Introduction
As the world's ageing population increases,
ST-segment elevation myocardial infarction (STEMI) incidence is
expected to increase to be the most common and fatal cardiac
emergency (1). Acute myocardial
infarction (AMI) is often caused by the interruption of coronary
artery blood flow and myocardial ischemic necrosis resulting from
decreased stability of coronary atherosclerotic plaque, ulcer,
rupture and other thrombosis (2).
Therefore, early, rapid and complete opening of the infarcted
artery is key to improving the prognosis of patients with STEMI
(2,3). Percutaneous coronary intervention (PCI)
is a non-surgical method used in extensive myocardial reperfusion
therapy (4). Early risk
stratification and identification of high-risk patients with STEMI
are of great significance in prognosis, and also in guiding
diagnosis and treatment decisions (5). Currently, the Global Registry of Acute
Coronary Events (GRACE) score is widely used as an acute risk
stratification tool in the evaluation of prognosis in patients with
acute coronary syndrome (ACS) (6).
GRACE score parameters include age, systolic blood pressure, pulse,
serum creatinine, Killip classification at admission, cardiac
arrest at admission, markers of myocardial necrosis and changes in
ST-segment (7). These eight
independent risk factors of prognosis are not only a predictive
value for risk stratification and nosocomial adverse outcomes in
patients with ACS, but also has a significant predictive power in
both short- and long-term major adverse cardiovascular events
(MACE), including all-cause mortality (8). Jakimov et al (9) showed that the GRACE score at admission
is an independent predictor of MACE over a 30-day follow-up period.
Xiang et al (10) showed that
the GRACE score at admission was an independent predictor of
long-term MACE in patients with AMI. The GRACE score is a
comprehensive assessment system guiding clinical diagnosis,
treatment and prognosis evaluation. However, there are some
limitations, including a lack of biomarkers that reflect thrombosis
and inflammation (11,12).
Previous findings have shown that thrombosis and
inflammation play central roles in the occurrence, progression,
rupture and thrombosis of atherosclerotic plaques (13). Platelets form an important link
between inflammatory reactions and thrombosis (14). At admission, large platelets actively
participate in metabolism and enzyme activity compared with smaller
platelets, which have greater thrombosis potential (14). The mean platelet volume (MPV) is easy
to measure, is a stable parameter of platelet activation and
aggregation, and plays an important role in predicting the adverse
outcomes in patients with STEMI (15,16).
Lymphocytes are one of the earliest cell types involved in
atherosclerotic plaque formation and are important biomarkers in
determining the inflammatory state of the body (17). Lymphocytes have multiple functions,
including producing immunoglobulin M antibodies, recognizing and
oxidizing low-density lipoprotein, and preventing atherosclerosis
(17,18). Therefore, in the present study, the
mean platelet volume to lymphocyte ratio (MPVLR) was used as a new
potential biomarker for inflammation and thrombosis. Recent studies
have reported that high MPVLR values at admission are associated
with various short-term and long-term adverse outcomes in patients
with STEMI after PCI (19,20). However, there are no previous studies
evaluating the association between MPVLR and GRACE score, and the
combined value of MPVLR with GRACE score in predicting the
prognosis of patients with STEMI after PCI. Therefore, aims of the
present study were to assess the potential association between
MPVLR and GRACE score, and to investigate whether combined MPVLR
and GRACE score is a powerful predictor of short-term MACE in
patients with STEMI after PCI.
Materials and methods
Study population
This study was retrospective and conducted at the
First Affiliated Hospital of Shihezi University Medical College
from October 2017 to January 2019, enrolling 556 patients
(including 321 males and 235 females, aged between 20 and 90 years)
diagnosed with STEMI who underwent primary PCI within 12 h. The
study was approved by The Ethics Committee of The First Affiliated
Hospital of Shihezi University School of Medicine.
STEMI was diagnosed based on the American College of
Cardiology (21) and included the
following criteria: i) Chest pain symptoms occurring within 24 h
prior to admission and lasting for >30 min; ii) an
electrocardiogram showing ST-segment elevation in ≥2 consecutive
leads and/or an abnormal Q wave and new left bundle-branch block;
and iii) serum biochemical marker creatinine kinase-myocardial band
isoenzyme (CK-MB) and/or cardiac troponin T (cTnT) is positively
elevated within 24 h after onset of the symptoms. The following
patients were excluded to avoid any factors that could have
affected MPVLR: i) Patients with autoimmune diseases (n=8); ii)
congenital heart diseases (n=4); iii) cancer (n=17); iv) acute and
chronic infectious diseases (n=13); v) severe liver and kidney
dysfunction diseases (n=19); vi) those taking steroid drugs within
3 months (n=10): vii) those who previously underwent PCI (n=13);
viii) patients with incomplete clinical data (n=5); ix) medication
is not regularly taken; and x) poor compliance. Out of 556 patients
initially enrolled, 464 patients met the inclusion criteria, and
all provided written informed consent. ‘Prodromal angina’ (PA) was
defined as a chest pain episode typically limited to 24 h before
infarction (22). No-reflow was
defined as the absence of effective perfusion of myocardial tissue
(TIMI flow-grade lower than 3) after coronary artery recanalization
without obvious spasm, dissection and residual stenosis (23).
Study procedures and clinical
data
Peripheral venous blood samples (4 ml) were
collected from patients prior to PCI. Hematological and biochemical
analyses were performed using fresh whole blood and plasma within
30 min of collection. The hematological parameters included testing
for the neutrophil count, lymphocyte count, highly sensitive
c-reactive protein (hsCRP) and MPV, which were measured using an
XT-4000 automated hematology analyzer (Sysmex Corporation). The
biochemical indicators analyzed included blood glucose, cTnT, CK-MB
and N-terminal pro-brain natriuretic peptide (NT-proBNP). CK-MB,
cTnT and NT-proBNP were determined using a Roche E601 immunology
analyzer (Roche Diagnostics), while blood glucose was measured
using an Hitachi7180 automatic biochemical analyzer (Hitachi,
Ltd.). MPVLR was calculated based on the ratio of mean platelet
volume to lymphocyte count at admission (20). Using a computer program on the
national chest pain center platform (https://datacs.chinacpc.org), the first attending
physician recorded the GRACE scores of all the patients at
admission. All the patients underwent Philips iE33 transthoracic
echocardiography (Philips Healthcare) to assess left ventricular
ejection fraction (LVEF) within 24 h after PCI.
Prior to PCI, all the patients received 300 mg
clopidogrel (Sanofi S.A.), 300 mg aspirin (Bayer), and after PCI
received daily doses of 75 mg clopidogrel and 100 mg aspirin. The
use and dosage of other cardiac drugs were determined by the
clinician according to the clinical guidelines formulated by the
American College of Cardiology and the condition of the patient
(21). The success of PCI was
assessed via thrombolysis in myocardial infarction flow grade level
3 after coronary artery therapy and residual stenosis of <30%
(20). Coronary angiography, PCI and
reperfusion therapy strategies were performed by experienced
cardiologists.
Primary endpoint and follow-up
Follow up of patients was completed by reviewing
hospital records, outpatient visits and telephone contact. The main
outcome was that MACE occurred during the follow-up period. MACE
included: Cardiogenic or all-cause mortality, malignant arrhythmias
(ventricular tachycardia, ventricular fibrillation, grade III
atrioventricular block), recurrent myocardial infarction, recurrent
angina, acute heart failure and stroke (neurological disorders
related to recent ischemic or hemorrhagic events) (24).
