Introduction
Sepsis is a systemic inflammatory response that is
caused by an overactive host response to infection (1). Although a series of advances have been
made in medical therapy for sepsis, the mortality rate remains high
(2) and is a challenge that has to
be regularly overcome in the clinic (3). Sepsis causes excessive tissue damage
and death in approximately 30-50% of patients (4), and sepsis/septic shock are often
accompanied by intestinal barrier dysfunction (5). Previous studies have revealed that
intestinal barrier dysfunction plays an important role in the
development of multiple organ dysfunction syndrome in sepsis
(6,7). Under normal physiological conditions,
the intestinal epithelial barrier can effectively prevent viral
microorganisms and endotoxins from transferring from the intestinal
lumen to the blood (8). When sepsis
occurs, an excessive inflammatory response contributes to
intestinal epithelial cell apoptosis (9). Tight junctions (TJs) between epithelial
cells are disrupted and paracellular gaps are widened (10), leading to impaired intestinal mucosal
integrity. Furthermore, damage to the intestinal mucosa exacerbates
bacterial translocation, leading to virulence factors that further
activate the host immune inflammatory defence mechanisms,
eventually resulting in multiple organ failure and life-threatening
clinical symptoms (11). Destruction
of the intestinal barrier integrity may be an important pathway
which leads to the multiple organ dysfunction caused by sepsis
(12). Protecting against intestinal
epithelial barrier damage and maintaining intestinal epithelial
barrier integrity reduces intestinal permeability and bacterial
translocation. This protection is essential to improving the
prognosis and survival of patients with sepsis (13).
Rhubarb is used as a traditional Chinese medicine to
treat intestinal diseases, and has been demonstrated to relieve
intestinal damage in septic rats (14). Currently, a number of active
ingredients have been extracted from rhubarb, including emodin,
aloe-emodin, chrysophanol and physcion (15). It is not clear which of these
components provides a protective effect on the intestinal mucosa in
sepsis. Among them, emodin is the main effective component of
rhubarb and is an anthraquinone derivative extracted from the
rhizome of rhubarb (16). Emodin has
numerous biological effects, such as antimicrobial,
anti-inflammatory, anti-viral, antibacterial, anti-tumour and
anti-fibrotic activities, as well as being able to affect diastolic
blood pressure (17). Ning et
al (18) showed that emodin can
alleviate intestinal mucosal damage in experimental severe acute
pancreatitis by inhibiting inflammatory cytokines, including
interleukin (IL)-1β and IL-18. Wang et al (11), found that rhubarb monomers could
promote the expression of the TJ proteins claudin-5, zonula
occludens (ZO)-1 and occludin and that this protected the
intestinal mucosal barrier of rats with sepsis induced by cecal
ligation and puncture (CLP). However, there is still no evidence
showing the mechanism of action by which emodin protects the
intestinal epithelial barrier integrity during sepsis. Since the
integrity of the intestinal epithelial barrier is predominantly
maintained by intestinal epithelial TJs, the mechanism by which the
intestinal epithelial barrier can be improved has been a focus of
research (19). Various TJ proteins
are expressed differently in intestinal tissue. The mRNA expression
levels of claudin-3 are higher in the duodenum than the distal
colon, while claudin-5 shows the reverse relationship (20,21). In
the present study, it was hypothesized that emodin provides a
protective function on intestinal epithelial TJ barrier integrity
in CLP-induced septic rats. A possible mechanism was related to the
expression of the TJ proteins claudin-3, ZO-1 and occludin.
Materials and methods
Ethics approval
The present study was approved by the Ethics
Committee of Putuo Hospital affiliated with Shanghai University of
Traditional Chinese Medicine. Specific pathogen-free male
Sprague-Dawley rats (n=60) weighing 280±20 g and aged 8-10 weeks
were obtained from the Shanghai Slack Laboratory Animal Co., Ltd.
All rats were acclimatized in housing conditions at 24±2˚C, 50%
humidity and 12 h light-dark cycles. Rats were fed with
conventional laboratory feed and water ad libitum. All
animal experiments were in accordance with the Experimental Animal
Centre Committee of Putuo Hospital (certification no. SYNK
2018-0032).
Animals and experimental design
Following the random number Table method, rats were
divided into the sham group, CLP group and CLP + emodin group, with
20 rats in each group. The CLP + emodin group was intragastrically
administered with 35 mg kg-1 emodin [high-performance
liquid chromatography (HPLC) ≥98%; Shanghai Gefan Biotechnology
Co., Ltd.] in a 0.5% sodium carboxy-methylcellulose suspension
(prepared to contain emodin at 5 g L-1) once daily at 09:00 a.m.,
for 5 days (22). The sham and CLP
groups were intragastrically administered an equal amount of 0.5%
sodium carboxy-methylcellulose solution. Following 2 h after the
last gavage, the sepsis model was induced in the CLP and CLP +
emodin groups. The sham group was treated as previously described
(23), briefly: The rats were
anaesthetized, and a 1 cm incision was made along the midline of
the abdomen. The caecum was removed, and the caecum was returned to
the abdominal cavity 2 min later. The CLP groups were treated as
previously described (24), briefly:
The rats were fasted for 12 h before surgery. The rats were then
fixed on a console and were anaesthetized by intraperitoneal
injection of 2.5% sodium pentobarbital (30 mg kg-1). An
incision of ~1 cm was cut along the midline of the abdomen, the
mesentery and caecum were separated, and the caecal root was
ligated using a 4/0 silk thread. A 1 cm syringe needle was used to
puncture the cecum at the ligation site, a small amount of faeces
was squeezed out, and the peritoneum and skin were stitched.
Finally, 10 rats from each group were sacrificed at 12 h and 24 h
after model establishment. Prior to sacrifice, all rats were
anesthetized using an intraperitoneal injection of 2.5% sodium
pentobarbital (50 mg kg-1). Rats were sacrificed by
cervical dislocation immediately following blood collection. The
blood was collected from the abdominal aorta, and the ileum tissues
were separated from the ileocecal area.
Haematoxylin and eosin (H&E)
staining for histopathology
The ileum tissues were fixed with 10% formalin at
room temperature for 7 days. The samples were dehydrated, embedded
in paraffin, sliced into 4-µm sections and stained with H&E.
Samples were stained with hematoxylin for 10 min and stained with
eosin for 2 min at room temperature. Subsequently, the sections
were examined under a light microscope at magnification, x400. The
images were observed by two pathologists who were blinded to the
experimental conditions of the present study. Intestinal mucosal
injuries were assessed using Chiu's score grading (25).
Transmission electron microscopy
A small segment of the ileum was spread on filter
paper to make a specimen of 1.5x1.5 cm and fixed with 2.5%
glutaraldehyde at 4˚C for 7 days. Then, in conjunction with the
Electron Microscopy Testing Centre of Shanghai University of
Traditional Chinese Medicine, the ileum specimens were adjusted to
~1 mm3, incubated with 1% osmium acid at room
temperature for 2 h, and finally dehydrated with ethanol and
acetone. The fixed tissue was embedded in epoxy resin and cut into
50-nm ultrathin sections. The sections were then stained with
uranyl acetate at room temperature for 2 h. The tissue sections
were observed under a transmission electron microscope and images
were captured at magnification, x8,200 to determine whether the
microvilli arrangement on the surface of intestinal mucosal
epithelial cells and the ultrastructures of organelles such as
epithelial space, TJs and mitochondria in epithelial cells were
normal.
Detection of diamine oxidase (DAO)
activity in intestinal tissue
Blood taken from the abdominal aorta of each rat was
centrifuged at 1,006.2 x g at 4˚C for 15 min. The supernatant was
collected and stored at -80˚C until examination. The activity of
DAO was determined using a DAO kit (Nanjing Jiancheng
Bioengineering Institute). The procedure followed the
manufacturer's protocol. Optical density was measured using a
microplate reader at 340 nm within 20 sec. A standard curve was
used to calculate the activity of DAO and to statistically analyse
the data.
