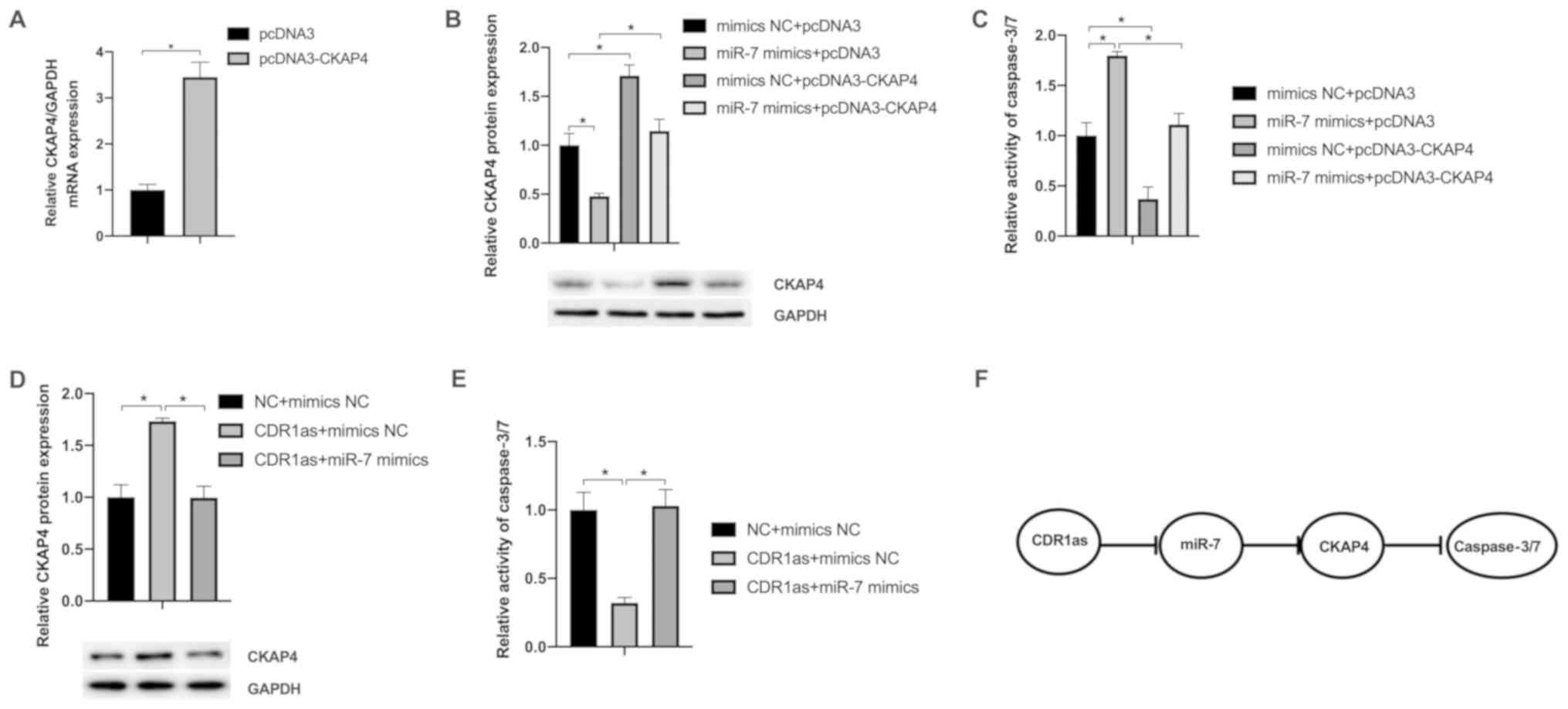
|
1
|
Scott RA, Bridgewater SG and Ashton HA:
Randomized clinical trial of screening for abdominal aortic
aneurysm in women. Br J Surg. 89:283–285. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reimerink JJ, van der Laan MJ, Koelemay
MJ, Balm R and Legemate DA: Systematic review and meta-analysis of
population-based mortality from ruptured abdominal aortic aneurysm.
Br J Surg. 100:1405–1413. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Cao X, Cai Z, Liu J, Zhao Y, Wang X, Li X
and Xia H: miRNA-504 inhibits p53-dependent vascular smooth muscle
cell apoptosis and may prevent aneurysm formation. Mol Med Rep.
16:2570–2578. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chaikof EL, Brewster DC, Dalman RL,
Makaroun MS, Illig KA, Sicard GA, Timaran CH, Upchurch GR Jr and
Veith FJ: Society for Vascular Surgery. The care of patients with
an abdominal aortic aneurysm: The society for vascular surgery
practice guidelines. J Vasc Surg. 50 (4 Suppl):S2–S49.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ding L, Ren J, Zhang D, Li Y, Huang X, Ji
J, Hu Q, Wang H, Ni Y and Hou Y: The TLR3 agonist inhibit drug
efflux and sequentially consolidates low-dose cisplatin-based
chemoimmunotherapy while reducing side effects. Mol Cancer Ther.
