Introduction
Alcohol consumption accounts for 3.8% of global
mortality and 4.6% of disability-adjusted life-years lost due to
premature death (1). Among the
various harmful effects of alcohol, alcoholic liver disease induces
a wide spectrum of liver abnormalities, including simple steatosis,
alcoholic hepatitis/steatohepatitis, progressive fibrosis and
ultimately alcoholic cirrhosis and/or hepatocellular carcinoma
(2).
The latest worldwide survey of coronary
revascularization shows that 583,000 coronary-artery bypass
operations were performed in 1995(3). According to European statistics, the
annual rate of use of balloon angioplasty is approximately 739
procedures per million population (4). Approximately 60 percent of patients
treated with balloon angioplasty or bypass surgery have multivessel
disease that could be treated by either procedure. Coronary artery
bypass grafting is the most effective treatment for patients with
liver failure that is complicated with severe coronary heart
disease, and who cannot be treated using coronary stent
intervention (5). However, coronary
artery bypass grafting is contraindicated for patients with liver
failure. Recently, a case of severe coronary heart disease with
liver failure was successfully treated.
Case report
A 62-year old man was admitted to the Tianjin First
Central hospital following 4 years of xanthochromia and 4 years of
liver cirrhosis. The patient was admitted to the Department of
Liver Transplantation in September 2017 with alcoholic liver
cirrhosis and end-stage liver disease. A period of 5 years
previously, the patient had exhibited skin and scleral
xanthochromia with no obvious cause. This was accompanied by
