Introduction
Approximately 30% of patients with cirrhosis have
oesophageal varices at the time of diagnosis; this proportion
increases with time and reaches 90% after ~10 years (1). Patients with oesophageal varices have a
high tendency to develop bleeding. Only 10-20% of variceal bleeding
occurs from gastric varices, but the associated outcome is worse
than that of bleeding from oesophageal varices (2-5).
Patients surviving a variceal bleed are at high risk of rebleeding
(>60% in the first year) and the mortality of each rebleeding
episode is ~20% (6). Therefore,
prevention of recurrent variceal bleeding is important for patients
with cirrhosis.
For secondary prophylaxis of variceal bleeding, the
goal of improving outcomes is evolving, since therapy in these
cases attempts to reduce the risk of death, and thus prevent the
onset of complications of cirrhosis that may lead to death
(1). The treatment effectiveness of
secondary preventions, including endoscopic ligation or endoscopic
sclerotherapy (ES), drug therapies [non-selective β-blockers (NSBB)
with or without isosorbide mononitrate (ISMN)] and transjugular
intrahepatic portosystemic shunts (TIPS) is an area of interest,
but at present, a firm consensus as to the most effective treatment
has not been reached. Several randomized controlled trials (RCTs)
have investigated treatment outcomes in terms of mortality,
complications and adverse effects (7-12).
A previous study compared endoscopic variceal ligation (EVL) with a
combination of EVL and nadolol and identified that adverse effects
more frequently occurred in the EVL plus nadolol group (0.03 vs.
33%) (12). Another trial compared
nadolol plus ISMN alone with EVL plus the drug combination and
observed that the combination of EVL and drugs led to more adverse
effects (62 vs. 32%), but there were no significant differences in
either mortality or the causes of death (11). However, a previous direct
meta-analysis comprising 925 patients comparing endoscopic therapy
with a combination of BB and endoscopic therapy identified that
mortality at 24 months was significantly lower in the combined
treatment group (13). In addition
to inconsistent results among the previous trials and analyses, to
the best of our knowledge, there has been no previous network
meta-analysis to compare treatment outcomes. Therefore, the present
study was performed to compare the effectiveness of standard
treatments for the secondary prevention of variceal bleeding in
patients with cirrhosis through a network meta-analysis. The
specific treatments studied were TIPS, endoscopic therapy (EVL
alone or ES alone), a combination of EVL and ES, a combination of
EVL/ES and NSBB (propranolol and nadolol) with or without ISMN, as
well as a combination of NSBB and ISMN.
Materials and methods
Literature search
Searches were performed in the electronic PubMed,
Cochrane Library, Embase and Web of Science databases in February
2018. The following search terms were used: ‘Cirrhotic patients’,
‘patients with cirrhosis’, ‘liver cirrhosis’, ‘haemorrhage’,
‘bleeding’, ‘rebleeding’, ‘variceal’, ‘oesophageal varices’,
‘endoscopic variceal ligation’, ‘endoscopic band ligation’,
‘endoscopic ligation’, ‘endoscopic sclerotherapy’, ‘sclerotherapy’,
‘endoscopic therapy’, ‘vasoconstrictors’, ‘venodilators’,
‘adrenergic beta antagonist’, ‘adrenergic-beta antagonist’,
‘adrenergic beta-antagonist’, ‘adrenergic-beta-antagonist’,
‘nitrate’, ‘beta-blocker’, ‘isosorbide mononitrate’, ‘placebo’,
‘TIPS’, ‘transjugular intrahepatic portosystemic shunt’ and
‘randomized controlled trial’. Manual searches of reference lists
of relevant articles were also performed to identify additional
studies. Only RCTs were included.
Inclusion and exclusion criteria
RCTs, irrespective of publication status, were
included if they investigated endoscopic therapy with various
combinations of NSBB and ISMN, or TIPS alone among adult patients
with cirrhosis, who had at least one previous episode of upper
gastrointestinal bleeding. Trials fulfilling the following criteria
were excluded: i) Focus on primary prevention of variceal bleeding;
ii) inclusion of pediatric patients or patients without cirrhosis;
iii) comparison of only one of the aforementioned treatment
regimens with other treatment(s), as it was impossible to make a
network comparison; or iv) a clearly irrelevant topic, e.g.
nutrition after variceal bleeding.
Study selection
Only RCTs whose reports were available in English or
Chinese were included. If a trial was designed with more than two
treatment arms, at least two of the arms had to match the scope of
the present study.
Data extraction
According to the newly published guidelines of the
American Association for the Study of Liver Diseases (AASLD) and
consensus (14), therapies for
secondary prophylaxis must account for the presence or absence of
other complications of cirrhosis. In patients with a low risk of
death (those with variceal haemorrhage as the sole complication of
cirrhosis), the objective of therapy should be the prevention of an
additional complication, whereas in patients with a high risk of
death (those with variceal haemorrhage and other decompensating
events), the objective of therapy should be to improve survival
(15,16). Mortality (overall mortality,
mortality due to rebleeding and mortality due to liver failure),
treatment failure and complications (bleeding from gastroesophageal
ulcer) were analyzed.
Data of treatment failure were analyzed when clearly
stated in the literature, with exclusion of data that satisfied
certain criteria but lacked declaration of treatment failure.
Authors of the included trials were not approached for further data
due to the large number of RCTs selected and acquisition of
adequate data associated with treatment outcomes. The primary
outcomes were overall mortality, mortality due to rebleeding,
including but not limited to recurrent variceal bleeding and
mortality due to liver failure. Overall mortality was defined as
death that occurred during the trial treatment or follow-up caused
by disease progression or treatment complications. Secondary
outcomes were treatment failure and bleeding from gastroesophageal
ulcers, including but not limited to post-banding ulcers.
Methodological quality
A bias assessment was performed for the included
trials by evaluating randomization, completion of trials and
blinding. The major targets were concealment randomization,
participant blinding, health care provider blinding, data collector
blinding, outcome assessor blinding and early trial cessation.
Randomization was considered concealed if it
involved a third independent party or person not involved in the
treatment of patients, opaque sealed envelopes or a similar method.