Statistical analysis
The Kolmogorov-Smirnov test was used to determine
the normality of each of the random samples. Mean and standard
deviation were used to describe the numerical variables following a
normal distribution. However, the median and interquartile range
were used to describe numerical variables not following normal
distribution. The t-test or Mann-Whitney U test were used to
compare the numerical variables between two groups, while one-way
ANOVA was used to compare the numerical variables among multiple
groups. When differences between the two groups were needed to be
compared using ANOVA, Tukey's post-hoc test was used if equal
variances were assumed, and Games-Howell post-hoc test was used if
equal variances were not assumed. Non-normally distributed data
were analyzed with the Kruskal-Wallis non-parametric test, followed
by Dunn's post-hoc test. Frequencies and percentages were used to
describe nominal variables, and comparisons between groups
performed using χ2 test or Fisher exact probability
method. Spearman's rank correlation was utilized to determine the
correlation between MPVLR and GRACE scores. A receiver operating
characteristic curve (ROC) was used to analyze the value of MPVLR,
GRACE score and their combination in predicting MACE and
angiographic no-reflow occurrence. Delong's test was used to
compare the area under ROC curve (AUC). Kaplan-Meier analysis
method was used to estimate the MACE-free survival rate based on
the cut-off values of MPVLR and GRACE scores. The log-rank test was
used to compare the MACE free survival rate between groups. Cox
regression models were used to evaluate independent risk factors
for short-term MACE in patients with STEMI after PCI. Univariate
analysis P<0.1 factors were included in multivariate Cox
regression analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline clinical characteristics
The present study enrolled 464 patients with STEMI
after PCI and the median follow-up period was 22 days (range:
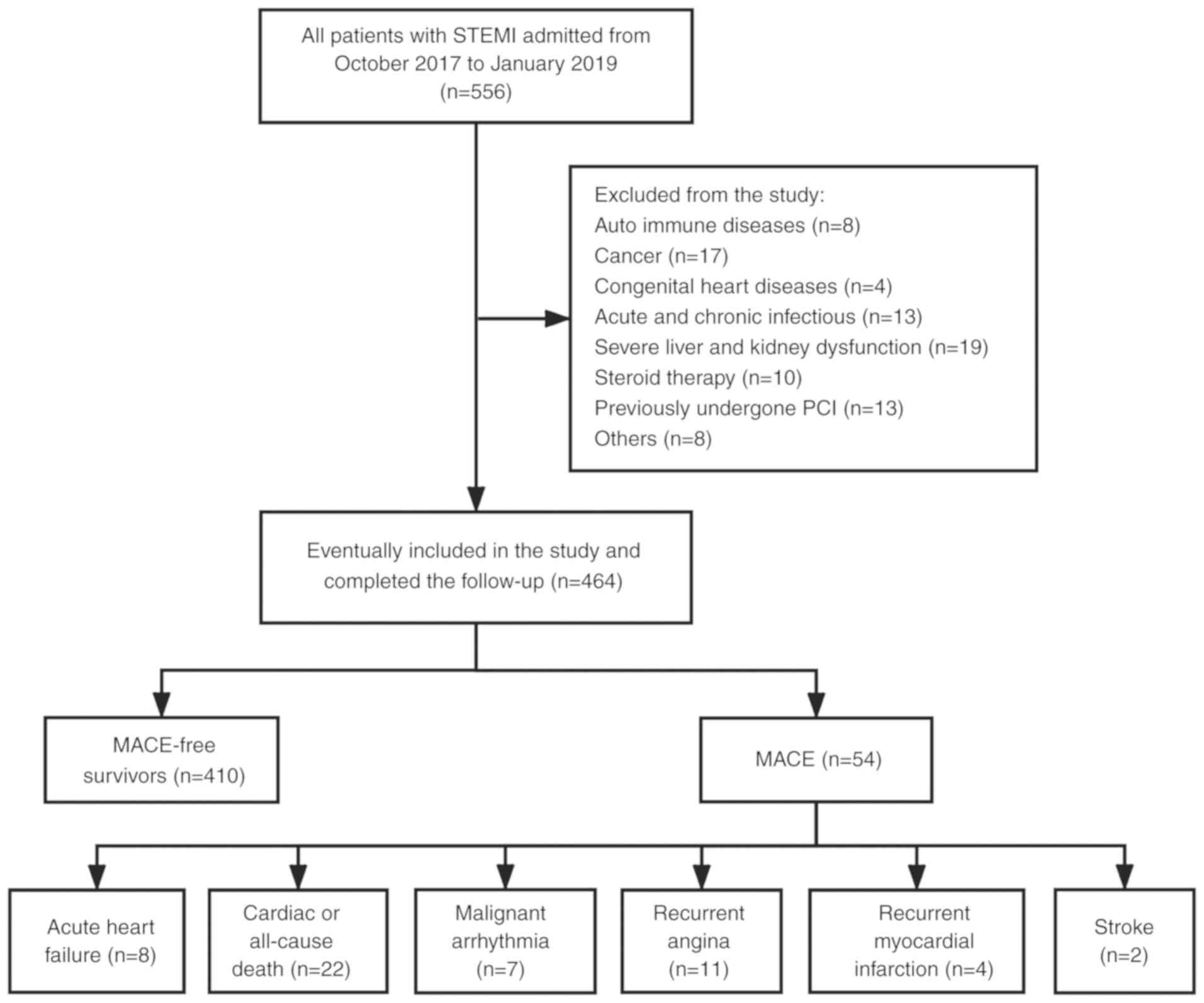
1-30). Fig. 1 shows the patient
selection flow chart. ROC analyses results indicated that the
cut-off values of MPVLR and GRACE score for differentiating
short-term MACE were 5.38 (sensitivity=85.2%; specificity=64.6%;
P<0.001) and 145 (sensitivity=88.9%; specificity=62.7%;
P=0.005). Based on the optimal cut-off values of MPVLR and GRACE
score, patients were segregated into four groups (25): i) Low MPVLR + low GRACE score (Group
1; MPVLR ≤5.38; GRACE score ≤145; n=181); ii) low MPVLR + high
GRACE score (Group 2; MPVLR ≤5.38; GRACE score >145; n=91); iii)
high MPVLR + low GRACE score (Group 3; MPVLR >5.38; GRACE score
≤145; n=93); and iv) high MPVLR + high GRACE score (Group 4; MPVLR
>5.38; GRACE score >145; n=99). The basic clinical and
procedural characteristics of the four groups of patients are shown
in Table I. Patients in Group 4 were
significantly older, had a higher admission GRACE score, Killip
class, neutrophil count, neutrophil to lymphocyte ratio (NLR), MPV,
MPVLR, peak of cTnT, NT-proBNP and hsCRP, and lower levels of LVEF
and lymphocyte count compared to patients in Group 1. Patients in
Group 4 also had a higher proportion of diabetes mellitus and PA
compared with the other three groups. Moreover, there were no
significant differences in coronary angiography and postoperative
medication among the four groups.
 | Table IComparison of baseline clinical
characteristics of patients divided into groups based on the
different MPVLR combined with GRACE scores. |
Table I
Comparison of baseline clinical
characteristics of patients divided into groups based on the
different MPVLR combined with GRACE scores.