Detection of the urine
lactulose/mannitol (L/M) ratio in intestinal tissue
The rats were given a mannitol (HPLC ≥98%; Shanghai
Gefan Biotechnology Co., Ltd.) and lactulose (HPLC ≥98%; Shanghai
Gefan Biotechnology Co., Ltd.) suspension [50 mg mannitol and 100
mg lactulose dissolved in 2 ml double distilled
(dd)H2O], administered intragastrically 2 ml/rat, 2 h
after establishing the sepsis model. Urine was collected within 6 h
and was centrifuged at 8,049.6 x g at 4˚C for 4 min. The
supernatant was stored at -80˚C and analyzed by mass spectrometry
in collaboration with the Shanghai University of Traditional
Chinese Medicine Science and Technology Innovation Centre.
Standards were prepared by diluting mannitol, lactulose and
deuterated mannitol with ddH2O to varying
concentrations. The samples were diluted 10 times with
ddH2O; then, 50 µl of sample was added to 450 µl of
acetonitrile (containing 1 µg ml-1 internal standard),
vortexed at room temperature for 1 min, and centrifuged at 14,310.4
x g at 4˚C for 5 min. A total of 10 µl of the supernatant was
sampled. The instrument parameters were set, including the mass
spectrometry conditions. Electrospray ion source nozzle position
was 3:7, atomizing gas flow rate was 10 l min-1, gas
curtain gas flow rate was 12 l min-1, collision gas flow
rate was 12 l min-1, ion source voltage was -4500 V, ion
source temperature was 300˚C, and ion selective channel m/z (mass
to charge ratio) were as follows: Lactulose, 341.1/160.7 (collision
energy, -13; declustering potential, -74); mannitol, 181.1/88.9
(collision energy, -21; declustering potential, -70); and
deuterated mannitol, 183.0/88.9 (collision energy, -21;
declustering potential, -67). Isocratic elution at a flow rate of
0.3 ml min-1 and a volume of 10 µl per sample were used.
The chromatogram was recorded and the sample content was calculated
from the standard curve. Finally, the urine L/M ratio and
statistics were calculated.
Immunohistochemistry
Ileum tissue was fixed in 10% formalin at room
temperature for 7 days. The tissue pieces were cut and embedded in
paraffin. Sections were cut to a thickness into 5-µm and placed on
slides. The samples were dehydrated by gradient alcohol. The
alcohol concentration was 100, 95, 90, 80, 70, 50, and 30%, and
dehydration was performed for ~35 min at room temperature. A 1%
sodium citrate antigen repair solution was used to soak the slides
in a 100˚C water bath for 30 min. When cooled to room temperature,
the samples were incubated with 3% hydrogen peroxide for 10 min and
rinsed with 1x PBS (3x5 min) before incubation with 5% BSA at 37˚C
for 10 min. Rabbit antibodies against ZO-1 (1:100; cat. no.
40-2200; Invitrogen; Thermo Fisher Scientific, Inc.), occludin
(1:80; cat. no. ab216327; Abcam) and claudin-3 (1:80; cat. no.
ab15102; Abcam) )were added and incubated at 4˚C overnight. The
slides were washed with 1x PBS (3x5 min), and horseradish
peroxidase conjugated anti-rabbit immunoglobulin G secondary
antibody (cat. no. SA1022; Boster Biological Technology) was added
and incubated at 37˚C for 30 min. The slides were washed with 1X
PBS (3x5 min), StreptAvidin-Biotin Complex was added and incubated
at 37˚C for 30 min. The slides were washed with 1X PBS (3x5 min),
and 3,3'-diaminobenzidine dye was added at room temperature for 1-3
sec under a fluorescent microscope. The slides were washed with 1X
PBS (3x5 min) and counterstained with haematoxylin at room
temperature for ~15 sec. Then, the slides were dehydrated with
gradient alcohol and the alcohol concentration was 30, 50, 70, 80,
90, 95 and 100%, and dehydration was performed for ~20 min at room
temperature. Xylene was subsequently added to the slides at room
temperature for ~10 min, glass coverslips were added and images
were captured with a fluorescent microscope (magnification,
x200).
Western blot analysis
Ileum tissues were removed from -80˚C storage and
cut into 50-mg pieces. A total of 1 ml protein lysis buffer (1:1)
composed of RIPA buffer (cat. no. P0013C; Beyotime Institute of
Biotechnology) and EDTA-free protease inhibitor cocktail (cat. no.
04693132001; Roche; Merck KGaA) was added to extract the proteins
using a tissue homogenizer. The supernatant was then collected by
centrifugation at 8,049.6 x g at 4˚C for 5 min. Total protein was
determined using a bicinchoninic acid protein quantitation kit
(cat. no. P0010S; Beyotime Institute of Biotechnology). Samples
were mixed with 1X SDS-PAGE protein loading buffer (cat. no. P0015;
Beyotime Institute of Biotechnology) and denatured by heating at
100˚C for 15 min. Next, the samples (60 µg protein) were separated
on SDS-PAGE with 10% gel (cat. no. P0012A; Beyotime Biotechnology)
until the bromophenol blue had migrated out of the end of the gel.
Proteins were then transferred on to a PVDF membrane (EMD
Millipore) and blocked in 5% milk at room temperature for 2 h. The
membranes were incubated overnight with rabbit antibodies against
GAPDH (0.925 mg/ml; 1:3,000 dilution; cat. no. ab181602; Abcam),
ZO-1 (0.25 mg/ml; 1:1,000 dilution; cat. no. 40-2200; Invitrogen;
Thermo Fisher Scientific, Inc.), occludin (0.604 mg/ml, 1:500
dilution; cat. no. ab216327; Abcam), and claudin-3 (500 µl, 1:1,000
dilution; cat. no. ab15102; Abcam). The membranes were washed with
1X TBS-Tween-20 (TBS-T; 0.1% Tween-20; 3x10 min) and placed in goat
anti-rabbit secondary antibody (1:2,000 dilution, cat. no.
111-005-003; Jackson ImmunoResearch Laboratories, Inc.) at room
temperature for 2 h. The membranes were washed with 1X TBST (3x10
min) and developed using the Clarity Western ECL substrate (cat.
no. 170-5060; Bio-Rad Laboratories, Inc.). Finally, the density of
the bands was quantified using ImageJ software (version 1.51;
National Institutes of Health). Data were normalized against a
housekeeping gene GAPDH and the expression levels of the other
groups were presented as the relative fold difference compared to
the sham group (26).
Reverse transcription-quantitative PCR
(RT-qPCR)
The ileum tissues were removed from -80˚C storage
and cut into 50-mg pieces. Total RNA was extracted with
TRIzol® reagent (cat. no. 9109; Takara Biotechnology
Co., Ltd.) following the manufacturer's instructions. The
concentration of the RNA samples was determined using
spectrophotometric optical density measurement at 260 nm. A total
of 1 μg of total RNA from each sample was reverse transcribed into
cDNA using a PrimeScript RT Reagent kit (cat. no. RR047A; Takara
Bio, Inc.). The following heat cycle was used for RT: 42˚C for 2
min, 37˚C for 15min at and 85˚C for 5 sec. PCR was performed using
a PCR machine (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Each reaction volume consisted of 20 µl and contained 5 µl of
sample cDNA, 10.4 µl TB Green Premix Ex Taq PCR master mix (cat.
no. RR420A; Takara Bio, Inc.), 0.5 µl each of forward and reserve
primers and 3.6 µl ddH2O. β-actin was used as a
reference gene. The primer sequences of the target genes are shown
in Table I.
 | Table IPrimers used in the present
study. |
Table I
Primers used in the present
study.
