Introduction
After ischemia and hypoxia in myocardial cells, the
metabolism is inhibited significantly, the biological processes
such as DNA transcription and translation as well as protein
assembly and transport are restricted, and the cell viability is
reduced. Furthermore, the raised production of reactive oxygen
species (ROS), enhanced permeability of inner and outer
mitochondrial membranes, release of cytochrome c, calcium
overload and opening of mitochondrial permeability transition pore
can lead to reduced generation of adenosine triphosphate (ATP) and
energy metabolism disturbance in mitochondria and activate cell
apoptosis and necrosis pathways (1,2).
Mitochondrion is a major organelle for energy metabolism through
cell respiration, and NAD+ and NADP+ are
involved in the electron transfer and ATP synthesis in respiratory
chain complex. Besides, the level of mitochondrial membrane
potential can reflect the integrity of mitochondrial membrane
structure and function, and mitochondrial damage is the key link of
cellular hypoxic-ischemic damage (3).
The role of glucagon-like peptide-1 (GLP-1), a
natural hypoglycemic hormone, in regulating blood glucose has been
relatively well investigated, and theoretical bases for the
cardiovascular protective effects of GLP-1 have been provided
through studies (4,5). Liraglutide is an analogue of GLP
produced via gene recombination and belongs to GLP-1 receptor
agonist. It shows homology of 97% with natural physiological GLP-1
and favorable safety. A study manifested that liraglutide can
promote the survival of cardiomyocytes (6), but there are few studies on the role of
liraglutide in inhibiting cell apoptosis.
Autophagy is an intracellular route of
‘self-digestion’ that can maintain cellular homeostasis. It can not
only degrade the misfolded or denatured proteins and polymers in
cells but also break down the impaired mitochondria and other
organelles (7). Moreover, autophagy,
also known as ‘autophagic flux’, is regarded as a dynamic process.
Studies have manifested that autophagy exerts certain effects in
the occurrence and development of ischemic cardiomyopathy.
Liraglutide can regulate the autophagy levels of liver cells and
pancreatic β-cells (8), but whether
liraglutide can exert mycardial protective effect by enhancing the
autophagic flux in cardiomyocytes has not been confirmed yet.
This investigation observed the protective effects
of different concentrations of liraglutide on cardiomyocytes under
anoxic conditions by culturing primary cardiomyocytes under
CoCl2-induced chemical hypoxia.
Materials and methods
Experimental materials
H9C2 cells were purchased from Shanghai Institute of
Biochemistry and Cell Biology (Shanghai, China). The cell
suspension was transferred into a 10 ml centrifuge tube and added
with 5 ml of Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS) (both from Gibco; Thermo Fisher
Scientific, Inc.). After centrifugation at 4˚C, 950 x g for 10 min,
the supernatant was discarded, and an appropriate amount of DMEM
containing 10% FBS was added, pipetted and mixed evenly. Then the
cell density was adjusted to 1x105/ml, the cells were
seeded into a 25 ml culture flask for culture in an incubator with
5% CO2 at 37˚C, and the medium was replaced after 24
h.
Model and grouping
The hypoxia model was established using
CoCl2 (500 µmol/l for 24 h). The H9C2 cells were divided
into blank control group (Cont group), liraglutide groups (Lira
group) (1, 10, 100 and 1,000 nmol/l). CoCl2 group
(CoCl2 group) and CoCl2 + liraglutide groups
(CoCl2 + Lira group) (1, 10, 100 and 1,000 nmol/l).
Cell counting kit-8 (CCK-8)
Cells in the logarithmic growth phase were digested
and collected, prepared into cell suspension with a concentration
of 1x105/ml, and inoculated into a 96-well plate (100
µl/well). In the experiment, 3 duplicated wells and blank controls
were set. After inoculation overnight, the good cell adherence was
confirmed under a microscope. Next, 20 µl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma-Aldrich; Merck KGaA) was added into each well after the
cells were treated by groups, followed by culture at 37˚C for 4 h.
Later, the supernatant was absorbed carefully, and 150 µl of
dimethyl sulfoxide (DMSO) was added into each well and mixed by
shaking. Finally, the optical density (OD) value at the wavelength
of 570 nm in each well was measured using a microplate reader.
Lactate dehydrogenase (LDH)
Cells in the logarithmic growth phase were digested
and collected, prepared into cell suspension with a concentration
of 1x105/ml, and inoculated into the 96-well plate (100
µl/well). Three duplicated wells and control group were set for the
experiment. After cell adherence, the culture plate was placed in
an incubator with 5% CO2 for culture at 37˚C for 24 h.
Subsequently, the supernatant was taken for each group (20
µl/well), and corresponding reagents were added according to the
kit instructions, followed by mixing, placing at room temperature
for 3 min and zeroing using double distilled water and cuvette
(optical path, 1 cm) at the wavelength of 440 nm. Finally, the OD
value in each tube was determined using the microplate reader.
Pyruvic acid (1 gmol) produced in the reaction system after
reaction with matrix in 1,000 ml of culture solution at 37˚C for 15
min was defined as 1 unit. The content of LDH in the medium was
calculated according to the formula.
