Introduction
Lung cancer is the most frequent malignancy
worldwide and one of the leading causes of global mortality arising
from cancer (1). It can be divided
into two subtypes: Small cell lung cancer and non-small cell lung
cancer (NSCLC), which are defined based on their histological
characteristics (2). NSCLC is
considered to be the most common type of lung cancer, accounting
for approximately 85% of all lung tumors (3). According to statistics, most patients
with NSCLC are diagnosed with advanced tumors due to limited
strategies available for early diagnosis (4). Although advancements have been made in
terms of therapeutic methods such as surgery, chemotherapy and
radiotherapy, the 5-year overall survival of NSCLC is only 11%
(5). Thus, there is a need for more
efficient strategies to improve the diagnosis, prognosis and
therapy for patients with NSCLC.
MicroRNAs (miRNAs/miRs) are found to be deregulated
in various human cancers (6). These
small noncoding RNAs can regulate gene expression at the
post-transcriptional level and are involved in various cell
processes such as proliferation, migration, invasion,
differentiation and apoptosis (7,8).
Emerging studies focused on the clinical significance of miRNAs for
their diagnostic and prognostic value (9). In addition, the aberrant expression of
miRNAs was reported to play a regulatory role in tumor progression
in different types of human cancer (10). Several functional miRNAs with
critical roles have also been identified in NSCLC. Li et al
(3) found that upregulated
expression of miR-421 could predict poor prognosis of NSCLC and
contribute to tumor cell proliferation, migration and invasion.
Jiang et al (11) showed
evidence of miR-940 acting as a tumor suppressor by inhibiting
NSCLC cell invasion and epithelial-mesenchymal transition via the
transforming growth factor-β signaling pathway. The aforementioned
studies indicated the considerable potential of miRNAs as
biomarkers and therapeutic targets in NSCLC.
Considering the therapeutic potential of miRNAs for
the treatment of human cancers, it is crucial to deliver specific
miRNAs to targeted areas using a noninvasive approach with
relatively high safety and effectiveness. Ultrasound-targeted
microbubble destruction (UTMD) is considered a novel strategy for
gene delivery (12). During UTMD,
the gene is integrated into a microbubble and is then released when
the microbubble reaches the targeted area and collapses (13). Microbubble destruction resulting from
ultrasound induces an increase in capillary permeability and
induces irreversible holes in target cell membranes, contributing
to gene transfer into the nucleus and enhanced expression and
transfection of the target gene (14). Additionally, gene transfer by UTMD
can avoid degradation by lytic enzymes (15). The application of UTMD has been
highlighted in cancer treatment, which greatly contributes to
targeted cancer therapy (16).
The abnormal expression of miR-767 is closely
related to DNA hypomethylation in NSCLC. miR-767 was identified as
an upstream regulator of tet methylcytosine dioxygenase (TET) 1 and
TET3, which are established tumor suppressors in various tumors,
including NSCLC (17,18). The biological function of miR-767 has
been identified in human melanoma (19) and glioma (20). However, the precise role of miR-767
in NSCLC remains to be elucidated. The aim of the present study was
to investigate the functional role of miR-767 in NSCLC progression.
Furthermore, the present study assessed the transfection efficiency
of UTMD-mediated transfection of miR-767 into NSCLC cells and the
feasibility of UTMD-mediated miR-767 therapy.
Materials and methods
Clinical sample collection
Samples from 108 patients who were diagnosed with
NSCLC in Zibo City Linzi District People's Hospital (Shandong,
China) between May 2014 and April 2016 were used in the study. The
patients included 62 males and 46 females with a mean age of
63.3±13.9 years (range 25-80 years). None of the patients had
received any anti-tumor therapy prior to radical resection surgery.
Prior to surgery, serum samples were obtained from blood collected
from the patients and stored at -80˚C for further use. During
surgery, 108 paired tumor and adjacent normal tissues were isolated
and stored in liquid nitrogen. In addition, 50 age (mean 62.8±14.2
years) and gender (male:female ratio, 29:21)-matched healthy
volunteers were enrolled in the study to provide healthy serum
control samples. All participants signed informed consent before
sampling. The experimental procedures were approved by the Ethics
Committee of Zibo City Linzi District People's Hospital (approval
no. 20140922).
Cell culture and transfection
Human NSCLC cell lines A549, H1299 and PC9 and
bronchial epithelial cell line 16HBE were purchased from the
Shanghai Cell Bank of China. Cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in a humidified
incubator with 5% CO2 at 37˚C. miR-767 inhibitor
(5'-CAUGCUCAGACAACCAUGGUGCA-3') and miRNA negative control (miR-NC;
5'-CAGUACUUUUGUGUAGUACAA-3') were synthesized by Shanghai
GenePharma Co., Ltd. miR-767 inhibitor (100 nM) or miR-NC (100 nM)
was transfected into A549 and H1299 cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h following the manufacturer's protocols.
Cells transfected with the transfection reagent alone served as the
mock group.