| Variable | Low MPVLR + low
GRACE score (Group 1, n=181) | Low MPVLR + high
GRACE score (Group 2, n=91) | Low MPVLR + high
GRACE score (Group 3, n=93) | High MPVLR + high
GRACE score (Group 4, n=99) | P-value |
|---|
| Baseline
characteristics | | | | | |
|
Age,
years | 53.68±7.91 | 55.43±8.26 | 53.06±8.94 |
59.42±9.99a-c | <0.001 |
|
Male, n
(%) | 105 (58.01) | 55 (60.44) | 51 (54.84) | 57 (57.58) | 0.897 |
|
Current
smokers, n (%) | 94 (51.93) | 52 (57.14) | 41 (44.09) | 53 (53.54) | 0.337 |
|
Hypertension,
n (%) | 121 (66.85) | 63 (69.23) | 65 (69.89) | 81 (81.82) | 0.062 |
|
Diabetes
mellitus, n (%) | 49 (27.07) | 27 (29.67) | 35
(37.63)a,b | 45
(45.45)a-c | 0.012 |
|
Dyslipidaemia,
n (%) | 52 (28.73) | 15 (16.48) | 23 (24.73) | 32 (32.32) | 0.179 |
|
Prodromal
angina, n (%) | 60 (33.15) | 28 (30.77) | 30 (32.26) | 17
(17.17a-c | 0.032 |
|
Killip class
≥II, n (%) | 66 (36.46) | 35 (38.46) | 40
(43.01)a,b | 55
(55.56)a-c | 0.017 |
|
GRACE
score | 129.52±13.95 |
156.82±12.70a |
120.32±17.32a,b |
167.24±14.86a-c | 0.001 |
| Laboratory
data | | | | | |
|
Neutrophil
count, x109/l | 6.03±2.68 | 6.21±3.42 |
6.44±2.88a |
7.43±3.75a-c | 0.004 |
|
Lymphocyte
count, x109/l | 2.84±0.64 | 2.81±0.77 |
1.59±0.30a,b |
1.57±0.37a,b | <0.001 |
|
NLR | 2.25±1.20 | 2.44±1.70 |
4.24±2.10a,b |
5.09±3.05a-c | <0.001 |
|
MPV, fl | 10.45±0.97 |
10.85±0.73a |
11.02±0.93a |
11.00±1.10a | <0.001 |
|
MPVLR | 3.83±0.81 | 4.08±0.90 |
7.21±1.66a,b |
7.83±2.95a-c | <0.001 |
|
Peak CK-MB,
U/l | 146 (84-249) | 86 (64-186) | 144 (66-233) | 162 (53-375) | 0.113 |
|
Peak cTnT,
ng/ml | 3.64
(2.25-5.70) | 4.29
(2.31-5.89)a | 4.20
(2.82-6.09)a | 6.85
(2.72-8.99)a-c | <0.001 |
|
NT-proBNP,
pg/ml | 885
(494-2,499) | 1,366
(741-3,215)a | 1,239
(764-2,519)a | 3,610
(1,750-6,800)a-c | <0.001 |
|
Glu,
mmol/l | 7.32±3.93 | 7.73±3.54 | 7.97±4.40 | 7.92±3.22 | 0.476 |
|
hsCRP,
mg/l | 2.60
(1.40-9.38) | 1.40
(0.87-3.20)a | 1.72
(0.85-5.50)a | 2.70
(1.64-5.45)b,c | 0.002 |
|
LVEF | 59.52±9.24 | 59.46±10.81 |
57.56±8.53a,b |
52.65±10.19a-c | <0.001 |
| Culprit vessel, n
(%) | | | | | 0.327 |
|
Right
coronary artery | 80 (44.20) | 30 (32.97) | 38 (40.86) | 37 (37.37) | |
|
Left
circumflex artery | 23 (12.71) | 10 (10.98) | 12 (12.90) | 13 (13.13) | |
|
Left
anterior descending artery | 78 (43.09) | 49 (53.58) | 43 (46.24) | 47 (47.47) | |
|
Left main
coronary artery | 0 (0) | 2 (2.20) | 0 (0) | 2 (2.03) | |
|
Number of
implanted stents, n | 1.21±0.57 | 1.31±0.59 | 1.17±0.50 | 1.22±0.56 | 0.942 |
| Postoperative
medication, n (%) | | | | | |
|
Clopidogrel | 167 (92.23) | 86 (94.51) | 88 (94.62) | 92 (92.93) | 0.848 |
|
Aspirin | 173 (95.58) | 85 (93.41) | 89 (95.70) | 96 (96.97) | 0.709 |
|
Statin | 153 (84.53) | 79 (86.81) | 78 (83.87) | 90 (90.91) | 0.436 |
|
Beta-blocker | 144 (79.56) | 68 (74.73) | 73 (78.49) | 80 (80.81) | 0.753 |
|
ACEI or
ARB | 90 (49.72) | 43 (47.25) | 49 (52.69) | 55 (55.56) | 0.669 |
|
Calcium
channel blocker | 40 (22.10) | 18 (19.78) | 22 (23.68) | 30 (30.30) | 0.332 |
Comparison of baseline clinical
characteristics of patients in the MACE and MACE-free groups
Table II shows the
baseline clinical characteristics of patients in the MACE and
MACE-free groups. Compared to the MACE-free group, the MACE group
patients were older, had higher neutrophil count, NLR, MPV, peak of
cTnT, NT-proBNP, hsCRP, and also a higher proportion of diabetes
and killip class≥II. However, patients with MACE had a lower
proportion of PA, lymphocyte count and LVEF. Moreover, the MPVLR
and GRACE score of patients in the MACE group were significantly
higher compared with the MACE-free group.
 | Table IIComparison of baseline clinical
characteristics of patients in the MACE and MACE-free groups. |
Table II
Comparison of baseline clinical
characteristics of patients in the MACE and MACE-free groups.
| Variable | MACE group
(n=54) | MACE-free group
(n=410) | P-value |
|---|
| Baseline
characteristics | | | |
|
Age,
years | 71.19±11.29 | 58.11±10.69 | <0.001 |
|
Male, n
(%) | 32 (59.26) | 236 (57.56) | 0.812 |
|
Current
smokers, n (%) | 34 (62.96) | 206 (50.24) | 0.079 |
|
Hypertension,
n (%) | 44 (81.48) | 286 (69.76) | 0.074 |
|
Diabetes
mellitus, n (%) | 28 (51.85) | 128 (31.22) | 0.003 |
|
Dyslipidaemia,
n (%) | 19 (35.19) | 103 (25.12) | 0.114 |
|
Prodromal
angina, n (%) | 11 (20.37) | 144 (35.12) | 0.031 |
|
Killip class
≥II, n (%) | 30 (55.56) | 166 (40.49) | 0.035 |
|
GRACE
score | 165.31±17.32 | 138.56±24.06 | <0.001 |
| Laboratory
data | | | |
|
Neutrophil
count, x109/l | 8.19±3.17 | 6.49±3.18 | <0.001 |
|
Lymphocyte
count, x109/l | 1.70
(1.38-2.00) | 2.30
(1.80-2.90) | <0.001 |
|
NLR | 4.61
(3.47-6.40) | 2.44
(1.53-4.33) | <0.001 |
|
MPV, fl | 10.90
(10.60-11.80) | 10.70
(10.10-11.40) | 0.002 |
|
MPVLR | 6.39
(5.64-8.10) | 4.61
(3.66-6.00) | <0.001 |
|
Peak CK-MB,
U/l | 166 (41-300) | 137 (69-245) | 0.914 |
|
Peak cTnT,
ng/ml | 7.15
(3.67-9.70) | 4.09
(2.47-6.02) | <0.001 |
|
NT-proBNP,
pg/ml | 3,800
(1,736-4,602) | 1,209
(650-2,609) | <0.001 |
|
Glu,
mmol/l | 8.35±3.72 | 7.59±3.83 | 0.171 |
|
hsCRP,
mg/l | 3.25
(1.58-8.28) | 2.25
(1.10-5.71) | 0.041 |
|
LVEF | 48.94±8.46 | 58.79±9.61 | <0.001 |
| Culprit vessel, n
(%) | | | |
|
Right
coronary artery | 18 (33.33) | 160 (39.02) | 0.419 |
|
Left
circumflex artery | 8 (14.81) | 67 (16.34) | 0.775 |
|
Left
anterior descending artery | 27 (50.00) | 180 (43.90) | 0.397 |
|
Left main
coronary artery | 1 (1.85) | 3 (0.73) | 0.403 |
|
Number of
implanted stents, n | 1.25±0.65 | 1.22±0.55 | 0.770 |
| Postoperative
medication, n (%) | | | |
|
Clopidogrel | 50 (92.59) | 383 (93.41) | 0.820 |
|
Aspirin | 51 (94.44) | 392 (95.61) | 0.699 |
|
Statin | 46 (85.19) | 354 (86.34) | 0.817 |
|
Beta-blocker | 42 (77.78) | 323 (78.78) | 0.866 |
|
ACEI or
ARB | 26 (48.15) | 211 (51.46) | 0.647 |
|
Calcium
channel blocker | 12 (22.22) | 98 (23.90) | 0.785 |
Clinical adverse outcomes
During the follow-up period, 54 (11.64%) patients
experienced MACE. These included 22 (4.74%) cardiac or all-cause
mortality, seven (1.51%) malignant arrhythmia, 11 (2.37%) recurrent
angina, four (0.86%) recurrent myocardial infarction, eight (1.72%)
acute heart failure and two (0.43%) strokes. The present results
suggested that the incidence of MACE and angiographic no-reflow
during follow-up were significantly increased in the high MPVLR +
high GRACE score group compared with the other three groups. In
terms of cardiac or all-cause mortality and recurrent angina, these
were significantly increased in patients in the high MPVLR + high
GRACE score group compared with the other three groups. However,
malignant arrhythmia, recurrent angina, acute heart failure and
stroke were similar in the four groups (Table III).