Caspase-3 activity
Cells in each group were collected and washed with
phosphate-buffered saline (PBS). Then the cells were lysed with
trypsin via ice bath, and the cell lysate was extracted to aspirate
the cell culture solution for later use. After that, the adherent
cells were digested using trypsin and collected into the spare cell
culture solution. Finally, Ac-DEVD-pNA (2 mM) was added, mixed and
incubated at 37˚C for 60-120 min. The absorbance could be detected
when there was relatively apparent color change.
ROS concentration
Cells cultured to a density of 6-8x104/ml
in each group were taken and added with culture solution. After
passage for 24 h, different concentrations of stimuli were added
for reaction for 8 h, then the culture solution was discarded, and
the probe CM-H2DCFDA (final concentration: 5 pmol/l) for total ROS
in cells was added for incubation in the dark at 37˚C for 30 min.
Next, the probe was washed with PBS, and a laser scanning confocal
microscope (excitation wavelength: 488 nm and emission wavelength:
515 nm) was employed for detection, under which green fluorescence
was visible. Screenshots were taken with 8-10 cells/high-power
field (x600), and the fluorescence intensity was analyzed via
software.
Nitric oxide (NO) concentration
The concentration was detected using the NO kit
provided by Applygen Technologies Inc. DMEM solution was changed
before pharmacological preconditioning, and the supernatant of the
cells cultured was taken and centrifuged at 4˚C, 950 x g for 10 min
for the measurement of NO content. All the operations were
conducted in strict accordance of the methods in the kit
instructions. Finally, the OD value at the wavelength of 540 nm was
measured by adding Griess Reagent I and Griess Reagent II in
sequence.
Mitochondrial membrane potential
After treatment, the cells were collected and
resuspended in 0.5 ml of cell culture solution with serum
available. Subsequently, 0.5 ml of JC-1 staining solution was added
and mixed by inverting several times, followed by incubation in the
incubator away from light at 37˚C for 30 min and centrifugation at
4˚C, 1,050 x g for 10 min. Then the supernatant was discarded, and
the cells were washed with 1X JC-1 staining buffer twice. Later,
the cells were resuspended in 1X JC-1 staining buffer and
centrifuged at 4˚C, 10,500 x g for 10 min, and the aforementioned
washing was repeated once after the supernatant was discarded.
Finally, the fluorescence intensity of the cells was detected using
a flow cytometer (FACSCalibur; BD Biosciences).
Polymerase chain reaction (PCR)
The treated cells in each group were collected to
extract the total ribonucleic acid (RNA) using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.). After the concentration of the
samples was measured, the reverse transcription system was added
according to the concentration to perform the reverse
transcription. The former 40 cycles were utilized to synthesize the
cDNA, and the reverse transcription reaction conditions were set
for PCR amplification. Real-time fluorescence signal was collected
after each cycle, and the amplification and melting curves were
recorded.
Western blotting (WB)
Cells in each group were fetched and washed twice
with D-Hank's solution, which was then absorbed using absorbent
paper. Next, 150 µl of ice-cold lysis buffer was added into each
group and then placed on ice for lysis for 30 min. The proteins in
each group were collected into an Eppendorf (EP) tube using a cell
scraper, followed by centrifugation at 4˚C, 10,500 x g for 10 min.
Then, the supernatant was sucked and transferred into a new EP
tube, and 5X loading buffer was added and mixed after the protein
concentration was determined via bicinchoninic acid (BCA) method
(Pierce; Thermo Fisher Scientific, Inc.), followed by heating at
100˚C for 6 min. Then 30 µl of proteins was added into prepared
separation gel and spacer gel loading wells, which were subjected
to electrophoresis in the electrophoresis buffer under a proper
voltage. Then, the gel was stuck closely to a polyvinylidene
fluoride (PVDF) membranes (Millipore), followed by transfer in
transmembrane solution at 0˚C under a constant voltage (100 V) for
60 min. After the PVDF membranes were blocked in 5% skim milk
powder at room temperature for 1 h, it was clipped according to the
molecular weight and then sealed in primary antibodies in a
refrigerator at 4˚C overnight. The PVDF membrane was taken out the
next day and rinsed with Tris-buffered saline-Tween-20 (TBST),
followed by addition of secondary antibody IgG (1:5,000) for
incubation at room temperature for 1 h. After that, the membrane
was rinsed with TBST again, and Tannon 5200 immunofluorescence
development system was applied for image development as well as
measurement and calculation of gray scale.
Statistical analysis
The data are presented as mean ± SD (standard
deviation) and percentage to those in control group, and analyzed
using Statistical Product and Service Solutions (SPSS) 13.0
software (SPSS Inc.). The t-test was used for analyzing measurement
data. Differences between two groups were analyzed by using the
Student's t-test. Comparison between multiple groups was done using
One-way ANOVA test followed by Post Hoc Test (Least Significant
Difference). P<0.05 indicates that the difference in data is
statistically significant.