Trials were not considered to feature early cessation unless
premature termination was specifically announced in the
article.
Statistical analysis
The odds ratio (OR) was used to denote the results
with a 95% CI, indicating the strength of association between
treatments and outcomes. An OR<1 represents the benefit of the
comparison group compared with the control group. Pooled ORs and
their 95% CIs were also calculated. Statistical significance was
established with a two-sided P<0.05 or a CI that did not include
a value of 1. The risk ratio was not used to measure outcomes due
to limited data regarding the number of events among the selected
trials.
To assess the comparability of the included trials,
a heterogeneity analysis was performed. Since inconsistency is a
source of heterogeneity in network meta-analyses, a generalized
Cochran's Q statistic (Qtotal) and I2
statistic were adopted for assessment of homogeneity and
consistency assumptions. Statistical heterogeneity was considered
significant when P<0.10 for the Q-test or I2<50%.
The network meta-analysis used fixed-effects models with
I2 values of 0% for overall mortality, mortality due to
rebleeding, mortality due to liver failure and bleeding from a
gastroesophageal ulcer, and I2=29.4% for treatment
failure.
All treatments were ranked according to P-score,
which is on a scale from 0 (worst) to 1 (best). P-scores are based
solely on the point estimates and standard errors of the most
frequent network meta-analysis estimates under normality
assumption, and can easily be calculated as means of one-sided
P-values. They measure the extent of certainty that a treatment is
better than another treatment, averaged over all competing
treatments (17). Sensitivity
analysis was performed by removal of trials with a mean follow-up
of <6 months. The network meta-analyses were performed using R
3.3.1 along with the ‘netmeta’ package by Schwarzer et al
(18).
Results
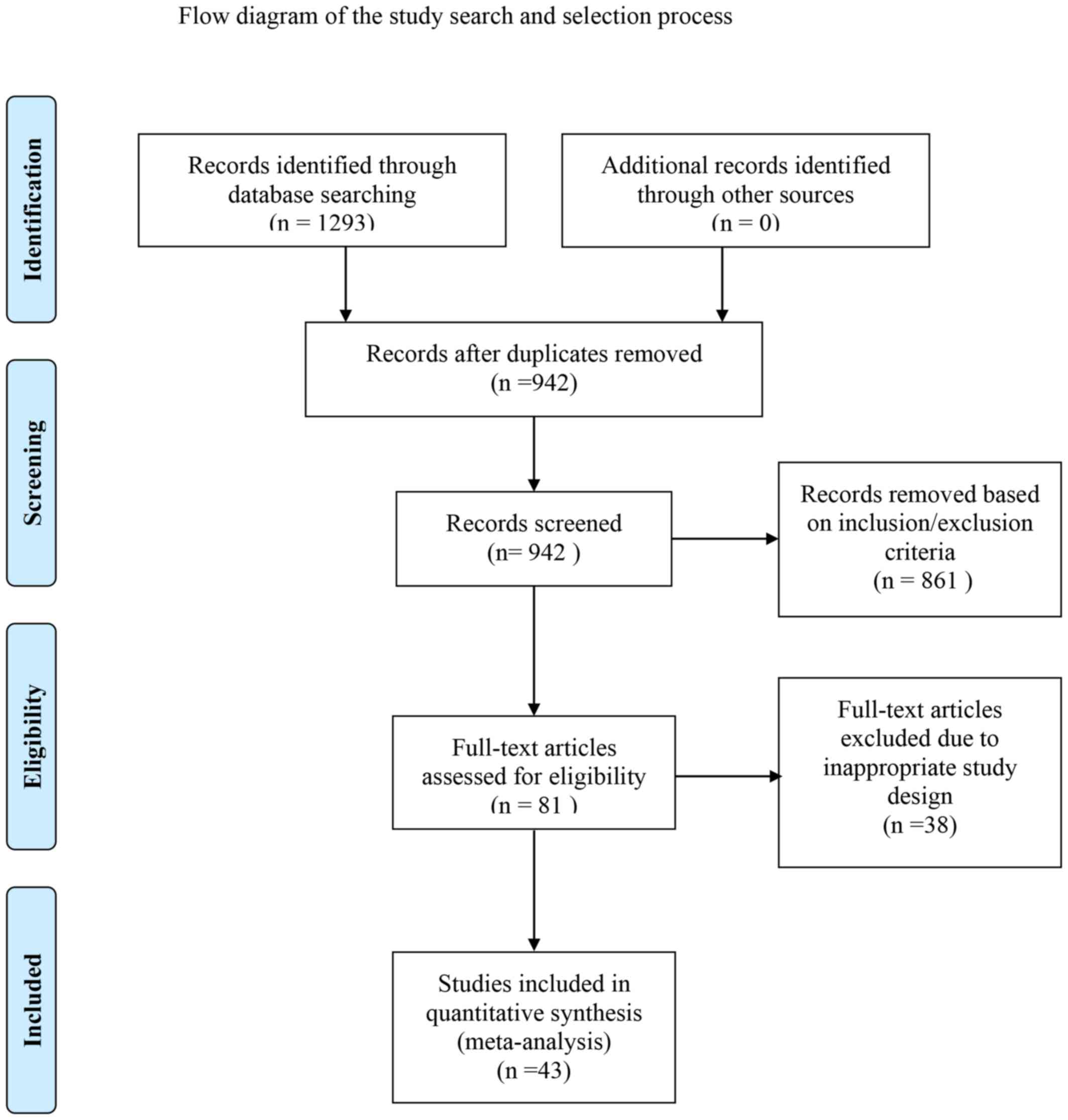
Search results
Electronic and manual searches identified 1,293
records in total. Following screening of titles and abstracts, 861
references were excluded and the remainder was subjected to
full-text screening. Among the excluded studies were duplicates,
non-RCTs, trials investigating other treatments, or those covering
different topics or focusing on primary prevention of variceal
bleeding, due to inadequate data for the present study or
randomizing patients without cirrhosis. A previous trial
investigating the effects of carvedilol plus EVL was excluded, as
it assessed hemodynamic responses but not mortality (19). The screening process followed the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
(PRISMA) guidelines and is depicted in a flow chart in Fig. 1.
