1. Introduction
Imaging techniques are being increasingly used in
the non-invasive evaluation of various cutaneous diseases. They
possess a complementary role in the in vivo assessment of
cutaneous lesions and can support clinicians in their diagnosis and
therapeutic decisions. There are several imaging methods apart from
classical photography including manual dermoscopy, videodermoscopy
or sequential digital dermoscopy (SDD), computer-aided diagnosis
(CAD), total body photography, high-frequency ultrasonography
(HFUS), reflectance confocal microscopy, multiphoton tomography,
electrical impedance spectroscopy, Raman spectroscopy and
quantitative dynamic infrared imaging (1). They can be classified as follows: Τools
useful in skin tumor screening in daily clinical routine, tools for
the examination of preselected lesions in order to obtain an
automated diagnostic score, tools for the examination of
preselected lesions at specialized centers and devices at an
experimental stage of development (2). The aim of this review was to collect
and analyze data concerning non-invasive diagnostic strategies for
cutaneous tumors, such as naevi and basal cell carcinoma (BCC)
diagnosis, focusing on two main methods, namely videodermoscopy and
HFUS. This study was approved by the Clinical Research Ethics
Committee of ‘St. Spiridon’ County Emergency Clinical Hospital
(Iasi, Romania) and by the Research Ethics Committee of ‘Grigore T.
Popa’ University of Medicine and Pharmacy (Iasi, Romania). Written
informed consent was obtained from all patients prior to
publication.
Videodermoscopy and HFUS can be used in daily
clinical dermatologist practice in order to reduce invasive
procedures, like biopsies and fine needle aspirations (2,3).
Videodermoscopy using computer technology may be used to store
images, indirectly evaluate and extract features in order to
distinguish melanoma from other pigmented benign skin lesions. HFUS
offers in vivo preoperative details and represents a useful
method to investigate superficial macular and nodular cutaneous
lesions with depth lower than 1.5 cm. One benefit is that it helps
in the evaluation of lesions when dimensions are too small to be
assessed by other imaging methods, such as computed tomography or
magnetic resonance imaging (2,3).
2. Videodermoscopy and CAD in naevi and
BCC
The term ‘dermoscopy’ (synonym: epiluminescence
microscopy) refers to surface microscopy and currently indicates
all methods that offer visualization at 10-fold magnification of
skin tumoral lesions using optical magnification and fluid
immersion or polarized lighting. Videodermoscopy represents digital
dermoscopy and this method allows dermoscopic visualization of
tumoral structures in higher magnification. Dermoscopy improves the
ability of physicians to differentiate the various types of
melanocytic naevi from one another and to diagnose melanoma. The
main principle of dermoscopy screening is the examination of all
cutaneous lesions, not only pigmentary or nonpigmentary lesions
chosen by the patient or preselected for clinical examination.
Argenziano et al (4) proposed a new dermoscopic classification
of naevi into seven groups:
i) Globular/congenital naevi with globular pattern
in children reveal brown globules inside the lesion and especially
at the periphery. It may sometimes also show hypopigmentation or a
structureless brown pigmentation. In adults, these types of naevi
appear elevated, with a papillomatous surface and dermoscopically
present a cobblestone pattern or sometimes a fried-egg pattern
characterized by a regular pigment network with globules,
cobblestone or structureless area in the center and a flat portion
at the periphery.
ii) Reticular naevi are late-acquired melanocytic
naevi and can be considered small (<6 mm) or large (≥6 mm),
having a flat or elevated surface with a brown to black color.
Dermoscopically, they present a regular pigment network with or
without areas of hypopigmentation and/or structureless brown to
black coloration. Large lesions may have multiple hypo- and/or
hyperpigmented areas.
iii) Pigmented Spitz or Reed naevi are brown-black,
flat to elevated, symmetrical lesions which are characterized by a
star-burst pattern seen by dermoscopy with radiating pattern
appearance (multiple streaks of pigmentation and symmetrical large
globules at the periphery). In no pigmented lesions are dotted
vessels and reticular depigmentation seen.
iv) Blue naevi by naked eye examination are blue to
black papules, nodules with variable size and dermoscopy reveals a
homogeneous pattern of structureless blue coloration.
v) Site-related naevi: a) Acral naevus: Brown to
black, flat to slightly elevated tumoral lesion on palms/soles
showing in parallel, parallel-furrow, lattice-like or fibrillar
pattern. b) Facial naevus in children may appear as a flat or
elevated, brown symmetrical lesion, smaller than 15 mm with a
dermoscopic pseudoreticular pattern. In adults, it may appear as an
elevated and skin colored lesion with the presence of comma vessels
in dermoscopy.
vi) Naevi with special features: a) Irritated naevus
shows a reticular, globular or structureless pattern with grey and
red pigmented areas. b) Naevus with eczematous halo may present
reticular, globular or structureless pattern with yellow areas and
eczematous changes within a naevus. c) Combined naevi show at least
two of the following patterns: reticular, globular, homogeneous or
starburst. d) Recurrent naevus with atypical pigmentation pattern
and scar-like structures. e) Halo naevus has a globular pattern
with blue pepper-like granules and/or white scar-like areas.
vii) Unclassifiable melanocytic lesions: one of the
previous type of naevi which shows atypical features (in this cases
a melanoma cannot be excluded) (4).
Melanocytic naevi represent direct precursor lesions
for melanoma and 20-30% of melanomas develop on a nevus (5). A high total nevus count is a risk for
melanoma and ongoing surveillance of naevi for malignant
degeneration is needed (6). Melanoma
is a high-risk skin cancer and it has potential to metastasize,
while basal cell carcinoma has the potential to infiltrate the
surrounding tissue. Melanoma screening strategies have the
objective of a total body examination and Breitbart et al
(7) reported that improving melanoma
prognosis is based on early detection. Two meta-analyses have
demonstrated that dermoscopy can improve the sensitivity for
recognition of cutaneous melanomas in comparison to examination
with the naked eye (7,8). Videodermoscopy increases the
effectiveness of melanoma screening methods and consists of a
modern method of capturing and sequential monitoring of digital
dermoscopic images of single or multiple cutaneous lesions. The
initial images printed or stored electronically are used in
comparison with future examinations. The utility of videodermoscopy
for monitoring pigmented skin lesions was for the first time
reported by Stolz et al (9)
and Braun et al (10).