Results
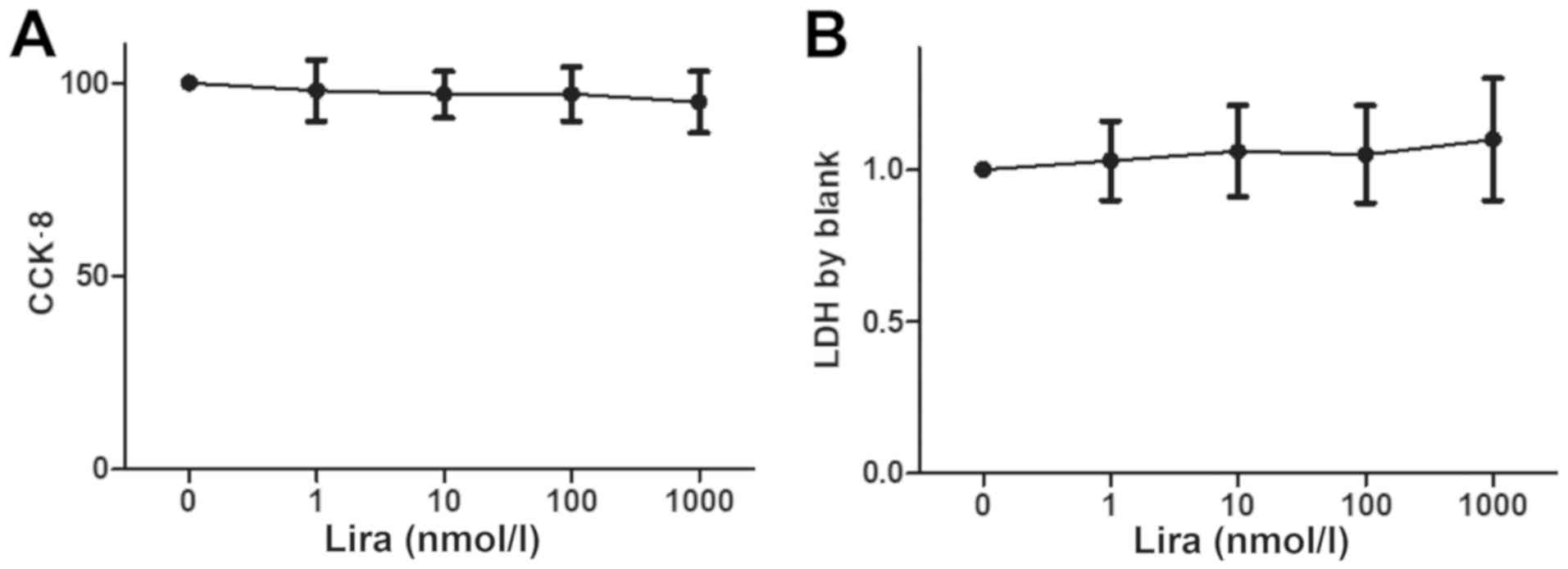
Impact of different concentrations of
liraglutide on H9C2 cell viability and cytotoxicity
Cell viability was detected via CCK-8, and the LDH
concentration was measured to determine the impact on cytotoxicity.
The results showed that there was no obvious decline in cell
viability and cytotoxicity between Lira group and Cont groups
(Fig. 1).
Liraglutide relieves apoptosis of
hypoxic H9C2 cells
The cell viability was detected via CCK-8, the LDH
concentration was measured to determine the impact on cytotoxicity,
the RNA expression of caspase-3 was determined via PCR, and the
activation level of caspase-3 was detected using the kit. According
to the results, the cell viability was weakened markedly in
CoCl2 group compared to that in Cont group (Fig. 2A; P<0.05), while it was
strengthened in Lira groups in comparison to that in
CoCl2 group. Moreover, 100 nmol/l group had higher cell
viability than 10 nmol/l group, displaying a statistically
significant difference (P<0.05). Cell viability in 1,000 nmol/l
group was higher than that in 100 nmol/l group, but the difference
was not statistically significant. Cytotoxicity was increased
evidently in CoCl2 group compared with that in Cont
group (Fig. 2B; P<0.05), while it
was decreased after treatment with liraglutide in comparison to
that in CoCl2 group. Moreover, 100 nmol/l group
manifested a more apparent change than 10 nmol/l group, with a
statistically significant difference (P<0.05), but no
statistical difference was detected between 1,000 nmol/l group and
100 nmol/l group. Both RNA expression and activation level of
caspase-3 were raised remarkably in CoCl2 group, and the
changes were more prominent than those in Cont group (Fig. 2C and D; P<0.05). Liraglutide reduced the RNA
expression and activation level of caspase-3 in a dose-dependent
manner. 100 nmol/l group had more obvious changes than 10 nmol/l
group (P<0.05), while there were no statistically significant
differences between 1,000 nmol/l group and 100 nmol/l group.
Therefore, 100 nmol/l was selected as the therapeutic concentration
for subsequent research. It was proven that liraglutide is able to
protect the hypoxic cells.