 | Table IIIComparison of adverse outcomes among
the four groups based on the MPVLR and GRACE score cut-off. |
Table III
Comparison of adverse outcomes among
the four groups based on the MPVLR and GRACE score cut-off.
| Variable | Low MPVLR + low
GRACE score (Group 1, n=181) | Low MPVLR + high
GRACE score (Group 2, n=91) | High MPVLR + low
GRACE score (Group 3, n=93) | High MPVLR + high
GRACE score (Group 4, n=99) | P-value |
|---|
| Angiographic
no-reflow, n (%) | 8 (4.42) | 13
(14.29)a | 12
(12.90)a | 26
(26.26)a-c | <0.001 |
| MACE during
follow-up, n (%) | 6 (3.30) | 10
(11.00)a | 13
(13.98)a | 25
(25.25)a-c | <0.001 |
| Cardiac or
all-cause death | 2 (1.10) | 3 (3.30) | 4 (4.29) | 13
(13.13)a-c | <0.001 |
| Malignant
arrhythmia | 1 (0.55) | 2 (2.20) | 1 (1.08) | 3 (3.03) | 0.307 |
| Recurrent
angina | 0 (0.00) | 3 (3.30) | 2 (2.15) | 6
(6.07)a-c | 0.003 |
| Recurrent
myocardial infarction | 1 (0.55) | 0 (0.00) | 2 (2.15) | 1 (1.01) | 0.424 |
| Acute heart
failure | 2 (1.10) | 2 (2.20) | 3 (3.23) | 1 (1.01) | 0.552 |
| Stroke | 0 (0.00) | 0 (0.00) | 1 (1.08) | 1 (1.01) | 0.366 |
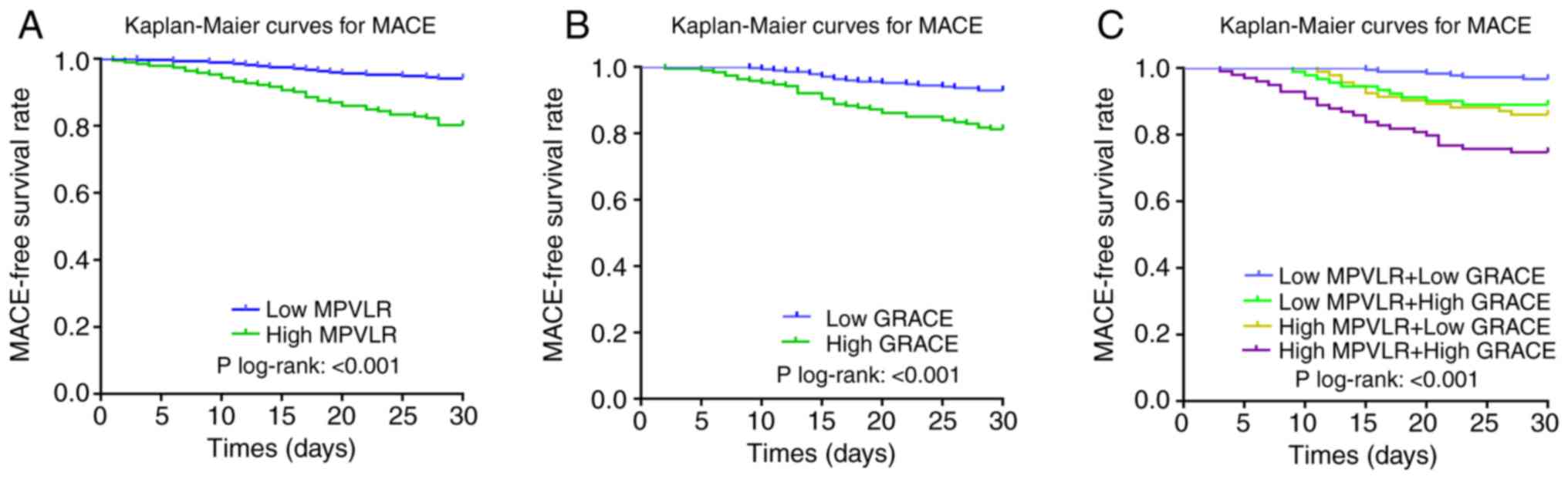
Kaplan-Meier survival curves based on the cut-off
values of MPVLR and GRACE score are shown in Fig. 2A and B, respectively. The rate of MACE in the
high MPVLR group (19.79 vs. 5.88%; log-rank, P<0.001; Fig. 2A) and the high GRACE score group
(18.42 vs. 6.93%; log-rank, P<0.001; Fig. 2B) increased significantly compared
with the control group during the follow-up period. In addition,
Kaplan-Meier survival curves based on the combined biomarkers
(MPVLR and GRACE scores) are shown in Fig. 2C. There was a significant intergroup
difference in short-term MACE among the four groups, and the
short-term MACE in the high MPVLR+ high GRACE score group was
increased compared with the other three groups (high MPVLR + high
GRACE score vs. high MPVLR + low GRACE score vs. low MPVLR + high
GRACE score; 25.25 vs. 13.98 vs. 11.00%, respectively; P<0.001;
Fig. 2C).
Independent predictors factors for
short-term MACE
Cox proportional hazard analysis was used to
construct model 1 and model 2 for prediction of the risk factors
for short-term MACE after PCI in patients with STEMI (Table IV). Univariate analysis results
suggested that age, prodromal angina, Killip class ≥II, NLR, hsCRP,
NT-proBNP, the peak of cTnT, LVEF and combined MPVLR with GRACE
score were all associated with a 30-day MACE. After adjusting the
covariates in model 1, high GRACE score (HR, 1.706; 95% CI,
1.435-3.058; P<0.001) and high MPVLR (HR, 1.668; 95% CI,
1.202-2.170; P<0.001) were significant independent predictors of
a 30-day MACE. Multivariate Cox analysis in model 2 showed that the
combination of high GRACE score with high MPVLR (HR, 2.455; 95% CI,
1.736-3.188; P<0.001) was a powerful predictor of a 30-day
MACE.
 | Table IVCox regression analysis of risk
factors for MACE in patients during follow-up. |
Table IV
Cox regression analysis of risk
factors for MACE in patients during follow-up.