Characteristics of the studies
included
A total of 43 trials (20-62)
with a total sample size of 3,787 patients with cirrhosis were
included for quantitative network meta-analysis. In total, 5
references were published or available as abstracts (22,35,39,42,43) and
the remainder were available in full text. A previous trial had 4
treatment arms (44), among which 3
(EVL alone, EVL plus propranolol plus ISMN and propranolol plus
ISMN) were included from the present study. Another had 3 arms
(22), of which 2 (EVL alone and
propranolol plus ISMN) were included, and the arm of carvedilol
treatment alone was excluded. All of the other trials were designed
with 2 treatment arms. The proportion of patients with cirrhosis
was 100% in all of the trials. The baseline characteristics of the
trials are presented in Table I.
Patient inclusion and exclusion criteria varied slightly across
trials, but patients were generally eligible if they were adults
with cirrhosis with at least one episode of endoscopic-proven
oesophageal or gastric variceal bleeding. Exclusion criteria
included hepatocarcinoma, non-bleeding varices, existing
multi-organ failure and lack of cirrhosis. A total of 30 of the 43
studies had a mean follow-up time of >2 years, as presented in
Table I. In total, five trials were
excluded from the sensitivity analysis due to follow-up times that
were unknown or <6 months (43,47,55,60,61).
TIPS alone was used as the comparative treatment in the forest
plots, since it is a recommended surgery for secondary prophylaxis
according to the newest UK guidelines (5).
 | Table ICharacteristic of randomized
controlled trials included in the network meta-analysis. |
Table I
Characteristic of randomized
controlled trials included in the network meta-analysis.
| First author
(year) | Treatment
groups | Patients (n) | Mean age
(years) | Females (%) | Varices, grade or
size, n | Child-Pugh class
(A/B/C) | Mean Pugh
score | Mean follow-up
(days) | No. of
subjectsa | (Refs.) |
|---|
| Sauerbruch
(2015) | TIPS | 90 | 55 | 31 | NA | 45% A | 6.9 | 906 | 27/NA/11/NA/NA | (20) |
| |
Propranolol+ISMN | 95 | 55 | 34 | NA | 49% A | 7 | 906 | 25/NA/9/NA/NA | |
| Luo (2015) | TIPS | 37 | 51 | 49 | NA | 0/25/12 | 8.76 | 690 | 12/1/9/NA/0 | (21) |
| |
EVL+Propranolol | 36 | 50 | 33 | NA | 0/24/12 | 8.89 | 637 | 17/3/10/NA/2 | |
| Kumar (2015) | EVL | 56 | 44 | NA | NA | NA | 8.6 | 500 | 9/NA/NA/NA/NA | (22) |
| |
Propranolol+ISMN | 39 | 44 | NA | NA | NA | 8.6 | 500 | 8/NA/NA/NA/NA | |
| Kong (2015) | EVL | 20 | 54 | 30 | F2:10; F3:10 | 9/11/0 | NA | 488 | 0/NA/NA/NA/NA | (23) |
| | ES | 18 | 53 | 50 | F2:9; F3:11 | 8/10/0 | NA | 488 | 0/NA/NA/NA/NA | |
| Kumar (2009) |
EVL+Propranolol+ISMN | 88 | 42 | 15 | MG 3.1:88 | 35/31/10 | 3.2 | 458 | 2/NA/NA/NA/NA | (24) |
| | EVL | 89 | 41 | 12 | MG 3.2:89 | 26/34/15 | 3.2 | 458 | 3/NA/NA/NA/NA | |
| Sauer (2002) | TIPS | 43 | 54 | 37 | MG 2.4:43 | 15/16/12 | 7.9 | 1,498 | 8/1/4/1/NA | (25) |
| | EVL | 42 | 55 | 45 | MG 2.6:42 | 10/19/13 | 8.2 | 1,315 | 7/2/2/5/NA | |
| Gülberg (2002) | TIPS | 28 | 57 | 29 | NA | 11/15/2 | NA | 730 | 4/1/2/NA/NA | (26) |
| | EVL | 26 | 56 | 27 | NA | 10/12/4 | NA | 730 | 4/2/1/NA/NA | |
| Escorsell
(2002) |
Propranolol+ISMN | 46 | 56 | 20 | NA | 0/28/16 | NA | 470 | 11/4/2/NA/NA | (27) |
| | TIPS | 47 | 57 | 30 | NA | 0/30/17 | NA | 436 | 13/3/5/NA/NA | |
| Villanueva