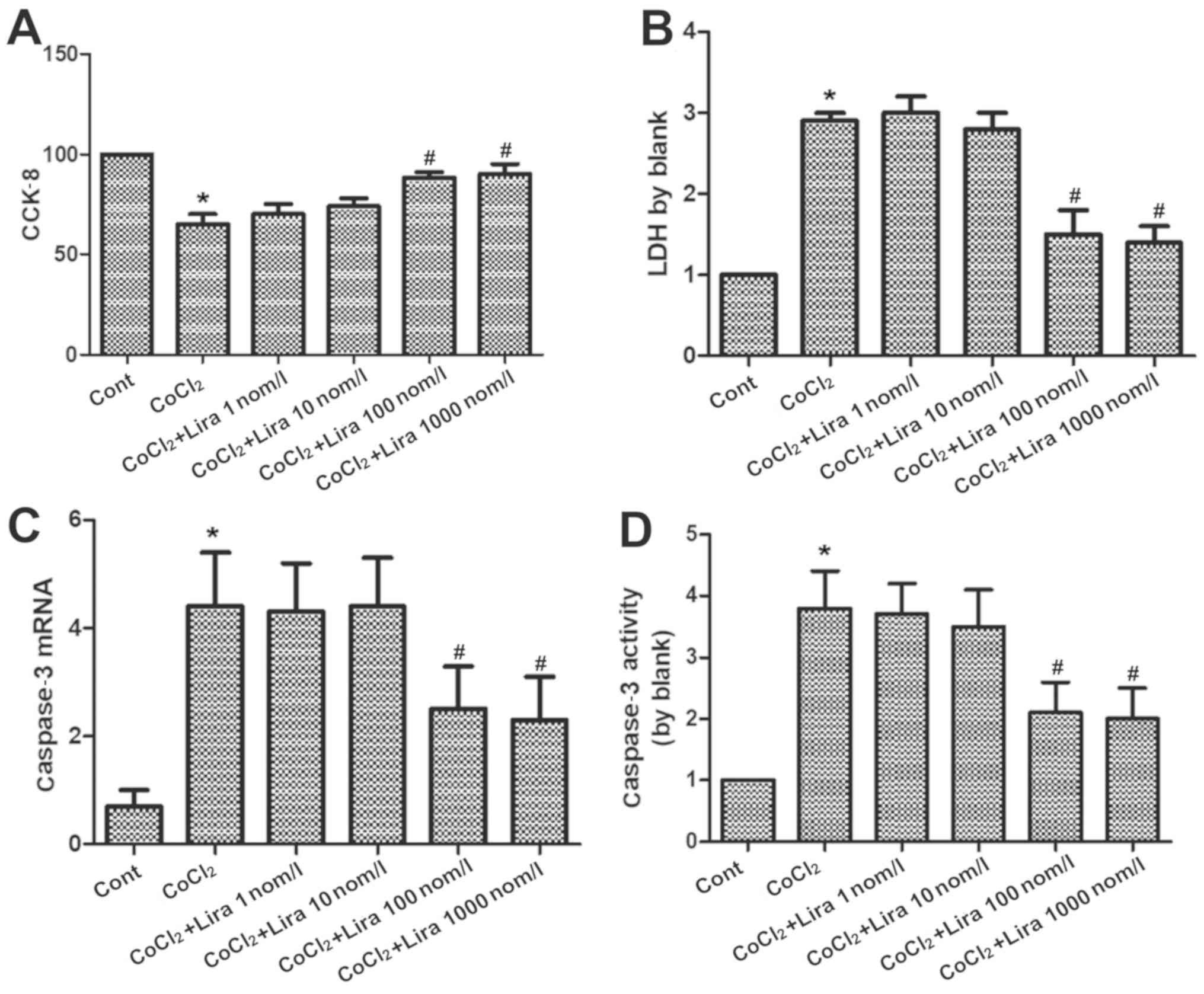 | Figure 2Impact of liraglutide on apoptosis of
hypoxic cardiomyocytes. (A) Cell viability in Cont group, CoCl2
group and CoCl2 + Lira group (1, 10, 100 and 1,000 nmol/l) detected
via CCK-8. (B) Cytotoxicity in Cont group, CoCl2 group and CoCl2 +
Lira group (1, 10, 100 and 1,000 nmol/l) detected via LDH. (C)
Caspase-3 mRNA detected in each group via PCR. (D) Caspase-3
activity detected in each group. *P<0.05 vs. Cont
groups; #P<0.05 vs. CoCl2 groups. CCK-8, cell
counting kit-8; LDH, lactate dehydrogenase. |
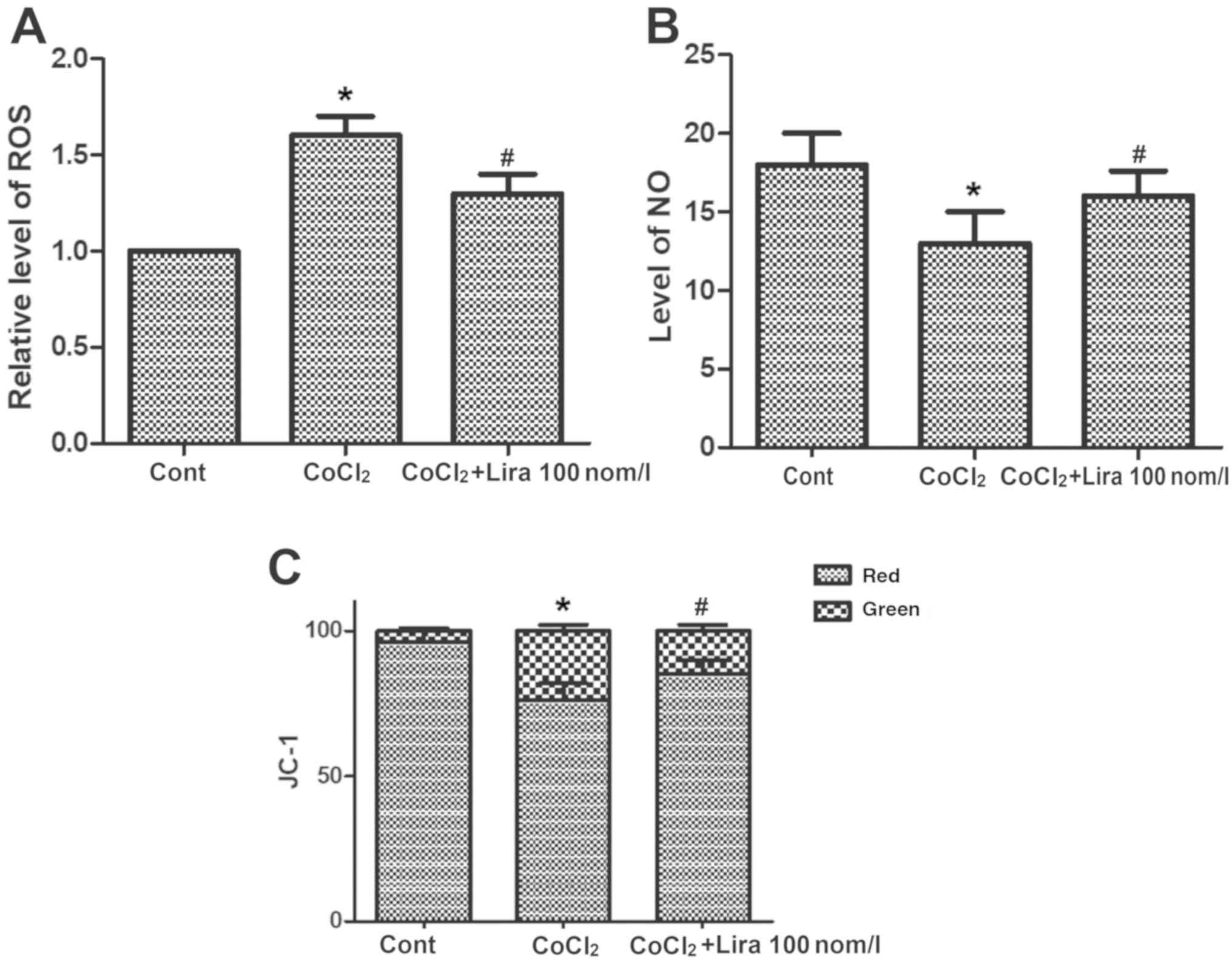
Liraglutide ameliorates oxidative
stress in hypoxic H9C2 cells
The concentrations of ROS and NO and change in
mitochondrial membrane potential were detected. CoCl2
increased the concentration of ROS notably (Fig. 3A; P<0.05), while the
CoCl2-induced ROS increase could be relieved by
liraglutide. CoCl2 group exhibited obviously declined NO
concentration in cells (Fig. 3B;
P<0.05) and destroyed mitochondrial membrane potential, and the
mitochondrial membrane potential was decreased markedly compared
with that in Cont group (Fig. 3C;
P<0.05). In Lira group, however, the lowered NO concentration
triggered by CoCl2 was improved notably, and the
mitochondrial membrane potential was stabilized, displaying
apparent changes in comparison with CoCl2 group
(P<0.05). These results verified that liraglutide is capable of
ameliorating the oxidative stress in hypoxic cells.
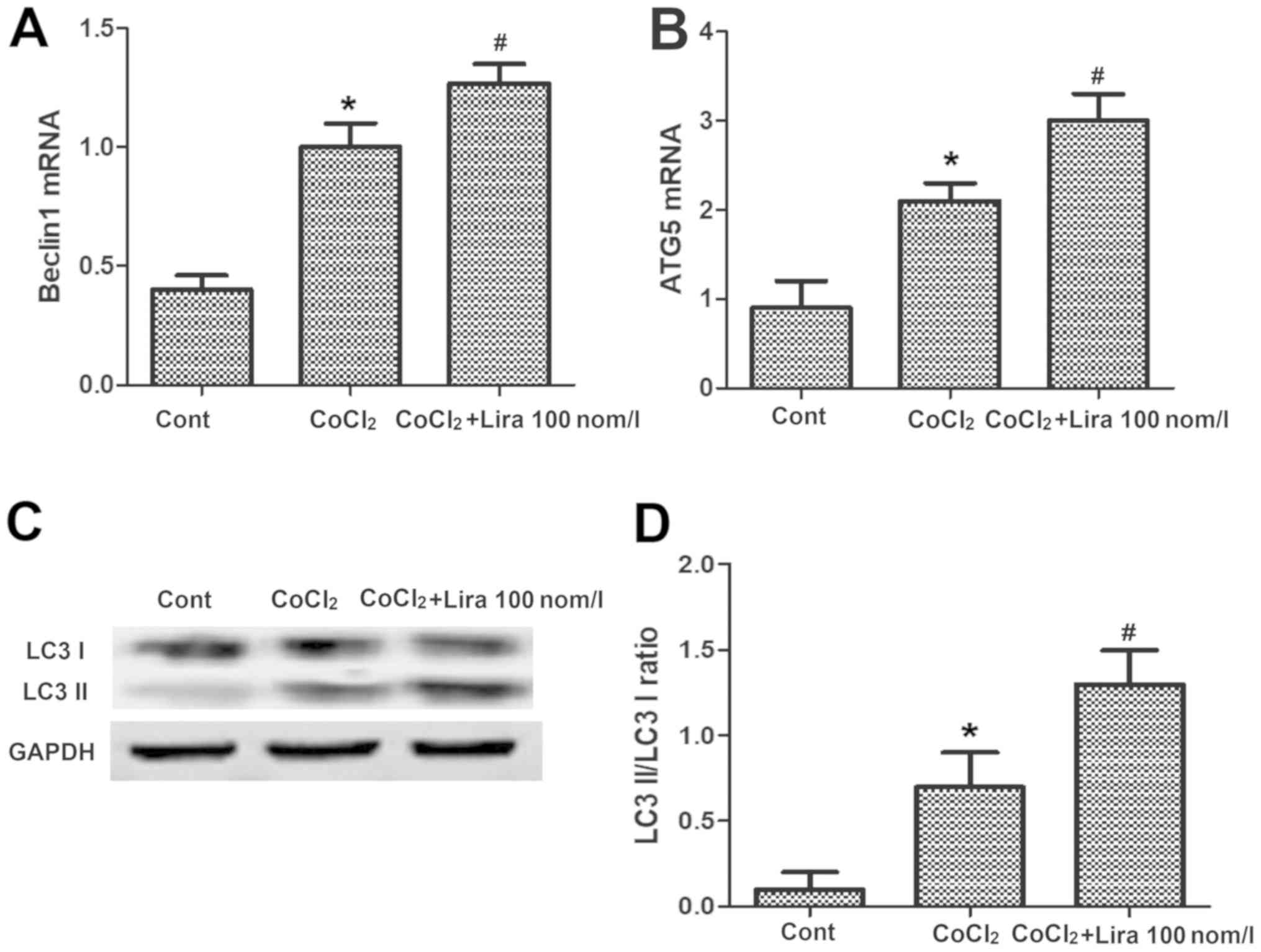
Liraglutide induces autophagy of