| | Primer
sequence |
|---|
| Gene [NCBI
reference] | Forward | Reverse |
|---|
| ZO-1
[NM_001106266] |
5'-CAGGCCATTACGAGCCTCTC-3' |
5'-AGGCTGTGGCTTGGTAGCTG-3' |
| occludin
[NM_031329] |
5'-CCAATGGCCTACTCCTCCAA-3' |
5'-CATCCACGGACAAGGTCAGA-3' |
| claudin-3
[NM_031700] |
5'-ATTCATCGGCAGCAGCATC-3' |
5'-CCAGCAGCGAGTCGTACATC-3' |
| β-actin
[NM_031144] |
5'-TGTCACCAACTGGGACGATA-3' |
5'-GGGGTGTTGAAGGTCTCAAA-3' |
The PCR conditions were 1 min at 95˚C, followed by
40 cycles of 5 sec at 95˚C and 30 sec at 60˚C. The last stage was
followed by an incubation of 15 sec at 95˚C, 1 min at 60˚C and 15
sec at 95˚C to establish a PCR melting curve. Relative
quantification of PCR products was normalized against the sham
group to calculate the relative expression in the CLP group and CLP
+ emodin group. Relative expression levels of ZO-1, occludin, and
claudin-3 mRNA levels were analyzed using the 2-ΔΔCq
method (27) with QuantStudio_Flex
Real-Time PCR System software (version 1.3; Thermo Fisher
Scientific, Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
software (version 24.0; IBM Corp.), and the measurement data are
expressed as the mean ± SD. All data were tested for homogeneity of
variances. One-way ANOVAs were used for comparisons between groups.
Fisher's least significant difference test was used for multiple
comparisons between groups. The pathological scores were compared
using Kruskal-Wallis test with post-hoc Nemenyi tests for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Emodin alleviates the pathological
changes of intestinal epithelial TJ barrier injury in septic rats
Emodin relieves the pathological damage to the intestinal
epithelial barrier in septic rats
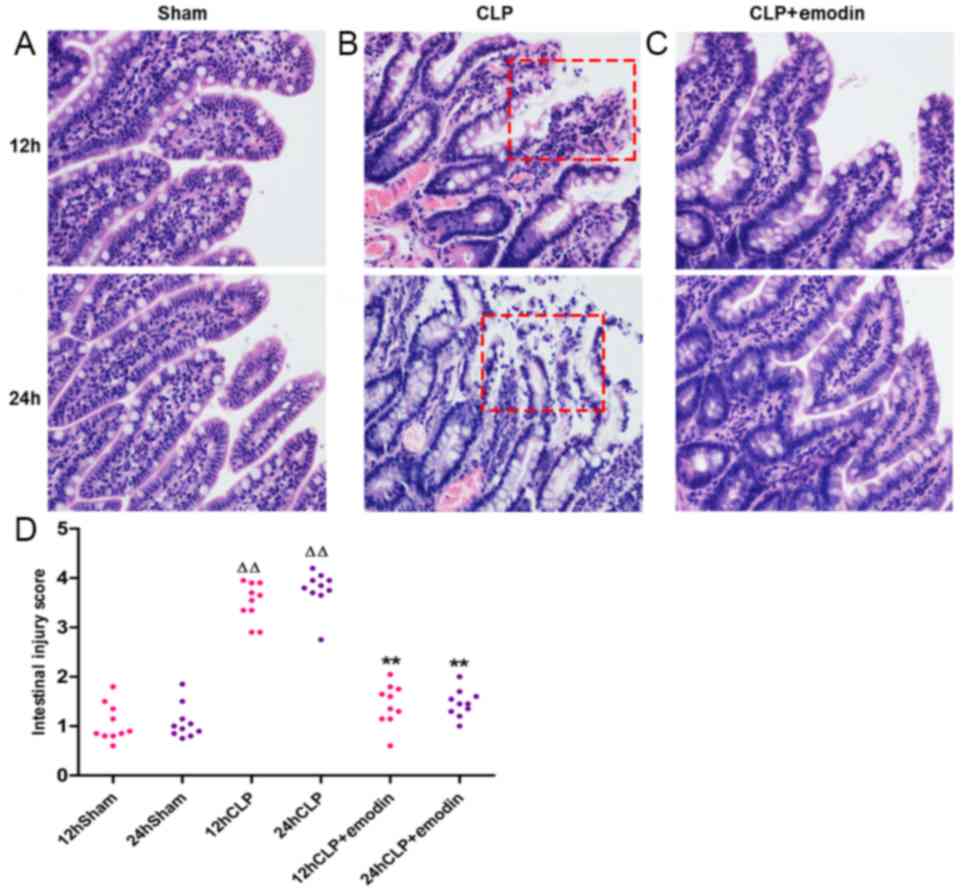
As observed by light microscopy, the sham group
(Fig. 1A) had intact intestinal
mucosa, and the intestinal villi were neatly arranged. After CLP,
the intestinal mucosa of the CLP group (Fig. 1B) presented different degrees of
congestion and oedema, inflammatory cell infiltration, irregular
arrangement of intestinal villi and an enlarged villus gap. The
intestinal mucosa apical epithelial space was further enlarged, the
top epithelium of the villus was detached, and the intestinal
lamina propria was disrupted. Compared with the damage in the CLP
group, the emodin pre-treatment group (Fig. 1C) showed reduced intestinal mucosal
damage. Using the Chiu scoring system to semi-quantitatively
analyse the intestinal mucosal morphology of each group, it was
found that the CLP group had a significantly higher score than that
of the sham group, and the damage at 24 h in the model group was
the highest (P<0.01; 12 and 24 h, respectively; Fig. 1D). After pre-treatment with emodin,
the CLP + emodin group had a significantly lower score compared
with the CLP model group (P<0.01; 12 and 24 h, respectively).
These data indicated that emodin may alleviate the pathological
damage to intestinal mucosa in rats with sepsis caused by CLP.
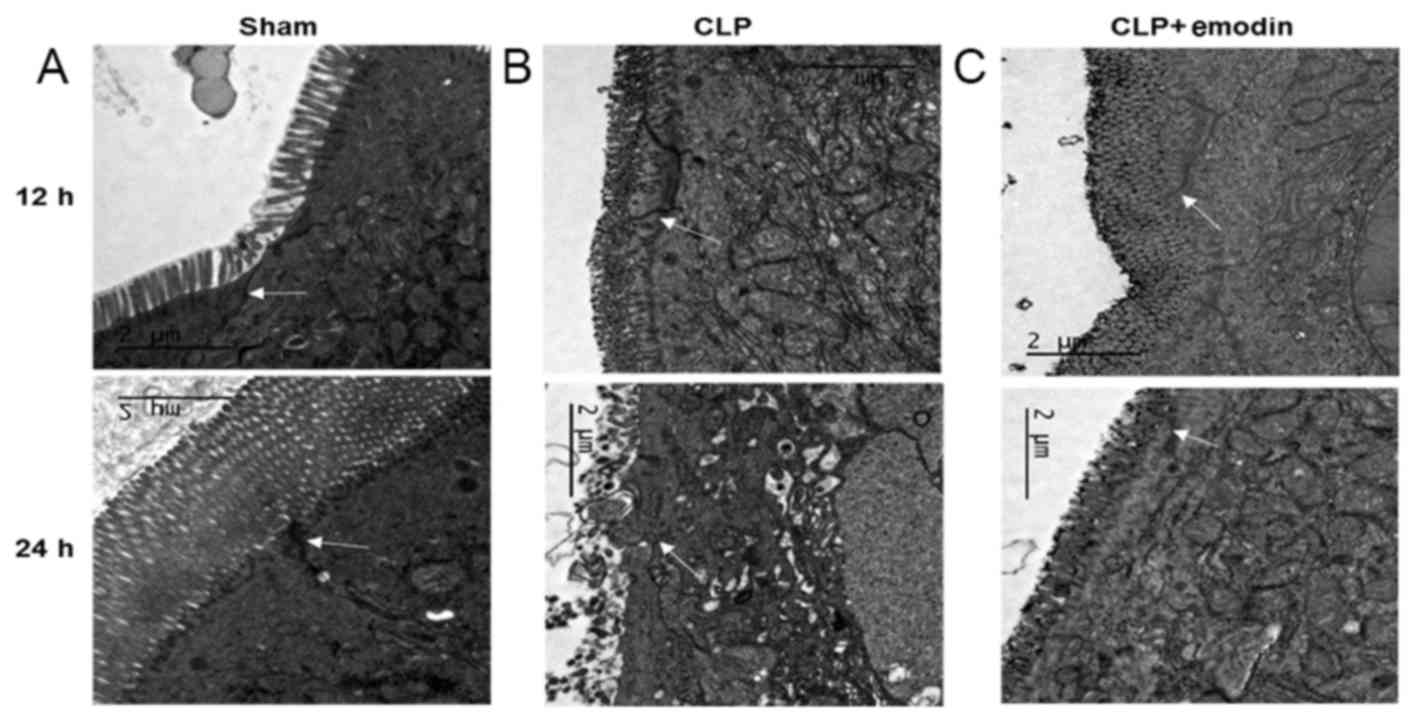
Emodin improves the pathological changes of TJ
barrier ultrastructures in septic rats. Electron microscopy showed
that in the sham operation group (Fig.