(2001) | EVL | 72 | 58 | 35 | G1: 1; G2:49;
G3:22 | 11/43/18 | 8.4 | 666 | 30/10/12/23/7 | (28) |
| | Nadolol+ISMN | 72 | 60 | 40 | G1: 2; G2:41;
G3:29 | 19/39/14 | 7.9 | 605 | 23/4/10/12/0 | |
|
Pomier-Layrargues | EVL | 39 | 54 | 31 | NA | NA | 9.8 | 581 | 16/3/9/NA/NA | (29) |
| (2001) | TIPS | 41 | 53 | 29 | NA | NA | 9.6 | 678 | 17/0/10/NA/NA | |
| Narahara
(2001) | TIPS | 38 | 51 | 16 | NA | NA | 6.8 | 1,116 | 11/NA/7/NA/0 | (30) |
| | ES | 40 | 55 | 25 | NA | NA | 7.4 | 1,047 | 7/NA/NA/NA/NA | |
| Hou (2001) | EVL | 47 | 55 | 34 | NA | 14/17/16 | 8.2 | 351 | 6/NA/4/NA/NA | (31) |
| | EVL+ES | 47 | 59 | 23 | NA | 11/23/13 | 8 | 336 | 7/NA/5/NA/NA | |
| Meddi (1999) | TIPS | 18 | 61 | 28 | NA | NA | 7.6 | 545 | 4/NA/NA/NA/NA | (32) |
| | ES | 20 | 58 | 30 | NA | NA | 7.6 | 545 | 2/NA/NA/NA/NA | |
| Villarreal
(1999) | TIPS | 22 | 58 | 32 | NA | 5/10/7 | 8.6 | 760 | 3/0/1/0/NA | (33) |
| | ES | 24 | 55 | 8 | NA | 3/14/7 | 8.8 | 503 | 8/6/1/7/NA | |
| Sauer (1997) | TIPS | 42 | 54 | 64 | NA | 15/18/9 | 7 | 530 | 12/0/7/NA/0 | (34) |
| | ES | 41 | 60 | 51 | NA | 12/18/11 | 7 | 584 | 11/5/3/NA/3 | |
| Sanyal (1997) | TIPS | 41 | NA | NA | NA | NA | NA | 1,000 | 12/NA/2/NA/NA | (35) |
| | ES | 39 | NA | NA | NA | NA | NA | 1,000 | 7/NA/2/NA/NA | |
| Villanueva
(1996) | Nadolol+ISMN | 43 | 58 | 33 | G1:3; G2:30;
G3:10 | 9/27/7 | NA | 545 | 4/0/2/3/1 | (36) |
| | ES | 43 | 60 | 33 | G1:2; G2:31;
G3:10 | 11/22/10 | NA | 545 | 9/2/5/16/7 | |
| Li (2010) | ES | 36 | 54 | 58 | G1:0; G2:0;
G3:36 | NA | 7.36 | 181.5 | 7/NA/NA/4/7 | (37) |
| | EVL | 27 | 51 | 26 | G1: 0; G2:0;
G3:27 | NA | 6.86 | 181.5 | 1/NA/NA/0/0 | |
| Holster (2016) |
EVL+Propranolol | 35 | 54 | 49 | NA | 13/18/4 | 7.3 | 708 | 9/NA/0/12/2 | (38) |
| | TIPS | 37 | 56 | 34 | NA | 13/19/5 | 7.5 | 708 | 12/NA/3/14/1 | |
| van Buuren
(2000) | TIPS | 19 | NA | NA | NA | NA | NA | 632 | NA/NA/NA/1/NA | (39) |
| | EVL | 18 | NA | NA | NA | NA | NA | 632 | NA/NA/NA/0/NA | |
| Cabrera (1996) | ES | 32 | 56 | 28 | NA | 14/16/2 | 7.22 | 454 | 5/3/NA/10/5 | (40) |
| | TIPS | 31 | 56 | 35 | NA | 14/13/4 | 7.1 | 454 | 6/0/NA/0/0 | |
| Dobrucali
(1998) | EVL | 50 | 55 | 22 | G2:1; G3:5;
G4:44 | 18/23/9 | NA | 908 | NA/NA/4/NA/NA | (41) |
| | ES | 28 | 42 | 43 | G2:2; G3:3;
G4:23 | 7/13/8 | NA | 908 | NA/NA/0/NA/NA | |
| Holster (2013) | TIPS | 35 | 54 | 44 | NA | NA | 7.4 | 545 | 8/NA/NA/NA/NA | (42) |
| | EVL | 36 | 54 | 44 | NA | NA | 7.4 | 545 | 7/NA/NA/NA/NA | |
| Merli (1994) | TIPS | 21 | NA | NA | NA | NA | NA | NA | 2/NA/NA/NA/NA | (43) |
| | ES | 17 | NA | NA | NA | NA | NA | NA | 1/NA/NA/NA/NA | |
| Ahmad (2009) |
Propranolol+ISMN | 35 | 52 | 40 | G1+G2:12;
G3:23 | 2/19/14 | 9.11 | 182 | 6/4/4/9/0 | (44) |
| | EVL | 39 | 53 | 36 | G1+G2:15;
G3:24 | 7/23/9 | 8.28 | 182 | 8/2/3/12/3 | |
| |
EVL+propranolol+ISMN | 37 | 50 | 19 | G1+G2:15;
G3:22 | 4/27/6 | 8.32 | 182 | 7/2/3/8/2 | |
| Argonz (2000) | EVL | 41 | 53 | 22 | G2:27; G3:14 | 14/23/4 | NA | 337 | 16/9/1//1 | (45) |
| | EVL+ES | 39 | 53 | 23 | G2:22; G3:17 | 11/26/2 | NA | 386 | 12/4/3//2 | |
| Avgerinos
(1997) | EVL | 37 | 55 | 16 | S:1; M:19;
L:17 | 23/11/3 | 7.7 | 472 | 8/0/NA/5/2 | (46) |
| | ES | 40 | 56 | 20 | S:3; M:19;
L:18 | 27/10/3 | 8 | 454 | 8/2/NA/4/4 | |
| Avgerinos
(2004) | ES | 25 | 52 | 24 | G1:3; G2:13;
G3:9 | 6/8/11 | 9.4 | 42 | 7/4/NA/13/NA | (47) |
| | EVL | 25 | 56 | 20 | G1:3; G2:12;
