Introduction
Pancreatic cancer is one of the most malignant
cancers and is the fourth most common cause for cancer-related
deaths worldwide (1). The incidence
of pancreatic cancer is rising in many countries. Among the
different types of pancreatic cancer, pancreatic ductal
adenocarcinoma (PDAC) accounts for more than 80% of all diagnosed
cases of pancreatic cancer; PDAC is considered incurable (2,3). Over
the past few decades, significant progress has been made in the
diagnosis and treatment of different types of solid cancer, which
has greatly increased the chances of patients being cured. However,
although the survival rates have increased over the past decade,
the prognosis of pancreatic cancer is still poor, with a
5-year-survival rate below 10% following diagnosis (4). Lack of obvious and unique symptoms and
reliable biomarkers for early diagnosis as well as invasive
metastasis which results in the reduced sensitivity and response to
treatment are the main reason for the poor prognosis. Thus, it is
critical to discover novel therapeutic targets and develop novel
and effective therapeutic strategies to advance the current therapy
for PDAC.
MicroRNAs (miRNAs) are single-stranded small
non-coding RNAs which contain approximately 22 nucleotides. miRNAs
are reported to play an important role in the modulation of
expression levels of their target genes via binding to the 3'
untranslated region (5). It has been
proven that miRNAs exert a pivotal role in cancer through
regulating cancer cell proliferation, differentiation, invasion,
migration and cell apoptosis (6,7). In
pancreatic cancer cells, various miRNAs were found to act as
oncogenic mediators by downregulation or inhibition of the
expression of tumor-suppressor genes, while some of the miRNAs
serve as tumor suppressors that downregulate expression of
oncogenic genes, and thus regulate the proliferation, growth and
metastasis of cancer cells. Previous research by other groups
showed that the expression levels of miR-20a, miR-21, miR-99a and
miR-191 were significantly increased in pancreatic patient serum
while miR-375, miR-148, miR-96 and miR-497 expression levels were
lower in pancreatic patient serum when compared with levels in
normal pancreatic tissues (8,9). Among
these miRNAs, miR-25 located on chromosome 7 (7q22), constituted
with the miR-106b, miR-93 and miR106b/25 cluster, was reported to
play critical roles in different types of human cancers. miR-25 was
found to play an oncogenic role in breast cancer (10), ovarian cancer (11,12),
gastric cancer (13,14), hepatocellular carcinoma and lung
cancer (15,16), where their expression was
significantly increased and promoted tumor cell growth and
proliferation, while in prostate and colon cancers, miR-25
expression was downregulated and acted as a tumor suppressor
(17). However, its role in
pancreatic cancer has not been widely investigated.
In the present study, the expression and the
biological roles of miR-25 in PDAC tumors and pancreatic cancer
cells were explored. To date, only one study reported that the
expression of miR-25 is increased in pancreatic cancer patient
serum and miR-25 was suggested to be used as a potential biomarker
for the diagnosis of pancreatic cancer (18). However, the function and the
mechanism involved in its regulation of pancreatic cancer
proliferation and development are still unknown. The present study
revealed that miR-25 expression is much higher in both PDAC tumor
tissues and pancreatic cancer cell lines. miR-25 was found to play
a promotive role in pancreatic cell proliferation and increased
G1-to-S phase transition by targeting Abl interactor 2
(ABI2). Thus, these finding suggest that miR-25 may be a
diagnostic biomarker and therapeutic target for PDAC.
Materials and methods
Tissue samples and cell lines
A total of 25 pairs of pancreatic ductal
adenocarcinoma (PDAC) and normal adjacent tissues were collected
from patients (12 males and 13 females; age rage 39-76 years) at
the West China Hospital (Chengdu, Sichuan, China) between January
2012 and September 2017. Specimens were collected from patients who
did not undergo preoperative treatments such as chemotherapy. The
present study was approved by the Ethics Committee of the West
China Hospital and is in line with the 2004 Helsinki Declaration's
code of Ethics. Written informed consents were obtained from all
patients or from their legal guardians. Pathological examinations
were performed prior to the start of the study to verify the
presence of sufficient cancer cells in the tumor tissue samples and
that non-cancerous tissues were not contaminated with cancer cells.