Microbubble preparation and miRNA
transfection
Microbubbles were obtained using a previously
reported method (21), which was
performed by sonication of 0.4 mg/ml
1,2-distearoyl-3-trimethylammoni-umpropane (Avanti Polar Lipids,
Inc.) with perfluoropropane gas, 1 mg/ml polyethyleneglycol-2000
stearate (Avanti Polar Lipids, Inc.) and 2 mg/ml
distearoylphosphatidylcholine (Avanti Polar Lipids, Alabaster,
Inc). miR-767 inhibitor or miR-NC was incubated with the
microbubbles for 30 min at 37˚C. The mixture was then added into
H1299 and A549 cells for transfection using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from clinical samples and cells were
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
protocol. RNA was reverse transcribed into cDNA using the
PrimeScript RT reagent kit (Takara Bio, Inc.) following the
manufacturer's instructions. qPCR was performed using a SYBR-Green
PCR Master Mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) and
a 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction conditions were as follows: 95˚C
for 10 min, followed by 40 cycles of 95˚C for 20 sec, 60˚C for 10
sec, 72˚C for 15 sec. The primer pairs used for the qPCR were:
miR-767 forward, 5'-GCCGAGTGCACCATGGTTGT-3' and reverse,
5'-CTCAACTGGTGTCGTGGA-3' and U6 forward, 5'-CTCGCTTCGGCAGCACA-3'
and reverse, 5'-AACGCTTCACGAATTTGCGT-3'. miR-767 expression was
quantified using the 2-ΔΔCq method
and normalized to the internal reference gene U6(22).
Cell proliferation assay
NSCLC cell proliferation was examined using a Cell
Counting kit (CCK)-8 assay (Beyotime Institute of Biotechnology).
Tumor cells were seeded at a density of 4x105 cells/well
in 96-well plates and cultured at 37˚C for 72 h. CCK-8 solution was
added into the cells every 24 h with a further 4 h incubation. Cell
proliferation was evaluated by reading the absorbance at a
wavelength of 450 nm using a microplate reader (Molecular
Devices).
Cell migration and invasion assay
NSCLC cell migration and invasion were measured
using Transwell chambers with a pore size of 8 µm (Corning Inc.).
Chambers were pre-coated with Matrigel (Corning, Inc.) for the
invasion assay. Following 48 h of cell transfection, cells (seeded
at a density of 5x105 cells/well) in serum-free
RPMI-1640 medium were seeded into the upper chamber. The lower
chambers contained culture medium supplemented with 10% FBS. After
24 h of incubation at 37˚C, cells in the lower chambers were
stained with crystal violet at room temperature for 20 min and
observed under an LX71 inverted light microscope (Olympus
Corporation). Cells were counted using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.).
Statistical analysis
Data are expressed as the mean ± SD and analyzed
using SPSS 18.0 (SPSS Inc.) and GraphPad Prism 5.0 (GraphPad
Software, Inc.). Differences between groups were calculated using
one-way ANOVA followed by Tukey's post hoc test, non-parametric
Wilcoxon signed-rank test or Mann-Whitney U test when appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of miR-767 in NSCLC
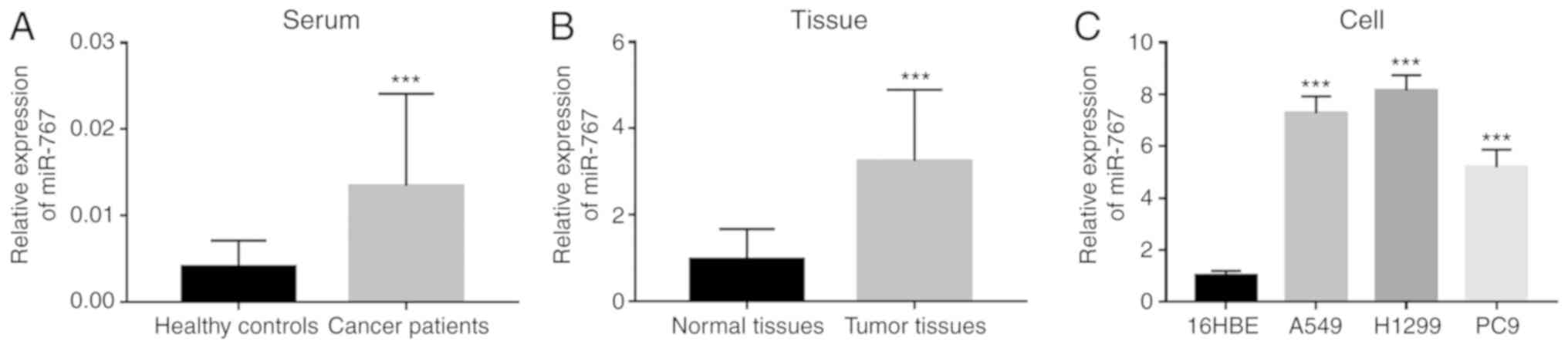
miR-767 expression was measured in clinical samples,
including serum and tissue specimens. Significantly increased
expression of miR-767 was observed in both serum and tissues of
NSCLC patients compared with corresponding normal controls
(Fig. 1A and B). In addition, upregulated expression of
miR-767 was also found in four NSCLC cell lines (A549, H1299 and
PC9) compared with 16HBE cells (Fig.
1C).