Haenssle et al (11) claimed
that SDD was especially useful in the evaluation of high risk
patients and this investigation improved the sensitivity and
positive predictive value of melanoma screening at follow-up
examinations in comparison with dermoscopy alone. Multiple studies
reported values of sensitivity examination between 83 and 84%
(10). Other benefits of SDD consist
in identifying initial featureless melanomas, monitoring the naevi
and decreasing the number of unnecessary biopsies. Indirect
advantages represent improving the patient-dermatologist
relationship and may help also in improving follow-up visit
compliance (12,13). However, there are limitations of SDD,
such as increasing the time of examination, costs, requiring expert
examiners, the possibility of occurrence of taking image problems
and equally possibility of monitoring melanomas instead of
biopsies. Dermoscopic algorithms including the ABCD rule, pattern
analysis or the 7-point checklist may not reliably differentiate
in situ melanomas from melanocytic naevi (14). Similarly, there is a lack of
consensus regarding which lesions should be analyzed (15,16).
Kittler et al (17) recommend
that short-term follow-up (3 months) should be indicated in
suspicious lesions that do not present features of melanoma and
biopsy is recommended when lesions morphology changes. The
selection of pigmented skin lesions for SDD follows clinical and
classical dermoscopic examination of all cutaneous lesions. Lesions
with macroscopic signs of atypia (asymmetric form, inhomogeneous
color, irregular borders) and/or dermatoscopic signs of atypia,
such as prominent pigmentary network, focal eccentric
hyper/hypopigmentation or multiple components have indication for
short follow-up. Poorly accessible locations may also have
indication for follow-up. Long-term follow-up (6-12 months) should
be indicated for patients with multiple atypical naevi or high-risk
individuals (17). Risk factors for
the development of melanoma mentioned in epidemiologic studies
include a positive patient or family history of melanoma, the
number of typical and atypical naevi, the Fitzpatrick skin,
intermittent UV exposure and the presence of other non-melanoma
skin cancer (18).
In a study of pigmented lesions, which had dynamic
alterations in follow-up, the reported ratio of excised cutaneous
melanomas to benign naevi was 1:79 in the group of patients with
multiple typical naevi, while in the group with multiple atypical
naevi, the ratio was 1:15. Similarly, melanomas could be diagnosed
earlier using SDD rather than clinical examination and conventional
dermoscopy (mean registered Breslow depth was 0.41 vs. 0.62 mm;
P=0.04) (19). Therefore, periodic
control examinations using SDD are useful particularly in
evaluating patients with multiple atypical naevi. Studies in cases
of patients without an increased risk of melanoma did not
demonstrate advantages from skin pigmented lesions dermoscopic
follow-up because many benign lesions were excised and no melanomas
were diagnosed (20).
Dermoscopically false positive and false negative
tumors exist and false positive diagnosis may lead to unnecessary
excisions. Otherwise false negative diagnosis is much more
dangerous, since it might fail to notice a cancer, with serious
consequences for both the patient and the dermatologist. On the one
hand, irritated or regressive seborrheic keratosis,
melanoacanthoma, solar lentigo, thrombosed angioma, dermatofibroma,
benign adnexal tumors and certain type of naevi such as Clark,
Spitz, recurrent, combined, blue or sclerosing are the most
frequent benign tumors that may display dermatoscopic
characteristics suggestive of malignancy. Dermoscopy and,
similarly, SDD may reveal certain features for benignity: solar
lentigo has a broad network; Clark nevus has a clinical context;
Spitz naevi are characteristic for young age, recurrent naevi
present pigmentation within the scar, combined naevi may show a
bluish central area, blue naevi have usually a certain onset and
sclerosing naevi most frequent have location on the upper back. On
the other hand, malignant tumors that might mimic benign tumors are
represented by melanoma with the following clinical types: In
situ, nevoid, amelanotic, spitzoid, regressive or verrucous;
squamous cell carcinoma and basal cell carcinoma with non-pigmented
clinical forms. Dermoscopy and SDD may give the following clues to
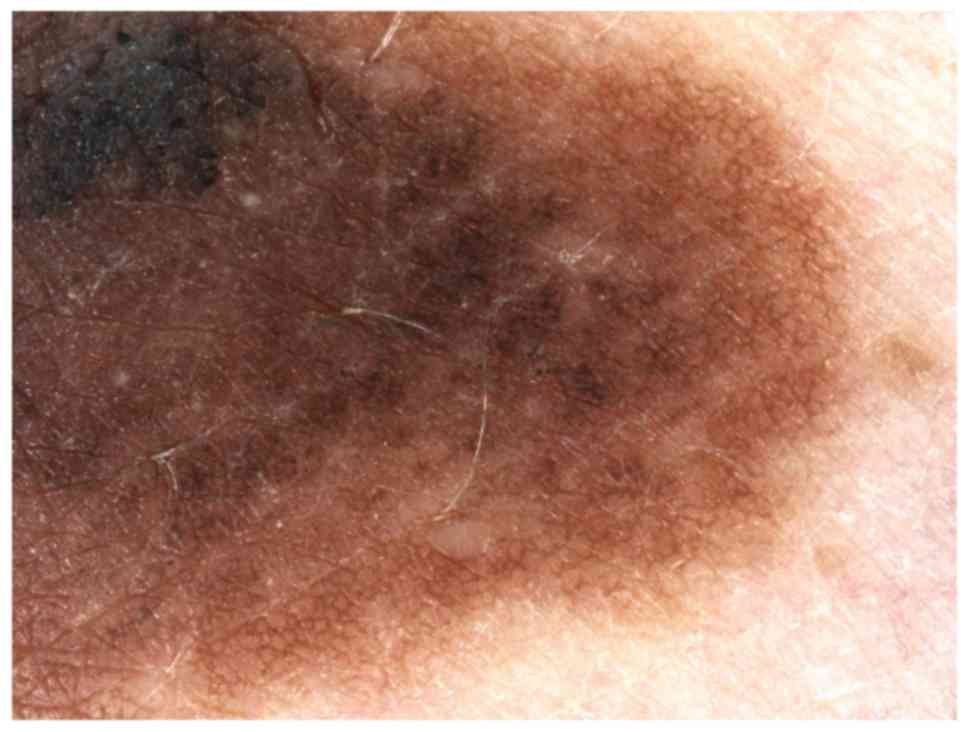
recognize the melanoma subtypes: Irregular hyperpigmented areas
appear in melanoma in situ, history of growth is
characteristic for nevoid melanoma, pink color and irregular linear
vessels accompanied by dotted vessels are suggestive for amelanotic
melanoma, blue-black sign appears in verrucous melanoma and
peppering or scar-like depigmentation may reveal a regressive
melanoma (21) (Fig. 1).
CAD represents artificial intelligence-based
techniques that are using a computer to analyze images and to
determine the risk of malignancy. In primary care, CAD may help
general practitioners to identify high-risk lesions and may reduce
unnecessary excisions without missing melanoma cases. Computer
analysis of single or multiple pigmented skin lesions includes
preprocessing images with artefacts (hair, air bubbles, specular
reflections), image enhancement, respectively, color calibration
and illumination correction. The other steps include lesions border
detection, comparison of segmentation algorithms, feature
extraction, registration and change detection, lesions
classification, use of CAD systems with digital dermoscopy analysis
instruments and 3D lesions analysis (22).