G3:10 | 7/6/12 | 9.2 | 42 | 5/2/NA/6/NA | |
| Avgerinos
(1993) | ES | 40 | 59 | 20 | S:3; M:19;
L:18 | 30/8/2 | 7.3 | 1,004 | 9/2/3/NA/NA | (48) |
| | ES+Propranolol | 45 | 58 | 36 | S:4; M:19;
L:22 | 33/8/4 | 7.6 | 1,035 | 8/3/3/NA/NA | |
| Baroncini
(1997) | ES | 54 | 61 | 31 | F3:36; F2:18 | 18/22/14 | 8 | 534 | 12/3/NA/NA/NA | (49) |
| | EVL | 57 | 63 | 33 | F3:42; F2:15 | 17/24/16 | 7.7 | 496 | 12/1/NA/NA/NA | |
| Chen (2016) | EVL | 48 | 56 | 33 | G1:18; G2:26;
G3:4 | 19/29/0 | NA | 182 | 1/1/NA/0/NA | (16) |
| | EVL+ES | 48 | 54 | 35 | G1:19; G2:23;
G3:6 | 20/28/0 | NA | 182 | 3/2/NA/0/NA | |
| García-Pagán
(2009) | Nadolol+ISMN | 78 | 56 | 32 | L:73 | 18/42/18 | 8.1 | 454 | 15/8/1/22/0 | (50) |
| |
EVL+Nadolol+ISMN | 80 | 57 | 19 | L:73 | 16/46/18 | 8.2 | 454 | 16/5/4/17/4 | |
| Hou (1995) | ES | 67 | 61 | 21 | F3:49;
F2+F1:18 | 15/29/23 | 8.2 | 293 | 11/4/6/NA | (51) |
| | EVL | 67 | 60 | 19 | F3:55;
F2+F1:12 | 17/21/29 | 8.8 | 287 | 14/0/13/NA/NA | |
| Hou (2000) | ES | 70 | 60 | 19 | F3:51;
F2+F1:19 | 17/34/19 | 8 | 1,918 | 11/4/NA/NA/2 | (52) |
| | EVL | 71 | 60 | 21 | F3:57;
F2+F1:14 | 20/26/25 | 8.4 | 1,818 | 9/1/NA/NA/3 | |
| Lo (1995) | ES | 59 | 54 | 17 | NA | 7/24/28 | 8.9 | 290 | 19/9/6/NA/NA | (53) |
| | EVL | 61 | 57 | 21 | NA | 9/22/30 | 9.5 | 310 | 10/4/6/NA/NA | |
| Lo (1997) | EVL | 37 | 53 | 14 | F3:27; F2:10 | 2/13/22 | NA | 30 | 7/3/3/NA/NA | (54) |
| | ES | 34 | 55 | 12 | F3:26; F2:8 | 3/11/20 | NA | 30 | 12/6/3/NA/NA | |
| de la Peña
(2005) | EVL+Nadolol | 43 | 60 | 23 | G1:10; G2:26;
G3:7 | 6/20/11 | NA | 529 | 5/0/2/2/1 | (55) |
| | EVL | 37 | 60 | 27 | G1:7; G2:17;
G3:13 | 6/25/12 | NA | 454 | 4/1/2/7/3 | |
| Peña (1999) | ES | 46 | 59 | 35 | G1:10; G2:31;
G3:5 | 12/22/13 | NA | 545 | 10/NA/3/11/2 | (56) |
| | EVL | 42 | 59 | 19 | G1:6; G2:25;
G3:11 | 10/22/10 | NA | 484 | 8/NA/2/5/3 | |
| Romero (2006) | Nadolol+ISMN | 57 | 51 | 35 | NA | 40/44/16 | 7 | 348 | 11/9/NA/18/0 | (57) |
| | EVL | 52 | 53 | 33 | NA | 32/58/10 | 7 | 363 | 10/6/NA/12/3 | |
| Stiegmann
(1992) | ES | 65 | 53 | 22 | G1:9; G2:31;
G3:25 | 20/32/11 | 9.9 | 303 | 29/8/NA/NA/NA | (58) |
| | EVL | 64 | 51 | 17 | G1:8; G2:35;
G3:21 | 22/30/12 | 9.4 | 303 | 18/3/NA/NA/NA | |
| Umehara (1999) | EVL+ES | 25 | 58 | NA | F3:9; F2:16 | 10/11/4 | NA | 611 | 3/0/NA/NA/NA | (59) |
| | EVL | 26 | 59 | NA | F3:8; F2:18 | 7/13/6 | NA | 629 | 4/0/NA/NA/NA | |
| Viazis (2002) | EVL | 36 | 64 | 42 | S:9; M:17;
L:10 | 4/18/14 | NA | 50 | NA/NA/NA/NA/NA | (60) |
| | ES | 37 | 62 | 46 | S:10; M:15;
L:12 | 6/16/15 | NA | 59 | NA/NA/NA/NA/NA | |
| Vinel (1992) | ES | 35 | 57 | 43 | F3:30; F2:5 | NA | 9.1 | 92 | 5/3/NA/NA/NA | (61) |
| | ES+Propranolol | 39 | 55 | 23 | F3:20; F2:19 | NA | 9.2 | 102 | 5/4/NA/NA/NA | |
Bias assessment
Risk of bias assessment for the RCTs included was
performed following the PRISMA recommendations; the results are
presented in Table SI. A total of
26 (61%) trials (20-22,24-30,34,36,38,44,47,16,50,56,61)
adopted concealed randomization via sealed opaque envelopes, by
using central randomization or through an independent person not
involved in the treatment of the patients. Only one trial declared
early cessation (54). A total of
two trials were open labelled (20,24) and
two trials reported using outcome assessors under blinded
conditions (27,16). Blinding of the remaining trials was
not specified.