Knockdown of miR-767 inhibits NSCLC
cell proliferation, migration and invasion
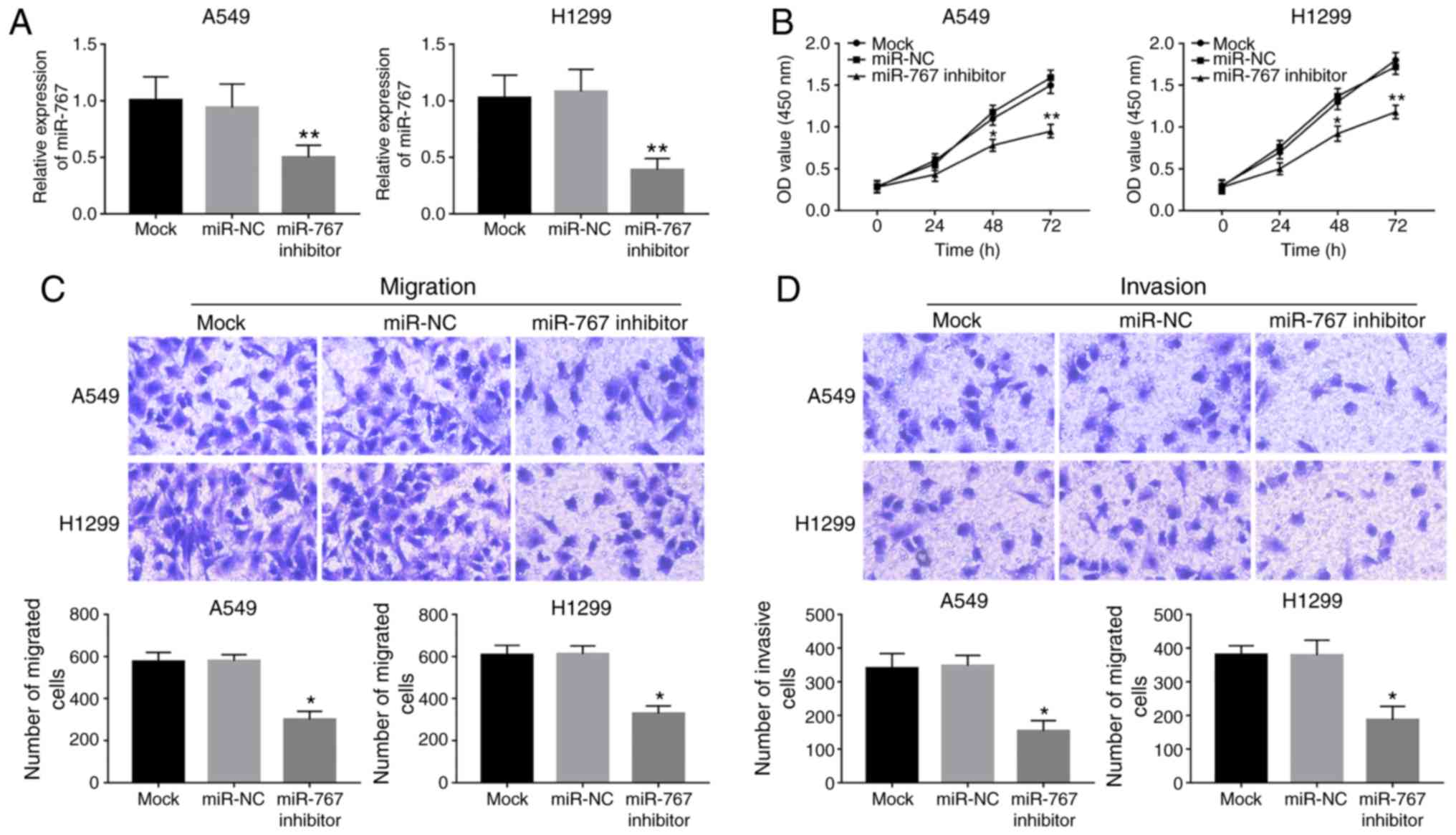
To investigate the biological function of miR-767 in
NSCLC progression, in vitro regulation of miR-767 was
attained by cell transfection in A549 and H1299 cells. As shown in
Fig. 2A, expression of miR-767 was
significantly reduced by miR-767 inhibitor transfection in both
cell lines. Knockdown of miR-767 in A549 and H1299 cells led to a
significant decrease in cell proliferation compared with the mock
group (Fig. 2B). Furthermore, cell
migration and invasion were suppressed by the inhibition of miR-767
in A549 and H1299 cells compared with mock and miR-NC groups
(Fig. 2C and D).