A review of 42 studies evaluating digital
dermoscopy-based CAD systems (Derm-CAD) in 9,602 lesions (with
detection of 1,220 melanomas, 83 basal cell carcinomas and 9
squamous cellular carcinomas) evaluated the accuracy of CAD systems
for diagnosing cutaneous invasive melanoma and atypical
intraepidermal melanocytic naevi (20). The results showed that in highly
selected patient populations all CAD types demonstrate high
sensitivity. CAD systems were useful as a back-up for specialist
diagnosis to assist in minimizing the risk of missing melanomas.
Nonetheless, the evidence base was too poor to understand whether
CAD system outputs translate to different clinical decision-making
in practice (23).
SDD can be improved by telediagnosis, which may
provide a better examination of pigmented cutaneous tumoral lesions
carried out by primary care physicians, not expert in that kind of
diagnosis, thus reducing the number of consultations in specialized
centers. Telediagnosis is usually used in highly specialized
centers, while in smaller and non-specialized units surgical
treatment is usually performed in cases of pigmented lesions
suspicious for malignancy (24).
BCC is the most common type of skin cancer in the
world and can be associated with significant morbidity, especially
if left untreated. BCC can present a variety of clinical
morphologies, such as erythematous patches to ulcerated nodules.
Depending on the subtype of BCC and the degree of pigmentation, the
clinical differential diagnosis can range from benign inflammatory
conditions to melanoma and dermoscopy has dramatically improved the
clinicians' diagnostic accuracy for both clinical pigmented or
non-pigmented tumoral types. There are multiple histopathologic
subtypes of BCC including superficial, nodular, morpheaform or
sclerosing or infiltrative BCC. Fibroepithelioma of Pinkus,
microcystic adnexal and basosquamous cell BCC are similarly defined
as histopathologic subtypes. The main dermoscopic criteria of BCC
are represented by classical arborizing vessels; fine or short
arborizing vessels, focused dots, large blue gray ovoid nests,
multiple blue gray globules, concentric structures, leaf-like
areas, spoke-wheel areas, small erosions or ulceration, pink-white
areas and short white streaks. The evaluation of vascular pattern
may facilitate discrimination between basal cell carcinomas
subtypes and it is reported that aggressive tumoral forms have less
or no pink coloration and a lack of central vessels (25). Digital dermoscopy of superficial
basal cell carcinoma usually reveals short, fine ‘microarborizing’
telangiectasia, multiple small erosions, shiny white-to-red,
translucent, opaque structureless areas and brown-colored pigmented
structures. Nodular basal cell carcinoma displays large arborizing
vessels, large ulcerations and blue-gray ovoid nests in pigmentary
forms. While vessels of BCC are bright red and arborizing, vessels
outside the tumors belonging to the normal dermal plexus have a
blurred aspect and a darker hue. Sclerodermiform BCC presents
branching vessels, which are usually finer, more scattered and show
fewer branches compared to the classic vessels of nodular BCC.
Moreover, the underlying fibrosis induces a whitish background,
whereas nodular BCC typically reveals a translucent pinkish color.
The differentiation between tumoral vessels and the vascular
pattern of normal skin is needed in order to estimate the lateral
extension of BCCs, which may have clinically ill-defined borders
(26-28).
SDD is similarly used for monitoring superficial CBC
response to very popular non-ablative treatments. Clinical
evaluation after therapy may not be reliable, but dermoscopy offers
information on the possible residual tumoral lesions (29). The disappearance of the BCC
dermatoscopic criteria has shown histopathologic clearance, while
the presence of the same or new BCC dermoscopic criteria correlates
with persistence or tumor reoccurring. Criteria such as arborizing
vessels, ulceration or blue-gray ovoid nests and maple leaf-like
areas with pigmented structures may predict residual disease. Red
or white structureless areas and superficial fine telangiectasia
correspond to equivocal features (30). The detection of blue-gray globules
has been reported to indicate early recurrence of CBC (23). In a study with a series of BCCs
treated with imiquimod, criteria like arborizing vessels,
spoke-wheel areas, maple leaf-like areas were reported to also
decrease in size and number after treatment initiation, whereas
structures such as multiple blue-gray globules and ovoid nests were
detected for a longer period of time (31).
3. High frequency ultrasonography of
melanocytic naevi and basal cell carcinoma
Ultrasonography has been used in dermatology for
nearly 40 years. Alexander and Miller introduced ultrasonography as
a non-invasive technique to appreciate normal skin thickness and in
1980 it was used to assess skin nodules and cutaneous diseases
(32). This procedure is a method
allowing the in vivo histologic evaluation of the cutaneous
structure. It is based on the phenomenon of transonic wave
reflection in the form of an imaging gray scale for interpretation,
in accordance with the skin characteristics represented by the
percentage of collagen, keratin and water in tissues. The
indications of this imaging technique are multiple and they include
the evaluation of benign or malignant tumors, melanocytic lesions,
as well as inflammatory diseases. Important data regarding size,
structure, elasticity and vascular flow of cutaneous lesions can be
obtained by using conventional 2D ultrasound, HFUS, Doppler
ultrasound, contrast enhanced ultrasound and elastography.
Elastography represents a non-invasive technique that offers
information on the soft tissue elasticity and a reduced elasticity
is correspondent to hypervascularization and tumor congestion
(32,33). Tumor macrocirculation can be
evaluated using color Doppler flow map for vessel enhancement and
pulse Doppler is indicated in differentiating between vein and
arteries in study of velocity (3).
HFUS using 20-100 MHz transducers constitutes a
modern procedure that is used for skin investigation. Studies show
that HFUS is superior to clinical examination alone, since it
provides information in measurement of size and appreciation of
contour, structure and assessment of skin lesion depth (31). The high frequency transducer offers
an 80-micrometer axial resolution and a 200-micrometer lateral
resolution. Using transducers with 20 MHz, epidermis is a
hyperechogenic entry line, which varies according to age,
anatomical area or the topical therapy. The dermis is markedly
echogenic and sharply demarcated from the hypoderm, which is
hypoechoic. Adipose panniculi are separated by echogenic
conjunctive vascular septae. Skin tumor HFUS allows the assessment
of macular and nodular lesions with depth smaller than 1.5 cm.
Epidermis, dermis, hypodermis, dermoepidermic and dermohypodermic
junctions are identified and allow correlation of the
ultrasonographic depth and the histologic index. This correlation
is limited in presence of perilesional inflammatory infiltrate
(1,34,35).