2A), the intestinal epithelial cells were closely arranged, the
microvilli on the surface of epithelial cells were neatly arranged;
the TJ stents and desmosomes were clear and intact, and the
paracellular spaces were narrow. However, in the CLP group
(Fig. 2B), the microvilli on the
surface of intestinal epithelial cells were unevenly arranged, the
lengths were irregular and there were different degrees of
shedding. In addition, the gaps between epithelial cells were
widened, and the boundaries of the TJ scaffolds and desmosomes
unclear or had disappeared. After pre-treatment with emodin
(Fig. 2C), the TJ gaps were
significantly reduced, the epithelial cells were tightly connected
and microvilli injury appeared to be alleviated. These results
indicated that emodin alleviated the sepsis-induced pathological
changes in the ultrastructures of the intestinal TJ barrier.
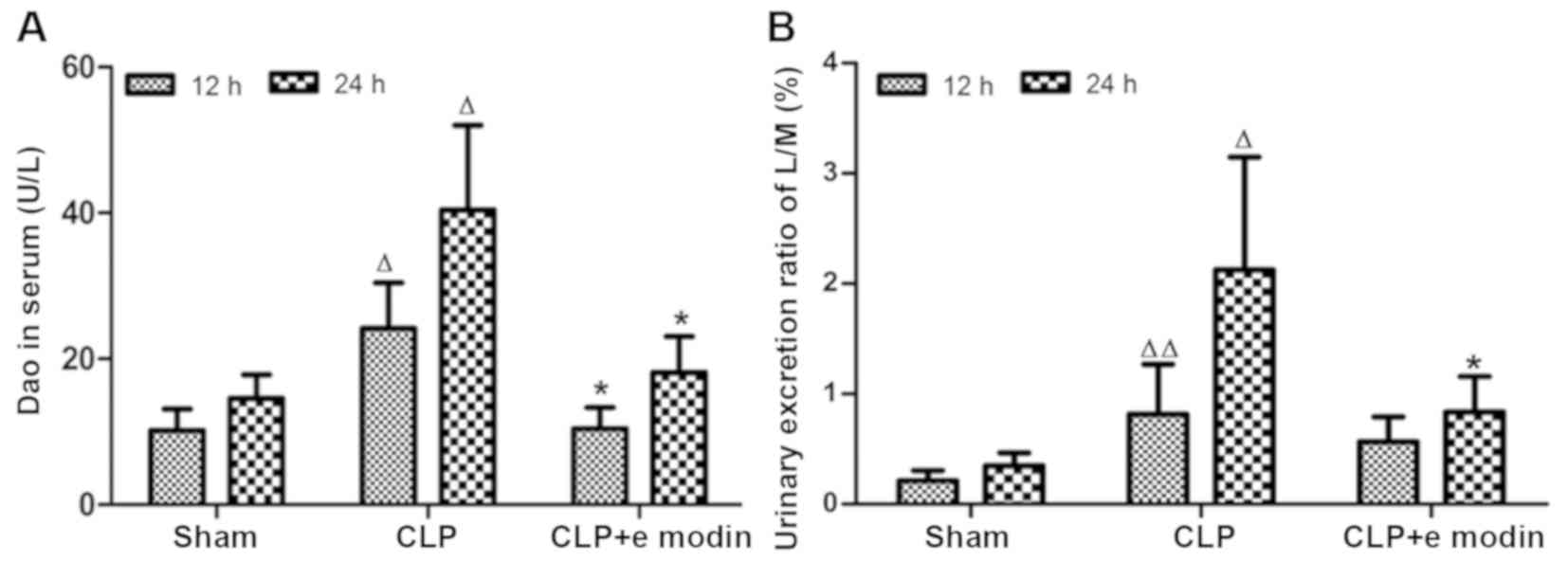
Emodin maintains the integrity of the
intestinal epithelial barrier in sepsis by reducing serum DAO
levels
The plasma DAO results (Fig. 3A) indicated that compared with the
corresponding sham groups, the DAO levels in the CLP groups
increased significantly (P<0.05; 12 and 24 h, respectively) and
peaked at 24 h. After emodin intervention, the activity of DAO was
significantly lower in the CLP + emodin treatment group when
compared with that of the corresponding CLP groups at 12 and 24 h
(P<0.05; 12 and 24 h, respectively). The above data indicated
that emodin could inhibit the increase in DAO activity in the serum
of rats with CLP-induced sepsis and had a protective effect in
intestinal mucosal injury.
Emodin maintains the integrity of the
intestinal epithelial barrier in sepsis by reducing the L/M ratio
in urine
The results of high-performance liquid
chromatography-based mass spectrometry for the determination of
mannitol and lactulose in urine (Fig.
3B) showed that the urine L/M ratio in the sham group did not
change significantly with time. In the CLP group, the L/M ratio was
significantly increased compared with that of the sham group
(P<0.01; 12 and 24 h, respectively). Compared with the L/M ratio
in the CLP group, the L/M ratio in the CLP + emodin group was
significantly reduced at 24 h (P<0.05; 24 h). These data
suggested that emodin could inhibit the increase in intestinal
epithelial barrier permeability in septic rats and help to maintain
the integrity of the epithelial barrier.