anorexia, nausea and intermittent fever, although the patient
exhibited no signs of abdominal pain, diarrhea, hematemesis,
unconsciousness or other symptoms, and no specific treatment was
provided.
A period of 4 years previously (January
2014-December 2018), the patient had been hospitalized in the
Tianjin First Central Hospital for anorexia and nausea. Abdominal
ultrasonography indicated liver cirrhosis, and the patient received
discontinuous hepatoprotective treatment.
A period of 2 years previously, the patient had
exhibited abdominal distention and edema of the lower extremities.
The effect of conservative treatment was poor, leading to the
patient being admitted to the Department of Liver Transplantation
for surgical treatment.
Considering that the patient had liver failure, a
long-term smoking history, poor lung function and poor overall
health, non-cardiopulmonary bypass treatment was selected, which
exerts a small effect on lung function and circulation, and can be
performed via an opening in the middle of the chest under routine
anesthesia. The left internal mammary artery was bridged to the
left anterior descending artery (LAD), and the great saphenous vein
was bridged from the ascending aorta to the posterior descending
branch. Conventional whole-liver allogeneic orthotopic liver
transplantation was subsequently performed during the surgery. The
donor liver was anastomosed to the superior vena cava, inferior
vena cava and portal vein, and the hepatic artery and biliary tract
were subsequently anastomosed. A T-shaped drainage tube was
inserted. This treatment was approved by the Ethics Committee of
Tianjin First Central Hospital and was in conformity with the