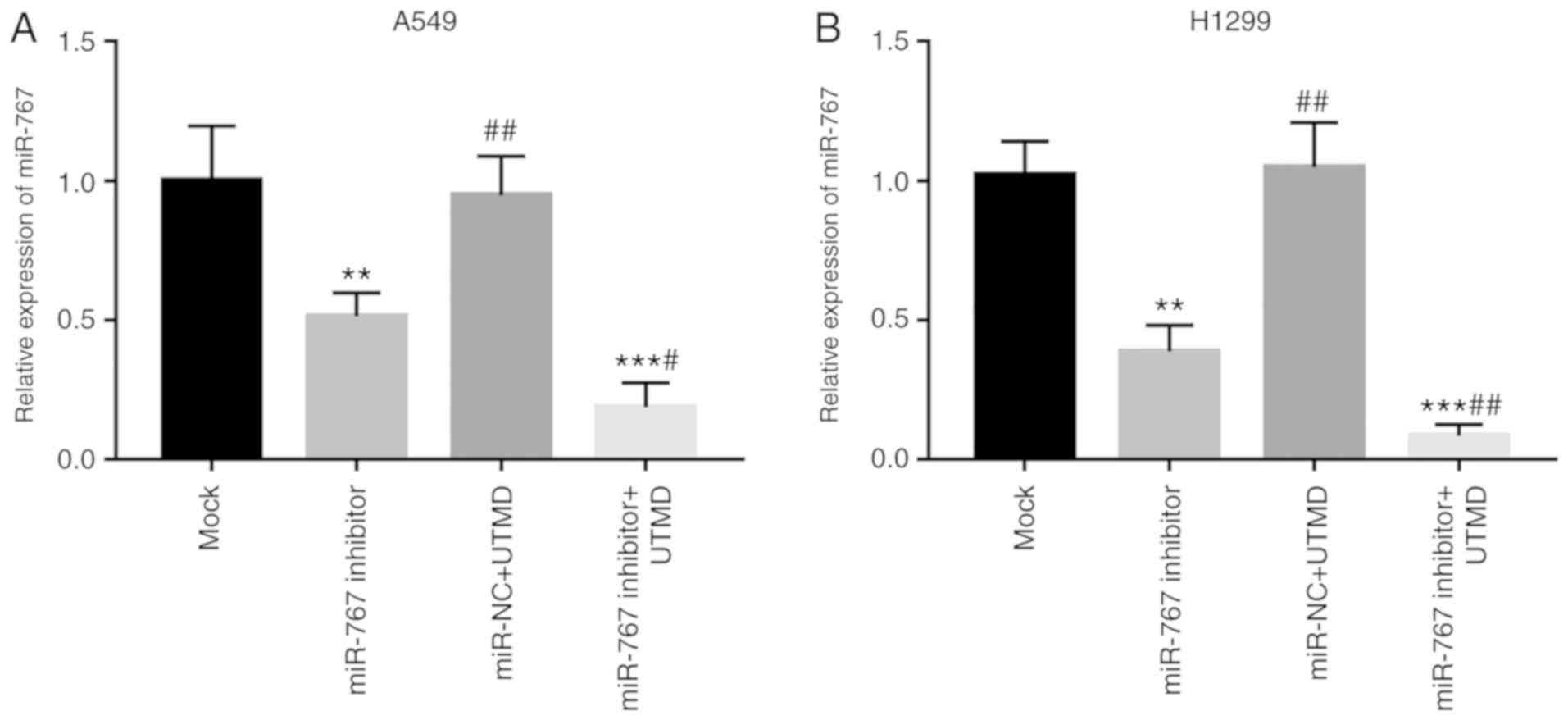
UTMD improves the transfection
efficiency of miR-767
Given the enhancement of gene transfer by UTMD, the
present study focused on the effects of UTMD on miR-767
transfection efficiency. Transfection of miR-767 inhibitor with
UTMD significantly decreased miR-767 expression compared with mock
and miR-767 inhibitor groups in A549 and H1299 cells (Fig. 3).
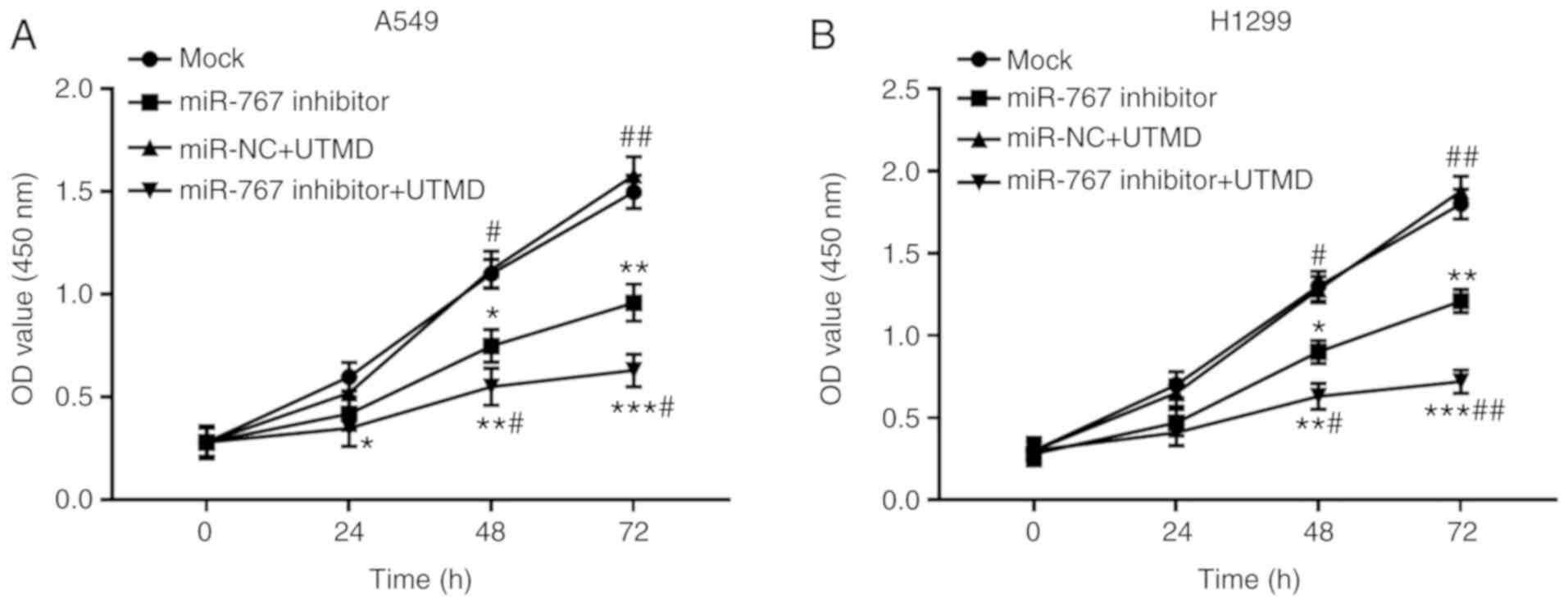
UTMD-mediated miR-767 inhibition
suppresses the proliferation of NSCLC cells
Since the transfection efficiency of miR-767 was
enhanced by the application of UTMD, tumor progression was
hypothesized to be influenced by UTMD mediation. As shown in