| | Multivariate
analysis |
|---|
| | Univariate
analysis | Model 1 | Model 2 |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.088
(1.062-1.234) | <0.001 | 1.055
(1.025-1.131) | <0.001 | 1.048
(1.017-1.113) | 0.002 |
| Diabetes
mellitus | 1.453
(0.921-1.964) | 0.236 | - | - | - | - |
| Prodromal
angina | 0.946
(0.795-0.991) | 0.042 | 0.910
(0.839-0.963) | 0.026 | 0.885
(0.816-0.947) | 0.021 |
| Killip class
≥II | 1.786
(1.036-2.886) | 0.036 | 1.721
(1.324-2.320) | 0.019 | 1.611
(1.266-1.897) | 0.026 |
| NLR | 1.315
(1.144-1.545) | <0.001 | 1.152
(1.089-1.418) | 0.020 | 1.128
(1.102-1.407) | 0.012 |
| hsCRP | 1.485
(1.122-1.676) | 0.042 | 1.233
(1.076-1.538) | 0.033 | 1.184
(1.076-1.510) | 0.019 |
| NT-proBNP | 1.032
(1.001-1.095) | <0.001 | 1.008
(1.000-1.039) | 0.027 | 1.005
(1.002-1.027) | 0.013 |
| Peak cTnT | 1.471
(1.086-1.787) | <0.001 | 1.325
(0.987-1.880) | 0.521 | 1.252
(0.932-1.780) | 0.475 |
| LVEF | 0.928
(0.863-0.953) | <0.001 | 0.911
(0.895-0.947) | <0.001 | 0.903
(0.880-0.928) | <0.001 |
| MPVLR >5.38 | 2.987
(2.247-4.568) | <0.001 | 1.668
(1.202-2.170) | <0.001 | - | - |
| GRACE >145 | 3.102
(1.691-4.926) | <0.001 | 1.706
(1.435-3.058) | <0.001 | - | - |
| Low MPVLR+ high
GRACE | 2.105
(1.358-6.281) | 0.013 | - | - | 1.625
(1.168-2.609) | 0.007 |
| High MPVLR + low
GRACE | 2.558
(1.637-7.002) | 0.022 | - | - | 1.806
(1.392-2.809) | 0.018 |
| High MPVLR + high
GRACE | 5.382
(3.745-8.753) | <0.001 | - | - | 2.455
(1.736-3.188) | <0.001 |
Correlation between MPVLR and GRACE
score
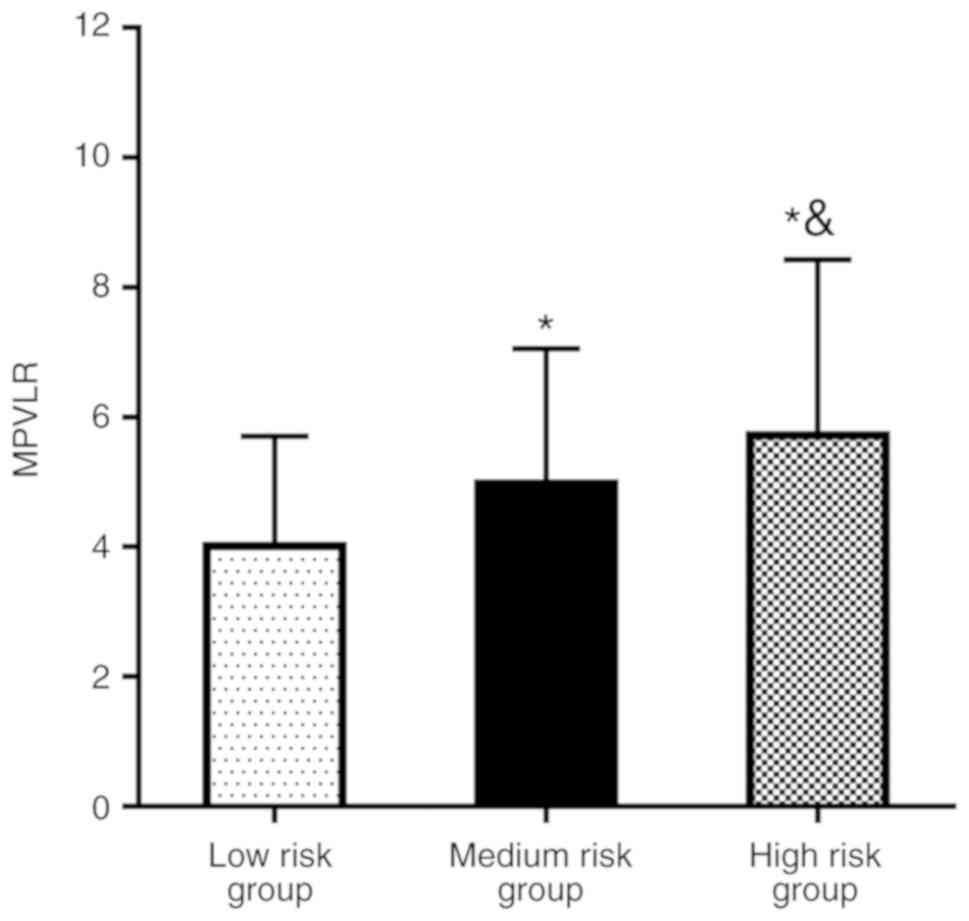
Based on the GRACE score, patients were categorized
into three groups: High-risk group (GRACE score >140; n=213),
medium-risk group (108< GRACE score ≤140; n=161) and low-risk
group (GRACE score ≤108; n=90). It was demonstrated that, with
increased GRACE risk stratification, the MPVLR level of each group
increased significantly (P<0.05; Fig.
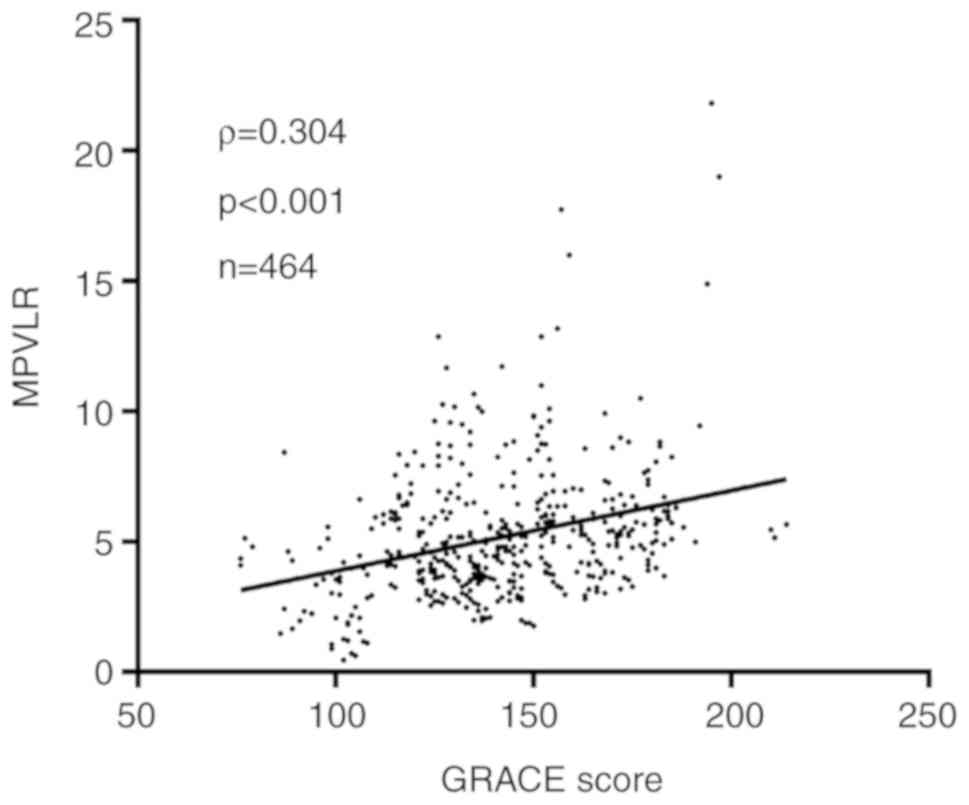
3). In addition, Spearman's rank correlation results indicated
that there was a significant linear correlation between MPVLR with
GRACE score (ρ=0.304; P<0.001; Fig.
4).