guidelines of National Institute of Health. Preoperative written
informed consent was obtained from the patient.
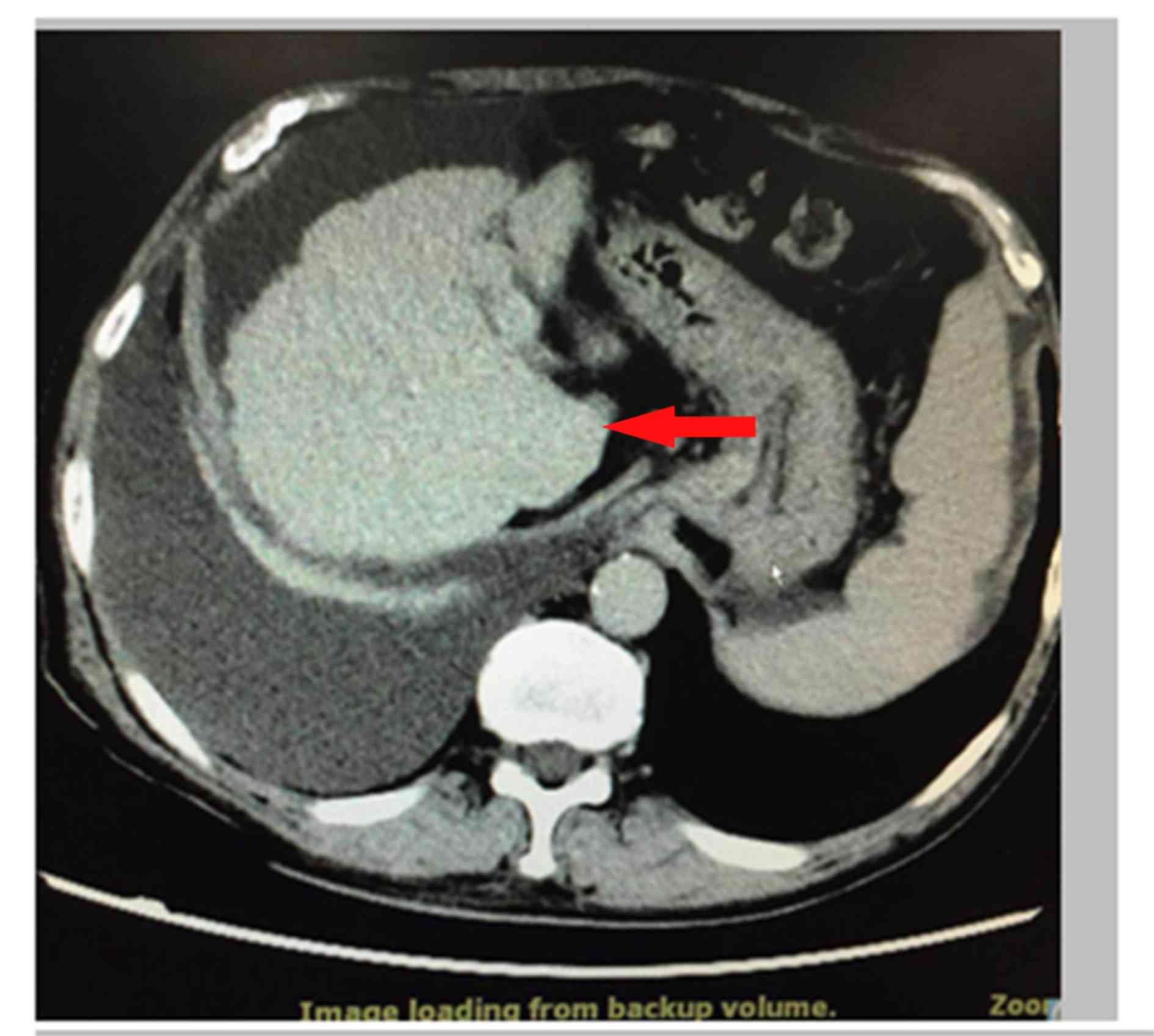
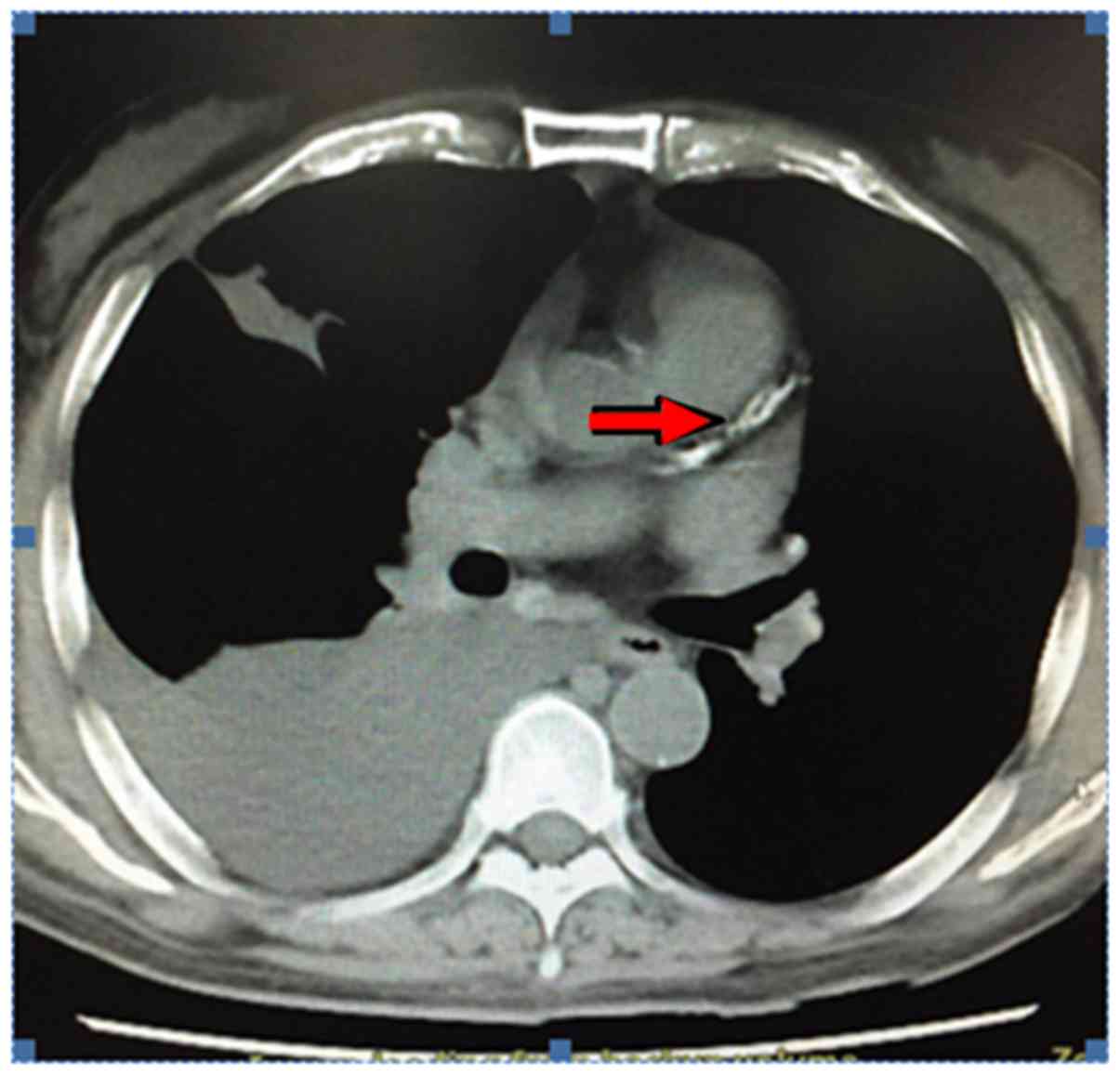
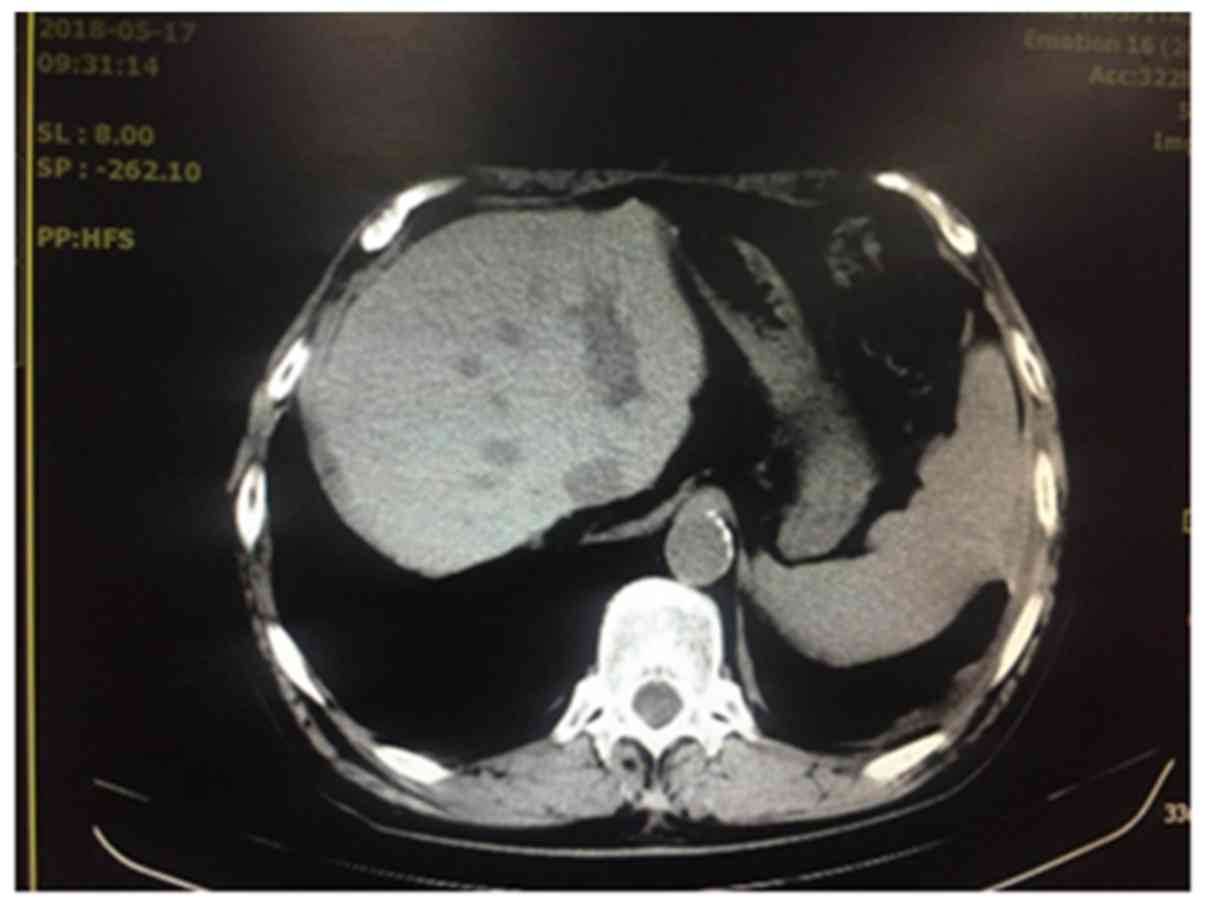
After hospitalization, a chest and abdominal CT was
performed for bilateral pleural effusion, cirrhosis and ascites
(Figs. 1 and 2). The blood routine results obtained on
September 14th 2017 were as follows, normal ranges are displayed in
brackets: White blood cell count (WBC), 2.72x109 cells/l
(4-10x109 cells/l); red blood cell count (RBC),
2.56x1012 cells/l (3.5-5.5x1012 cells/l);
hemoglobin count (HGB), 93.00 g/l (120-160 g/l); hematocrit count
(HCT), 27.30% (40-48%); and platelet count, 50x109
cells/l (100-300 cells/l). The biochemistry results obtained on the
same day were as follows: Total protein, 53.0 g/l (60-80 g/l);
albumin, 26.1 g/l (35-55 g/l); alanine aminotransferase, 15.3 U/l
(10-64 U/l); aspartate aminotransferase, 25.5 U/l (8-40 U/l);
bilirubin, 59.36 µmol/l (5.1-20.5 µmol/l); direct bilirubin, 34.49
µmol/l (0-8 µmol/l); indirect bilirubin, 24.87 µmol/l (0-13.6
µmol/l)); potassium, 3.81 mmol/l (3.5-5.3 mmol/l); sodium, 137.2
mmol/l (137-147 mmol/l); blood urea nitrogen, 2.61 mmol/l (3.1-8.0
mmol/l); chromium, 71.00 µmol/l (59-104 µmol/l); alkaline
phosphatase, 101.1 U/l (45-125 U/l); γ-glutamyltransferase, 8.9 U/l
(10-60 U/l); and Globulin, 26.9 g/l (20-40 g/l). The results of
arterial blood gas analysis obtained on the same day were as
follows: CO2, 29.5 mmHg (35-48 mmHg); PO2,
62.0 mmHg (83-108 mmHg); lactate, 3.03 mmol/l (0.5-1.6 mmol/l); and
blood ammonia, 70 µmol/l (18-60 µmol/l).