After surgical resection, tissues were quickly frozen in liquid
nitrogen and stored at -80˚C until use. Three human PDAC cell lines
were kindly provided by the West China Hospital, which included
Panc-1, Bxpc-3 and Aspc-1 cells. Sw1990 and HPDE6c7 (human
pancreatic non-tumor cell line) cells were obtained from American
Type Culture Collection (ATCC). HPDE6c7 was used as a normal
control cell line. All human PDAC cell lines were maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.), while the control cell line was cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in
a humidified 5% CO2 incubator.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNAs were isolated from the PDAC tissues, and
HPDE6c7, Panc-1, Bxpc-3, Sw1990 and Aspc-1 cell lines, using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's instructions. Then 2 µg of total
RNA was reverse transcribed into cDNA using the miScript II RT kit
(Qiagen). The following thermocycling conditions were used for cDNA
synthesis: 37˚C for 60 min and 85˚C for 5 min. qPCR for miR-25 was
carried out using a miScript SYBR Green PCR kit (Qiagen). The
following thermocycling conditions were used for the qPCR: Initial
denaturation at 93˚C for 15 sec; 45 cycles of 67˚C for 45 sec, 93˚C
for 15 sec, 67˚C for 1 min, 95˚C for 15 sec; a final extension at
75˚C for 10 min. U6 was used as the internal reference to normalize
the miR-25 expression using the
2-ΔΔCq method (19). The relative expression of AB12 was
analyzed by RT-qPCR using SYBR Green PCR Mix (Aidlab) with GAPDH as
internal control using the 2-ΔΔCq
method (19). The sequences for
primers used for RT-qPCR were: miR-25-forward,
5'-TCTGGTCTCCCTCACAGGAC-3' and miR-25-reverse,
5'-CATGGGTCGCCTACTCAC-3'; ABI2-forward,
5'-CTCGCAATGTCAAGATCCATTGGC-3' and ABI2-reverse,
5'-TTACTCGCCGCACTGAAGTCAC-3'.
Cell transfection
miR-25 mimics, miR-25 inhibitor, NC and inhibitor NC
were commercially obtained from GenePharma (Shanghai, China).
miR-25 related solutions (20 µM) were prepared by diluting the
original stocks with DEPC water. Approximately 5-6x105
cells in logarithmic growth phase were plated into a 6-well plate
in complete medium. Aspc-1 cells were transfected with miR-25
mimics (50 nM), NC (50 nM), miR-25 inhibitor (150 nM) and inhibitor
NC (150 nM) using transfection reagent HiPerFect (Qiagen) with
serum-free RPMI-1640 medium.
Cell proliferation assay and cell
cycle analysis
Cell proliferation was detected using the CCK-8
assay. Briefly, transfected cells were plated into 96-well plates
with 2x103 cells in each well and then the cells were
cultured for 0, 24, 48 and 72 h. Then, the CCK-8 assay was
performed by adding 10 μl of Cell Counting Kit-8 (CCK-8, Beyotime)
reagent into each well. The cells in the 96-well plate were
incubated in a 37˚C, 5% CO2 incubator for 2 h. Then the
cell viability/proliferation was determined by measuring of the
absorbance at 450 nm using a microplate reader.
5-Ethynyl-2'-deoxyuridine (EdU) incorporation assay was conducted
to assess the proliferation of the transfected cells. Briefly,
actively proliferating Aspc-1 cells were incorporated with EdU and
then the incorporation was evaluated using a Cell-Light™ EdU Cell
Proliferation Detection kit (RiboBio) according to the
manufacturer's protocols. Cell immunostaining was observed and
acquired with an epifluorescence microscope (magnification x100;
Axioplan II; Carl Zeiss AG) equipped with a charge-coupled device
camera. Digital images were analyzed and quantified with ImageJ 3.1
[National Institutes of Health (NIH), Bethesda, MD, USA). Flow
cytometry was performed to analyze the cell cycle. Briefly,
transfected cells were digested with trypsin, and then collected by
centrifugation at 450 x g for 5 min at room temperature and washed
twice with cold PBS. Cells were resuspended with fixing buffer
containing 70% ethanol and fixed for 30 min at room temperature.
Then cells were stained with 10 µg/ml propidium iodide (PI) (Sigma)
for 15 min at room temperature, and cell cycle stages were analyzed
using flow cytometry (BD Biosciences).
Bioinformatics analysis of miR-25
targets
miRanda (http://www.microrna.org/microrna/home.do), miRDB
(http://mirdb.org/), miRWalk (http://mirwalk.umm.uni-heidelberg.de/) and TargetScan
(http://www.targetscan.org/vert_72/)
were used to predict the potential targets of miR-25. Among all the
candidates searched, ABI2 (ab1 interactor 2) was predicted
to be a target of miR-25, and a highly conserved binding site for
miR-25 seed sequence was found in the 3'-UTR region of ABI2
mRNA from position 7039 to 7045.
Luciferase reporter assay
Luciferase-related ABI2 plasmids were constructed.
First, PCR was used to amplify the 3'-UTR sequence of wild-type
ABI2 and a target-site mutant, and then the PCR products were
ligated into a dual-luciferase reporter vector (Promega), and the
products were named as pGL3-ABI2-3'-UTR-WT (wild-type vector) and
pGL3-ABI2-3'-UTR-Mut (mutant vector). For cell transfection, Aspc-1
cells at the logarithmic growth phase were seeded into 96-well
plates at a density of 1.5x103 cells/well. After being
cultured overnight, the Aspc-1 cells were co-transfected with the
WT or Mut vector, miR-25 mimics, NC, miR-25 inhibitor, or inhibitor
NC using Attractene Transfection Reagent (Qiagen). After
transfection for 48 h, the luciferase activity was determined by
determining the ratio of firefly to Renilla luciferase
activity with a dual-luciferase reporter system (Promega).