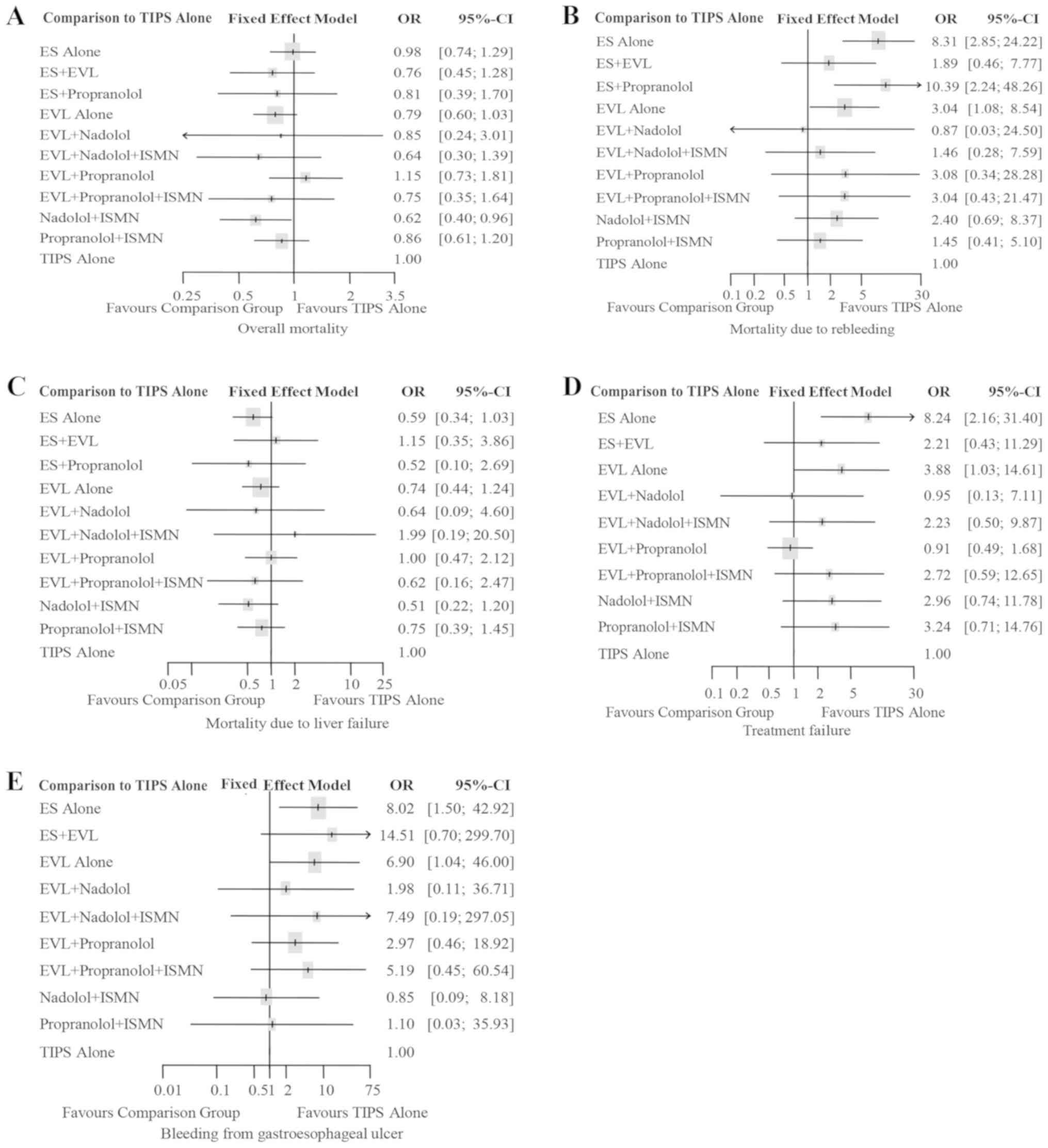
Overall mortality
In total, 40 trials with a total of 3,599 patients
reported overall mortality, involving all 11 treatment regimens.
Fig. S1A illustrates the evidence
networks connecting the regimens. Nadolol plus ISMN also had the
highest P-score (P-score=0.8162, Table
II) with the largest probability to reduce mortality when
compared with the other treatments. No statistical heterogeneity
was observed (Heterogeneity I2=0%; Cochran's test
P=0.9618, Table III) in this
outcome measure. The fixed-effects model analysis suggested that
nadolol plus ISMN was significantly more effective than TIPS alone
(OR=0.62, 95% CI: 0.40-0.96, Table
III), as presented in Fig. 2A.
Pairwise comparisons indicated that nadolol plus ISMN and EVL alone
were significantly more effective than ES alone in reducing overall
mortality (OR=0.63, 95% CI: 0.42-0.94; OR=0.80, 95% CI: 0.65-0.99,
respectively, Table III), while
differences among other treatments were not statistically
significant.
 | Table IIRanking for efficacy of 11 potential
treatment options. |
Table II
Ranking for efficacy of 11 potential
treatment options.
| | Overall
mortality | Mortality due to
rebleeding | Mortality due to
liver failure | Treatment
failure | Bleeding from
gastroesophageal ulcer |
|---|
| Rank | P-score | Treatment | P-score | Treatment | P-score | Treatment | P-score | Treatment | P-score | Treatment |
|---|
| 1 | 0.8162 | Nadolol+ISMN | 0.8276 | TIPS alone | 0.7536 | Nadolol+ISMN | 0.8071 |
EVL+propranolol | 0.7536 | Nadolol+ISMN |
| 2 | 0.7135 |
EVL+nadolol+ISMN | 0.7265 | EVL+nadolol | 0.6964 | ES alone | 0.7938 | TIPS alone | 0.6964 | ES alone |
| 3 | 0.5983 | ES+EVL | 0.6931 |
EVL+nadolol+ISMN | 0.6651 | ES+propranolol | 0.7932 | EVL+nadolol | 0.6651 | ES+propranolol |
| 4 | 0.5887 | EVL alone | 0.6833 |
Propranolol+ISMN | 0.6044 |
EVL+propranolol+ISMN | 0.5630 |
EVL+nadolol+ISMN | 0.6044 |
EVL+propranolol+ISMN |
| 5 | 0.5843 |
EVL+propranolol+ISMN | 0.6047 | ES+EVL | 0.5755 | EVL+nadolol | 0.5189 | ES+EVL | 0.5755 | EVL+nadolol |
| 6 | 0.5218 | ES+propranolol | 0.5041 | Nadolol+ISMN | 0.5230 | EVL alone | 0.4520 |
EVL+propranolol+ISMN | 0.5230 | EVL alone |
| 7 | 0.4847 | EVL+nadolol | 0.4377 |
EVL+propranolol | 0.5226 |
Propranolol+ISMN | 0.4328 | Nadolol+ISMN | 0.5226 |
Propranolol+ISMN |
| 8 | 0.4744 |
Propranolol+ISMN | 0.4331 |
EVL+propranolol+ISMN | 0.3439 |
EVL+propranolol | 0.3680 |
Propranolol+ISMN | 0.3439 |
EVL+propranolol |
| 9 | 0.2811 | ES alone | 0.3934 | EVL alone | 0.3022 | TIPS alone | 0.2513 | EVL alone | 0.3022 | TIPS alone |
| 10 | 0.2624 | TIPS alone | 0.1125 | ES alone | 0.2966 | ES+EVL | 0.0199 | ES alone | 0.2966 | ES+EVL |
| 11 | 0.1747 |
EVL+propranolol | 0.0842 | ES+propranolol | 0.2167 |
EVL+nadolol+ISMN | | | 0.2167 |
EVL+nadolol+ISMN |
 | Table IIINetwork meta-analysis results of
pairwise comparisons (Odds ratios with 95% CI). |
Table III
Network meta-analysis results of
pairwise comparisons (Odds ratios with 95% CI).