Combination of MPVLR with GRACE score
in predicting clinical adverse outcomes
ROC curves assessed and compared the predictive
efficacy of MPVLR, GRACE score and their combination in predicting
adverse clinical outcomes after PCI in patients with STEMI. As
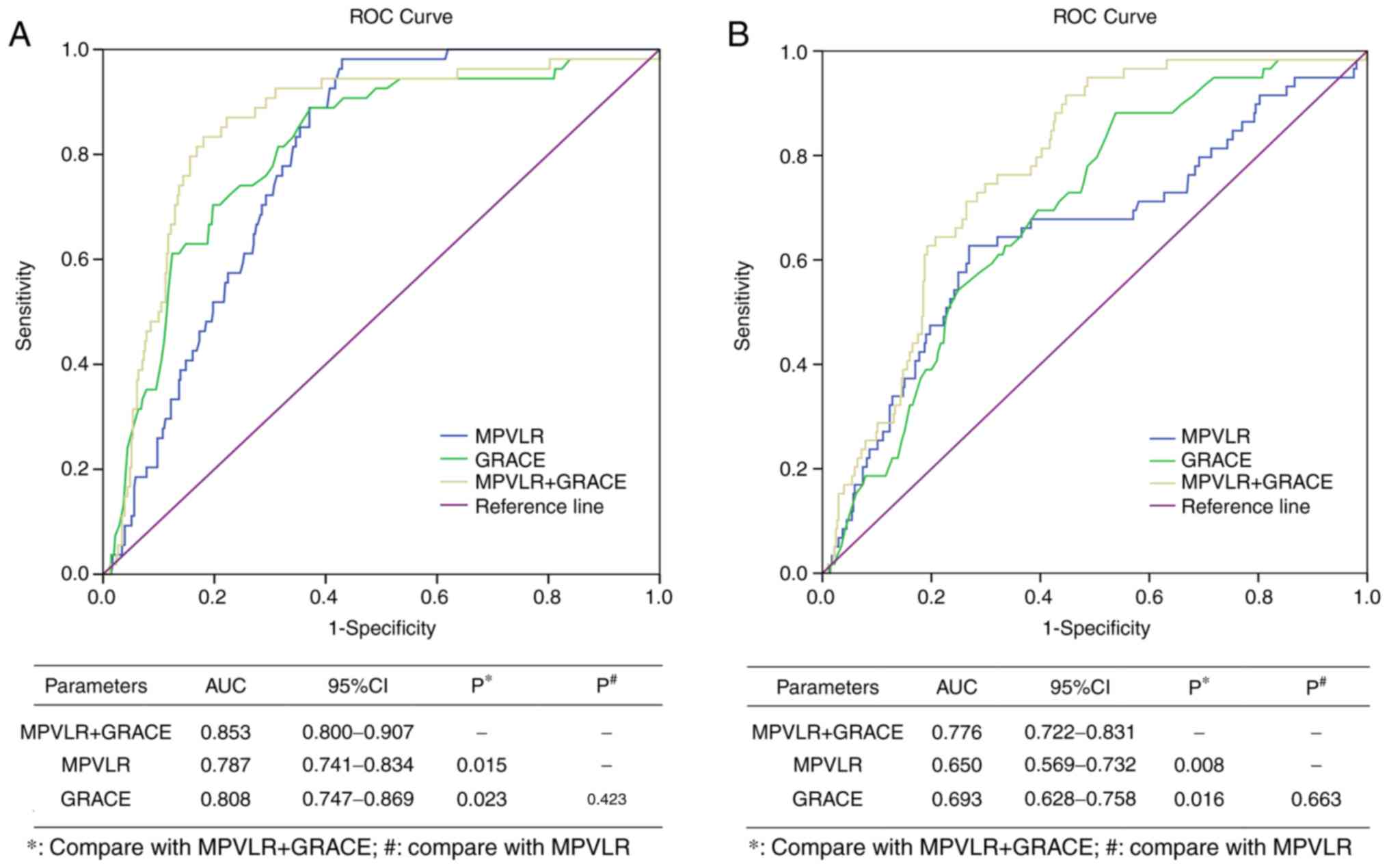
presented in Fig. 5A, a combination
of GRACE score with MPVLR (AUC, 0.853; 95% CI, 0.800-0.907) had
improved predictive efficacy for short-term MACE, compared with
individual MPVLR (AUC, 0.787; 95% CI, 0.741-0.834) and GRACE score
(AUC, 0.808; 95% CI, 0.747-0.869; P<0.05). In addition, GRACE
score together with MPVLR (AUC, 0.776; 95% CI, 0.722-0.831)
significantly improved the prediction efficiency of angiographic
no-reflow (Fig. 5B; P<0.05)
compared with single prediction with MPVLR (AUC, 0.650; 95% CI,
0.569-0.732) and GRACE score (AUC, 0.693; 95% CI, 0.628-0.758).
Collectively, the present results suggested that the combination of
MPVLR and GRACE score may improve the prediction of clinical
adverse outcomes in patients with STEMI after PCI.
Discussion
The present study, not only investigated the
potential association between GRACE score and MPVLR, but also
compared the predictive value of GRACE score, MPVLR and GRACE score
combined with MPVLR for no-reflow and short-term MACE in patients
with STEMI after PCI. In addition, the present study also examined
the potential mechanism between the loss of PA and the increase in
MPVLR and GRACE scores. The present results suggested that MPVLR is
a simple, non-invasive, economical and feasible biomarker, which
can account for the deficiency of the GRACE score system. It was
also indicated that MPVLR has a practical clinical value in
predicting the prognosis of patients with STEMI. The present
findings demonstrated that MPVLR combined with GRACE score has a
more powerful predictive potential for short-term adverse outcomes
in patients with STEMI after PCI, compared with an individual MPLVR
or GRACE score.
MPV is an important indicator of platelet activation
and aggregation (26). Large
platelets with more active metabolism can accelerate the formation
of coronary thrombosis, and play a considerable role in the
pathological and physiological process of AMI (27-29).
Previous clinical studies have shown that higher MPV at the time of
admission is associated with all-cause mortality and MACE incidence
in patients with STEMI (30,31). Goncalves et al (32) showed that patients with ACS have
larger MPV and larger platelet metabolism and enzyme activities
were higher, which increased the occurrence of adverse outcomes via
the release of inflammatory mediators, increased thrombosis,
aggravated microvascular dysfunction, inflammation and myocardial
injury, microcirculation insufficiency, large infarction area and
deterioration of cardiac function. Moreover, Núñez et al
found that lymphocytes are involved in the growth, development,
rupture and thrombosis of atherosclerotic plaques (33). The decrease in lymphocyte count is
related to the body's physiological stress, increased inflammatory
response and increased apoptosis (33). In addition, lymphocyte count was
found to be important biomarkers of inflammatory reactions in
patients with STEMI, and is associated with MACE and angiographic
no-reflow (34). The present results
suggested that patients in the MACE group had a lower lymphocyte
count compared with the MACE-free group. Moreover, patients in the
high MPVLR and high GRACE score group showed significantly lower
rates of prodromal angina compared with the other three groups.
These results are consistent with previous results from Gok et
al (35). However, the
relationship between PA absence and increases in MPVLR and GRACE
scores is not fully understood. PA may be a process of ischemic
preconditioning, which can delay the death of myocardial cells and
has a protective effect on the myocardia ischemic injured before
reperfusion (22,36). PA not only reduces myocardial infarct
size but also protects microcirculation after reperfusion (35,37).
Therefore, the absence of PA in patients with STEMI often indicates
that the disease is more serious, and the corresponding GRACE score
is higher and with poorer prognosis (38). In addition, inflammation plays an
important role in myocardial ischemia-reperfusion injury (38). Therefore, the reason for the higher
MPVLR in patients with STEMI in the absence of PA may be associated
with the anti-inflammatory effect and inhibition of platelet
activation of PA (39).
MPVLR is the ratio of MPV to lymphocyte count, and
an increase in MPV and/or a decrease in lymphocyte count can result
in increased MPVLR (20). MPVLR is a
comprehensive biomarker for thrombosis and inflammation (29). MPVLR combines the advantages of MPV
and lymphocytes in indicating the physiological stress in STEMI
patients, overcome the shortcomings of each one of them (20). In addition, MPVLR functions as an
indicator of the body's inflammatory response and the degree of
thrombosis (19). Ornek and Kurtul
(40) showed that MPVLR predicted
impaired coronary collateral circulation in patients with stable
coronary artery disease. Moreover, Kilic and Kurtul (41) showed that elevated MPVLR is
associated with the complexity and severity of coronary
atherosclerosis in patients with ACS. The present results indicated
that MPVLR levels were positively correlated with GRACE score and
that patients in the high MPVLR group had a higher incidence of
MACE. Therefore, MPVLR may be used as an auxiliary indicator to
predict adverse outcome in patients with STEMI.