Fig. 4, proliferation of A549 and
H1299 cells was significantly inhibited by UTMD-mediated miR-767
knockdown compared with the mock group. As expected, a further
suppression in cell proliferation was observed in cells transfected
with miR-767 inhibitor + UTMD compared with cells transfected with
miR-767 inhibitor alone.
UTMD-mediated miR-767 inhibition
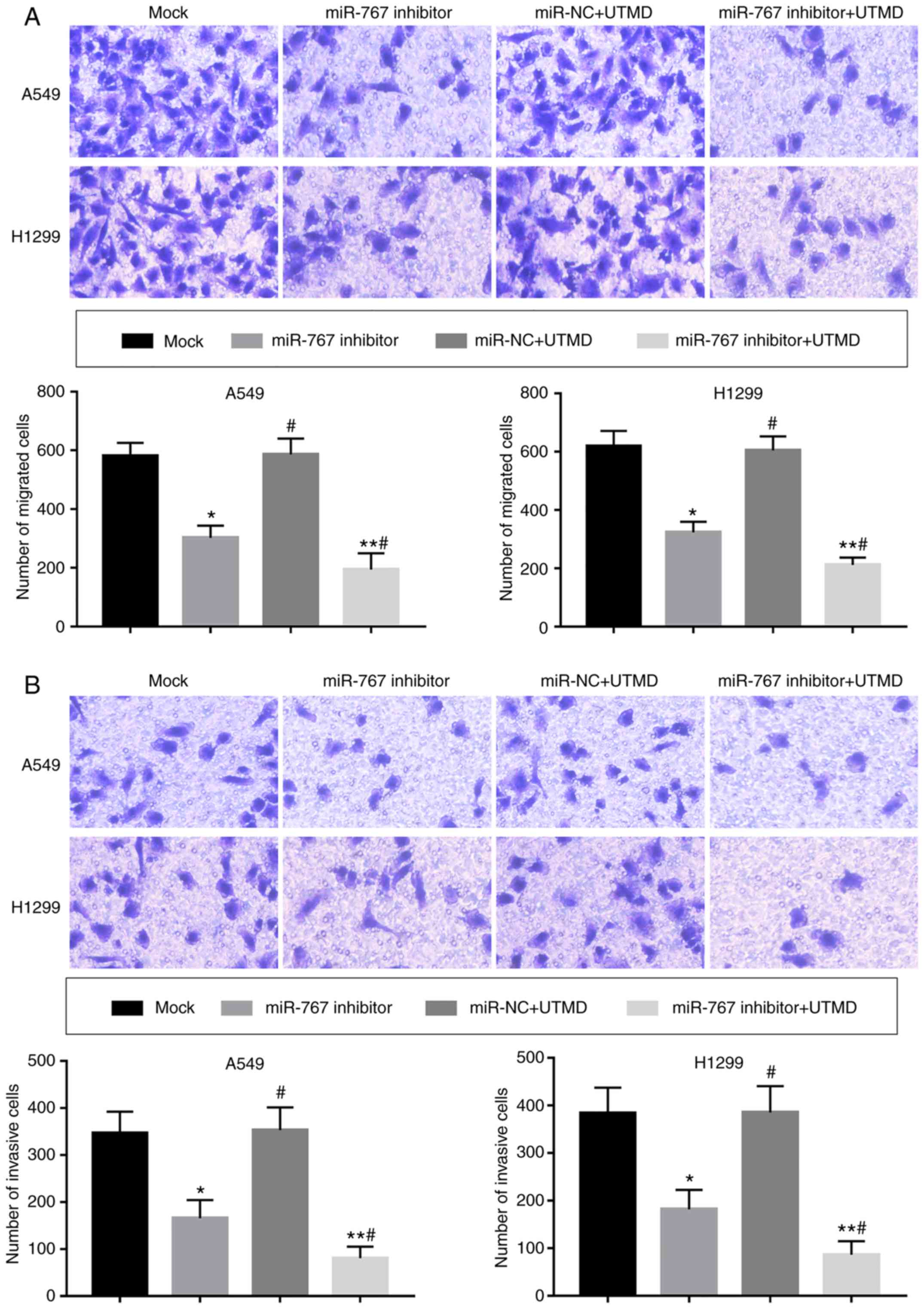
inhibits the migration and invasion of NSCLC cells
A significant decrease in cell migration and
invasion was observed following in vitro transfection with
UTMD-mediated miR-767 inhibitor compared with mock and miR-767
inhibitor groups (Fig. 5).