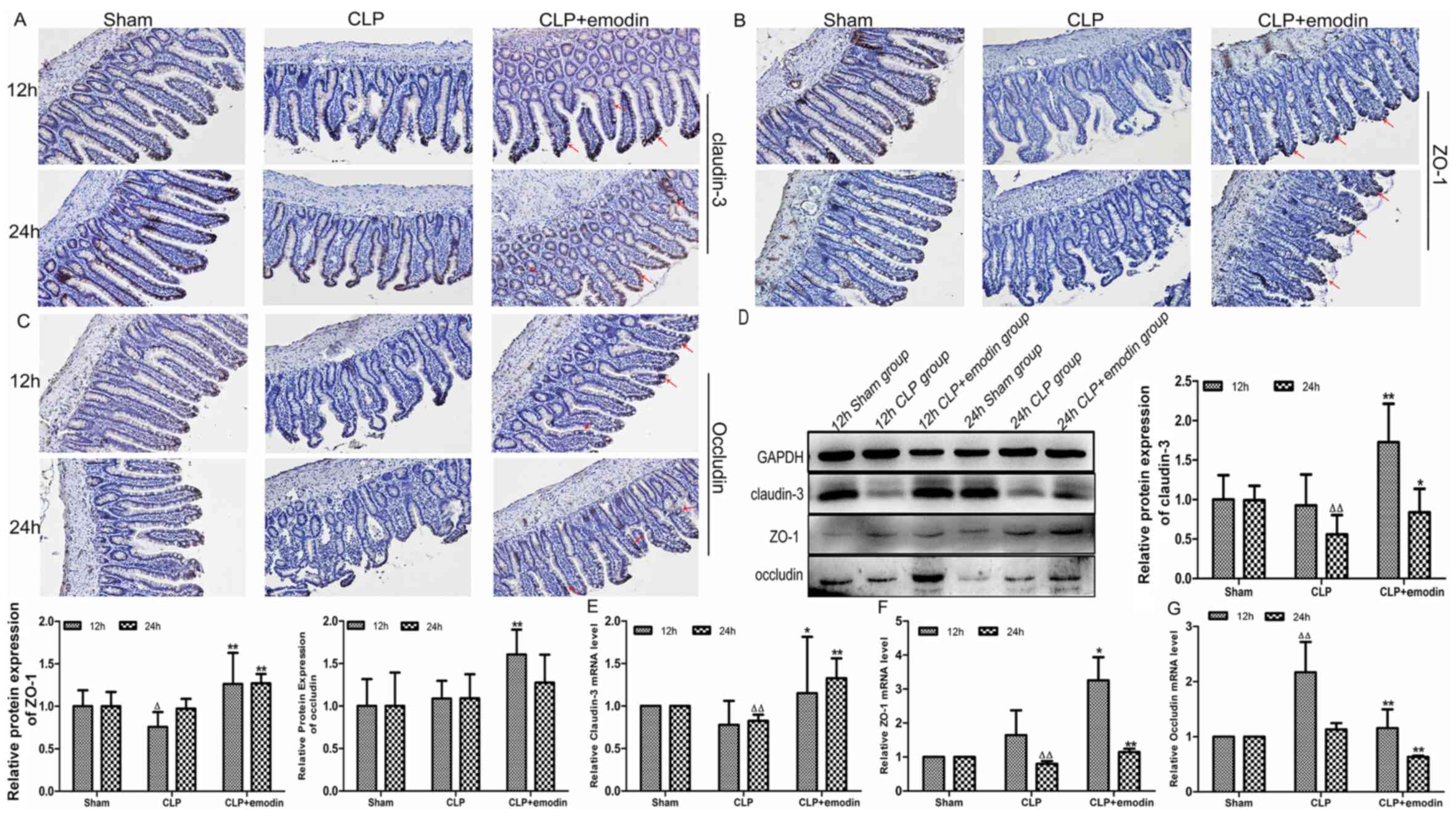
Emodin promotes the expression of the
TJ protein claudin-3 in the intestinal epithelium of CLP-induced
septic rats
As shown in Fig. 4,
immunohistochemical microscopy images showed the expression of
claudin-3 in the ileums of septic rats (Fig. 4A). In the sham group, claudin-3
expression (brown) was uniformly distributed in the intestinal
epithelial cells at the apex of each connection and it was tightly
packed with smooth edges. Claudin-3 was unevenly distributed, with
rough edges and faded staining in the CLP group. However, in the
CLP + emodin group, claudin-3 staining was darker and continuously
distributed. Western blotting (Fig.
4D) and RT-qPCR analysis (Fig.
4E) showed that compared with the expression levels in the sham
group, the expression of claudin-3 was decreased in the CLP group,
and there was a significant difference at 24 h (P<0.01in the
western blotting and RT-qPCR, respectively). With the emodin
intervention, claudin-3 protein levels were significantly increased
in the emodin pre-treatment groups at 12 and 24 h compared to the
CLP treatment alone (P<0.01 and P<0.05; 12 and 24 h,
respectively). The trend in claudin-3 mRNA levels was consistent
with that of the protein levels (P<0.05 and P<0.01; 12 and 24
h, respectively). These data indicated that emodin could inhibit
the increase in intestinal barrier permeability in sepsis by
promoting the expression of claudin-3 protein and mRNA.
Emodin promotes the expression of the
TJ protein ZO-1 in the intestinal epithelium of CLP-induced septic
rats
As depicted in Fig.
4, immunohistochemical microscopy images showed ZO-1 expression
in the ileums of septic rats (Fig.
4B). In the sham group, ZO-1 expression (brown) was
continuously and evenly distributed as well as being tightly
packed, with smooth edges and a strong positive expression. ZO-1
was unevenly distributed, with rough edges and faded staining in
the CLP group. However, in the CLP + emodin group, ZO-1 staining
was darker and presented a continuous distribution. Western
blotting (Fig. 4D) and RT-qPCR
analysis (Fig. 4F) showed that ZO-1
protein expression at 12 h (P<0.05) and mRNA levels at 24 h
(P<0.01) were significantly decreased in the CLP group compared
with the sham group. After emodin intervention, the protein
expression levels of ZO-1 were augmented in the emodin
pre-treatment groups at 12 and 24 h compared with the CLP treatment
alone group, and there was a statistically significant difference
(P<0.01; 12 and 24 h, respectively). The mRNA levels of ZO-1
were consistent with that of the protein expression (P<0.05 and
P<0.01; 12 and 24 h, respectively). These results indicated that
emodin may inhibit the increase in intestinal barrier permeability
in sepsis by promoting the expression of ZO-1.
Emodin partially promotes expression
of the intestinal epithelial TJ protein occludin in CLP-induced
septic rats
As depicted in Fig.
4, immunohistochemical microscopy images showed occludin
expression in the ileums of septic rats (Fig. 4C). In the sham group, occludin
expression (brown) was uniformly distributed in the intestinal
epithelial cells at the apex of each connection and tightly packed
within smooth edges. Occludin was unevenly distributed, with rough
edges and darker colours in the CLP group. However, in the CLP +
emodin group, occludin staining was darker and with a continuous
distribution. Western blot assays showed that compared with the
sham group, there was no obvious change in the expression levels in
the CLP group (Fig. 4D). RT-qPCR
analysis showed that compared with levels in the sham group, the
levels of occludin mRNA were significantly raised in the CLP group
at 12 h (P<0.01; Fig. 4G). After
emodin pre-treatment, the protein levels of occludin were increased
in the CLP + emodin group, and there was a significant difference
at 12 h (P<0.01). However, occludin mRNA was significantly
decreased at 12 h and 24 h (P<0.01). These data suggested that
emodin can inhibit the increase in intestinal barrier permeability
in sepsis by promoting the expression of occludin protein.
Discussion
The present study confirmed the protective effect of
emodin on CLP-induced intestinal epithelial barrier injury in
sepsis. It was found that emodin inhibited the increase in
intestinal barrier permeability induced by CLP. Emodin may also
maintain the integrity of the intestinal epithelial TJ barrier by
promoting the expression of TJ proteins, including claudin-3, ZO-1
and occludin. CLP-induced sepsis is the most commonly used animal
modelling method for sepsis (28).
This model accurately represents the progression and properties of
human sepsis. The model has a haemodynamic and metabolic phase
similar to abdominal infections, accompanied by the presence of
different stages of inflammation, as well as a prolonged and
reduced cytokine release (11).
In the present study, CLP was used to construct a
classical model of septic intestinal injury. The results showed
that the intestinal mucosa appeared to have different degrees of
pathological changes in the CLP group at 12 and 24 h following
treatment, including intestinal epithelial inflammatory cell
infiltration, intestinal villi that were not aligned and even the
occurrence of shedding, all of which were consistent with previous
reports (29,30). However, it was found that the
intestinal mucosal injury score of the 24 h CLP group was higher
than that of the 12 h CLP group, and the pathological changes in
the intestinal mucosa were more severe at 24 h. The reason for this
phenomenon may be related to the CLP modelling method. With the
progression of CLP, the bacteria and toxins in the intestinal
cavity invade distant organs and the circulatory system through the
portal system, the intestinal lymphatic pathway, and the direct
osmotic reabsorption pathway at 24 h (31,32). The
liver and lungs receive venous blood and lymph, respectively, from
the gastrointestinal tract. Inflammatory effectors are activated by
intestinal bacteria and toxins, releasing cytokines and
inflammatory mediators, which in turn further stimulates serious
systemic inflammation and aggravates intestinal mucosal damage
(14). DAO is an important indicator
for measuring intestinal mucosal damage, and a reduction in DAO
indicates that the integrity of the intestinal mucosa is destroyed
(33). Lactulose is a disaccharide
with a molecular weight of 342, and mannitol is a monosaccharide
with a molecular weight of 182. Both of these sugars are not easily
metabolised in the body. Generally, lactulose and mannitol are
excreted in the urine and can be accurately quantified. The urine
L/M ratio is a classic method for detecting intestinal barrier
function and intestinal barrier permeability (34). In the present study, DAO levels and
the L/M ratio were examined to evaluate the integrity of the
intestinal barrier in sepsis. The results reflected that DAO levels
and the L/M ratio were increased in the CLP group at 12 and 24 h.