The GRACE score is the largest and best-known
database prospective study used for ACS (42). Patients were enrolled from 30
countries across North and South America, Australia, New Zealand,
Asia and Europe, and it has been widely used to identify high-risk
patients with AMI and assess prognosis (43). The GRACE score system, however, has
some limitations, such as it does not consider the thrombotic
activity and inflammatory status of the body (44). Hence, there is a lack of biomarkers
related to adverse outcomes. Thus, there is a need for objective
biomarkers for the comprehensive evaluation of prognosis in AMI
patients. Previous studies have identified several biomarkers
outside the scoring system, such as NT-proBNP (45), hsCRP (46), neutrophil count (44), homocysteine and the
fibrinogen-albumin ratio (47,48);
these have significantly improved the predictive efficacy of GRACE
risk score system for adverse outcomes in patients with ACS. The
present study investigated the association between MPVLR and GRACE
score, and found that MPVLR combined with GRACE score may be used
as a powerful and stable predictor for short-term MACE prediction
after PCI in patients with STEMI.
The present study had some limitations. First, the
present study was a single-center retrospective study with limited
sample size and possible selection bias. Moreover, the prognostic
value of other biomarkers for patients with STEMI were not
investigated. In addition, the changes in MPVLR were not
dynamically observed and there was no assessment of whether a
similar predictive value was present in MPVLR after treatment.
In conclusion, MPVLR and GRACE score combination on
admission showed significant predictive value for short-term MACE
after PCI in patients with STEMI. Therefore, this combination may
be used to identify high-risk patients with poor prognosis and aid
treatment in the early disease stage. The combination of MPVLR,
which is a non-invasive, simple, economical and feasible biomarker,
and GRACE score provides a new perspective for the assessment,
treatment and prognosis of patients with STEMI.
Acknowledgements
The authors would like to thank Professor Guihua Li,
Director of The Department of Emergency (First Affiliated Hospital,
Shihezi University School of Medicine) for providing valuable
suggestions for the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC, MS and GL contributed to the conception and
design of the study, and drafted the manuscript. TZ and YM
contributed to the collection, collation and statistical analysis
of data. WZ, HZ and HH contributed to the feasibility analysis of
the study. XC and GL revised the manuscript. GL is responsible for
study supervision and management.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The First Affiliated Hospital of Shihezi University
School of Medicine. All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah ASV, Sandoval Y, Noaman A, Sexter A,
Vaswani A, Smith SW, Gibbins M, Griffiths M, Chapman AR, Strachan
FE, et al: Patient selection for high sensitivity cardiac troponin
testing and diagnosis of myocardial infarction: Prospective cohort
study. BMJ. 359(j4788)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bressi E, Mangiacapra F, Ricottini E,
Cavallari I, Colaiori I, Di Gioia G, Creta A, Capuano M, Viscusi MM
and Di Sciascio G: Impact of Neutrophil-to-Lymphocyte ratio and
Platelet-to-lymphocyte ratio on 5-year clinical outcomes of
patients with stable coronary artery disease undergoing elective
percutaneous coronary intervention. J Cardiovasc Transl Res.
11:517–523. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen X, Shao M, Zhang T, Meng YB, Zhang W,
Huang Z, Xu XG and Li GH: Prognostic value of elevated mean
platelet volume in acute myocardial infarction: A meta-analysis
including 8,945 patients. Int J Clin Exp Med. 12:10151–10163.
2019.
|
|
4
|
Turk J, Fourny M, Yayehd K, Picard N,
Ageron FX, Boussat B, Belle L, Vanzetto G, Puymirat E, Labarère J
and Debaty G: Age-related differences in reperfusion therapy and
outcomes for ST-segment elevation myocardial infarction. J Am
Geriatr Soc. 66:1325–1331. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lin A, Devlin G, Lee M and Kerr AJ:
Performance of the GRACE scores in a New Zealand acute coronary
syndrome cohort. Heart. 100:1960–1966. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shuvy M, Beeri G, Klein E, Cohen T, Shlomo
N, Minha S and Pereg D: Accuracy of the global registry of acute
coronary events (GRACE) risk score in contemporary treatment of
patients with acute coronary syndrome. Can J Cardiol. 34:1613–1617.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gray HH and Henderson RA: The GRACE
score's performance in predicting in-hospital and 1-year outcome.
Heart. 97:1461–1462. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen YH, Huang SS and Lin SJ: TIMI and
GRACE risk scores predict both short-term and long-term outcomes in
Chinese patients with acute myocardial infarction. Acta Cardiol
Sin. 34:4–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jakimov T, Mrdović I and Filipović B,
Zdravković M, Djoković A, Hinić S, Milić N and Filipović B:
Comparison of RISK-PCI, GRACE, TIMI risk scores for prediction of
major adverse cardiac events in patients with acute coronary
syndrome. Croat Med J. 58:406–415. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiang L, Wang M, You T, Jiao Y, Chen J and
Xu W: Prognostic value of ventricular wall motion score and global
registry of acute coronary events score in patients with acute
myocardial infarction. Am J Med Sci. 354:27–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
González-Pacheco H, Bojalil R,
Amezcua-Guerra LM, Sandoval J, Eid-Lidt G, Arias-Mendoza A,
Azar-Manzur F, Álvarez-Sangabriel A, Altamirano-Castillo A,
Briseño-Cruz JL, et al: Derivation and validation of a simple
inflammation-based risk score system for predicting in-hospital
mortality in acute coronary syndrome patients. J Cardiol.
73:416–424. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang JJ, Fan Y, Zhu Y, Zhang JD, Zhang SM,
Wan ZF, Su HL and Jiang N: Biomarkers enhance the long-term
predictive ability of the KAMIR risk score in Chinese patients with
ST-elevation myocardial infarction. Chin Med J (Engl). 132:30–41.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ridker PM, Everett BM, Thuren T, MacFadyen
JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker
SD, et al: Antiinflammatory therapy with canakinumab for
atherosclerotic disease. N Engl J Med. 377:1119–1131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fuentes QE, Fuentes QF, Andrés V, Pello
OM, Font de Mora J and Palomo GI: Role of platelets as mediators
that link inflammation and thrombosis in atherosclerosis.