hypoxic H9C2 cells
PCR was performed to detect the messenger RNA (mRNA)
expression of Beclin1 and autophagy-related-5 (Atg-5), and WB assay
was conducted to determine the expression of autophagy-related
proteins. It was indicated that after the cell hypoxia was
simulated by adding CoCl2, the mRNA expression of
autophagy-related genes Beclin1 and Atg-5 were raised evidently in
comparison with those in Cont group (Fig. 4A and B; P<0.05). After the addition of
liraglutide, the mRNA expression of Beclin1 and Atg-5 were further
increased, obviously higher than those in CoCl2 group
(Fig. 4A and B; P<0.05). The results of WB assay
revealed that the light chain 3 (LC3) II/LC3 I ratio was increased.
After treatment with liraglutide, the expression of Beclin1 and
Atg-5 were further elevated, and the LC3 II/LC3 I ratio was raised
compared with those in CoCl2 group (Fig. 4C and D; P<0.05), demonstrating that
liraglutide can induce the increased autophagy of hypoxic
cells.
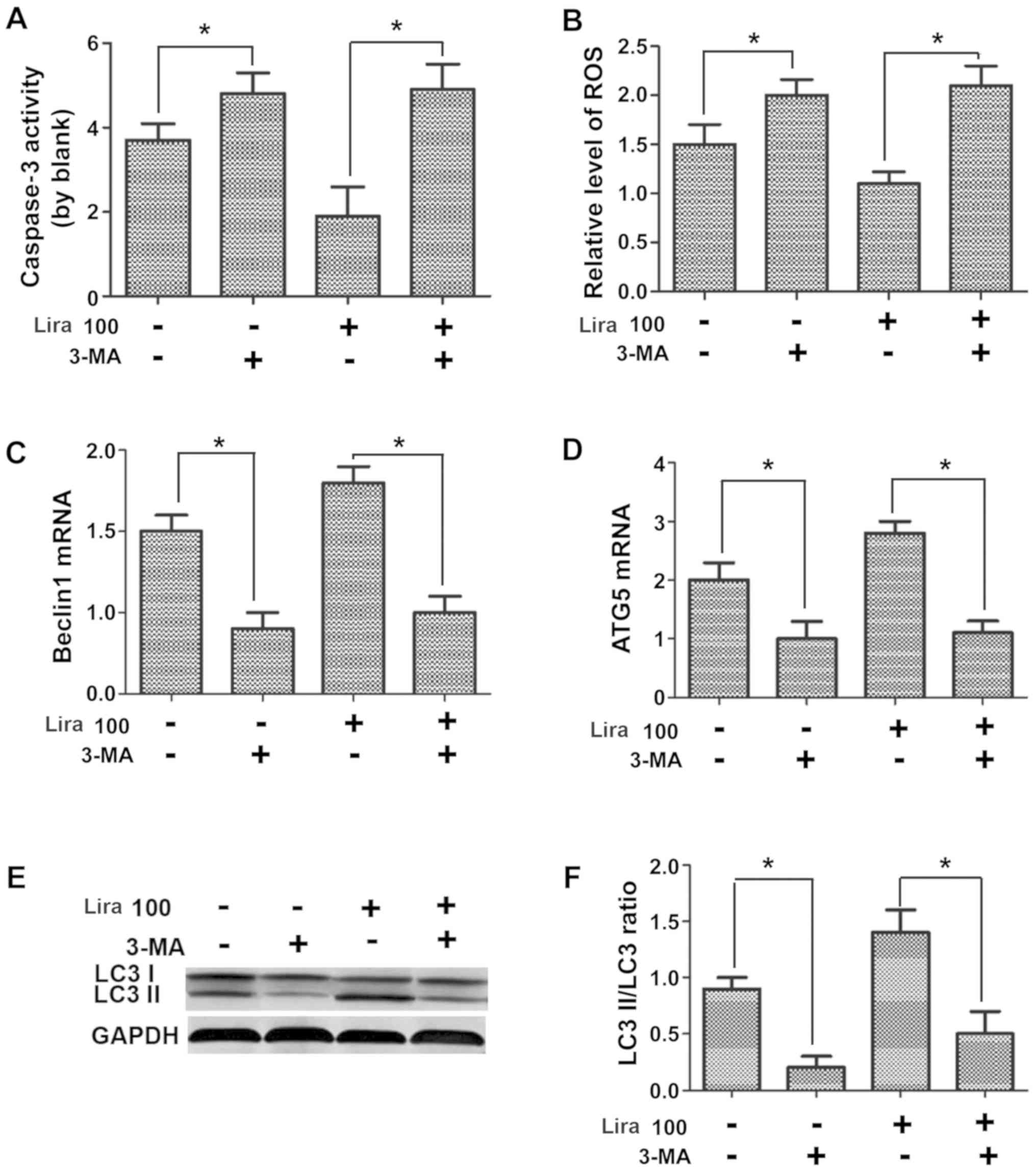
Autophagy inhibitor represses the
protective effects of liraglutide on hypoxic H9C2 cells
Caspase-3 activity and expression of ROS and NO were
detected, the mRNA expression of Beclin1 and Atg-5 were measured
via PCR, and the expression of autophagy-related proteins were
determined through WB assay. According to the results, compared
with those in Lira group, caspase-3 activity and ROS expression
were increased (Fig. 5A and B; P<0.05), while the NO expression was
reduced (Fig. 5C; P<0.05) in
