'OREAL China
Skin Grant (grant no. S2016131409).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DS performed the experiments of the current study,
analyzed the data and drafted the manuscript. SZ designed the
current study and supervised data analysis. JZ, LM and LW
participated in study design and analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ezzedine K, Eleftheriadou V, Whitton M and
van Geel N: Vitiligo. Lancet. 386:74–84. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rodrigues M, Ezzedine K, Hamzavi I, Pandya
AG and Harris JE: Vitiligo Working Group : New discoveries in the
pathogenesis and classification of vitiligo. J Am Acad Dermatol.
77:1–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ghafourian A, Ghafourian S, Sadeghifard N,
Mohebi R, Shokoohini Y, Nezamoleslami S and Hamat RA: Vitiligo:
Symptoms, pathogenesis and treatment. Int J Immunopathol Pharmacol.
27:485–489. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang F, Li X, Wang C, Xu Q, Wang W, Luo
Y, Tao L, Gao Q, Guo J, Chen S, et al: PM2.5 spatiotemporal
variations and the relationship with meteorological factors during
2013-2014 in Beijing, China. PLoS One. 10(e0141642)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim JY, Lee EY, Choi I, Kim J and Cho KH:
Effects of the particulate matter2.5 (PM2.5)
on lipoprotein metabolism, uptake and degradation, and embryo
toxicity. Mol Cells. 38:1096–1104. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang C, Tu Y, Yu Z and Lu R: PM2.5 and
cardiovascular diseases in the elderly: An overview. Int J Environ
Res Public Health. 12:8187–8197. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hammond D, Croghan C, Shin H, Burnett R,
Bard R, Brook RD and Williams R: Cardiovascular impacts and
micro-environmental exposure factors associated with continuous
personal PM2.5 monitoring. J Expo Sci Environ Epidemiol.
24:337–345. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Y, Ma Z, Zheng C and Shang Y: Ambient
temperature enhanced acute cardiovascular-respiratory mortality
effects of PM2.5 in Beijing, China. Int J Biometeorol.
59:1761–1770. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao J, Bo L, Gong C, Cheng P, Kan H, Xie
Y and Song W: Preliminary study to explore gene-PM2.5 interactive
effects on respiratory system in traffic policemen. Int J Occup Med
Environ Health. 28:971–983. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li Q, Liu H, Alattar M, Jiang S, Han J, Ma
Y and Jiang C: The preferential accumulation of heavy metals in
different tissues following frequent respiratory exposure to PM2.5
in rats. Sci Rep. 5(16936)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ding A, Yang Y, Zhao Z, Hüls A, Vierkötter
A, Yuan Z, Cai J, Zhang J, Gao W, Li J, et al: Indoor PM2.5
exposure affects skin aging manifestation in a Chinese population.
Sci Rep. 7(15329)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Y, Zheng L, Tuo J, Liu Q, Zhang X,
Xu Z, Liu S and Sui G: Analysis of PM2.5-induced cytotoxicity in
human HaCaT cells based on a microfluidic system. Toxicol In Vitro.
43:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Deng X, Zhang F, Rui W, Long F, Wang L,
Feng Z, Chen D and Ding W: PM2.5-induced oxidative stress triggers
autophagy in human lung epithelial A549 cells. Toxicol In Vitro.
27:1762–1770. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bekki K, Ito T, Yoshida Y, He C,
Arashidani K, He M, Sun G, Zeng Y, Sone H, Kunugita N and Ichinose
T: PM2.5 collected in China causes inflammatory and oxidative
stress responses in macrophages through the multiple pathways.
Environ Toxicol Pharmacol. 45:362–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chu M, Sun C, Chen W, Jin G, Gong J, Zhu
M, Yuan J, Dai J, Wang M, Pan Y, et al: Personal exposure to PM2.5,
genetic variants and DNA damage: A multi-center population-based
study in Chinese. Toxicol Lett. 235:172–178. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou W, Yuan X, Zhang L, Su B, Tian D, Li
Y, Zhao J, Wang Y and Peng S: Overexpression of HO-1 assisted
PM2.5-induced apoptosis failure and autophagy-related cell
necrosis. Ecotoxicol Environ Saf. 145:605–614. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jian Z, Li K, Song P, Zhu G, Zhu L, Cui T,
Liu B, Tang L, Wang X, Wang G, et al: Impaired activation of the
Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress
response: A possible mechanism for melanocyte degeneration in
vitiligo. J Invest Dermatol. 134:2221–2230. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jian Z, Tang L, Yi X, Liu B, Zhang Q, Zhu
G, Wang G, Gao T and Li C: Aspirin induces Nrf2-mediated
transcriptional activation of haem oxygenase-1 in protection of
human melanocytes from H2O2-induced oxidative stress. J Cell Mol
Med. 20:1307–1318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang L, Liu G, Lin Z, Wang Y, He H, Liu T
and Kamp DW: Pro-inflammatory response and oxidative stress induced
by specific components in ambient particulate matter in human
bronchial epithelial cells. Environ Toxicol. 31:923–936.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yu N, Zhu KJ, Ma SJ, Tang Hao and Tan XN:
The total flavonoids of clerodendrum bungei suppress A549 cells
proliferation, migration, and invasion by impacting Wnt/β-catenin
signaling. World J Tradit Chin Med. 4:15–20. 2017.
|
|
21
|
Ichikawa A, Takagi H, Suda K and Yao T:
New methodological approach for the rapid and sensitive detection
of melanocytes and melanocytic tumours. The DOPA-GA method.