Pigmentary tumoral lesions, such as naevi, melanomas and
nonpigmentary tumoral lesions, including carcinomas, can be
assessed and described in detail using ultrasound. Most of the skin
tumoral lesions appear as hypoechoic cutaneous or as subcutaneous
thickening on HFUS. Melanocytic naevi are hypoechoic, symmetrical
and usually well delimited from the adjacent dermis; they may
present many small echoes. Junctional naevi are very thin, whereas
dermal naevi are thicker. In congenital pigmentary naevi,
ultrasonography is a useful tool in monitoring and early detection
of possible malignant transformation. Acoustic shadowing and
retrolesional echogenicity is suggestive for melanoma (36). Similarly, cutaneous ultrasound can
support the differential diagnosis between blue naevi and
metastases of melanoma. Blue naevi appear hypoechoic, homogeneous,
‘dish-shaped’ lesions and are located in the superficial dermis,
whereas melanoma metastases are hypoechoic, heterogeneous lesions,
‘potato-shaped’ located in the hypodermis (37). Malignant melanoma has a high
mortality rate and the histological depth of tumor or Breslow index
represent the most important factor for prognosis and therapy. Thin
malignant melanoma is represented by irregular hypoechogenic aspect
band and HFUS can reveal the degree of dermic penetration with
possible visible areas of vertical growth towards dermis and
inflammatory infiltrate with hypoechogenic aspects. Similarly,
visible areas of tumoral regression accompanied by fibrosis of
hyperehogenic aspect can be identified (33). Color and Power Doppler studies may
help to evaluate vascularity of lesions. Nodular malignant melanoma
is described as a hypoechogenic nodular tumor and HFUS quantifies
the degree of the dermis invasion. Regarding therapeutic management
and prognosis, melanomas with depth index smaller than 1 mm defined
as thin melanomas have a good prognosis, with a 95 to 100% chance
of a 5-year-survival after surgical excision with a margin of 1 cm.
Ultrasonography is useful in melanoma patients staging (T stage)
and both conventional and Doppler ultrasound are helpful in the
quantification of N stage melanoma identifying possible positive
adenopathy before performing sentinel lymph node biopsy.
According to literature, BCC is the most common skin
cancer representing 75-90% of all skin cancers (35). Echography helps in the clinical
diagnosis and shows hypoechoic masses, which replace the collagen
(more hyperechoic) with tumor cells (lower density). It may
estimate tumor size (depth and diameter), delimitation of
presurgical margins and helps in surgical planning (study of
peritumoral blood vessels). It may also provide information on
invasion of adjacent structures (cartilage and/or bone) and
similarly help to evaluate the response to non-surgical treatments
and study of recurrences. High frequency 20 MHz ultrasound shows an
oval solid hypoechoic or anechoic tumor with irregular borders and
hyperechoic points. Usually, BCC is well delimited from the
surrounding dermis and dermis invasion can be quantified. Color
Doppler shows moderate increase in intra and peritumoral
vascularization. The hyperechoic spots may be useful in order to
differentiate BCC from other types of skin cancer. On histologic
analysis, the hyperechoic points appear to correlate with the
presence of horn cysts, microcalcifications or clusters of
apoptotic cells in the center of nests of basal cell carcinomas.
Wortsman described ultrasound as a first-line imaging modality for
the management of facial cutaneous BCC (38). Preoperative imaging in facial BCC may
aid surgical therapeutic plan, especially in high risk areas of
recurrence such as eyes, nose and ears, in case of incomplete
excisions. Risk factors for incomplete excision are head location,
multiple tumors, morpheiphorm and infiltrative subtypes (39-42).
A study conducted on 56 patients with BCC reported
lower ultrasonographic index in comparison with histologic index,
but a moderate correlation index was obtained and statistically
significant differences were not identified (43). HFUS can be similarly useful in
detecting subclinical satellite lesions. In another study with 46
subjects diagnosed with BCC (18 patients); superficial spreading
melanoma (8 patients) and nodular melanoma (20 patients), the
ultrasonographic depth index was comparable to the histological
one, with a very good sensitivity (98-99%) (43). BCC with depth index smaller than 1.5
mm may benefit from photodynamic therapy (35). HFUS provides real-time data on depth
and lateral invasion and their examination show hypoechoic
inhomogeneous tumors with possible ulceration (43). In addition, HFUS offers more accurate
depth index in comparison to conventional ultrasonography (39,44).
The benefits of using HFUS consist of repeatability,
the lack of risk for patients, being a non-invasive method with
minimal costs and also the intake of morphological details of
cutaneous lesions that cannot be obtained from clinical or
histological examination (41,45).
Depth, area and demarcation from the adjacent structures can be
described with the possibility of identifying a preoperatory
prognosis. It is also admitted that HFUS images could give
important information on internal structure especially the collagen
and keratin pattern. In a retrospective study in which the skin
tumor evaluation protocol was completed with HFUS, the accuracy of
clinical diagnosis was estimated to increase from 73 to 97%
(46). The estimation of tumor
margins is important in therapy planning and may avoid incomplete
excision and surgical reintervention. In addition, imaging findings
can be useful in the follow-up after cryotherapy or laser treatment
(36) and it is an easily and well
accepted method for follow-up of patients (36).