The DAO levels in the 24 h CLP group were markedly higher than
those at 12 h. This trend was consistent with the extent of
intestinal mucosal damage. These results also indirectly confirmed
the hypothesis that intestinal mucosal damage is more severe at 24
h after CLP compared to at 12 h.
Emodin has been shown to reduce septic jejunal
damage by inhibiting apoptosis of intestinal epithelial cells
through anti-inflammatory effects (35). In the present study, light microscopy
and electron microscopy were used to observe the pathological
changes in the intestinal mucosal epithelium. The results showed
that emodin can significantly reduce the pathological damage to
intestinal mucosa and reduce the Chiu's ileal score. Under light
microscopy, the intestinal mucosal tissue showed oedema and was
infiltrated with inflammatory cells. The structure of intestinal
villi was also complete. Electron microscopy showed that the
microvilli on the surface of the intestinal epithelium were neatly
arranged, the TJ structures of the epithelial cells were intact,
the gaps were narrowed and the junctions were tight. In addition,
Chiu's intestinal injury score was also significantly reduced, all
of which suggested that emodin can attenuate CLP-induced intestinal
epithelial barrier damage in sepsis. The DAO activity and urine L/M
ratio are important indicators for the clinical detection of
increased permeability of the intestinal wall (36). After emodin pre-treatment, DAO
activity in the blood and the L/M ratio in the urine showed a
downward trend compared with the CLP treatment group alone, and
this trend was more obvious at 24 h. This suggested that emodin may
inhibit the increase in intestinal permeability in septic rats and
maintain the integrity of the intestinal epithelial TJ barrier.
The intact intestinal epithelial TJ barrier plays a
key role in sepsis (37). The
barrier can prevent intestinal pathogens from entering the
intestinal tract through the paracellular pathway. The integrity of
the intestinal epithelial TJ barrier is maintained by an
intercellular junction complex formed by TJs, adhesion junctions,
desmosomes and gap junctions (38).
These ligation complexes form a semi-permeable diffusion barrier
between individual cells. They can prevent the abnormal passage of
antigens, solutes and water. TJs provide the backbone of the
structural integrity of the barrier and also provide a physical
basis for the epithelial barrier to ions and solutes (39). TJs are located at the apical ends of
the lateral membrane of epithelial cells (40) and have been shown to contain at least
40 proteins (9), such as claudins,
ZO-1 and occludin (41). There are
two main ways to regulate TJ transport, the pore pathway and the
leak pathway (42). The first
pathway is characterized by a small pore, which is thought to be
created by claudins. The pore pathway is closely related to claudin
protein activity (43) and allows
the passage of small solutes and the transport of specific ions.
Different claudin proteins have different functions. In particular,
claudin-3, a barrier-forming protein, has no charge selectivity and
is ubiquitously expressed in the jejunum, ileum and colon of rats
(44). Previous studies suggested
that the permeability of the pore pathway can directly regulate
myosin light chain kinase (MLCK) by tumour necrosis factor (TNF)-α
(45). When sepsis occurs, TNF-α
regulates the upregulation of MLCK promoter activity through the
NF-κB signalling pathway, thereby inducing increased expression of
MLCK (46). Increased expression of
MLCK can further promote increases in the permeability of the pore
pathway, which affects the expression of claudin proteins (42). Garcia-Hernandez et al
(47), found that claudin-3 was
expressed at lower levels in inflammatory bowel disease. Haines
et al (19), found that the
protein expression levels of claudin-3 were positively correlated
with intestinal epithelial tightness. In the present study,
immunohistochemistry, western blotting and RT-qPCR were used to
analyse the expression profile of claudin-3 in intestinal tissues.
The results showed that the grading grey scales of claudin-3
protein and the mRNA levels of claudin-3 were lower in the CLP
group compared with those of the corresponding sham group, with a
significant difference at 24 h. The low expression of claudin-3 in
the CLP model group is consistent with that of existing research
(48). These results indicated that
CLP may reduce intestinal barrier integrity by reducing the
expression of the barrier-forming protein claudin-3.
The leak pathway controls the paracellular transport
of large uncharged solutes (proteins and bacterial LPS but not
bacterial cells). Under normal physiological conditions, LPS in the
intestinal lumen has no effect on intestinal wall integrity. When
sepsis occurs, LPS and inflammatory cytokines (such as TNF-α) in
the intestinal lumen can enter the body through the leak pathway
(47). ZO-1 and occludin are related
to the leak pathway and are both involved in the maintenance and
regulation of the leakage channel flux barrier. ZO-1 is critical
for the assembly of TJs and epithelial barrier functions. Claudins
and occludin proteins are linked to cytoskeletal actomyosin fibres
by members of the ZO-1 protein family (49). In addition to providing a bridge
between occludin and claudins and the cytoskeleton, ZO-1 may also
affect the expression levels of transmembrane proteins at the
transcriptional and post-translational levels (50). Occludin, a TJ protein, is mainly
located in the TJs of cells after phosphorylation. When occludin is
abnormally distributed and expressed in the structure of the small
intestine, there is an increase in the intercellular space and an
increase in permeability between endothelial cells (31). Zhang et al (51), found that the expression levels of
the intestinal epithelial TJ proteins, ZO-1 and occludin, were both
decreased in an LPS-induced sepsis model. In the present study,
immunohistochemical staining, western blotting and RT-qPCR were
used to detect the expression profile of ZO-1 and occludin in the
intestinal epithelial barrier in sepsis. The grey scales of ZO-1
protein showed a downward trend in the CLP group, and there was a
significant difference at 12 h. The mRNA levels of ZO-1
significantly decreased in the CLP group at 24 h, but were
increased at 12 h. This phenomenon also occurs in different tissues
of other animal models (52). The
decrease in ZO-1 protein at 12 h could not be explained by a
reduction in ZO-1 transcription, which may be a secondary factor to
some combination of posttranslational regulation or degradation
(52). However, occludin protein
expression did not change during sepsis, but mRNA levels showed an
upward trend. It is possible that occludin protein is modulated via
post-transcriptional or post-translational regulations (53).
Decreased expression levels of TJ proteins are
strongly associated with impaired intestinal barrier function
(54). Thus, the protective effect
of emodin against CLP-induced intestinal barrier injury can be
partially explained by the increased expression of TJ proteins. In
recent years, numerous scientific reports described that emodin has
an anti-inflammatory effect and can reduce the expression of the
inflammatory factor TNF-α (55-57).
Compared with expression in the corresponding CLP group, the
protein expression levels of claudin-3 in the CLP + emodin group
showed a continuous and uniform distribution, with a smoother edge
and an enhanced positive signal. In addition, the protein and mRNA
expression levels of claudin-3 were significantly increased in the
CLP + emodin group compared with the CLP alone treatment group. It
is possible that emodin can reduce TNF-α levels in the sepsis model
(58) and further inhibit MLCK
activation. Further studies are required to clarify the detailed
mechanisms of action. These data indicated that emodin may inhibit
the increase in intestinal barrier permeability by promoting the
transcription and translation of the TJ protein claudin-3.