Dermatology. 219:195–201. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mohamad N, Gutiérrez A, Núñez M, Cocca C,
Martín G, Cricco G, Medina V, Rivera E and Bergoc R: Mitochondrial
apoptotic pathways. Biocell. 29:149–161. 2005.PubMed/NCBI
|
|
23
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee C: Collaborative power of Nrf2 and
PPARγ activators against metabolic and drug-induced oxidative
injury. Oxid Med Cell Longev. 2017(1378175)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Colucci R, Dragoni F and Moretti S:
Oxidative stress and immune system in vitiligo and thyroid
diseases. Oxid Med Cell Longev. 2015(631927)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Repnik U, Česen MH and Turk B: The
endolysosomal system in cell death and survival. Cold Spring Harb
Perspect Biol. 5(a008755)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Slimen IB, Najar T, Ghram A, Dabbebi H,
Ben Mrad M and Abdrabbah M: Reactive oxygen species, heat stress
and oxidative-induced mitochondrial damage. A review. Int J
Hyperthermia. 30:513–523. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kupsco A and Schlenk D: Oxidative stress,
unfolded protein response and apoptosis in developmental toxicity.
Int Rev Cell Mol Biol. 317:1–66. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shi Q, Zhang W, Guo S, Jian Z, Li S, Li K,
Ge R, Dai W, Wang G, Gao T and Li C: Oxidative stress-induced
overexpression of miR-25: the mechanism underlying the degeneration
of melanocytes in vitiligo. Cell Death Differ. 23:496–508.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hu R, Xie XY, Xu SK, Wang YN, Jiang M, Wen
LR, Lai W and Guan L: PM2.5 exposure elicits oxidative stress
responses and mitochondrial apoptosis pathway activation in HaCaT
keratinocytes. Chin Med J (Engl). 130:2205–2214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shi MH, Wu Y, Li L, Cai YF, Liu M, Gao XH
and Chen HD: Meta-analysis of the association between vitiligo and
the level of superoxide dismutase or malondialdehyde. Clin Exp
Dermatol. 42:21–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li QL, Wu YH, Niu M, Lu XJ, Huang YH and
He DH: Protective effects of tacalcitol against oxidative damage in
human epidermal melanocytes. Int J Dermatol. 56:232–238.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Karsli N, Akcali C, Ozgoztasi O, Kirtak N
and Inaloz S: Role of oxidative stress in the pathogenesis of
vitiligo with special emphasis on the antioxidant action of
narrowband ultraviolet B phototherapy. J Int Med Res. 42:799–805.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bao L, Li J, Zha D, Zhang L, Gao P, Yao T
and Wu X: Chlorogenic acid prevents diabetic nephropathy by
inhibiting oxidative stress and inflammation through modulation of
the Nrf2/HO-1 and NF-ĸB pathways. Int Immunopharmacol. 54:245–253.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Goldstein NB, Koster MI, Hoaglin LG,
Spoelstra NS, Kechris KJ, Robinson SE, Robinson WA, Roop DR, Norris
DA and Birlea SA: Narrow band ultraviolet B treatment for human
vitiligo is associated with proliferation, migration, and
differentiation of melanocyte precursors. J Invest Dermatol.
135:2068–2076. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L
and Li M: Interferon-gamma inhibits melanogenesis and induces
apoptosis in melanocytes: A pivotal role of CD8+ cytotoxic T
lymphocytes in vitiligo. Acta Derm Venereol. 95:664–670.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim HJ, Bae IH, Son ED, Park J, Cha N, Na
HW, Jung C, Go YS, Kim DY, Lee TR and Shin DW: Transcriptome
analysis of airborne PM2.5-induced detrimental effects on human
keratinocytes. Toxicol Lett. 273:26–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Videira IF, Moura DF and Magina S:
Mechanisms regulating melanogenesis. An Bras Dermatol. 88:76–83.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lo Cicero A, Delevoye C, Gilles-Marsens F,
Loew D, Dingli F, Guéré C, André N, Vié K, van Niel G and Raposo G:
Exosomes released by keratinocytes modulate melanocyte
pigmentation. Nat Commun. 6(7506)2015.PubMed/NCBI View Article : Google Scholar
|