On November 7, 2017, prior to surgery, the routine
blood examination results were as follows: WBC, 2.54x109
cells/l (4-10x109 cells/l); RBC, 2.46x1012
cells/l (3.5-5.5x1012 cells/l); HGB, 88.00 g/l (120-160
g/l); HCT, 26.30% (40-48%); and HLT, 53x109 cells/l
(100-300/l). The results for preoperative coagulation function
obtained on the same day were as follows: Kaolin partial
thromboplastin time, 54.9 sec (26-42 sec); prothrombin time, 17.4
sec (8.8-13.8 sec); international normalized ratio, 1.54 (0.8-1.2);
and prothrombin activity, 50% (80-120%). The results for
preoperative pulmonary function obtained on the same day were as
follows: Restriction of ventilation function, reduced slightly;
obstruction, reduced slightly; small airway function, moderately
reduced; and dispersion function, severely reduced. The results for
pleural effusion obtained on the same day were as follows: 4.7 cm
on the right side and 2.1 cm on the left side. The patient was in
an alcoholic liver cirrhosis decompensation period, so symptomatic
treatment was administered. Namely, magnesium isoglycyrrhizinate
injections (200 mg/day) to improve liver function, human serum
albumin (10-20 g/day) to treat hypoproteinemia and enteral
nutrition emulsion (500 ml/day) to improve the nutritional status
of patients. During hospitalization, the patient exhibited
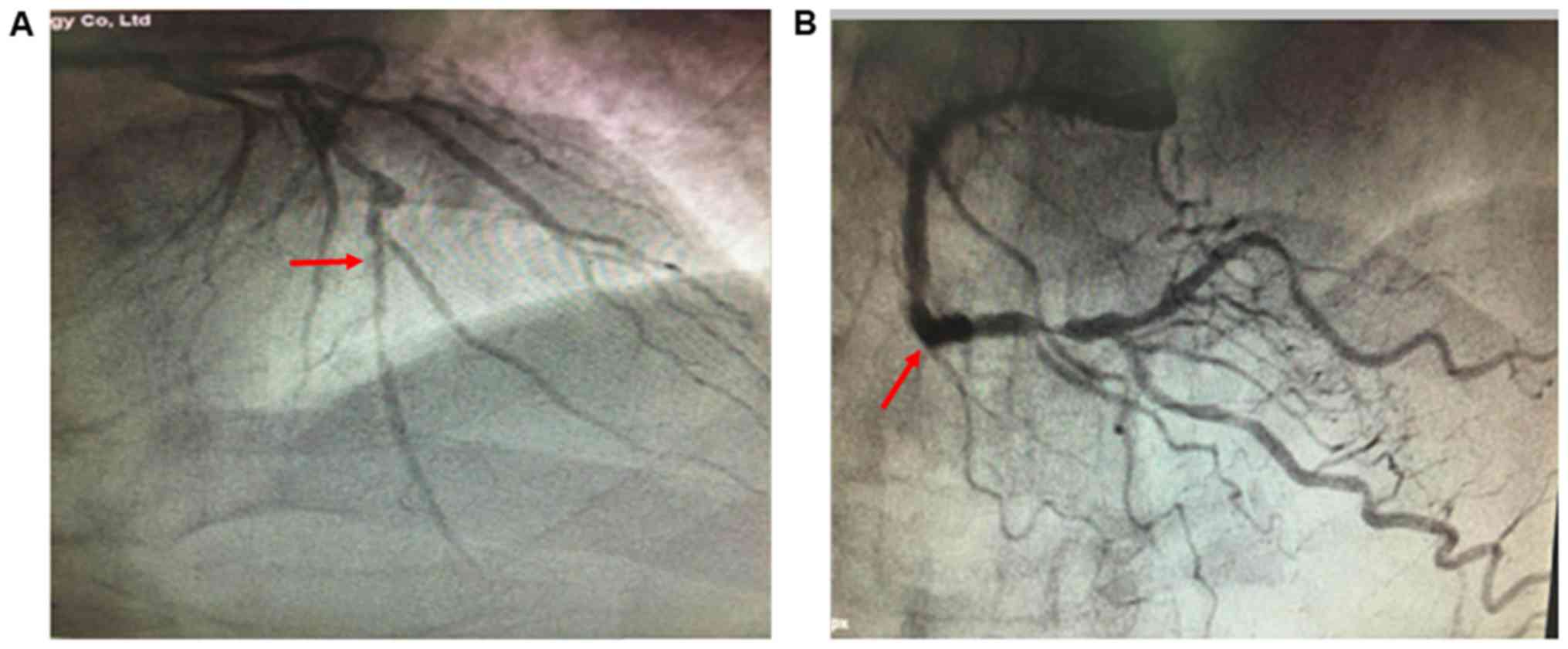
recurrent angina pectoris. On October 17th 2017, according to
coronary angiography, the areas from the left main artery to the
anterior descending artery were calcified, and left main artery
stenosis was at 50%; the proximal to distal anterior descending
artery exhibited diffuse lesions, and stenosis was at 70-90%.
Aneurysms were also identified in the center of the anterior
descending artery. The circumflex arteries were small and no
obvious stenosis was observed. Distal right coronary artery
stenosis was at 90% (Fig. 3).
The preoperative diagnosis was: i) Alcoholic
cirrhosis and decompensated liver cirrhosis; ii) coronary heart
disease and unstable angina; iii) chronic obstructive pulmonary
disease; and iv) Grade C liver function (classified via the
Child-Pugh classification) (4) with clear indications
for liver transplantation. The patient exhibited angina pectoris
and could not tolerate routine liver transplantation surgery, and