Western blot analysis
Cell lysates were prepared by digestion of the
collected cells with ice-cold RIPA buffer (Beyotime Institute of
Biotechnology) containing10 nM PMSF. The protein concentration of
each sample was measured and equal amount of proteins from each
sample were separated on 10% SDS polyacrylamide gels (SDS-PAGE) and
then the proteins were transferred to polyvinylidene fluoride
membranes. The membranes were blocked with 5% non-fat milk/TBST for
1 h and incubated with anti-ABI2 antibody (1:500; cat. no.
ab108340; Abcam) at 4˚C overnight. After being washed with cold
TBST 4 times (5 min each time), the membranes were incubated with
HRP-conjugated goat anti-rabbit immunoglobulin G secondary antibody
(1:2,000; cat. no. ab6721; Abcam) for 1 h. The protein expression
was visualized via chemiluminescence (Millipore). The ABI2 protein
expression level was analyzed using Image J software (NIH). GAPDH
(1:1,000; cat. no. ab8245; Abcam) expression served as the
control.
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used to conduct
the statistical analysis and all the data are presented as mean ±
SD. The statistical analysis between two groups was conducted by
the independent Student t-test. Differences among more than two
groups were analyzed by one-way ANOVA test, followed by
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistical significant difference.
Results
miR-25 is significantly upregulated in
PDAC tissues and cell lines
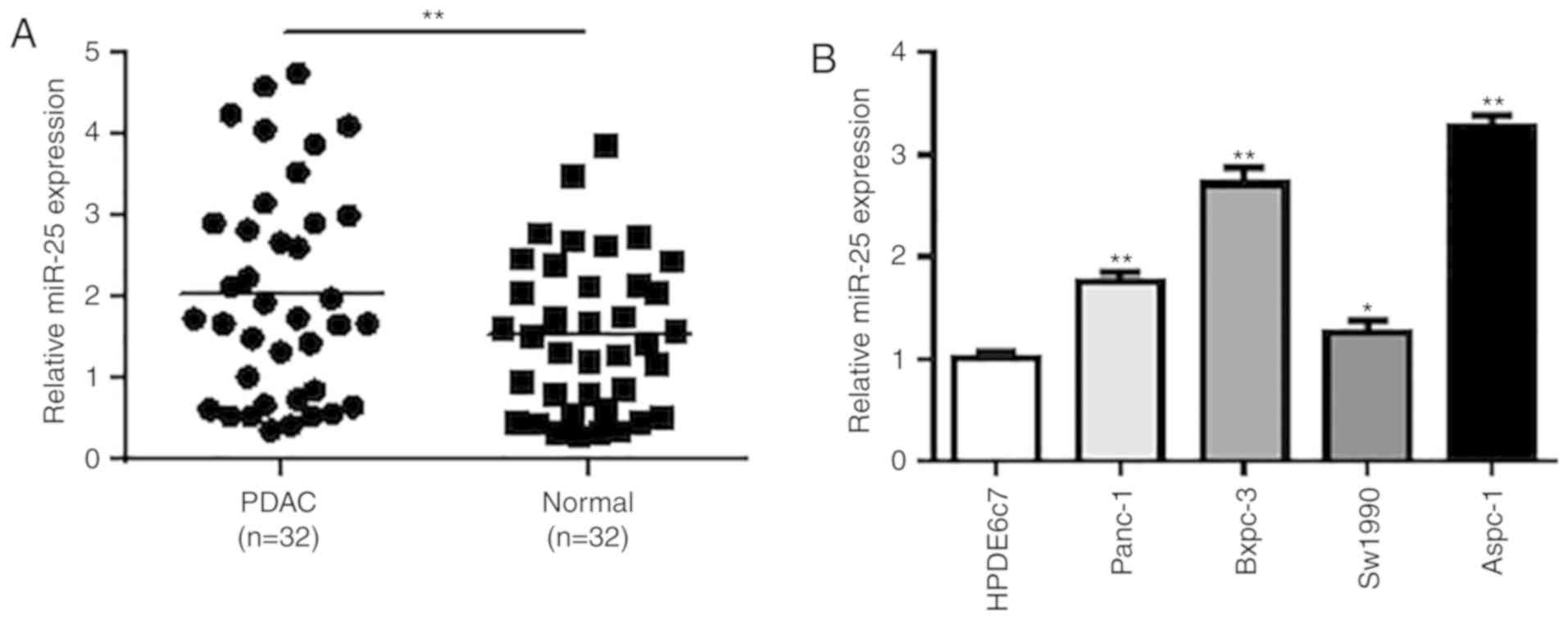
We first analyzed the expression of miR-25 in 25
pairs of PDAC tissues and adjacent normal pancreatic tissues using
RT-qPCR. The results showed that miR-25 expression was
significantly upregulated in human PDAC tissues when compared with
that noted in the adjacent normal tissues (P<0.01; Fig. 1A). Then, we detect the miR-25
expression in different PDAC cancer cell lines and normal cell
lines. The RT-qPCR results demonstrated that the miR-25 expression
was profoundly elevated in all four PDAC cell lines (Panc-1,
Bxpc-3, Aspc-1 and Sw1990) compared with normal HPDE6c7 cells
(P<0.05, P<0.01; Fig. 1B).