hypoxic cardiomyocytes after adding 3-MA inhibitor. The detection
of expression of autophagy-related genes indicated that 3-MA could
prominently repress the increased mRNA expression of Beclin1 and
Atg-5 induced by liraglutide (Fig.
5D and E; P<0.05), and the
LC3 II/LC3 I ratio was decreased notably (Fig. 5F; P<0.05), confirming that the
inhibition on cell autophagy can suppress the protective effects of
liraglutide on hypoxic cells.
Discussion
CoCl2 is a very important hypoxia inducible factor.
It was shown in this study that liraglutide was able to alleviate
the CoCl2-induced cardiomyocyte injury, whose major mechanism was
to relieve intracellular oxidative stress, mitochondrial damage and
cardiomyocyte apoptosis by promoting cell autophagy.
As an analogue of human GLP-1, liraglutide can exert
potential cardiovascular protective effects through both GLP-1
receptor-dependent and -independent pathways (9), but its mechanism remains unclear.
Currently, most studies have argued that the GLP-1 receptor is
expressed in myocardial tissues (10). It has been pointed out in various
studies that in in vitro experiments, the GLP-1 receptor
agonist exerts the protective effects by directly activating the
GLP-1 receptor on the myocardium (11,12).
Ischemic injury can reduce the content of ATP in
myocardial cells, resulting in energy stress and excessive
production of ROS (2). Ischemia
causes hypoxia of myocardial cells, leading to serious or
irreversible cardiac injury (3).
Excessive ROS from mitochondria is closely related to the