no liver donor was available. Therefore, the patient was
transferred to the Department of Cardiac Surgery, and an off-pump
coronary artery bypass graft was performed on November 8, 2017.
Three Bridging (5) was built during
the surgery (left internal mammary artery-LAD; aorta-SVG-posterior
descending branch; and aorta-SVG-diagonal branch). On day 4
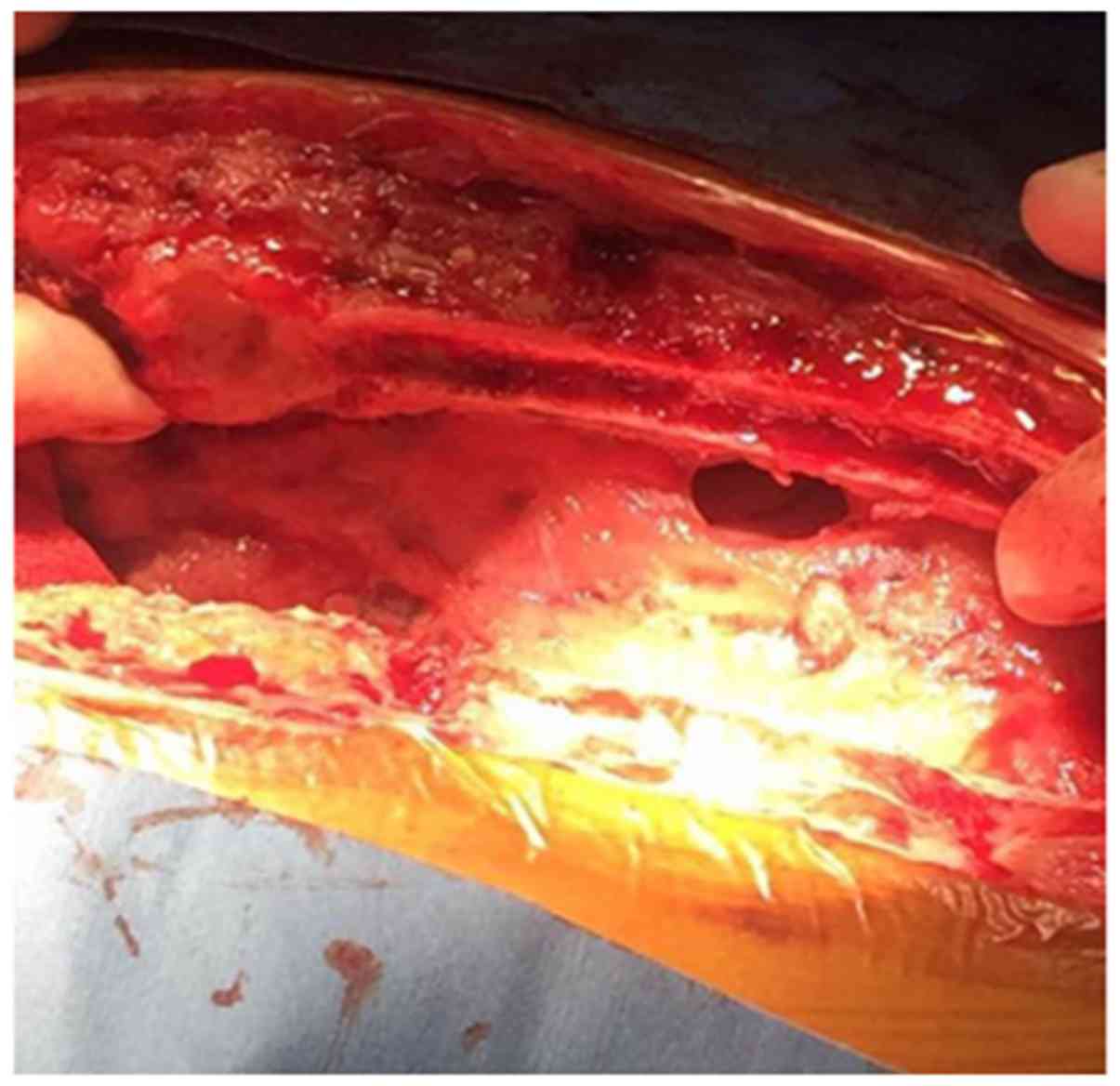
following surgery, a hemorrhagic effusion occurred in the middle
and lower segment of the anterior thoracotomy, and the daily
exudate amount was ~600 ml. Exudate also effused from the xiphoid
process when the wound was opened. Following bilateral chest
drainage, there was still a large amount of exudation from the
anterior chest wound. Debridement and bilateral pleural repair
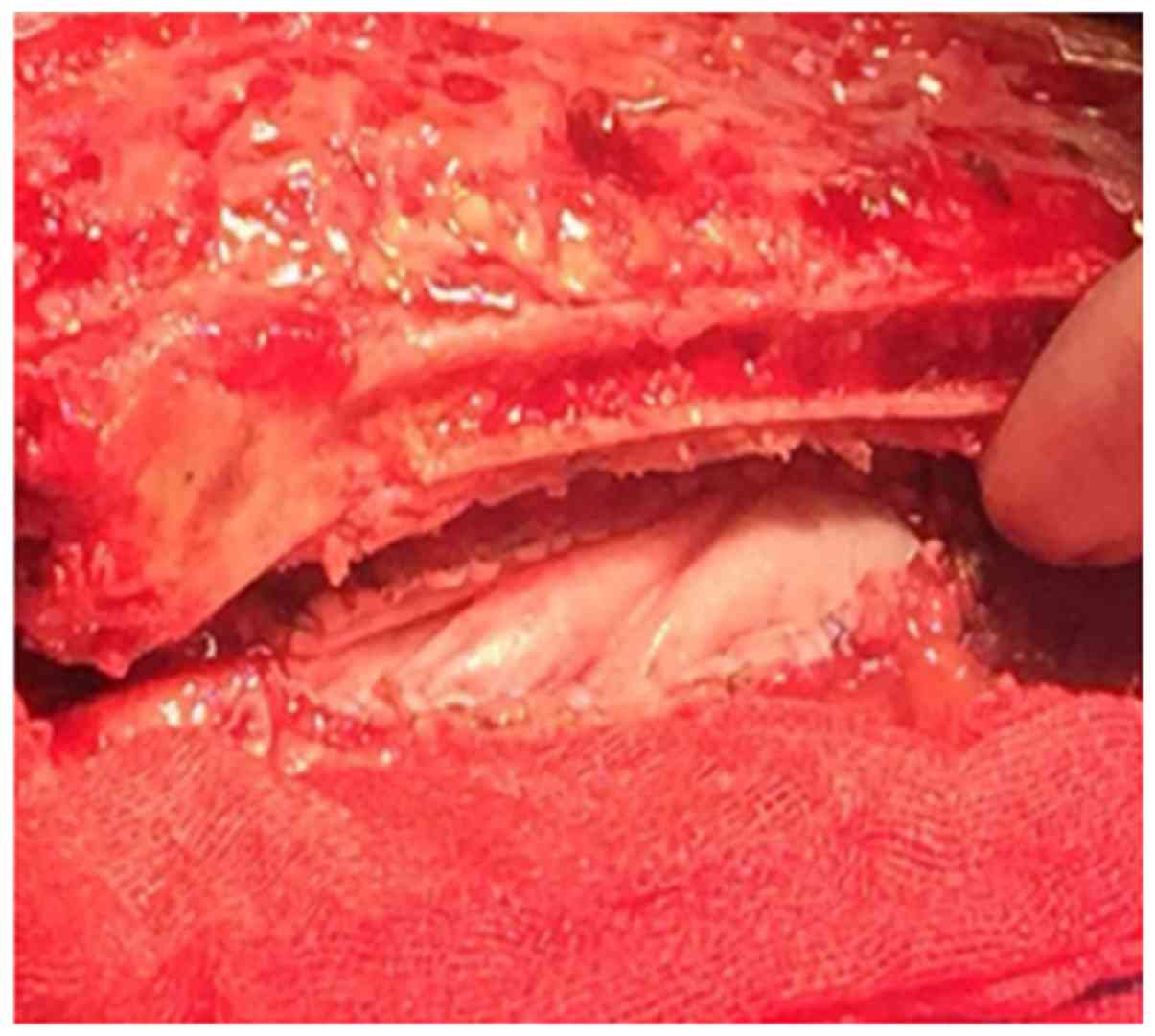
surgery were performed on November 21, 2017 (Figs. 4 and 5). Following surgery, the patient received
appropriate correction of hypoproteinemia and anti-infection
treatment (tigecycline, 50 mg/day, 21 days), however the wound did
not heal. General anesthesia allograft liver transplantation was
performed on December 8, 2017. Whole-liver orthotopic liver
transplantation was performed intraoperatively. The donor liver was
obtained from a brain-dead donor, and the cold ischemia period was
240 min. Intraoperatively, the superior vena cava, inferior vena
cava and portal vein were routinely anastomosed. One steel sternum
wire was removed, and the wound was fixed. Anti-rejection
(methylprednisolone 8 mg/day, cyclosporine 170 mg/day and
mycophenolate mofetil 2.0 g/day) and anti-infection treatments
(Tigecycline, 50 mg/day) were provided after surgery, and the
condition of the patient was stable following comprehensive
nutritional treatment (enteral nutrition emulsion, 500 ml/day for
60 days). A purulent secretion was identified from the anterior
chest wound at the level of the manubrium, and this was accompanied
by a high fever. Culture following a wound swab indicated the
presence of Staphylococcus aureus, and thus wound infection
was considered. Anterior chest wound debridement was performed on
January 8, 2018. Local necrotic bone was detected at the level of
the manubrium. One steel sternum wire was removed at the second
intercostal space. Recovery was good at the lower segment of
sternum, and a curette was used to clean the necrotic bones.