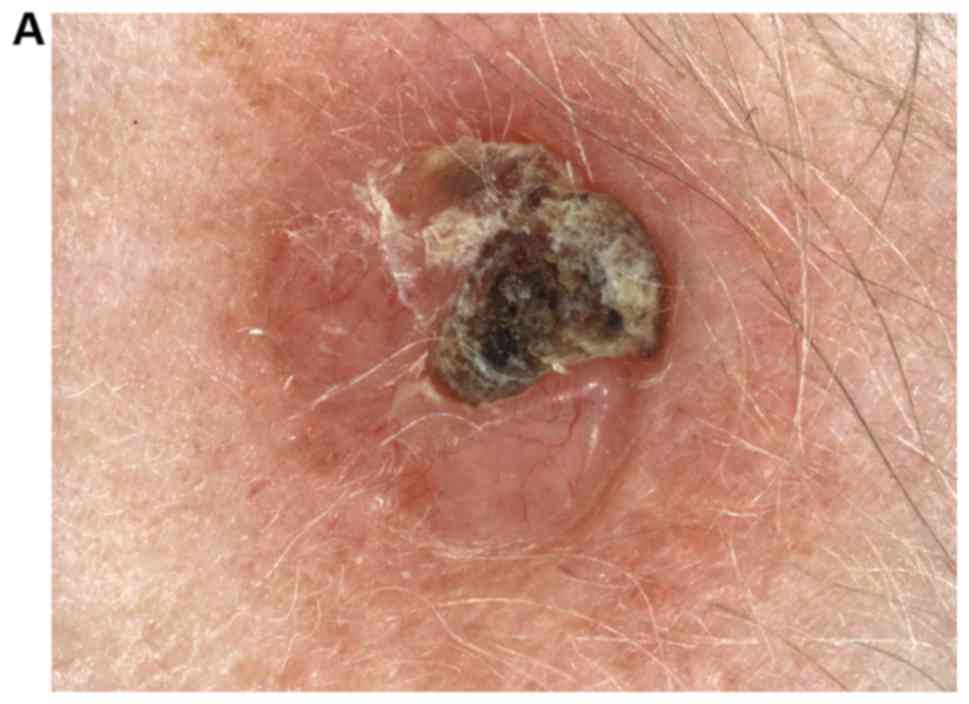
In contrast, ultrasonographic histologic
differentiation of skin tumors, either benign or malignant, is not
always possible according to literature data (46,47). It
has very good sensitivity, but low specificity (35). This technique requires modern devices
and it is a time-consuming technique for the examiner. HFUS is
operator sensitive and its accuracy depends on the examiner's level
of training in ultrasonography. Besides, errors may appear if the
pressure used during examination is not appropriate. Another limit
is that in situ tumors situated only in epidermis, such as
in situ melanoma and thin melanoma, may not be detectable
and ultrasound examination usually does not differentiate between
melanomas and clinically atypical naevi (34). In addition, the tumoral thickness is
not accurate if tumoral structures have important perilesional
inflammatory infiltrate. It has been reported that ultrasonographic
values could be slightly overestimated due to inflammatory
infiltrate associated to the tumor, as well as skin hypertrophied
sebaceous glands or hair follicles (48,49).
Similarly, the identification of small infiltrative dermic tumoral
lesions is another reported limitation of the technique (50) (Fig.
2).
Pellacani and Seidenari (49) used a combined approach based on
sonography and clinical-videomicroscopy in order to evaluate
preoperatively thick melanomas. Echographic thickness using 20 MHz
sonography was calculated for each tumoral lesion. Two clinical
features (nonpalpability for thin melanomas and clinical regression
for thick melanoma) and seven videomicroscopic features were
identified for distinction between thick and thin melanomas.
Central pigment network, central brown globules and blotches were
considered features of thin melanomas, while localized peripheral
pigment network, grayish polygonal areas, veil and vessels pattern
were features of thick ones. In this algorithm, a coefficient was
attributed to each variable, a score was obtained for each tumor
and a validated test for preoperative thickness prediction was
finally developed. This test enabled the distinction of thick
melanomas with 86.7% sensitivity and 100% specificity (49).
4. Other techniques
Reflectance confocal microscopy is a novel
non-invasive diagnostic technique based on focal point illumination
and visualization of different skin layers with possible
differentiation of benign skin lesions from malignant lesions,
which is also used for inflammatory skin disease diagnosis
(50-52).
Lentigo maligna is characterized by the disruption
of the typical honeycomb or cobblestone pattern in epidermis,
multiple large round pagetoid cells and large nucleated cells in
dermis (53,54). In melanoma, the epidermis has
irregular honeycomb or cobblestone patterns with large nucleated
pagetoid cells. Similarly, dermoepidermal junctions are
disorganized and the dermis has dense and sparse nests of cells,
plump bright and small bright cells with increased reticulated
fragmented bright collagen fibers. Pellacani and colleagues
(55) analyzed 351 melanocytic
lesions including melanoma and melanocytic naevi from 332 patients
to obtain diagnostic accuracy. A sensitivity of 92% and specificity
of 69% were reported (55). Spitz
nevus represents a melanocytic lesion difficult to diagnose. In
in vivo confocal microscopy, an atypical Spitz tumor is
indicated by large conflating tumor nests with isolated epithelioid
pleomorphic cells within the spinous layer, inhomogeneous nests,
combined melanocytic tumor and melanocytic proliferation with
elongated rete ridges (56).
In non-melanocytic tumors, such as BCC, confocal
microscopic features include cells with elongated nuclei orientated
at the same axis with separation of tumor islands from the
surrounding stroma. Reflectance microscopy examination of cystic
BCC may reveal solid tumoral masses appearing less bright than
surrounding stroma, large dark areas with bright structures inside
the center and periphery of tumoral lobules and numerous enlarged
vessels. Similarly, in the inner portion of tumoral masses a
peripheral palisade arrangement of elongated, polarized nuclei and
bright round-oval structures, thin filaments and large dendritic
cells may be observed (57). The
progression of BCCs may be repeatedly monitored using this
technique (58). Squamous cell
carcinoma shows parakeratosis and hyperkeratosis in the epidermis,
an atypical ‘honeycomb’ pattern with uneven dysplastic
keratinocytes and detached keratinocytes (59). Dilated and tortuous vessels,
perivascular inflammatory cells with shiny appearance may also be
observed. The papillary dermis exhibits dark areas corresponding to
blood vessels containing erythrocytes appearing as white central
elements and also bright perivascular elements of inflammatory
infiltrate may be noted (60). In
Bowen disease, an acanthotic epidermis with large cells with bright
center and dark peripheral halos may be observed. Similarly, there
are remarked cells with dark center and bright rim surrounded by a
dark hallo, which are related with dyskeratotic cells on
histological examination (61).
The limitations of the reflectance confocal
microscopy technique include the fact that diagnosed lesions are
limited to the upper dermis up to 250-300 µm and is operator
sensitive (53).
Multiphoton tomography is a tissue imaging method
based on the tissue exposure with intense near infrared laser
pulses resulting in two major signals (the autofluorescence and
second harmonic generation). These signals are used to image cells
and the extracellular matrix. Indications of the method include
early detection of skin cancer and other tissue pathologies,
obtaining non-invasive tissue histology within minutes. The use of
multiphoton imaging methods for skin tumor diagnosis and monitoring
shows considerable promise. Several studies (62,63) have
reported that tumors can be identified through a variety of
different contrast mechanisms and may be used in the investigation
of suspicious naevi or other neoplasms, such as melanoma, BCC or
squamous cell carcinoma, especially in locations such as the head
and neck, where surgeons need effective imaging tools to determine
the whole lesion margin in order to resect it completely. The
combination of multiphoton microscopy with other imaging
modalities, such as ultrasound imaging, confocal microscopy and
optical coherence tomography have been shown to be useful in skin
research (63).
Electrical impedance spectroscopy is a noninvasive
method that helps in diagnosing skin cancer using a handheld probe
with an electrode applied directly on the skin. The basic principle
is based on the utilization of electrical impedance variations to
differentiate between normal skin and tumoral lesions. It has
indication in lesions, which have clinical or dermoscopic
suspicious features (64).