Discussion
An increasing number of studies have reported the
important role of miRNAs in the treatment of various human cancers
(23-25).
The present study aimed to investigate the expressional patterns of
miR-767 in NSCLC clinical samples and cells and to analyze the
functional role of miR-767 in tumor progression. Furthermore, the
application of UTMD in miR-767 inhibitor transfection and its
effects on the regulation of miR-767 in NSCLC tumor progression
were also investigated. Significantly increased expression of
miR-767 was found in the serum, tissues and cell lines of NSCLC and
knockdown of miR-767 in tumor cells led to inhibited cell
proliferation, migration and invasion. The efficiency of the in
vitro transfection of miR-767 inhibitor significantly increased
by the use of UTMD, and UTMD-mediated miR-767 inhibition resulted
in further suppression in NSCLC cell proliferation, migration and
invasion.
Numerous miRNAs with aberrant expression have been
identified in human cancers (26).
The clinical significance of abnormal miRNAs has attracted
increasing attention in the diagnosis and prognosis of various
cancers (27). Previous findings
have shown several miRNAs with ectopic expressional patterns in
NSCLC, with demonstrated diagnostic and prognostic value (28). Increased expression of miR-223 in
NSCLC sputum samples was identified as a non-invasive diagnostic
biomarker for NSCLC detection (29).
The ectopic expression of miR-9, miR-16, miR-205 and miR-486 in
serum specimens collected from patients with NSCLC served as useful
diagnostic biomarkers for NSCLC (30). Upregulated expression of miR-411 was
shown to have relatively high diagnostic and prognostic value for
patients with NSCLC (31). In
addition to the clinical value of miRNAs, their functional roles in
tumor progression have also been investigated, providing further
evidence for the therapeutic potential of these miRNAs (32). In the pathogenesis of NSCLC, several
miRNAs, such as miR-612(33),
miR-421(3) and miR-4286(34), were reported to be involved in tumor
cell proliferation, migration and invasion and thus may serve as
potential therapeutic targets.
Aberrant expression of miR-767 was found to be
linked to the hypomethylation of NSCLC (17). In human melanoma, miR-767 was
identified as an oncogenic miRNA that promoted tumor cell
proliferation (19). In multiple
myeloma, miR-767 served as a mediator in the suppressive effects of
circ_0000190 on tumor progression (35). In glioma, tumor cell proliferation
and migration were inhibited by the overexpression of miR-767,
implying the potential of miR-767 as a novel therapeutic target
(25). Results from the current
study showed increased expression of miR-767 in serum and tissue
samples collected from patients with NSCLC. Similarly, the
expression of miR-767 was also elevated in NSCLC cell lines
compared with normal cells. Thus, miR-767 may have the potential to
serve as a biomarker for the screening of NSCLC. Further
experiments showed that knockdown of miR-767 induced by miR-767
inhibitor transfection suppressed NSCLC cell proliferation,
migration and invasion, indicating that miR-767 might serve as an
oncogenic miRNA in the tumor progression of NSCLC.
UTMD is considered an effective method of monitoring
the status of tumors and used as a novel tool to facilitate drug or
gene transfer by improving the permeability of tumor cells
(36). Lin et al (37) reported that UTMD promoted the
co-delivery of gemcitabine and miR-21, thereby improving the
treatment of pancreatic cancer. Ji et al (38) investigated the application of UTMD in
miR-133a delivery and found that UTMD-mediated miR-133a inhibited
tumor growth and improved the survival rate in a breast cancer
mouse model, suggesting the therapeutic potential of UTMD-mediated
miR-133a for breast cancer treatment. UTMD successfully enhanced
the transfer of miR-205, as evidenced by the significant increase
in miR-205 expression in prostate cancer cells, leading to
suppressed tumor cell proliferation, migration and invasion and
enhanced cell apoptosis (39).
However, to the best of our knowledge, the application of UTMD in
the treatment of NSCLC has rarely been reported.
In the present study, the transfection efficiency of
the miR-767 inhibitor was enhanced by the use of UTMD in NSCLC
cells. An increase in NSCLC cell proliferation, migration and
invasion induced by miR-767 inhibitor was further facilitated by
UTMD mediation. Therefore, UTMD contributed to in vitro
transfection of miR-767 inhibitor, which resulted in enhanced
effects of miR-767 on tumor progression of NSCLC. Although the
present study provided evidence for the role of miR-767 in NSCLC,
the related mechanisms behind its role remain unclear. Loriot et
al (17) suggested that miR-767
could directly regulate the expression of TET1 and TET3, which act
as tumor suppressors in human cancers (18). In human lung cancer cells, TET1 was
shown to inhibit tumor progression by regulating cell migration and
invasion (40). Thus, miR-767 might
exert its regulatory role in NSCLC progression by targeting TET1 or
TET3.