| A, Overall
mortality (Heterogeneity I2=0%, P=0.9618), fixed-effects
model |
|---|
| Group | ES alone | ES+EVL | ES+propranolol | EVL alone | EVL+nadolol |
EVL+nadolol+ISMN |
EVL+propranolol |
EVL+Propranolol+ISMN | Nadolol+ISMN |
Propranolol+ISMN | TIPS alone |
|---|
| ES alone | - | 1.29
[0.79,2.10] | 1.21
[0.61,2.40] | 1.25
[1.01,1.54] | 1.16
[0.33,4.07] | 1.53
[0.72,3.23] | 0.85
[0.50,1.45] | 1.30
[0.60,2.83] | 1.59
[1.06,2.38] | 1.14
[0.77,1.71] | 0.98
[0.74,1.29] |
| ES+EVL | 0.78
[0.48,1.27] | - | 0.94
[0.40,2.18] | 0.97
[0.62,1.52] | 0.90
[0.24,3.36] | 1.18
[0.50,2.78] | 0.66
[0.33,1.32] | 1.01
[0.42,2.43] | 1.23
[0.69,2.19] | 0.89
[0.49,1.60] | 0.76
[0.45,1.28] |
| ES+propranolol | 0.83
[0.42,1.64] | 1.06
[0.46,2.47] | - | 1.03
[0.50,2.11] | 0.96
[0.23,4.01] | 1.26
[0.46,3.48] | 0.70
[0.30,1.68] | 1.08
[0.38,3.03] | 1.31
[0.59,2.90] | 0.95
[0.43,2.09] | 0.81
[0.39,1.70] |
| EVL alone | 0.80
[0.65,0.99] | 1.03
[0.66,1.63] | 0.97
[0.47,1.99] | - | 0.93
[0.27,3.21] | 1.22
[0.59,2.53] | 0.68
[0.40,1.16] | 1.05
[0.49,2.22] | 1.27
[0.89,1.82] | 0.92
[0.63,1.35] | 0.79
[0.60,1.03] |
| EVL+nadolol | 0.86
[0.25,3.03] | 1.11
[0.30,4.16] | 1.04
[0.25,4.37] | 1.08
[0.31,3.71] | - | 1.32
[0.31,5.54] | 0.74
[0.19,2.83] | 1.12
[0.26,4.79] | 1.37
[0.38,4.98] | 0.99
[0.27,3.61] | 0.85
[0.24,3.01] |
|
EVL+nadolol+ISMN | 0.66
[0.31,1.39] | 0.84
[0.36,1.99] | 0.79
[0.29,2.19] | 0.82
[0.40,1.69] | 0.76
[0.18,3.19] | - | 0.56
[0.23,1.37] | 0.85
[0.30,2.43] | 1.04
[0.55,1.96] | 0.75
[0.33,1.70] | 0.64
[0.30,1.39] |
|
EVL+propranolol | 1.17
[0.69,2.00] | 1.51
[0.76,3.02] | 1.42
[0.60,3.38] | 1.46
[0.86,2.48] | 1.36
[0.35,5.23] | 1.79
[0.73,4.39] | - | 1.53
[0.62,3.76] | 1.86
[0.99,3.52] | 1.34
[0.76,2.36] | 1.15
[0.73,1.81] |
|
EVL+propranolol+ISMN | 0.77
[0.35,1.67] | 0.99
[0.41,2.38] | 0.93
[0.33,2.61] | 0.96
[0.45,2.03] | 0.89
[0.21,3.79] | 1.17
[0.41,3.33] | 0.65
[0.27,1.61] | - | 1.22
[0.53,2.80] | 0.88
[0.40,1.92] | 0.75
[0.35,1.64] |
| Nadolol+ISMN | 0.63
[0.42,0.94] | 0.81
[0.46,1.44] | 0.76
[0.34,1.69] | 0.79
[0.55,1.12] | 0.73
[0.20,2.65] | 0.96
[0.51,1.81] | 0.54
[0.28,1.01] | 0.82
[0.36,1.89] | - | 0.72
[0.43,1.21] | 0.62
[0.40,0.96] |
|
Propranolol+ISMN | [0.59,1.31]
0.87 | 1.13
[0.62,2.03] | 1.06
[0.48,2.34] | 1.09
[0.74,1.60] | 1.01
[0.28,3.71] | 1.33
[0.59,3.03] | 0.74
[0.42,1.31] | 1.14
[0.52,2.49] | 1.39
[0.82,2.34] | - | 0.86
[0.61,1.20] |
| TIPS alone | 1.02
[0.77,1.35] | 1.31
[0.78,2.22] | 1.23
[0.59,2.58] | 1.27
[0.97,1.67] | 1.18
[0.33,4.20] | 1.56
[0.72,3.37] | 0.87
[0.55,1.37] | 1.33
[0.61,2.89] | 1.62
[1.04,2.52] | 1.17
[0.83,1.63] | - |
| B, Mortaility due
to rebleeding (Heterogeneity I2=0%, P=0.9963),
fixed-effects model |
| Group | ES alone | ES+EVL | ES+propranolol | EVL alone | EVL+nadolol |
EVL+nadolol+ISMN |
EVL+propranolol |
EVL+Propranolol+ISMN | Nadolol+ISMN |
Propranolol+ISMN | TIPS alone |
| ES alone | - | 4.39 | 0.80 | 2.74 | 9.53 | 5.68 | 2.70 | 2.74 | 3.46 | 5.75 | 8.31 |
| ES alone | - | 4.39
[1.45,13.26] | 0.80
[0.27,2.41] | 2.74
[1.59,4.73] | 9.53
[0.38,237.86] | 5.68
[1.42,22.68] | 2.70
[0.23,31.58] | 2.74
[0.42,17.81] | 3.46
[1.44,8.31] | 5.75
[1.30,25.47] | 8.31
[2.85,24.22] |
| ES+EVL | 0.23
[0.08,0.69] | - | 0.18
[0.04,0.87] | 0.62
[0.24,1.63] | 2.17