Among all the four tumor cell lines, Aspc-1 cells had the highest
expression of miR-25 and thus Aspc-1 cells were selected for
further functional studies.
miR-25 promotes PDAC cell
proliferation
Since elevated expression of miR-25 was shown in
both PDAC patient tissues and PDAC cancer cell lines, we speculated
that miR-25 plays an important role in the regulation of the PDAC
cell activities. Hence, the effect of miR-25 on PDAC cell
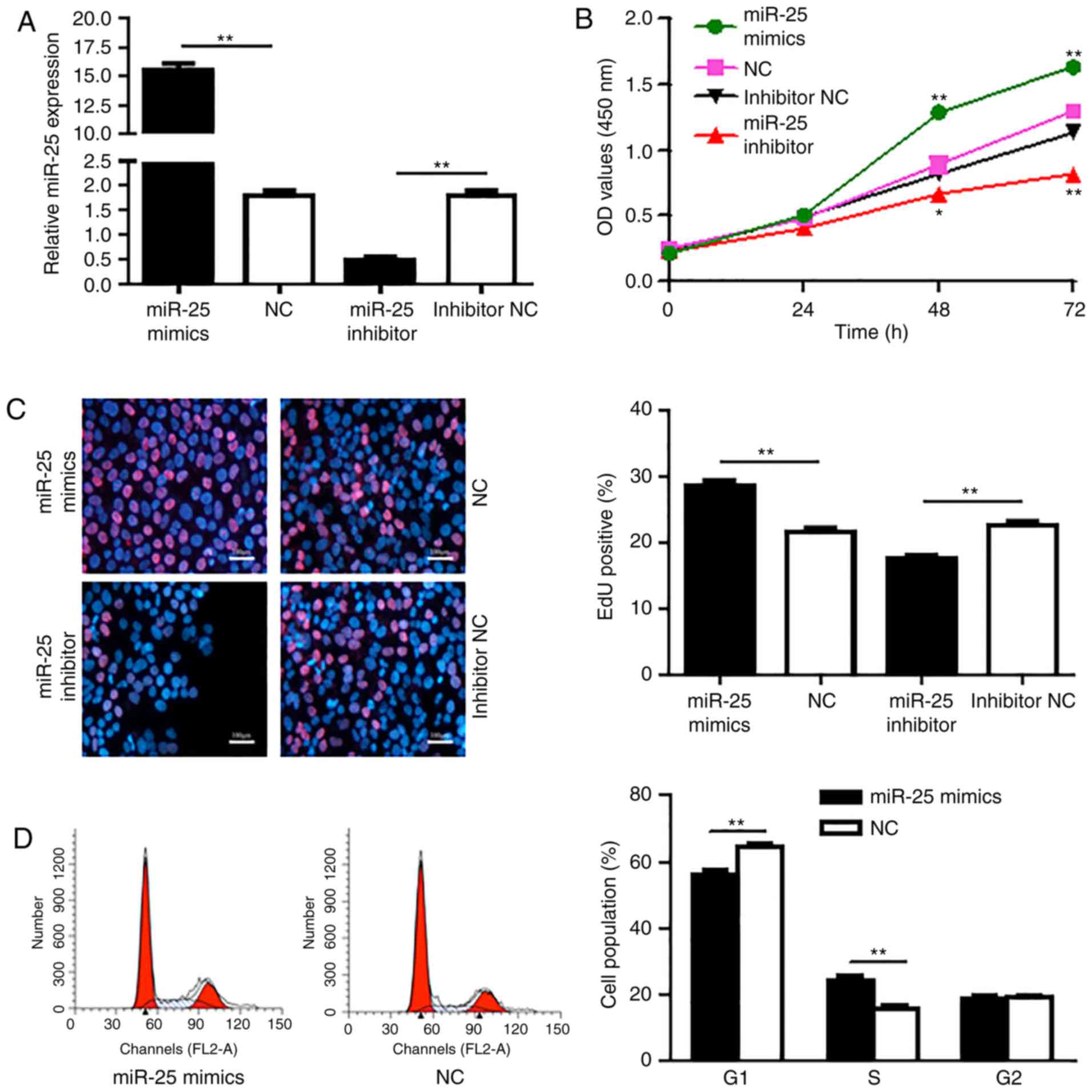
proliferation and growth was detected. To verify the role of miR-25
in the growth of PDAC cells, acquisition and loss of function
experiments for miR-25 was performed by transfection of Aspc-1
cells with miR-25 mimic, negative control (NC), miR-25 inhibitor
and inhibitor NC. RT-qPCR results showed that miR-25 mimic
transfection significantly increased the miR-25 level compared with
the Aspc-1 cells transfected with NC (P<0.01; Fig. 2A). While, miR-25 inhibitor
transfection markedly decreased the expression of miR-25 compared
with cells transfected with inhibitor NC (P<0.01).