In conclusion, the present study found increased
expression of miR-767 in serum and tissues from NSCLC patients and
cells compared with corresponding normal controls. Knockdown of
miR-767 inhibited tumor cell proliferation, migration and invasion.
Furthermore, the transfection efficiency of miR-767 inhibitor can
be enhanced by UTMD, resulting in a more significant suppression of
the biological functions of NSCLC cells. The results suggested that
miR-767 may serve as a promising diagnostic biomarker and a
potential therapeutic target in NSCLC, and UTMD-mediated miR-767
inhibitor delivery may be a novel and effective therapeutic
strategy for the treatment of NSCLC.
Acknowledgements
The authors would like to thank Dr Min Yang, Dr Yan
Su andDr Ruizhu Xie (Affiliated Hospital of Weifang Medical
University) for their help in clinical sample collection anddata
analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL made conceived and designed the current study,
acquired clinical samples and prepared the manuscript preparation.
YZ and WL analyzed and interpreted the data and revised the
manuscript. MX performed cell experiments.
Ethics approval and consent to
participate
All the participants signed the informed consent
before sampling. The experimental procedures were approved by the
Ethics Committee of Zibo City Linzi District People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Blandin Knight S, Crosbie PA, Balata H,
Chudziak J, Hussell T and Dive C: Progress and prospects of early
detection in lung cancer. Open Biol. 7(pii: 170070)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li O, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dejima H, Iinuma H, Kanaoka R, Matsutani N
and Kawamura M: Exosomal microRNA in plasma as a non-invasive
biomarker for the recurrence of non-small cell lung cancer. Oncol
Lett. 13:1256–1263. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I: EUROCARE-4 Working
Group: Recent cancer survival in Europe: A 2000-02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li Y, Zhang X, Yang Z, Li Y, Han B and
Chen LA: miR-339-5p inhibits metastasis of non-small cell lung
cancer by regulating the epithelial-to-mesenchymal transition.
Oncol Lett. 15:2508–2514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu J, Pan X and Hu Z: MiR-502 mediates
esophageal cancer cell TE1 proliferation by promoting AKT
phosphorylation. Biochem Biophys Res Commun. 501:119–123.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li J, Liang Y, Lv H, Meng H, Xiong G, Guan
X, Chen X, Bai Y and Wang K: miR-26a and miR-26b inhibit esophageal
squamous cancer cell proliferation through suppression of c-MYC
pathway. Gene. 625:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qiu Z, Li H, Wang J and Sun C: miR-146a
and miR-146b in the diagnosis and prognosis of papillary thyroid
carcinoma. Oncol Rep. 38:2735–2740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Song Y, Guo Q, Gao S and Hua K: miR-454-3p
promotes proliferation and induces apoptosis in human cervical
cancer cells by targeting TRIM3. Biochem Biophys Res Commun.
516:872–879. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiang K, Zhao T, Shen M, Zhang F, Duan S,
Lei Z and Chen Y: MiR-940 inhibits TGF-β-induced
epithelial-mesenchymal transition and cell invasion by targeting
Snail in non-small cell lung cancer. J Cancer. 10:2735–2744.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ji Y, Han Z, Shao L and Zhao Y:
Ultrasound-targeted microbubble destruction of calcium channel
subunit alpha 1D siRNA inhibits breast cancer via G protein-coupled
receptor 30. Oncol Rep. 36:1886–1892. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen H and Hwang JH: Ultrasound-targeted
microbubble destruction for chemotherapeutic drug delivery to solid
tumors. J Ther Ultrasound. 1(10)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tinkov S, Bekeredjian R, Winter G and
Coester C: Microbubbles as ultrasound triggered drug carriers. J
Pharm Sci. 98:1935–1961. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tu J, Zhang H, Yu J, Liufu C and Chen Z:
Ultrasound-mediated microbubble destruction: A new method in cancer
immunotherapy. Onco Targets Ther. 11:5763–5775. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu J and Li RK: Ultrasound-targeted
microbubble destruction in gene therapy: A new tool to cure human
diseases. Genes Dis. 4:64–74. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Loriot A, Van Tongelen A, Blanco J,
Klaessens S, Cannuyer J, van Baren N, Decottignies A and De Smet C:
A novel cancer-germline transcript carrying pro-metastatic miR-105
and TET-targeting miR-767 induced by DNA hypomethylation in tumors.
Epigenetics. 9:1163–1171. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Thienpont B, Galle E and Lambrechts D: TET
enzymes as oxygen-dependent tumor suppressors: Exciting new avenues
for cancer management. Epigenomics. 8:1445–1448. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang K and Guo L: MiR-767 promoted cell
proliferation in human melanoma by suppressing CYLD expression.