Necrotic bones were also identified at the right side of the
manubrium, and debridement and gauze packing treatments were
performed. The patient was transferred to a general ward to
continue recovery. The sternum wound gradually healed, and there
was intermittent purulent exudate near the xiphoid. Culturing
indicated the presence of S. aureus, so chronic
osteomyelitis was considered. Wound debridement was performed on
February 5, 2018 due to the osteomyelitis. Sternal and costal
cartilage necrosis was identified at the xiphoid process in the
lower segment of the wound. One steel sternum wire was removed at
the fourth intercostal space, and necrotic bone was scraped. A
total of three costal cartilages on the left side and two costal
cartilages were removed on the right side. Followings surgery,
dressings were changed daily, the condition of the patient
gradually stabilized and wound exudation gradually reduced. At 5
months after surgery, the wound had healed and the patient left
hospital. The results of an abdominal CT (Fig. 6) and an ultrasound test were normal.
The patient continued to receive methylprednisolone (8 mg/day),
cyclosporine (170 mg/day), and mycophenolate mofetil (2.0 g/day)
for anti-rejection therapy.
Discussion
Patients often exhibit coronary heart disease when
waiting for liver transplantation and some patients cannot receive
coronary stenting; therefore, coronary artery bypass grafting is
the only effective treatment (6-9).
Coronary artery bypass grafting is contraindicated in end-stage
liver disease due to symptoms of coagulation dysfunction,
refractory hypoalbuminemia, pleural effusion, edema of the lower
extremities, hepatic encephalopathy, hypersplenism, leukopenia,
thrombocytopenia and gastrointestinal bleeding (10,11). In
2004, a study performed at Northwestern University (Evanston, IL,
USA) reported five cases of patients with severe coronary heart
disease and hepatic failure who received simultaneous coronary
artery bypass grafting and liver transplantation, with an average
age of 57.8 years (6). No
perioperative death was exhibited in the aforementioned patients,
and at the 25-month follow-up the survival rate was 80% (12). In 1997, Pelosi et al (12) introduced 12 cases of liver
transplantation following coronary artery bypass graft. To the best
of our knowledge, no clinical literature report on coronary artery
bypass graft surgery for alcoholic liver cirrhosis and hepatic
failure exists. In the current case, the patient exhibited advanced
alcoholic liver cirrhosis, and life-threatening angina pectoris
occurred frequently while the patient waited for a donor liver. An
emergency coronary artery bypass graft was performed subsequently,
and wound infection (S. aureus) occurred following surgery.
During wound treatment, orthotopic liver transplantation was
performed, and the postoperative abdominal wound healed well. The
chest wound also healed after multiple debridements.
End-stage liver disease is a contraindication for
coronary artery bypass graft. If liver transplantation and CABG
cannot be performed simultaneously, surgery should be selected
carefully. End-stage liver disease leads to a number of
complications (13,14), and blood loss is often high; heavy
heparinization during coronary artery bypass grafting will
exacerbate the risk of bleeding (15). Hypoproteinemia also causes poor
nutritional status and poor tissue healing ability (16), and coronary artery bypass surgery
wounds can easily become infected and lead to secondary sternal
osteomyelitis (17). Systemic tissue
edema and pleural effusion can cause a decrease in respiratory
function, and pleural breakage may be difficult to repair due to
thoracotomy and internal mammary artery incision (18). Postoperative decompensation of liver
cirrhosis causes continuous production and outflow of pleural
fluid, which can lead to slow wound recovery (19). Additionally, hypersplenism leads to
severe leukopenia, anemia and other immune deficiencies, which can
lead to secondary infections (20).
Postoperative blood transfusion is also likely to induce hepatic
encephalopathy and increase infection risk (21). Patients can also exhibit angina
pectoris during the waiting period for liver transplantation, which
may not be effectively alleviated using drug therapy. Coronary
artery lesions can also develop into diffuse coronary calcification
stenosis, and medical interventional therapy cannot be subsequently
performed; in this case, salvage coronary artery bypass grafting
should be performed (22).