After transfection for 24, 48 and 72 h, the CCK-8
assay was conducted to evaluate the effect of miR-25 on the
proliferation of Aspc-1 cells. CCK-8 results showed that the
proliferation of Aspc-1 cells in the miR-25 mimetic group was
significantly higher at 48 and 72 h than that noted in the NC group
(P<0.01; Fig. 2B). In addition,
the proliferation of Aspc-1 cells in the miR-25 inhibitor group was
markedly lower than that in the inhibitor NC group (P<0.05,
P<0.01).
The main event in the S-phase of the cell cycle is
DNA replication (synthesis), the aim of which is to produce two
identical semi-conserved chromosomes, that transform into another
cell cycle and prepare for cell proliferation. Next, EdU
incorporation assay was performed to detect the effect of miR-25 on
Aspc-1 cell DNA synthesis. The results showed that upregulation of
miR-25 resulted in an increase in the population of EdU-positive
cells compared to cells treated with the NC-mimics (P<0.01),
while downregulation of miR-25 significantly reduced the population
of EdU-positive-cells compared to the cells treated with the
inhibitor NC (P<0.01), indicating that miR-25 promotes the DNA
synthesis in Aspc-1 cells (Fig.
2C).
In addition, malignant cells typically exhibit a
dysregulated cell cycle, a key tumor characteristic that promotes
tumor proliferation, growth and invasion. Here, we also detected
the effect of miR-25 on cell cycle phases in Aspc-1 cells by flow
cytometry. As shown in Fig. 2D,
Aspc-1 cells were transfected with miR-25 mimic and upregulation of
miR-25 was achieved by transfection of miR-25 mimic in Aspc-1 cells
and the elevated miR-25 resulted in an increase in the S phase cell
population (P<0.01) and a decrease in the G1 phase cell
population (P<0.01) compared to the NC mimic transfected cells.
These findings confirmed that miR-25 promotes PDAC cell
proliferation.
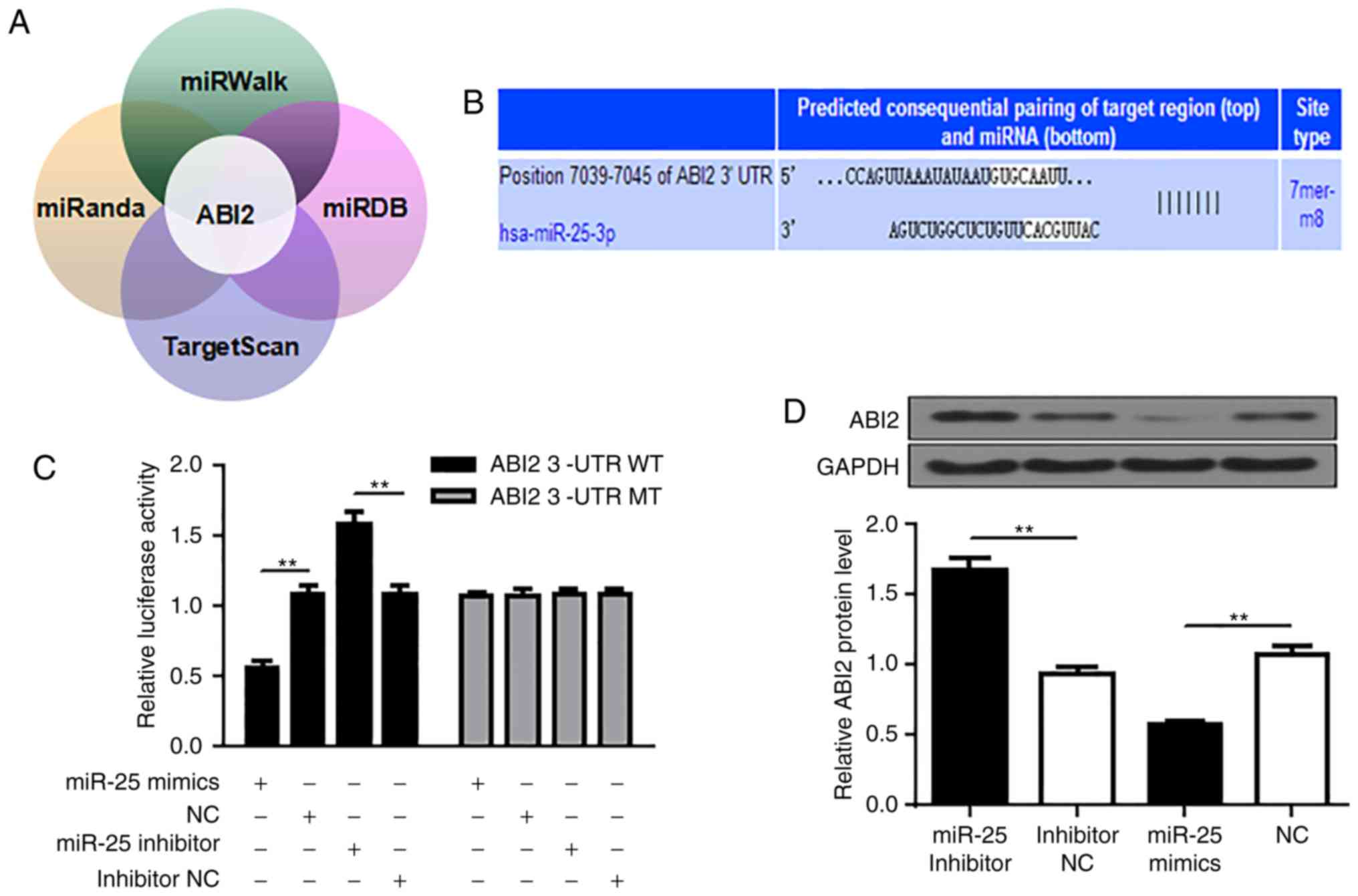
ABI2 is a direct target of miR-25
miRanda (http://www.microrna.org/microrna/home.do), miRDB
(http://mirdb.org/), miRWalk (http://mirwalk.umm.uni-heidelberg.de/) and TargetScan
(http://www.targetscan.org/vert_72/)
were used to search for the potential target genes for miR-25.