Gene. 641:272–278. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang J, Xu S, Xu J, Li Y, Zhang J, Zhang
J and Lu X: miR7675p inhibits glioma proliferation and metastasis
by targeting SUZ12. Oncol Rep. 42:55–66. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Leong-Poi H, Kuliszewski MA, Lekas M,
Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ and
Lindner JR: Therapeutic arteriogenesis by ultrasound-mediated
VEGF165 plasmid gene delivery to chronically ischemic skeletal
muscle. Circ Res. 101:295–303. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang L, Dou Y, Sui Z, Cheng H, Liu X, Wang
Q, Gao P, Qu Y and Xu M: Upregulated miRNA-182-5p expression in
tumor tissue and peripheral blood samples from patients with
non-small cell lung cancer is associated with downregulated Caspase
2 expression. Exp Ther Med. 19:603–610. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang L, Tang X, Shi X and Su L:
miR-532-5p promotes breast cancer proliferation and migration by
targeting RERG. Exp Ther Med. 19:400–408. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang J, Kong X, Shi Q and Zhao B:
MicroRNA-383-5p acts as a potential prognostic biomarker and an
inhibitor of tumor cell proliferation, migration, and invasion in
breast cancer. Cancer Biomark: Dec. 24:2019.doi: 10.3233/CBM-190704
(Epub ahead of print). PubMed/NCBI View Article : Google Scholar
|
|
26
|
Biswas S: MicroRNAs as therapeutic agents:
The future of the battle against cancer. Curr Top Med Chem.
18:2544–2554. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Azarbarzin S, Hosseinpour Feizi MA,
Safaralizadeh R, Ravanbakhsh R, Kazemzadeh M, Fateh A, Karimi N and
Moaddab Y: The value of miR-299-5p in diagnosis and prognosis of
Intestinal-type gastric adenocarcinoma. Biochem Genet. 54:413–420.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zheng W, Zhao J, Tao Y, Guo M, Ya Z, Chen
C, Qin N, Zheng J, Luo J and Xu L: MicroRNA-21: A promising
biomarker for the prognosis and diagnosis of non-small cell lung
cancer. Oncol Lett. 16:2777–2782. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bagheri A, Khorram Khorshid HR, Mowla SJ,
Mohebbi HA, Mohammadian A, Yaseri M, Solaymani-Dodaran M,
Sherafatian M and Tavallaie M: Altered miR-223 expression in sputum
for diagnosis of Non-small cell lung cancer. Avicenna J Med
Biotechnol. 9:189–195. 2017.PubMed/NCBI
|
|
30
|
Sromek M, Glogowski M, Chechlinska M,
Kulinczak M, Szafron L, Zakrzewska K, Owczarek J, Wisniewski P,
Wlodarczyk R, Talarek L, et al: Changes in plasma miR-9, miR-16,
miR-205 and miR-486 levels after non-small cell lung cancer
resection. Cell Oncol (Dordr). 40:529–536. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang SY, Li Y, Jiang YS and Li RZ:
Investigation of serum miR-411 as a diagnosis and prognosis
biomarker for non-small cell lung cancer. Eur Rev Med Pharmacol
Sci. 21:4092–4097. 2017.PubMed/NCBI
|
|
32
|
Ow SH, Chua PJ and Bay BH: miR-149 as a
potential molecular target for cancer. Curr Med Chem. 25:1046–1054.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kang X, Kong F, Wu S, Liu Q, Yang C, Wu X
and Zhang W: microRNA-612 suppresses the malignant development of
non-small-cell lung cancer by directly targeting
bromodomain-containing protein 4. Onco Targets Ther. 12:4167–4179.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ling C, Wang X, Zhu J, Tang H, Du W, Zeng
Y, Sun L, Huang JA and Liu Z: MicroRNA-4286 promotes cell
proliferation, migration, and invasion via PTEN regulation of the
PI3K/Akt pathway in non-small cell lung cancer. Cancer Me.
8:3520–3531. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Feng Y, Zhang L, Wu J, Khadka B, Fang Z,
Gu J, Tang B, Xiao R, Pan G and Liu J: CircRNA circ_0000190
inhibits the progression of multiple myeloma through modulating
miR-767-5p/MAPK4 pathway. J Exp Clin Cancer Res.
38(54)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang TY, Choe JW, Pu K, Devulapally R,
Bachawal S, Machtaler S, Chowdhury SM, Luong R, Tian L, Khuri-Yakub
B, et al: Ultrasound-guided delivery of microRNA loaded
nanoparticles into cancer. J Control Release. 203:99–108.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lin L, Fan Y, Gao F, Jin L, Li D, Sun W,
Li F, Qin P, Shi Q, Shi X and Du L: UTMD-promoted Co-delivery of
gemcitabine and miR-21 inhibitor by Dendrimer-entrapped gold
nanoparticles for pancreatic cancer therapy. Theranostics.
8:1923–1939. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ji Y, Han Z, Shao L and Zhao Y: Evaluation
of in vivo antitumor effects of low-frequency ultrasound-mediated
miRNA-133a microbubble delivery in breast cancer. Cancer Med.
5:2534–2543. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Qin D, Li H and Xie H: Ultrasoundtargeted
microbubble destructionmediated miR205 enhances cisplatin
cytotoxicity in prostate cancer cells. Mol Med Rep. 18:3242–3250.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Park SJ, Lee BR, Kim HS, Ji YR, Sung YH,
ShikChoi K, Park HD, Kim SH, Kim MO and Ryoo ZY: Inhibition of
migration and invasion by Tet-1 overexpression in human lung
carcinoma H460 cells. Oncol Res. 23:89–98. 2016.PubMed/NCBI View Article : Google Scholar
|