The present study also found that in the emodin
pre-treatment group, expression of ZO-1 and occludin proteins
showed a continuous and uniform distribution, with a smoother edge
and an enhanced positive signal, when compared with that of the
corresponding CLP group. Moreover, protein and mRNA expression
levels of ZO-1 were significantly increased in the CLP + emodin
group. However, while the expression of occludin protein was
increased, the mRNA was not. Although studies have found that
occludin overexpression is protective in Madin-Darby canine kidney
cells in vitro (59), in
vivo studies indicate that occludin gene defects do not affect
the integrity of intestinal epithelial TJs (60). Therefore, further investigation is
needed to determine whether occludin plays a role in intestinal
epithelial paracellular permeability. Together, these findings
suggested that emodin may inhibit the increase in intestinal
barrier permeability by promoting the transcription and translation
of ZO-1 and occludin proteins, at least to a certain degree.
In conclusion, emodin may alleviate intestinal
barrier damage caused by CLP, inhibit the increase in intestinal
barrier permeability and maintain the integrity of the intestinal
epithelial barrier. Intestinal epithelial TJ proteins are key
molecules for the determination of intestinal epithelial barrier
permeability. Based on results from previous studies and the
present study, it is speculated that the amelioration of intestinal
epithelial barrier damage by emodin can be attributed, at least in
part, to the promotion of claudin-3, ZO-1 and occludin expression.
However, the specific mechanism of action requires further
exploration. In the present study, other TJ proteins were not
tested, and the effect of emodin on other TJ proteins is not fully
understood. Despite these limitations, the protective effect of
emodin on CLP-induced intestinal epithelial TJ barrier injury was
investigated and an understanding of the protective effect of
emodin on regulating intestinal barrier dysfunction in sepsis was
provided.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
National Natural Science Foundation of China (grant no.
81573901).
Availability of data and material
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, RG and YS designed experiments and analyzed and
interpreted data. YL, RG, MZ, PC and JL performed experiments and
collected data. YS and RG reviewed articles and contributed
reagents and materials. YL wrote the manuscript. All authors read
and approved the final manuscript.
Ethics and approval
This study was approved by the Ethics Committee of
Putuo Hospital affiliated with Shanghai University of Traditional
Chinese Medicine. All animal experiments were in accordance with
the Experimental Animal Centre Committee of Putuo Hospital
(certification no. SYNK 2018-0032).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Condotta SA, Cabrera-Perez J, Badovinac VP
and Griffith TS: T-cell-mediated immunity and the role of TRAIL in
sepsis-induced immunosuppression. Crit Rev Immunol. 33:23–40.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cao Y, Chen Q, Wang Z, Yu T, Wu J, Jiang
X, Jin X and Lu W: PLK1 protects against sepsis-induced intestinal
barrier dysfunction. Sci Rep. 8(1055)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hua S, Liu X, Lv S and Wang Z: Protective
effects of cucurbitacin B on acute lung injury induced by sepsis in
rats. Med Sci Monit. 23:1355–1362. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu W, Wang XH, Yang XJ, Zhang XY and Qi
WJ: Intestinal barrier dysfunction and its related factors in
patients with sepsis. Zhonghua Yi Xue Za Zhi. 96:3568–3572.
2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
6
|
Yoseph BP, Klingensmith NJ, Liang Z, Breed
ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML and
Coopersmith CM: Mechanisms of intestinal barrier dysfunction in
sepsis. Shock. 46:52–59. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu J, Liu Z, Zhan W, Jiang R, Yang C, Zhan
H and Xiong Y: Recombinant TsP53 modulates intestinal epithelial
barrier integrity via upregulation of ZO-1 in LPS-induced septic
mice. Mol Med Rep. 17:1212–1218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lu WH, Jin XJ, Jiang XG, Wang Z, Wu JY and
Shen GG: Resuscitation with hydroxyethyl starch 130/0.4 attenuates
intestinal injury in a rabbit model of sepsis. Indian J Pharmacol.
47:49–54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li Q, Zhang Q, Wang C, Liu X, Li N and Li
J: Disruption of tight junctions during polymicrobial sepsis in
vivo. J Pathol. 218:210–221. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gu L, Li N, Gong J, Li Q, Zhu W and Li J:
Berberine ameliorates intestinal epithelial tight-junction damage
and down-regulates myosin light chain kinase pathways in a mouse
model of endotoxinemia. J Infect Dis. 203:1602–1612.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang L, Cui YL, Zhang Z, Lin ZF and Chen
DC: Rhubarb monomers protect intestinal mucosal barrier in sepsis
via junction proteins. Chin Med J (Engl). 130:1218–1225.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li MX, Liu JF, Lu JD, Zhu Y, Kuang DW,
Xiang JB, Sun P, Wang W, Xue J, Gu Y, et al: Plasmadiafiltration
ameliorating gut mucosal barrier dysfunction and improving survival
in porcine sepsis models. Intensive Care Med Exp.
4(31)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen G, Huang B, Fu S, Li B, Ran X, He D,
Jiang L, Li Y, Liu B, Xie L, et al: G protein-coupled receptor 109A
and host microbiota, odulate intestinal epithelial integrity during
sepsis. Front Immunol. 9(2079)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen DC and Wang L: Mechanisms of
therapeutic effects of rhubarb on gut origin sepsis. Chin J
Traumatol. 12:365–369. 2009.PubMed/NCBI
|
|
15
|
Li P, Lu Q, Jiang W, Pei X, Sun Y, Hao H
and Hao K: Pharmacokinetics and pharmacodynamics of rhubarb
anthraquinones extract in normal and disease rats. Biomed
Pharmacother. 91:425–435. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang G, Sun B, Zhu H, Gao Y, Li X, Xue D
and Jiang H: Protective effects of emodin combined with danshensu
on experimental severe acute pancreatitis. Inflamm Res. 59:479–488.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen G, Zhang J, Zhang H, Xiao Y, Kao X,
Liu Y and Liu Z: Anti-inflammatory effect of emodin on
lipopolysaccharide-induced keratitis in Wistar rats. Int J Clin Exp
Med. 8:12382–12389. 2015.PubMed/NCBI
|
|
18
|
Ning JW, Zhang Y, Yu MS, Gu ML, Xu J,
Usman A and Ji F: Emodin alleviates intestinal mucosal injury in
rats with severe acute pancreatitis via the caspase-1 inhibition.
Hepatobiliary Pancreat Dis Int, 2017. 16(4): p. 431-436.
|
|
19
|
Haines RJ, Beard RS Jr, Chen L, Eitnier RA
and Wu MH: Interleukin-1beta mediates beta-catenin-driven
downregulation of claudin-3 and barrier dysfunction in Caco2 cells.
Dig Dis Sci. 61:2252–2261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Holmes JL, Van Itallie CM, Rasmussen JE
and Anderson JM: Claudin profiling in the mouse during postnatal
intestinal development and along the gastrointestinal tract reveals
complex expression patterns. Gene Expr Patterns. 6:581–588.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rahner C, Mitic LL and Anderson JM:
Heterogeneity in expression and subcellular localization of
claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut.