ABI2 (ab1 interactor2) was predicted to be a target of
miR-25, and a highly conserved binding site for miR-25 seed
sequence was found in the 3'-UTR region of ABI2 mRNA from
position 7039 to 7045 containing the seed sequence (Fig. 3A and B). To confirm this prediction, wild-type
(WT) or mutated (MT) ABI2 3'-UTR was amplified by PCR and ligated
into a luciferase reporter vector and assayed for detection of
luciferase activity. As shown in Fig.
3C, overexpression of miR-25 profoundly inhibited the 3'-UTR of
WT rather than MT, whereas inhibition of miR-25 significantly
promoted the 3'-UTR of WT rather than MT (P<0.01). In addition,
consistent with the dual luciferase assay results, immunoblotting
results showed that upregulation of miR-25 reduced ABI2 protein
levels, while inhibition of miR-25 significantly increased ABI2
protein levels (P<0.01). (Fig.
3D). Thus, these observations indicate that miR-25 negatively
regulates the expression of ABI2 by directly binding to its
3'-UTR.
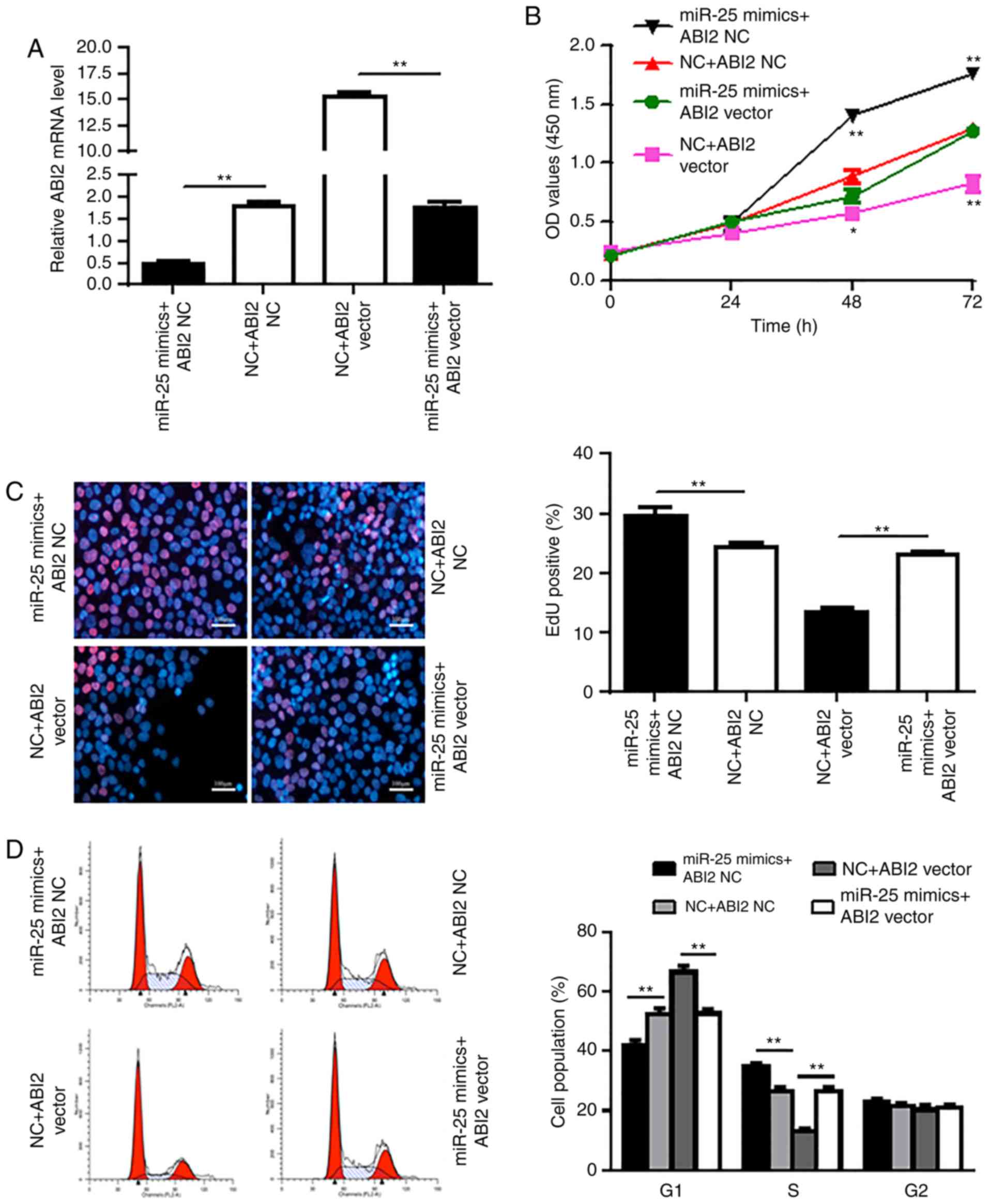
ABI2 overexpression attenuates the
oncogenic effect of miR-25
To further investigate the functional relationship
between miR-25 and ABI2, we designed the ABI2 vector and
transfected Aspc-1 cells with this vector and miR-25 mimic, which
was confirmed by RT-qPCR (Fig. 4A).