Raman spectroscopy represents a noninvasive tool
used in vivo in skin cancer diagnosis that captures unique
optical signals via molecular vibrations in tissue samples
(65). Zhang et al (66) suggested in a meta-analysis that Raman
spectroscopy could be an accurate tool for differentiating
melanoma, BCC and squamous cell carcinoma from normal tissue. In a
cohort with 645 confirmed lesions from 573 patients with skin
tumors, pretumoral lesions and benign skin lesions were included.
They were divided into a training cohort (n=518) and testing cohort
(n=127) and it was found that the diagnostic tumor specificity used
for fixed sensitivity was improved from 0.17-0.65 to
0.20-0.75(67).
Other technologies (stepwise two-photon-laser
spectroscopy, quantitative dynamic infrared imaging, in vivo
multiphoton tomography, infrared thermal image-analysis, epidermal
genetic information retrieval) are on the verge of becoming less
experimental, yet more clinically applicable for diagnosing skin
cancer.
5. Conclusions
The early accurate diagnosis of skin cancer is
essential to guide the appropriate management and to improve the
morbidity and survival rates. None of the presented imaging
techniques is able to provide a certain and final diagnosis or to
completely replace the histopathological examination. Up to date,
the need for a complete skin cancer screening fully provided by
automated devices has not been satisfied (2).
Videodermoscopy or SDD has distinct advantages over
malignant tumor screening. This technique improves sensitivity and
specificity of melanoma detection and represents a complementary
examination method to improve early detection of melanomas
particularly in patients at risk. Dermatologists should use a
combination of history, clinical examination and SDD in order to be
effective in the diagnosis of initial malignant tumor lesions.
Digital imaging applications in both naevi and BCCs have real
benefits: Objective non-invasive documentation of tumoral lesions,
digital dermatological image archives, telediagnosis, quantitative
description of clinical features of cutaneous lesions and
3-dimensional reconstruction. Dermoscopic malignant skin tumor
diagnosis is limited in the diagnosis of very early and mainly
featureless melanomas. Although automatic diagnosis systems are not
perfect yet, their most valuable functionality has already been
achieved in the capacity of description of lesion
characteristics.
HFUS represents a non-invasive, reliable method that
can be complementary utilized in the physical examination for the
assessment, diagnosis and management of cutaneous tumors.
Although the gold standard method of diagnosing skin
tumors is histopathological examination, non-invasive methods such
as SDD and HFUS, allow a multimodal approach and offer the
opportunity of presurgical tumor evaluation and the establishment
of prognostic factors and therapeutic management (68).
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Irina Radu, Individual Service Provider,
certified translator in Medicine and Pharmacy (certificate
credentials: series E no. 0048).
Funding
No funding was received.
Availability of data and materials
Imaging data were provided using
MicroDermVisiomed® system analysis and Dermascan C
USB® (20 MHz B-mode) equipments. The datasets used
and/or analyzed during the current study are available from the
corresponding author on reasonable request.
Authors' contributions
All authors contributed to the acquisition of the
data and critical revision of manuscript for important intellectual
content. AS conceived review on dermoscopy. IAG and LGS performed
videodermoscopy, HFUS techniques and wrote the manuscript. LS, IAP,
DV wrote and conceived review sections on dermoscopy and MC wrote
and conceived review on ultrasound. EPA, AIP and TT searched data
on the other imaging techniques. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Clinical Research
Ethics Committee of ‘St. Spiridon’ County Emergency Clinical
Hospital (Iasi, Romania) and by the Research Ethics Committee of
‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania). Written informed consent was obtained from all patients
prior to publication.
Patient consent for publication
Written informed consent was obtained from all
patients prior to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Korotkov K and Garcia R: Computerized
analysis of pigmented skin lesions: A review. Artif Intell Med.
56:69–90. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fink C and Haenssle HA: Non-invasive tools
for the diagnosis of cutaneous melanoma. Skin Res Technol.
23:261–271. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Crisan D, Badea AF, Crisan M, Rastian I
and Solovastru Gheuca L: Integrative analysis of cutaneous skin
tumours using ultrasonographic criteria. Preliminary results. Med
Ultrason. 16:285–290. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Argenziano G, Zalaudek I, Ferrara G,
Hofmann-Wellenhof R and Soyer HP: Proposal of a new classification
system for melanocytic naevi. Br J Dermatol. 157:217–227.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tsao H, Bevona C, Goggins W and Quinn T:
The transformation rate of moles (melanocytic nevi) into cutaneous
melanoma: A population-based estimate. Arch Dermatol. 139:282–288.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goldstein AM and Tucker MA: Dysplastic
Nevi and Melanoma. Cancer Epidemiol Biomarkers Prev. 22:528–532.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Breitbart EW, Waldmann A, Nolte S,
Capellaro M, Greinert R, Volkmer B and Katalinic A: Systematic skin
cancer screening in Northern Germany. J Am Acad Dermatol.
66:201–211. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bafounta ML, Beauchet A, Aegerter P and
Saiag P: Is dermoscopy (epiluminescence microscopy) useful for the
diagnosis of melanoma? Results of a meta-analysis using techniques
adapted to the evaluation of diagnostic tests. Arch Dermatol.
137:1343–1350. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Stolz W, Schiffner R, Pillet L, Vogt T,
Harms H, Schindewolf T, Landthaler M and Abmayr W: Improvement of
monitoring of melanocytic skin lesions with the use of a
computerized acquisition and surveillance unit with a skin surface
microscopic television camera. J Am Acad Dermatol. 35:202–207.
1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Braun RP, Lemonnier E, Guillod J, Skaria
A, Salomon D and Saurat JH: Two types of pattern modification
detected on the follow-up of benign melanocytic skin lesions by
digitized epiluminescence microscopy. Melanoma Res. 8:431–437.
1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Haenssle HA, Krueger U, Vente C, Thoms KM,
Bertsch HP, Zutt M, Rosenberger A, Neumann C and Emmert S: Results
from an observational trial: Digital epiluminescence microscopy
follow-up of atypical nevi increases the sensitivity and the chance
of success of conventional dermoscopy in detecting melanoma. J
Invest Dermatol. 126:980–985. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Argenziano G, Mordente I, Ferrara G,
Sgambato A, Annese P and Zalaudek I: Dermoscopic monitoring of
melanocytic skin lesions: Clinical outcome and patient compliance
vary according to follow-up protocols. Br J Dermatol. 159:331–336.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Robinson JK and Nickoloff BJ: Digital
epiluminescence microscopy monitoring of high-risk patients. Arch
Dermatol. 140:49–56. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kardynal A and Olszewska M: Modern
non-invasive diagnostic techniques in the detection of early
cutaneous melanoma. J Dermatol Case Rep. 8:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bauer J, Blum A, Strohhäcker U and Garbe
C: Surveillance of patients at high risk for cutaneous malignant
melanoma using digital dermoscopy. Br J Dermatol. 152:87–92.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Haenssle HA, Vente C, Bertsch HP,
Rupprecht R, Abuzahra F, Junghans V, Ellinghaus B, Emmert S,
Hallermann C, Rosenberger A, et al: Results of a surveillance
programme for patients at high risk of malignant melanoma using
digital and conventional dermoscopy. Eur J Cancer Prev. 13:133–138.