Platelets. 24:255–262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Linden MD and Jackson DE: Platelets:
Pleiotropic roles in atherogenesis and atherothrombosis. Int J
Biochem Cell Biol. 42:1762–1766. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Avci E, Kiris T, Çelik A, Variş E, Esin
FK, Köprülü D and Kadi H: Prognostic value of rising mean platelet
volume during hospitalization in patients with ST-segment elevation
myocardial infarction treated with primary percutaneous coronary
intervention. BMC Cardiovasc Disord. 18(226)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sage AP and Mallat Z: Multiple potential
roles for B cells in atherosclerosis. Ann Med. 46:297–303.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moriya J: Critical roles of inflammation
in atherosclerosis. J Cardiol. 73:22–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hudzik B, Szkodziński J, Lekston A,
Gierlotka M, Poloński L and Gąsior M: Mean platelet
volume-to-lymphocyte ratio: A novel marker of poor short- and
long-term prognosis in patients with diabetes mellitus and acute
myocardial infarction. J Diabetes Complications. 30:1097–1102.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kurtul A and Acikgoz SK: Usefulness of
mean platelet Volume-to-Lymphocyte ratio for predicting
angiographic No-Reflow and Short-Term prognosis after primary
percutaneous coronary intervention in patients with ST-Segment
elevation myocardial infarction. Am J Cardiol. 120:534–541.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
O'Gara PT, Kushner FG, Ascheim DD, Casey
DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM,
Franklin BA, et al: 2013 ACCF/AHA guideline for the management of
ST-elevation myocardial infarction: A report of the American
College of Cardiology Foundation/American Heart Association task
force on practice guidelines. Circulation. 127:e362–e425.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maruhashi T, Ishihara M, Inoue I, Kawagoe
T, Shimatani Y, Kurisu S, Nakama Y, Kagawa E, Dai K, Matsushita J
and Ikenaga H: Effect of prodromal angina pectoris on the infarct
progression in patients with first ST-elevation acute myocardial
infarction. Circ J. 74:1651–1657. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bayramoğlu A, Taşolar H, Kaya A, Tanboğa
İH, Yaman M, Bektaş O, Günaydın ZY and Oduncu V: Prediction of
no-reflow and major adverse cardiovascular events with a new
scoring system in STEMI patients. J Interv Cardiol. 31:144–149.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fan Z, Li Y, Ji H and Jian X: Prognostic
utility of the combination of monocyte-to-lymphocyte ratio and
neutrophil-to-lymphocyte ratio in patients with NSTEMI after
primary percutaneous coronary intervention: A retrospective cohort
study. BMJ Open. 8(e023459)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shin HC, Jang JS, Jin HY, Seo JS, Yang TH,
Kim DK and Kim DS: Combined use of neutrophil to lymphocyte ratio
and C-reactive protein level to predict clinical outcomes in acute
myocardial infarction patients undergoing percutaneous coronary
intervention. Korean Circ J. 47:383–391. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Celik T, Kaya MG, Akpek M, Gunebakmaz O,
Balta S, Sarli B, Duran M, Demirkol S, Uysal OK, Oguzhan A and
Gibson CM: Predictive value of admission platelet volume indices
for In-hospital major adverse cardiovascular events in acute
ST-segment elevation myocardial infarction. Angiology. 66:155–162.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kırış T, Yazici S, Günaydin ZY, Akyüz Ş,
Güzelburç Ö, Atmaca H, Ertürk M, Nazli C and Dogan A: The
prognostic impact of In-hospital change in mean platelet volume in
patients with Non-ST-Segment elevation myocardial infarction.
Angiology. 67:690–696. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Machado GP, Araujo GN, Carpes CK, Lech M,
Mariani S, Valle FH, Bergoli LCC, Gonçalves SC, Wainstein RV and
Wainstein MV: Comparison of Neutrophil-to-Lymphocyte ratio and mean
platelet volume in the prediction of adverse events after primary
percutaneous coronary intervention in patients with ST-elevation
myocardial infarction. Atherosclerosis. 274:212–217.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Monteiro Júnior JGM, Torres DOC, da Silva
MCFC, Martins CMH, da Silva IK, do Nascimento MEM, Dos Santos ACO,
Montarroyos UR and Filho DCS: Prognostic value of hematological
parameters in patients with acute myocardial infarction:
Intrahospital outcomes. PLoS One. 13(e0194897)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ranjith MP, DivyaRaj R, Mathew D, George B
and Krishnan MN: Mean platelet volume and cardiovascular outcomes
in acute myocardial infarction. Heart Asia. 8:16–20.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wasilewski J, Desperak P, Hawranek M,
Ciślak A, Osadnik T, Pyka Ł, Gawlita M, Bujak K, Niedziela J,
Krawczyk M and Gąsior M: Prognostic implications of mean platelet
volume on short- and long-term outcomes among patients with
non-ST-segment elevation myocardial infarction treated with
percutaneous coronary intervention: A single-center large
observational study. Platelets. 27:452–458. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Goncalves SC, Labinaz M, Le May M, Glover
C, Froeschl M, Marquis JF, O'Brien E, Shukla D, Ruchin P, Sookur D,
et al: Usefulness of mean platelet volume as a biomarker for
long-term outcomes after percutaneous coronary intervention. Am J
Cardiol. 107:204–209. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Núñez J, Miñana G, Bodí V, Núñez E,
Sanchis J, Husser O and Llàcer A: Low lymphocyte count and
cardiovascular diseases. Curr Med Chem. 18:3226–3233.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wagdy S, Sobhy M and Loutfi M:
Neutrophil/lymphocyte ratio as a predictor of In-hospital major
adverse cardiac events, New-onset atrial fibrillation, and
no-reflow phenomenon in patients with ST elevation myocardial
infarction. Clin Med Insights Cardiol. 10:19–22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gok M, Kundi H, Kiziltunc E, Evlice M,
Cetin M, Suleymanoglu M, Kurtul A and Ornek E: Relationship between
prodromal angina pectoris and Neutrophil-to lymphocyte ratio in
patients with ST elevation myocardial infarction. Heart Lung Circ.
28:901–907. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Heusch G and Gersh BJ: The pathophysiology
of acute myocardial infarction and strategies of protection beyond
reperfusion: A continual challenge. Eur Heart J. 38:774–784.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Orbegozo Cortés D, Su F, Santacruz C,
Hosokawa K, Donadello K, Creteur J, De Backer D and Vincent JL:
Ischemic conditioning protects the microcirculation, preserves
organ function, and prolongs survival in sepsis. Shock. 45:419–427.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang H, Qiu B, Zhang Y, Cao Y, Zhang X,
Wu Z, Wang S and Mei L: The value of Pre-infarction angina and
plasma D-Dimer in predicting No-reflow after primary percutaneous
coronary intervention in ST-Segment elevation acute myocardial
infarction patients. Med Sci Monit. 24:4528–4535. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kharbanda RK, Peters M, Walton B,
Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J and
MacAllister R: Ischemic preconditioning prevents endothelial injury
and systemic neutrophil activation during ischemia-reperfusion in
humans in vivo. Circulation. 103:1624–1630. 2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ornek E and Kurtul A: Relationship of mean
platelet volume to lymphocyte ratio and coronary collateral
circulation in patients with stable angina pectoris. Coron Artery
Dis. 28:492–497. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kilic A and Kurtul A: RETRACTED: Mean
platelet Volume-to-Lymphocyte ratio as a novel marker for severity
and complexity of coronary atherosclerosis in patients with acute
coronary syndrome. Angiology: Jan 1, 2017 doi:
10.1177/0003319717724274 (Epub ahead of print).
|
|
42
|
Tang EW, Wong CK and Herbison P: Global
registry of acute coronary events (GRACE) hospital discharge risk
score accurately predicts long-term mortality post acute coronary
syndrome. Am Heart J. 153:29–35. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Moady G, Iakobishvili Z, Beigel R, Shlomo
N, Matetzky S, Zahger D and Atar S: The predictive value of low
admission hemoglobin over the GRACE score in patients with acute
coronary syndrome. J Cardiol. 73:271–275. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang S, Wan Z, Zhang Y, Fan Y, Gu W, Li
F, Meng L, Zeng X, Han D and Li X: Neutrophil count improves the
GRACE risk score prediction of clinical outcomes in patients with
ST-elevation myocardial infarction. Atherosclerosis. 241:723–728.
2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Klingenberg R, Aghlmandi S, Räber L,
Gencer B, Nanchen D, Heg D, Carballo S, Rodondi N, Mach F,
Windecker S, et al: Improved risk stratification of patients with
acute coronary syndromes using a combination of hsTnT, NT-proBNP
and hsCRP with the GRACE score. Eur Heart J Acute Cardiovasc Care.
7:129–138. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shahzad S, Mateen S, Hasan A and Moin S:
GRACE score of myocardial infarction patients correlates with
oxidative stress index, hsCRP and inflammation. Immunobiology.
224:433–439. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fan Y, Wang J, Zhang S, Wan Z, Zhou D,
Ding Y, He Q and Xie P: Homocysteine enhances the predictive value
of the GRACE risk score in patients with ST-elevation myocardial
infarction. Anatol J Cardiol. 18:182–193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xiao L, Jia Y, Wang X and Huang H: The
impact of preoperative fibrinogen-albumin ratio on mortality in
patients with acute ST-segment elevation myocardial infarction
undergoing primary percutaneous coronary intervention. Clin Chim
Acta. 493:8–13. 2019.PubMed/NCBI View Article : Google Scholar
|