The ABI2 vector was found to significantly attenuate the
carcinogenic effect of miR-25 in Aspc-1 cells (P<0.05,
P<0.01) (Fig. 4B-D). These data
indicate that miR-25 promotes Aspc-1 cell proliferation by
negatively regulating its target gene ABI2.
Discussion
miRNAs are small non-coding RNAs, that have been
explored and proven to serve as potential diagnostic biomarker and
prognostic factor in multiple types of cancers (20). In addition, miRNAs may play an
oncogenic role or tumor-suppressor role in different tumors to
regulate cancer cell proliferation, growth and tumor metastasis
including pancreatic cancer (7,20). In
pancreatic cancer, several serum miRNAs have been investigated and
suggested as biomarker for both diagnosis and prognosis and the
expression profile of these miRNAs has been reported to be
associated with the development and progression of pancreatic
cancer (21,22). Among these miRNAs, miR-29a and miR-21
have been reported to promote pancreatic cancer growth (23), while miR-429 plays an inhibitory role
in pancreatic ductal adenocarcinoma growth via targeting of
TBK1(23). Due to the high mortality
rate of PDAC, a highly malignant disease, discovery of novel
biomarkers of PDAC and investigation of new therapeutic targets for
PDAC are critical and important for improving the survival time and
treatment efficacy.
Among all the miRNAs, miR-25 is one of the most
researched and well-described miRNA related to different human
cancers. It is 22 nucleotides in length and has been reported to be
overexpressed in different type of human carcinomas which include
breast cancer (10), ovarian cancer
(11,12), gastric cancer (13,14),
hepatocellular carcinoma and lung cancer (15,16).
However, the expression of miR-25 in PDAC tumor tissue and its
biological function as well as the molecular mechanism involved in
its biological role are still under investigation. In the present
study, our results showed that miR-25 serves as an important
regulator in the progression of PDAC. First, our data demonstrated
that the expression of miR-25 was significantly higher in PDAC
tissues when compared with normal tissues. Moreover, expression of
miR-25 was also upregulated in PDAC cell lines compared with that
noted in the normal immortalized human pancreatic duct epithelial
cell line HPDE6c7 which has been widely used for comparison with
PDAC cells in many research studies. Furthermore, upregulation of
miR-25 significantly enhanced cell proliferation and G1-to-S phase
transition in Aspc-1 cells, while downregulation of miR-25
inhibited the tumor cell proliferation and cell cycle transition.
These data revealed for the first time that miR-25 plays an
oncogenic role and promotes pancreatic cell proliferation and
growth through regulation of the pancreatic cancer cell cycle,
indicating its key regulatory role in promotion of the progression
of PDAC.
To further investigate the miR-25 target in PDAC,
luciferase reporter assay was performed. The results revealed that
miR-25 directly binds to the 3' untranslated region of ABI2. In
addition western blot analysis showed that miR-25 downregulated
ABI2 expression at the protein level. ABI-2, a member of the ABI
family of adaptor proteins, has been proven to be involved in
signaling pathways involving tyrosine kinase and Rac GTPase. One
previous study showed that deficiency of ABI2 led to defects in
cell migration (24). In the present
study, introduction of ABI2 mRNA into cells overexpressing miR-25
attenuated the miR-25 overexpression-induced cell proliferation and
cell cycle regulation. In conclusion, these results demonstrate
that miR-25 is an oncogenic miRNA that promotes PDAC cell
proliferation by targeting ABI2. Altogether, our study indicated
that miR-25 may have the potential to be investigated as a
biomarker for the diagnosis of PDAC and its oncogenic role in PDAC
suggest that it may be explored as a potential therapeutic target
for the treatment of PDAC. However, there are still limitations in
the present research study. Only one PDAC cell line was used in the
functional studies, and more cell lines will be needed to confirm
the role of miR-25 in the regulation of PDAC cell function and
activity. Moreover, an in vivo model should be established
in the future to further verify the function of miR-25 in PDAC.