During coronary artery bypass grafting, the damaged
pleura should be completely closed to prevent pleural effusion, and
to prevent interference with wound healing (23). The majority of pleura in patients are
thin, and it is difficult to repair these following pleural
breakage (24). Pneumothorax
occurred after opening the anterior thoracic wound, and pleural
effusion continued. The anterior thoracic wound was opened and
repaired, and the wound was sutured after pleural effusion
catheterization. The middle and lower part of the wound was
subsequently opened to change the dressing due to low protein,
malnutrition, secondary infection, persistent exudation of pleural
effusion, persistent reduction of protein and aggravated liver
cirrhosis (25-28).
For patients with normal heart function and severe
coronary artery disease, simultaneous liver transplantation during
coronary artery bypass grafting can be selected, to avoid the risk
of acute myocardial infarction during liver transplantation and
reduce the impact of liver failure on patient recovery following
the coronary artery bypass graft (12). In the present case, the angina
pectoris was life-threatening and could not be alleviated, so an
emergency coronary artery bypass graft was performed. As there was
no suitable liver donor, liver transplantation and coronary artery
bypass grafting could not be performed simultaneously. After
coronary artery bypass grafting, the wound did not heal and liver
function deteriorated, and a large quantity of pleural fluid
leaked. Liver transplantation was performed when the anterior
thoracic wound was unhealed. Immunosuppressive agents were used to
decrease the risk of infection. Chronic osteomyelitis and S.
aureus infection occurred in the anterior chest wound. The
wound healed following two rounds of necrotic tissue
debridement.
For patients with hepatic failure, when performing
thoracotomy, the bilateral pleura should not be opened. If pleural
damage and breakage is observed, an autologous or allogeneic
pericardial patch should be used to close the breakage (29), and this is essential to prevent
postoperative wound dehiscence, exudation and infection (30,31). A
sternal incision, intercostal incision or a robot can be used to
perform coronary artery bypass graft. In the current case, the
bilateral pleural crevasses were not repaired, and pleural fluid
continued to leak out after the operation. Then, tension
pneumothorax occurred, and emergent pleural repair and wound
suturing were performed. After suturing, the wounds could not heal
due to low protein, anemia and hypersplenism caused by liver
failure. During the operation, the superior and inferior vena cava
were exposed, and an abdominal cavity expander was used, as the
steel wire of the lower sternum was loosened, to avoid cutting the
sternum. After liver transplantation, the wound was sutured after
the sternum was fixed. Due to the use of immunosuppressive agents
after transplantation, S. aureus infection was indicated in
the upper sternum near the second costal cartilage at 1 month after
transplantation, and debridement of the local sternum and costal
cartilage was performed. During this period, the healed sternum
should be protected to avoid cracking. A period of 2 months after
transplantation, local infection in the lower segment of the
sternum occurred, and healed after debridement.
Multidisciplinary collaboration and comprehensive
supportive treatment after liver transplantation is the key to
successful postoperative recovery. Performing liver transplantation
during the period when the anterior chest wound is unhealed,
combined with the use of immunosuppressive agents, can increase the
risk of wound infection. After 5 days of intensive care treatment,
the liver function of the patient gradually recovered, and the
patient was transferred to an intensive care unit. The patient was
given adequate nutritional support through a naso-intestinal tube;
the recovery of gastrointestinal function accelerated, and the
liver function became normal with treatment. The secretions of the
anterior chest wound tested positive for S. aureus
infection. The wounds healed gradually after two rounds of
debridement. The patient recovered and was discharged 5 months
after the coronary artery bypass graft and 4 months after liver
transplantation. During the long course of treatment in the current
case, lessons can be learnt regarding the surgical choice, wound
management, postoperative liver function recovery, heart and kidney
function maintenance, and nutritional support, and the present
report may provide a reference for the use of thoracotomy in liver
failure.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National Key
Research and Development Program (grant no. 2016YFC1305104) and
Tianjin Clinical Research Center for Organ Transplantation Project
(grant no. 15ZXLCSY00070).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and ZS contributed to make substantial
contributions to the study conception and design, the acquisition
of data and the analysis and interpretation of data. JC and ZS
contributed to drafting the manuscript and critically revising the
manuscript for important intellectual content. ZS gave final
approval of the version to be published. ZS, KW and XK contributed
to data collection and data entry. WJ and LZ contributed to the
data analysis. CP and FX contributed to data interpretation. JC
prepared the manuscript, and WZ and HC performed the literature
analysis search. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study has been approved by the Ethics
Committee of Tianjin First Central Hospital and was in conformity
with the guidelines of National Institute of Health (permit no.
81004). All study participants provided written consent to be
involved in the present study. All study participants had given
their written informed consent for publication before participating
in the current study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rehm J, Mathers C, Popova S,
Thavorncharoensap M, Teerawattananon Y and Patra J: Global burden
of disease and injury and economic cost attributable to alcohol use
and alcohol-use disorders. Lancet. 373:2223–2233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marroni CA, Fleck AM Jr, Fernandes SA,
Galant LH, Mucenic M, de Mattos Meine MH, Mariante-Neto G and
Brandão ABM: Liver transplantation and alcoholic liver disease:
History, controversies, and considerations. World J Gastroenterol.
24:2785–2805. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fujihara J, Yasuda T, Kawai Y, Morikawa N,
Arakawa K, Koda Y, Soejima M, Kimura-Kataoka K and Takeshita H:
First survey of the three gene polymorphisms (PON1 Q192R, eNOS
E298D and eNOS C-786T) potentially associated with coronary artery
spasm in African populations and comparison with worldwide data.
Cell Biochemi Funct. 29:156–163. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Lip GYH, Collet JP, Haude M, Byrne R,
Chung EH, Fauchier L, Halvorsen S, Lau D, Lopez-Cabanillas N,
Lettino M, et al: 2018 joint european consensus document on the
management of antithrombotic therapy in atrial fibrillation
patients presenting with acute coronary syndrome and/or undergoing
percutaneous cardiovascular interventions: A joint consensus
document of the European heart rhythm association (EHRA), European
society of cardiology working group on thrombosis, European
association of percutaneous cardiovascular interventions (EAPCI),
and European association of acute cardiac care (ACCA) endorsed by
the heart rhythm society (HRS), Asia-pacific heart rhythm society
(APHRS), Latin America heart rhythm society (LAHRS), and cardiac
arrhythmia society of Southern Africa (CASSA). Europace.