pathogenesis of cardiovascular diseases, such as atherosclerosis,
myocardial infarction and heart failure (1,2). In this
study, we found that liraglutide relieved the ROS increase,
improved the lowered NO concentration and stabilized the
mitochondrial membrane potential, which indicated that liraglutide
is capable of ameliorating the oxidative stress in hypoxic
cells.
Autophagy, an intracellular protective mechanism,
can degrade the misfolded proteins and damaged organelles such as
mitochondria in the cells, thus enabling the mitochondria to
synthesize ATP and maintain energy homeostasis in cells (13). Whether the autophagy has protective
effects on hypoxic myocardium still remains controversial. Some
research teams argued that it is beneficial in increasing the
autophagy level in myocardium (14),
but it is also reported that the excess increase in autophagy will
damage the myocardium (15).
According to the experimental results in this
research, liraglutide elevated the autophagy level in myocardium
and had certain mycardial protective effects at the same time.
Therefore, it was considered in this study that properly increased
autophagy in CoCl2-induced hypoxic myocardium is conducive to
eliminating the damaged substances in cells, reducing oxidative
stress, ameliorating mitochondrial damage and producing
cardioprotective effects. Interestingly, the expression of Beclin
and Atg-5 also increased in CoCl2 treated cells. However, no
protective effect was found in CoCl2 treated cells. This phenomenon
further demonstrates the complexity of autophagy.