16:1068–1079. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang Z, Xie L, Han L, Qu X, Yang Y, Zhang
Y, He Z, Wang Y and Li J: Circular RNAs: Regulators of
cancer-related signaling pathways and potential diagnostic
biomarkers for human cancers. Theranostics. 7:3106–3117.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhao ZJ and Shen J: Circular RNA
participates in the carcinogenesis and the malignant behavior of
cancer. RNA Biol. 14:514–521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lasda E and Parker R: Circular RNAs:
Diversity of form and function. RNA. 20:1829–1842. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu B, Yang T, Wang Z, Zhang Y, Liu S and
Shen M: CircRNA CDR1as/miR-7 signals promote tumor growth of
osteosarcoma with a potential therapeutic and diagnostic value.
Cancer Manag Re. 10:4871–4880. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chan CYT, Cheuk BLY and Cheng SWK:
Abdominal aortic aneurysm-associated MicroRNA-516a-5p regulates
expressions of methylenetetrahydrofolate reductase, matrix
metalloproteinase-2, and tissue inhibitor of matrix
metalloproteinase-1 in human abdominal aortic vascular smooth
muscle cells. Ann Vasc Surg. 42:263–273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kimura H, Fumoto K, Shojima K, Nojima S,
Osugi Y, Tomihara H, Eguchi H, Shintani Y, Endo H, Inoue M, et al:
CKAP4 is a Dickkopf1 receptor and is involved in tumor progression.
J Clin Invest. 126:2689–2705. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Sun CM, Geng J, Yan Y, Yao X and Liu M:
Overexpression of CKAP4 is associated with poor prognosis in clear
cell renal cell carcinoma and functions via cyclin B signaling. J
Cancer. 8:4018–4026. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yajima N, Masuda M, Miyazaki M, Nakajima
N, Chien S and Shyy JY: Oxidative stress is involved in the
development of experimental abdominal aortic aneurysm: A study of
the transcription profile with complementary DNA microarray. J Vasc
Surg. 36:379–385. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liang B, Wang S, Wang Q, Zhang W, Viollet
B, Zhu Y and Zou MH: Aberrant endoplasmic reticulum stress in
vascular smooth muscle increases vascular contractility and blood
pressure in mice deficient of AMP-activated protein kinase-α2 in
vivo. Arterioscler Thromb Vasc Biol. 33:595–604. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu L, Gong X, Sun L, Zhou Q, Lu B and Zhu
L: The circular RNA Cdr1as act as an oncogene in hepatocellular
carcinoma through targeting miR-7 expression. PLoS One.
11(e0158347)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stather PW, Sylvius N, Sidloff DA, Dattani
N, Verissimo A, Wild JB, Butt HZ, Choke E, Sayers RD and Bown MJ:
Identification of microRNAs associated with abdominal aortic
aneurysms and peripheral arterial disease. Br J Surg. 102:755–766.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Geng HH, Li R, Su YM, Xiao J, Pan M, Cai
XX and Ji XP: The circular RNA Cdr1as promotes myocardial
infarction by mediating the regulation of miR-7a on its target
genes expression. PLoS One. 11(e0151753)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kumar L, Shamsuzzama Haque R, Baghel T and
Nazir A: Circular RNAs: The emerging class of non-coding RNAs and
their potential role in human neurodegenerative diseases. Mol
Neurobiol. 54:7224–7234. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang X, Yang D and Wei Y: Overexpressed
CDR1as functions as an oncogene to promote the tumor progression
via miR-7 in non-small-cell lung cancer. Onco Targets Ther.
11:3979–3987. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Emeto TI, Moxon JV, Au M and Golledge J:
Oxidative stress and abdominal aortic aneurysm: Potential treatment
targets. Clin Sci (Lond). 130:301–315. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xia J, Cao T, Ma C, Shi Y, Sun Y, Wang ZP
and Ma J: miR-7 suppresses tumor progression by directly targeting
MAP3K9 in pancreatic cancer. Mol Ther Nucleic Acids. 13:121–132.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Visani M, de Biase D, Marucci G, Cerasoli
S, Nigrisoli E, Bacchi Reggiani ML, Albani F, Baruzzi A and Pession
A: PERNO study group. Expression of 19 microRNAs in glioblastoma
and comparison with other brain neoplasia of grades I-III. Mol
Oncol. 8:417–430. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tuffy KM and Planey SL:
Cytoskeleton-associated protein 4: Functions beyond the endoplasmic
reticulum in physiology and disease. ISRN Cell Biol. 2012:1–11.
2012.
|
|
28
|
McCabe CD, Spyropoulos DD, Martin D and
Moreno CS: Genome-wide analysis of the homeobox C6 transcriptional
network in prostate cancer. Cancer Res. 69:1988–1996.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jang SW, Liu X, Fu H, Rees H, Yepes M,
Levey A and Ye K: Interaction of Akt-phosphorylated SRPK2 with
14-3-3 mediates cell cycle and cell death in neurons. J Biol Chem.
284:24512–24525. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang S, Wang Y, Jiang J, Wang R, Li L, Qiu
Z, Wu H and Zhu D: 15-HETE protects rat pulmonary arterial smooth
muscle cells from apoptosis via the PI3K/Akt pathway.
Prostaglandins Other Lipid Mediat. 91:51–60. 2010.PubMed/NCBI View Article : Google Scholar
|