Acknowledgements
Not applicable
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH and HL conceived and designed the study. HL, LZ,
SL, DY, JY and ML performed the experiments. WH and HL wrote the
manuscript. LZ, SL, DY, JY and ML reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the West China Hospital. All patients and healthy
volunteers provided written informed consent prior to their
inclusion within the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hariharan D, Saied A and Kocher HM:
Analysis of mortality rates for pancreatic cancer across the world.
HPB (Oxford). 10:58–62. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Garrido-Laguna I and Hidalgo M: Pancreatic
cancer: From state-of-the-art treatments to promising novel
therapies. Nat Rev Clin Oncol. 12:319–334. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Adamska A, Domenichini A and Falasca M:
Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies.
Int J Mol Sci. 18(E1338)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Friedman RC, Farh KKH, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Garofalo M and Croce CM: microRNAs: Master
Regulators as Potential Therapeutics in Cancer. Annu Rev Pharmacol
Toxicol. 51:25–43. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Y, Li M, Wang H, Fisher WE, Lin PH,
Yao Q and Chen C: Profiling of 95 microRNAs in pancreatic cancer
cell lines and surgical specimens by real-time PCR analysis. World
J Surg. 33:698–709. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Khan MA, Zubair H, Srivastava SK, Singh S
and Singh AP: Insights Into the Role of microRNAs in Pancreatic
Cancer Pathogenesis: Potential for Diagnosis, Prognosis, and
Therapy. Adv Exp Med Biol. 889:71–87. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie
T, Zhang J, Peng C, Lin Y and Chen J: MicroRNA-25 regulates
chemoresistance-associated autophagy in breast cancer cells, a
process modulated by the natural autophagy inducer
isoliquiritigenin. Oncotarget. 5:7013–7026. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang H, Zuo Z, Lu X, Wang L, Wang H and
Zhu Z: miR-25 regulates apoptosis by targeting Bim in human ovarian
cancer. Oncol Rep. 27:594–598. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng S, Pan W, Jin Y and Zheng J: miR-25
promotes ovarian cancer proliferation and motility by targeting
LATS2. Tumour Biol. 35:12339–12344. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao H, Wang Y, Yang L, Jiang R and Li W:
miR-25 promotes gastric cancer cells growth and motility by
targeting RECK. Mol Cell Biochem. 385:207–213. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li BS, Zuo QF, Zhao YL, Xiao B, Zhuang Y,
Mao XH, Wu C, Yang SM, Zeng H, Zou QM, et al: MicroRNA-25 promotes
gastric cancer migration, invasion and proliferation by directly
targeting transducer of ERBB2, 1 and correlates with poor survival.
Oncogene. 34:2556–2565. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Q, Zou C, Zou C, Han Z, Xiao H, Wei H,
Wang W, Zhang L, Zhang X, Tang Q, et al: MicroRNA-25 functions as a
potential tumor suppressor in colon cancer by targeting Smad7.
Cancer Lett. 335:168–174. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang C, Wang X, Su Z, Fei H, Liu X and Pan
Q: MiR-25 promotes hepatocellular carcinoma cell growth, migration
and invasion by inhibiting RhoGDI1. Oncotarget. 6:36231–36244.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Caiazza C and Mallardo M: The Roles of
miR-25 and its Targeted Genes in Development of Human Cancer.
MicroRNA. 5:113–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Deng T, Yuan Y, Zhang C, Zhang C, Yao W,
Wang C, Liu R and Ba Y: Identification of Circulating MiR-25 as a
Potential Biomarker for Pancreatic Cancer Diagnosis. Cell Physiol
Biochem. 39:1716–1722. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Song W, Li Q and Wang L and Wang L:
Modulation of FoxO1 expression by miR-21 to promote growth of
pancreatic ductal adenocarcinoma. Cell Physiol Biochem. 35:184–190.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun XJ, Liu BY, Yan S, Jiang TH, Cheng HQ,
Jiang HS, Cao Y and Mao AW: MicroRNA-29a Promotes Pancreatic Cancer
Growth by Inhibiting Tristetraprolin. Cell Physiol Biochem.
37:707–718. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Song B, Zheng K, Ma H, Liu A, Jing W, Shao
C, Li G and Jin G: miR-429 determines poor outcome and inhibits
pancreatic ductal adenocarcinoma growth by targeting TBK1. Cell
Physiol Biochem. 35:1846–1856. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Grove M, Demyanenko G, Echarri A, Zipfel
PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR,
Wetsel WC, et al: ABI2-deficient mice exhibit defective cell
migration, aberrant dendritic spine morphogenesis, and deficits in
learning and memory. Mol Cell Biol. 24:10905–10922. 2004.PubMed/NCBI View Article : Google Scholar
|