2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kittler H, Pehamberger H, Wolff K and
Binder M: Follow-up of melanocytic skin lesions with digital
epiluminescence microscopy: Patterns of modifications observed in
early melanoma, atypical nevi, and common nevi. J Am Acad Dermatol.
43:467–476. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gandini S, Sera F, Cattaruzza MS, Pasquini
P, Zanetti R, Masini C, Boyle P and Melchi CF: Meta-analysis of
risk factors for cutaneous melanoma: III. Family history, actinic
damage and phenotypic factors. Eur J Cancer. 41:2040–2059.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Haenssle HA, Korpas B, Hansen-Hagge C,
Buhl T, Kaune KM, Johnsen S, Rosenberger A, Schön MP and Emmert S:
Selection of patients for long-term surveillance with digital
dermoscopy by assessment of melanoma risk factors. Arch Dermatol.
146:257–264. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schiffner R, Schiffner-Rohe J, Landthaler
M and Stolz W: Long-term dermoscopic follow-up of melanocytic
naevi: Clinical outcome and patient compliance. Br J Dermatol.
149:79–86. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Papageorgiou V, Apalla Z, Sotiriou E,
Papageorgiou C, Lazaridou E, Vakirlis S, Ioannides D and Lallas A:
The limitations of dermoscopy: False-positive and false-negative
tumours. J Eur Acad Dermatol Venereol. 32:879–888. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dasgeb B, Morris MA, Mehregan D and Siegel
EL: Quantified ultrasound elastography in the assessment of
cutaneous carcinoma. Br J Radiol. 88(20150344)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lallas A, Apalla Z, Argenziano G, Longo C,
Moscarella E, Specchio F, Raucci M and Zalaudek I: The
dermatoscopic universe of basal cell carcinoma. Dermatol Pract
Concept. 4:11–24. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
de Giorgi V, Gori A, Savarese I, D'Errico
A, Grazzini M, Papi F, Maio V, Covarelli P, Urso C and Massi D:
Teledermoscopy in doubtful melanocytic lesions: Is it really
useful? Int J Dermatol. 55:1119–1123. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lupu M, Caruntu C, Popa MI, Voiculescu VM,
Zurac S and Boda D: Vascular patterns in basal cell carcinoma:
Dermoscopic, confocal and histopathological perspectives. Oncol
Lett. 17:4112–4125. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bakos RM, Bakos L, Cartell A, Manzoni AP
and Prati C: Radial streaking: Unusual dermoscopic pattern in
pigmented superficial basal cell carcinoma. J Eur Acad Dermatol
Venereol. 21:1263–1265. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lallas A, Argenziano G, Zendri E,
Moscarella E, Longo C, Grenzi L, Pellacani G and Zalaudek I: Update
on non-melanoma skin cancer and the value of dermoscopy in its
diagnosis and treatment monitoring. Expert Rev Anticancer Ther.
13:541–558. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Altamura D, Menzies SW, Argenziano G,
Zalaudek I, Soyer HP, Sera F, Avramidis M, DeAmbrosis K, Fargnoli
MC and Peris K: Dermatoscopy of basal cell carcinoma: Morphologic
variability of global and local features and accuracy of diagnosis.
J Am Acad Dermatol. 62:67–75. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schulze HJ, Cribier B, Requena L,
Reifenberger J, Ferrándiz C, Garcia Diez A, Tebbs V and McRae S:
Imiquimod 5% cream for the treatment of superficial basal cell
carcinoma: Results from a randomized vehicle-controlled phase III
study in Europe. Br J Dermatol. 152:939–947. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mun JH, Jwa SW, Song M, Ko HC, Kim BS, Kim
MB and Kim HS: Pitfalls of using dermatoscopy in defining surgical
margins of basal cell carcinoma. Dermatol Surg. 37:1704–1705.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Micantonio T, Fargnoli MC, Piccolo D and
Peris K: Letter: Changes in dermoscopic features in superficial
basal cell carcinomas treated with imiquimod. Dermatol Surg.
33:1403–1405. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Botar-Jid CM, Cosgarea R, Bolboacă SD,
Şenilă SC, Lenghel LM, Rogojan L and Dudea SM: Assessment of
cutaneous melanoma by use of very- high-frequency ultrasound and
real-time elastography. AJR Am J Roentgenol. 206:699–704.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mandava A, Ravuri PR and Konathan R:
High-resolution ultrasound imaging of cutaneous lesions. Indian J
Radiol Imaging. 23:269–277. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cammarota T, Pinto F, Magliaro A and Sarno
A: Current uses of diagnostic high-frequency US in dermatology. Eur
J Radiol. 27 (Suppl 2):S215–S223. 1998.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Crisan M, Crisan D, Sannino G, Lupsor M,
Badea R and Amzica F: Ultrasonographic staging of cutaneous
malignant tumors: An ultrasonographic depth index. Arch Dermatol
Res. 305:305–313. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Samimi M, Perrinaud A, Naouri M, Maruani
A, Perrodeau E, Vaillant L and Machet L: High-resolution
ultrasonography assists the differential diagnosis of blue naevi
and cutaneous metastases of melanoma. Br J Dermatol. 163:550–556.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Harland CC, Kale SG, Jackson P, Mortimer
PS and Bamber JC: Differentiation of common benign pigmented skin
lesions from melanoma by high-resolution ultrasound. Br J Dermatol.
143:281–289. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wortsman X: Sonography of facial cutaneous
basal cell carcinoma: A first-line imaging technique. J Ultrasound
Med. 32:567–572. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gambichler T, Moussa G, Sand M, Sand D,
Altmeyer P and Hoffmann K: Applications of optical coherence
tomography in dermatology. J Dermatol Sci. 40:85–94.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gambichler T, Regeniter P, Bechara FG,
Orlikov A, Vasa R, Moussa G, Stücker M, Altmeyer P and Hoffmann K:
Characterization of benign and malignant melanocytic skin lesions
using optical coherence tomography in vivo. J Am Acad Dermatol.
57:629–637. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guitera P, Li LX, Crotty K, Fitzgerald P,
Mellenbergh R, Pellacani G and Menzies SW: Melanoma histological
Breslow thickness predicted by 75-MHz ultrasonography. Br J
Dermatol. 159:364–369. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nassiri-Kashani M, Sadr B, Fanian F,
Kamyab K, Noormohammadpour P, Shahshahani MM, Zartab H, Naghizadeh
MM, Sarraf-Yazdy M and Firooz A: Pre-operative assessment of basal
cell carcinoma dimensions using high frequency ultrasonography and
its correlation with histopathology. Skin Res Technol.