Gastroenterology. 120:411–422. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun Y, Sun L, Liu S, Song J, Cheng J and
Liu J: Effect of emodin on Aquaporin 5 expression in rats with
sepsis-induced acute lung injury. J Tradit Chin Med. 35:679–684.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pereira RS, Bertoncheli CM, Adefegha SA,
Castilhos LG, Silveira KL, Rezer JFP, Doleski PH, Abdalla FH,
Santos KF, Leal CAM, et al: Sepsis induced by cecal ligation and
perforation (CLP) alters nucleotidase activities in platelets of
rats. Microb Pathog. 111:345–351. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi X, Zhang Y, Wang H and Zeng S: Effect
of triggering receptor expressed on myeloid cells 1 (TREM-1)
blockade in rats with cecal ligation and puncture (CLP)-induced
sepsis. Med Sci Monit. 23:5049–5055. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu J, Lyu B, Gan T, Wang L and Zhu M:
Electroacupuncture improves acute bowel injury recovery in rat
models. Exp Ther Med. 14:4655–4662. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo S, Nighot M, Al-Sadi R, Alhmoud T,
Nighot P and Ma TY: Lipopolysaccharide regulation of intestinal
tight junction permeability is mediated by TLR4 signal transduction
pathway activation of FAK and MyD88. J Immunol. 195:4999–5010.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang B, Liu C, Yang N and Wang X: A
comparative study of changes of autophagy in rat models of CLP
versus LPS induced sepsis. Exp Ther Med. 14:2194–2200.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gao M, Jiang Y, Xiao X, Peng Y, Xiao X and
Yang M: Protective effect of pioglitazone on sepsis-induced
intestinal injury in a rodent model. J Surg Res. 195:550–558.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou H, Liang H, Li ZF, Xiang H, Liu W and
Li JG: Vagus nerve stimulation attenuates intestinal epithelial
tight junctions disruption in endotoxemic mice through α7 nicotinic
acetylcholine receptors. Shock. 40:144–151. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang J, Zhang S, Wu J, Zhang J, Dong J,
Guo P, Tang S, Zhang W and Wu F: Imipenem and normal saline with
cyclophosphamide have positive effects on the intestinal barrier in
rats with sepsis. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 162:90–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fang H, Gong C, Fu J, Liu X, Bi H, Cheng
Y, Liu Y, Tang Y and Wang D: Evaluation of 2 rat models for sepsis
developed by improved cecal ligation/puncture or feces
intraperitoneal-injection. Med Sci Monit.
26(e919054)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen J, Huang C, Wang J, Zhou H, Lu Y, Lou
L, Zheng J, Tian L, Wang X, Cao Z, et al: Dysbiosis of intestinal
microbiota and decrease in paneth cell antimicrobial peptide level
during acute necrotizing pancreatitis in rats. PLoS One.
12(e0176583)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Warners MJ, Vlieg-Boerstra BJ, Verheij J,
van Hamersveld PHP, van Rhijn BD, Van Ampting MTJ, Harthoorn LF, de
Jonge WJ, Smout AJPM and Bredenoord AJ: Esophageal and small
intestinal mucosal integrity in eosinophilic esophagitis and
response to an elemental diet. Am J Gastroenterol. 112:1061–1071.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen YK, Xu YK, Zhang H, Yin JT, Fan X,
Liu DD, Fu HY and Wan B: Emodin alleviates jejunum injury in rats
with sepsis by inhibiting inflammation response. Biomed
Pharmacother. 84:1001–1007. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu GF, Guo M, Tian ZQ, Wu GZ, Zou XP and
Zhang WJ: Increased of serum high-mobility group box chromosomal
protein 1 correlated with intestinal mucosal barrier injury in
patients with severe acute pancreatitis. World J Emerg Surg.
9(61)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cuellar P, Hernández-Nava E, García-Rivera
G, Chávez-Munguía B, Schnoor M, Betanzos A and Orozco E: Entamoeba
histolytica EhCP112 dislocates and degrades claudin-1 and claudin-2
at tight junctions of the intestinal epithelium. Front Cell Infect
Microbiol. 7(372)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen J, Zhang R, Wang J, Yu P, Liu Q, Zeng
D, Song H and Kuang Z: Protective effects of baicalin on
LPS-induced injury in intestinal epithelial cells and intercellular
tight junctions. Can J Physiol Pharmacol. 93:233–237.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Colgan SP, Curtis VF, Lanis JM and Glover
LE: Metabolic regulation of intestinal epithelial barrier during
inflammation. Tissue Barriers. 3(e970936)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xing T, Camacho Salazar R and Chen YH:
Animal models for studying epithelial barriers in neonatal
necrotizing enterocolitis, inflammatory bowel disease and
colorectal cancer. Tissue Barriers. 5(e1356901)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Salvo Romero E, Alonso Cotoner C, Pardo
Camacho C, Casado Bedmar M and Vicario M: The intestinal barrier
function and its involvement in digestive disease. Rev Esp Enferm
Dig. 107:686–696. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Turner JR: Intestinal mucosal barrier
function in health and disease. Nat Rev Immunol. 9:799–809.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shen L, Weber CR, Raleigh DR, Yu D and
Turner JR: Tight junction pore and leak pathways: A dynamic duo.
Annu Rev Physiol. 73:283–309. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Barmeyer C, Fromm M and Schulzke JD:
Active and passive involvement of claudins in the pathophysiology
of intestinal inflammatory diseases. Pflugers Arch. 469:15–26.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dokladny K, Zuhl MN and Moseley PL:
Intestinal epithelial barrier function and tight junction proteins
with heat and exercise. J Appl Physiol (1985). 120(6):692–701.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang Y and Li J: Carbachol ameliorates
lipopolysaccharide-induced intestinal epithelial tight junction
damage by down-regulating NF-κβ and myosin light-chain kinase
pathways. Biochem Biophys Res Commun. 428:321–326. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Garcia-Hernandez V, Quiros M and Nusrat A:
Intestinal epithelial claudins: Expression and regulation in
homeostasis and inflammation. Ann N Y Acad Sci. 1397:66–79.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xing X, Jiang R, Wang L, Lei S, Zhi Y, Wu
Y, Zhu M, Huang L, Xia G and Chen Z: Shenfu injection alleviates
intestine epithelial damage in septic rats. Am J Emerg Med.
33:1665–1670. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sonika U, Goswami P, Thakur B, Yadav R,
Das P, Ahuja V and Saraya A: Mechanism of increased intestinal
permeability in acute pancreatitis: Alteration in tight junction
proteins. J Clin Gastroenterol. 51:461–466. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li W, Sun K, Ji Y, Wu Z, Wang W, Dai Z and
Wu G: Glycine regulates expression and distribution of claudin-7
and ZO-3 proteins in intestinal porcine epithelial cells. J Nutr.
146:964–969. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang S, Zheng S, Wang X, Shi Q, Wang X,
Yuan S, Wang G and Ji Z: Carbon monoxide-releasing molecule-2
reduces intestinal epithelial tight-junction damage and mortality
in septic rats. PLoS One. 10(e0145988)2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Eadon MT, Hack BK, Xu C, Ko B, Toback FG
and Cunningham PN: Endotoxemia alters tight junction gene and
protein expression in the kidney. Am J Physiol Renal Physiol.
303:F821–F830. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hwang I, An BS, Yang H, Kang HS, Jung EM
and Jeung EB: Tissue-specific expression of occludin, zona
occludens-1, and junction adhesion molecule A in the duodenum,
ileum, colon, kidney, liver, lung, brain, and skeletal muscle of
C57BL mice. J Physiol Pharmacol. 64:11–18. 2013.PubMed/NCBI
|
|
54
|
Yan H and Ajuwon KM: Butyrate modifies
intestinal barrier function in IPEC-J2 cells through a selective
upregulation of tight junction proteins and activation of the Akt
signaling pathway. PLoS One. 12(e0179586)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen D, Liu J, Lu L, Huang Y, Wang Y, Wang
M, Liu Y, Xie D, Chen J, Diao J, et al: Emodin attenuates
TNF-α-induced apoptosis and autophagy in mouse C2C12 myoblasts
though the phosphorylation of Akt. Int Immunopharmacol. 34:107–113.
2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Xia S, Ni Y, Zhou Q, Liu H, Xiang H, Sui H
and Shang D: Emodin attenuates severe acute pancreatitis via
antioxidant and anti-inflammatory activity. Inflammation.
42:2129–2138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Dong Y, Zhang L, Jiang Y, Dai J, Tang L
and Liu G: Emodin reactivated autophagy and alleviated inflammatory
lung injury in mice with lethal endotoxemia. Exp Anim. 68:559–568.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xia XM, Li BK, Xing SM and Ruan HL: Emodin
promoted pancreatic claudin-5 and occludin expression in
experimental acute pancreatitis rats. World J Gastroenterol.
18:2132–2139. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Cummins PM: Occludin: One protein, many
forms. Mol Cell Biol. 32:242–250. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Van Itallie CM and Anderson JM: Claudin
interactions in and out of the tight junction. Tissue Barriers.
1(e25247)2013.PubMed/NCBI View Article : Google Scholar
|