The autophagy inhibitor 3-MA was applied to further
prove that the protective effects of liraglutide on the
CoCl2-induced hypoxic myocardium is associated with cell autophagy
(16). It was found that after the
introduction of 3-MA, the autophagy level and the effects of
liraglutide on cardiomyocyte autophagy induced were repressed.
Furthermore, the analyses on oxidative stress, mitochondrial damage
and apoptosis change in cells revealed that the effects of
liraglutide in alleviating oxidative stress in cardiomyocytes,
protecting the mitochondria and resisting cell apoptosis were
inhibited significantly after the addition of 3-MA. The results
confirmed that the protective effects of liraglutide can be
inhibited by 3-MA, that is, the protective effects of liraglutide
on hypoxic cardiomyocytes are triggered by inducing cardiomyocyte
autophagy.
In conclusion, liraglutide ameliorates the
CoCl2-induced oxidative stress in hypoxic cardiomyocytes, relieve
mitochondrial damage and reduce apoptosis via inducing cell
autophagy.
Acknowledgements
Not applicable.
Funding
Not funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZP and SL designed the study and performed the
experiments, TW, YL and LW collected the data, JY and HD analyzed
the data, ZP and SL prepared the manuscript. All authors read and
approved the inal manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lai YF, Wang L and Liu WY: Nicotinamide
pretreatment alleviates mitochondrial stress and protects hypoxic
myocardial cells via AMPK pathway. Eur Rev Med Pharmacol Sci.
23:1797–1806. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheng Y, Liu DZ, Zhang CX, Cui H, Liu M,
Zhang BL, Mei QB, Lu ZF and Zhou SY: Mitochondria-targeted
antioxidant delivery for precise treatment of myocardial
ischemia-reperfusion injury through a multistage continuous
targeted strategy. Nanomedicine (Lond). 16:236–249. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Y, Qiu L, Liu X, Hou Z and Yu B: PINK1
alleviates myocardial hypoxia-reoxygenation injury by ameliorating
mitochondrial dysfunction. Biochem Biophys Res Commun. 484:118–124.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arturi F, Succurro E, Miceli S, Cloro C,
Ruffo M, Maio R, Perticone M, Sesti G and Perticone F: Liraglutide
improves cardiac function in patients with type 2 diabetes and
chronic heart failure. Endocrine. 57:464–473. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Inoue T, Inoguchi T, Sonoda N, Hendarto H,
Makimura H, Sasaki S, Yokomizo H, Fujimura Y, Miura D and
Takayanagi R: GLP-1 analog liraglutide protects against cardiac
steatosis, oxidative stress and apoptosis in streptozotocin-induced
diabetic rats. Atherosclerosis. 240:250–259. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chang G, Liu J, Qin S, Jiang Y, Zhang P,
Yu H, Lu K, Zhang N, Cao L, Wang Y, et al: Cardioprotection by
exenatide: A novel mechanism via improving mitochondrial function
involving the GLP-1 receptor/cAMP/PKA pathway. Int J Mol Med.
41:1693–1703. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Y, Liu J, Tao Z, Wu P, Cheng W, Du Y,
Zhou N, Ge Y and Yang Z: Exogenous HGF prevents cardiomyocytes from
apoptosis after hypoxia via up-regulating cell autophagy. Cell
Physiol Biochem. 38:2401–2413. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang J, Wu J, Wu H, Liu X, Chen Y, Wu J,
Hu C and Zou D: Liraglutide protects pancreatic β-cells against
free fatty acids in vitro and affects glucolipid metabolism
in apolipoprotein E-/- mice by activating autophagy. Mol
Med Rep. 12:4210–4218. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS,
Drucker DJ and Husain M: Cardioprotective and vasodilatory actions
of glucagon-like peptide 1 receptor are mediated through both
glucagon-like peptide 1 receptor-dependent and -independent
pathways. Circulation. 117:2340–2350. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wei Y and Mojsov S: Distribution of GLP-1
and PACAP receptors in human tissues. Acta Physiol Scand.
157:355–357. 1996.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu SY, Zhang Y, Zhu PJ, Zhou H and Chen
YD: Liraglutide directly protects cardiomyocytes against
reperfusion injury possibly via modulation of intracellular calcium
homeostasis. J Geriatr Cardiol. 14:57–66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bose AK, Mocanu MM, Carr RD, Brand CL and
Yellon DM: Glucagon-like peptide 1 can directly protect the heart
against ischemia/reperfusion injury. Diabetes. 54:146–151.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He C, Zhu H, Li H, Zou MH and Xie Z:
Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances
cardiac autophagy and protects against cardiomyocyte apoptosis in
diabetes. Diabetes. 62:1270–1281. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kobayashi S, Xu X, Chen K and Liang Q:
Suppression of autophagy is protective in high glucose-induced
cardiomyocyte injury. Autophagy. 8:577–592. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang J, Pi C and Wang G: Inhibition of
PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy
in hepatocellular carcinoma cells. Biomed Pharmacother.
103:699–707. 2018.PubMed/NCBI View Article : Google Scholar
|