19:e132–e138. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Manea A, Crisan D, Badea AF, Dumitrascu
ID, Baciut MF, Bran S, Mitre I, Crisan M and Baciut G: The value of
ultrasound diagnosis in the multidisciplinary approach of cutaneous
tumours. Case report. Med Ultrason. 1:108–110. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Catalano O, Caracò C, Mozzillo N and Siani
A: Locoregional spread of cutaneous melanoma: Sonography findings.
AJR Am J Roentgenol. 194:735–745. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Schmid-Wendtner MH and Dill-Müller D:
Ultrasound technology in dermatology. Semin Cutan Med Surg.
27:44–51. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wortsman X and Wortsman J: Clinical
usefulness of variable-frequency ultrasound in localized lesions of
the skin. J Am Acad Dermatol. 62:247–256. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Harland CC, Bamber JC, Gusterson BA and
Mortimer PS: High frequency, high resolution B-scan ultrasound in
the assessment of skin tumours. Br J Dermatol. 128:525–532.
1993.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bobadilla F, Wortsman X, Muñoz C, Segovia
L, Espinoza M and Jemec GB: Pre-surgical high resolution ultrasound
of facial basal cell carcinoma: Correlation with histology. Cancer
Imaging. 8:163–172. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Pellacani G and Seidenari S: Preoperative
melanoma thickness determination by 20-MHz sonography and digital
videomicroscopy in combination. Arch Dermatol. 139:293–298.
2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jambusaria-Pahlajani A, Schmults CD,
Miller CJ, Shin D, Williams J, Kurd SK and Gelfand JM: Test
characteristics of high-resolution ultrasound in the preoperative
assessment of margins of basal cell and squamous cell carcinoma in
patients undergoing Mohs micrographic surgery. Dermatol Surg.
35:9–16. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ianoși SL, Forsea AM, Lupu M, Ilie MA,
Zurac S, Boda D, Ianosi G, Neagoe D, Tutunaru C, Popa CM, et al:
Role of modern imaging techniques for the in vivo diagnosis of
lichen planus. Exp Ther Med. 17:1052–1060. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cioplea M, Caruntu C, Zurac S, Bastian A,
Sticlaru L, Cioroianu A, Boda D, Jugulete G, Nichita L and Popp C:
Dendritic cell distribution in mycosis fungoides vs. inflammatory
dermatosis and other T-cell skin lymphoma. Oncol Lett.
17:4055–4059. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Rao BK: Atlas of Confocal Microscopy in
Dermatology. 1st edition. NIDIskin LLC, New York, NY, p166,
2013.
|
|
54
|
Hofmann-Wellenhoff R, Pellacani G, Malvehy
J and Soyer HP (eds): Reflectance Confocal Microscopy for Skin
Diseases. Springer-Verlag, Berlin, 2012.
|
|
55
|
Pellacani G, Guitera P, Longo C, Avramidis
M, Seidenari S and Menzies S: The impact of in vivo reflectance
confocal microscopy for the diagnostic accuracy of melanoma and
equivocal melanocytic lesions. J Invest Dermatol. 127:2759–2765.
2007.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Diaconeasa A, Boda D, Solovan C, Enescu
DM, Vîlcea AM and Zurac S: Histopathologic features of Spitzoid
lesions in different age groups. Rom J Morphol Embryol. 54:51–62.
2013.PubMed/NCBI
|
|
57
|
Căruntu C, Boda D, Guţu DE and Căruntu A:
In vivo reflectance confocal microscopy of basal cell carcinoma
with cystic degeneration. Rom J Morphol Embryol. 55:1437–1441.
2014.PubMed/NCBI
|
|
58
|
Ghita MA, Caruntu C, Rosca AE, Kaleshi H,
Caruntu A, Moraru L, Docea AO, Zurac S, Boda D, Neagu M, et al:
Reflectance confocal microscopy and dermoscopy for in vivo,
non-invasive skin imaging of superficial basal cell carcinoma.
Oncol Lett. 11:3019–3024. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Rishpon A, Kim N, Scope A, Porges L,
Oliviero MC, Braun RP, Marghoob AA, Fox CA and Rabinovitz HS:
Reflectance confocal microscopy criteria for squamous cell
carcinomas and actinic keratoses. Arch Dermatol. 145:766–772.
2009.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lupu M, Caruntu A, Caruntu C, Boda D,
Moraru L, Voiculescu V and Bastian A: Non-invasive imaging of
actinic cheilitis and squamous cell carcinoma of the lip. Mol Clin
Oncol. 8:640–646. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ianoși SL, Batani A, Ilie MA, Tampa M,
Georgescu SR, Zurac S, Boda D, Ianosi NG, Neagoe D, Calina D, et
al: Non-invasive imaging techniques for the in vivo
diagnosis of Bowen's disease: Three case reports. Oncol Lett.
17:4094–4101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Seidenari S, Arginelli F, Bassoli S,
Cautela J, French PM, Guanti M, Guardoli D, Konig K, Talbot C and
Dunsby C: Multiphoton laser microscopy and fluorescence lifetime
imaging for the evaluation of the skin. Dermatol Res Pract.
2012(810749)2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lin SJ, Jee SH, Kuo CJ, Wu RJ Jr, Lin WC,
Chen JS, Liao YH, Hsu CJ, Tsai TF, Chen YF, et al: Discrimination
of basal cell carcinoma from normal dermal stroma by quantitative
multiphoton imaging. Opt Lett. 31:2756–2758. 2006.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Braun RP, Mangana J, Goldinger S, French
L, Dummer R and Marghoob AA: Electrical impedance spectroscopy in
skin cancer diagnosis. Dermatol Clin. 35:489–493. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhao J, Zeng H, Kalia S and Lui H: Using
Raman spectroscopy to detect and diagnose skin cancer in vivo.
Dermatol Clin. 35:495–504. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang J, Fan Y, Song Y and Xu J: Accuracy
of Raman spectroscopy for differentiating skin cancer from normal
tissue. Medicine (Baltimore). 97(e12022)2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zhao J, Zeng H, Kalia S and Lui H:
Wavenumber selection based analysis in Raman spectroscopy improves
skin cancer diagnostic specificity. Analyst (Lond). 141:1034–1043.
2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Crisan D, Gheuca Solovastru L, Crisan M
and Badea R: Cutaneous histiocytoma - histological and imaging
correlations. A case report. Med Ultrason. 16:268–270.
2014.PubMed/NCBI
|