21:192–193. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sacco RL, Adams R, Albers G, Alberts MJ,
Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J,
Harbaugh R, et al: Guidelines for prevention of stroke in patients
with ischemic stroke or transient ischemic attack: A statement for
healthcare professionals from the American heart
Association/American stroke association council on Stroke:
Co-sponsored by the council on cardiovascular radiology and
intervention: The American academy of neurology affirms the value
of this guideline. Stroke. 37:577–617. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Axelrod D, Koffron AJ, Dewolf A, Baker A,
Fryer J, Baker T, Frederiksen J, Horvath K and Abecassis M: Safety
and efficacy of combined orthotopic liver transplantation and
coronary artery bypass grafting. Liver Transpl. 10:1386–1390.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Hogan BJ, Gonsalkorala E and Heneghan MA:
Evaluation of coronary artery disease in potential liver transplant
recipients. Liver Transpl. 23:386–395. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Lee BC, Li F, Hanje AJ, Mumtaz K,
Boudoulas KD and Lilly SM: Effectively screening for coronary
artery disease in patients undergoing orthotopic liver transplant
evaluation. J Transplant. 2016(7187206)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sabzi F and Faraji R: Liver function tests
following open cardiac surgery. J Cardiovas Thorac Res. 7:49–54.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Goodman WG, Goldin J, Kuizon BD, Yoon C,
Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al:
Coronary-artery calcification in young adults with end-stage renal
disease who are undergoing dialysis. N Engl J Med. 342:1478–1483.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Raggi P, Boulay A, Chasan-Taber S, Amin N,
Dillon M, Burke SK and Chertow GM: Cardiac calcification in adult
hemodialysis patients: A link between end-stage renal disease and
cardiovascular disease? J Am Coll Cardiol. 39:695–701.
2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pelosi F, Klintmalm GB, Simon WB and
Roberts WC: Liver transplantation after coronary artery bypass
grafting. Am J Cardiol. 79:1405–1407. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Parikh A, Washburn K, Matsuoka L, Pandit
U, Kim JE, Almeda J, Mora-Esteves C, Halff G, Genyk Y, Holland B,
et al: A multicenter study of 30 days complications after deceased
donor liver transplantation in the model for end-stage liver
disease score era. Liver Transpl. 21:1160–1168. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Rahimi RS and Rockey DC: End-stage liver
disease complications. Curr Opin Gastroenterol. 29:257–263.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Filsoufi F, Rahmanian PB, Castillo JG,
Karlof E, Schiano TD and Adams DH: Excellent results of cardiac
surgery in patients with previous liver transplantation. Liver
Transpl. 13:1317–1323. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Liamis G, Filippatos TD, Liontos A and
Elisaf MS: Hyponatremia in patients with liver diseases: Not just a
cirrhosis-induced hemodynamic compromise. Hepatol Int. 10:762–772.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Johnson P, Frederiksen JW, Sanders JH,
Lewis V and Michaelis LL: Management of chronic sternal
osteomyelitis. Ann Thoracic Surg. 40:69–72. 1985.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Flege JB Jr: Pericardial incision for
internal mammary artery coronary bypass. Ann Thorac Surg. 44:424.
1987.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chertoff J and Nathoo S: Decompensated
liver cirrhosis presenting as a spontaneous left-sided bacterial
empyema. ACG Case Rep J. 3:124–126. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li LY, Chen HZ, Bao YC, Yu QS and Yang WM:
Successful treatment of hypersplenism in Wilson's disease by
partial splenic embolization. J Invest Surg. 31:75–81.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barge W, Khemani F, Vogle A and Aloman C:
Tu1572-clinical significant insomnia prevalence in cirrhotic
population is independent of hepatic encephalopathy and sleep
quality. Gastroenterology. 154 (Suppl 1)(S1255)2018.
|
|
22
|
Makikallio TH, Holm NR, Lindsay M, Spence
MS, Erglis A, Menown IB, Trovik T, Eskola M, Romppanen H, Kellerth
T, et al: Percutaneous coronary angioplasty versus coronary artery
bypass grafting in treatment of unprotected left main stenosis
(NOBLE): A prospective, randomised, open-label, non-inferiority
trial. Lancet. 388:2743–2752. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Light RW: Pleural effusions following
cardiac injury and coronary artery bypass graft surgery. Semin
Respir Crit Care Med. 22:657–664. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Verma N, Robinson JD and Gunn ML:
Pericardial rupture and cardiac herniation in blunt trauma. Radiol
Case Rep. 13:573–575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Alonso JC: Pleural effusion in liver
disease. Semin Respir Crit Care Med. 31:698–705. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bernardi M, Maggioli C and Zaccherini G:
Human albumin in the management of complications of liver
cirrhosis. Crit Care. 16(211)2012.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Bunchorntavakul C, Chamroonkul N and
Chavalitdhamrong D: Bacterial infections in cirrhosis: A critical
review and practical guidance. World J Hepatol. 8:307–321.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Eghtesad S, Poustchi H and Malekzadeh R:
Malnutrition in liver cirrhosis: The influence of protein and
sodium. Middle East J Dig Dis. 5:65–75. 2013.PubMed/NCBI
|
|
29
|
Vaidyanathan S, Gupta A and Sivakumar K:
Massive pericardial effusion, yet no signs of tamponade! Ann
Pediatr. Cardiol. 10:100–101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mao J, Wang Y, Philippe E, Cianciulli T,
Vesely I, How D, Bourget JM, Germain L, Zhang Z and Guidoin R:
Microstructural alterations owing to handling of bovine pericardium
to manufacture bioprosthetic heart valves: A potential risk for
cusp dehiscence. Morphologie. 101:77–87. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oldenburg WA, Almerey T, Selim M, Farres H
and Hakaim AG: Durability of carotid endarterectomy with bovine
pericardial patch. Ann Vasc Surg. 50:218–224. 2018.PubMed/NCBI View Article : Google Scholar
|