[0.08,59.65] | 1.29
[0.26,6.44] | 0.61
[0.04,8.50] | 0.62
[0.08,4.83] | 0.79
[0.24,2.60] | 1.31
[0.23,7.41] | 1.89
[0.46,7.77] |
| ES+propranolol | 1.25
[0.42,3.76] | 5.49
[1.15,26.14] | - | 3.42
[1.00,11.71] | 11.91
[0.40,357.18] | 7.10
[1.21,41.67] | 3.37
[0.23,49.96] | 3.42
[0.39,30.05] | 4.33
[1.06,17.67] | 7.19
[1.13,45.80] | 10.39
[2.24,48.26] |
| EVL alone | 0.37
[0.21,0.63] | 1.60
[0.61,4.19] | 0.29
[0.09,1.00] | - | 3.48
[0.15,82.91] | 2.07
[0.57,7.50] | 0.98
[0.09,11.36] | 1.00
[0.16,6.09] | 1.26
[0.62,2.57] | 2.10
[0.50,8.88] | 3.04
[1.08,8.54] |
| EVL+nadolol | 0.10
[0.00,2.62] | 0.46
[0.02,12.66] | 0.08
[0.00,2.52] | 0.29
[0.01,6.85] | - | 0.60
[0.02,18.24] | 0.28
[0.01,15.52] | 0.29
[0.01,11.04] | 0.36
[0.01,9.36] | 0.60
[0.02,19.66] | 0.87
[0.03,24.50] |
|
EVL+nadolol+ISMN | 0.18
[0.04,0.70] | 0.77
[0.16,3.85] | 0.14
[0.02,0.83] | 0.48
[0.13,1.74] | 1.68
[0.05,51.36] | - | 0.47
[0.03,7.51] | 0.48
[0.05,4.42] | 0.61
[0.21,1.78] | 1.01
[0.15,6.97] | 1.46
[0.28,7.59] |
|
EVL+propranolol | 0.37
[0.03,4.34] | 1.63
[0.12,22.53] | 0.30
[0.02,4.40] | 1.02
[0.09,11.71] | 3.53
[0.06,193.72] | 2.11
[0.13,33.29] | - | 1.01
[0.05,19.49] | 1.28
[0.10,16.33] | 2.13
[0.17,27.32] | 3.08
[0.34,28.28] |
|
EVL+propranolol+ISMN | 0.37
[0.06,2.38] | 1.60
[0.21,12.42] | 0.29
[0.03,2.57] | 1.00
[0.16,6.10] | 3.48
[0.09,133.93] | 2.08
[0.23,19.05] | 0.99
[0.05,18.94] | - | 1.27
[0.18,8.80] | 2.10
[0.29,15.46] | 3.04
[0.43,21.47] |
| Nadolol+ISMN | 0.29
[0.12,0.69] | 1.27
[0.38,4.19] | 0.23
0.06,0.94] | 0.79
[0.39,1.61] | 2.75
[0.11,70.92] | 1.64
[0.56,4.80] | 0.78
[0.06,9.91] | 0.79
[0.11,5.50] | - | 1.66
[0.33,8.26] | 2.40
[0.69,8.37] |
|
Propranolol+ISMN | 0.17
[0.04,0.77] | 0.76
[0.13,4.31] | 0.14
[0.02,0.89] | 0.48
[0.11,2.01] | 1.66
[0.05,53.91] | 0.99
[0.14,6.79] | 0.47
[0.04,6.00] | 0.48
[0.06,3.49] | 0.60
[0.12,2.99] | - | 1.45
[0.41,5.10] |
| TIPS alone | 0.12
[0.04,0.35] | 0.53
[0.13,2.17] | 0.10
[0.02,0.45] | 0.33
[0.12,0.93] | 1.15
[0.04,32.17] | 0.68
[0.13,3.54] | 0.32
[0.04,2.97] | 0.33
[0.05,2.32] | 0.42
[0.12,1.45] | 0.69
[0.20,2.44] | - |
| C, Mortality due to
liver failure (Heterogeneity I2=0%, P=0.8985),
fixed-effects model |
| Group | ES alone | ES+EVL | ES+propranolol | EVL alone | EVL+nadolol |
EVL+nadolol+ISMN |
EVL+propranolol |
EVL+Propranolol+ISMN | Nadolol+ISMN |
Propranolol+ISMN | TIPS alone |
| ES alone | - | 0.51
[0.15,1.68] | 1.13
[0.24,5.26] | 0.80
[0.49,1.29] | 0.92
[0.13,6.63] | 0.29
[0.03,2.97] | 0.59
[0.23,1.50] | 0.94
0.23,3.90] | 1.15
[0.52,2.55] | 0.79
[0.34,1.79] | 0.59
[0.34,1.03] |
| ES+EVL | 1.96
[0.60,6.46] | - | 2.21
[0.31,15.51] | 1.56
[0.53,4.65] | 1.82
[0.20,16.37] | 0.58
[0.05,7.24] | 1.15
[0.28,4.78] | 1.85
[0.32,10.65] | 2.26
[0.62,8.24] | 1.54
[0.41,5.87] | 1.15
[0.35,3.86] |
| ES+propranolol | 0.89
[0.19,4.16] | 0.45
[0.06,3.18] | - | 0.71
[0.14,3.56] | 0.82
[0.07,10.03] | 0.26
[0.02,4.21] | 0.52
[0.09,3.17] | 0.84
[0.10,6.83] | 1.02
[0.18,5.79] | 0.70
[0.12,4.01] | 0.52
[0.10,2.69] |
| EVL alone | 1.26
[0.78,2.03] | 0.64
[0.22,1.90] | 1.41
[0.28,7.11] | - | 1.16
[0.17,7.85] | 0.37
[0.04,3.62] | 0.74
[0.30,1.84] | 1.19
[0.30,4.66] | 1.44
[0.72,2.91] | 0.99
[0.46,2.14] | 0.74
0.44,1.24] |
| EVL+nadolol | 1.08
[0.15,7.75] | 0.55
[0.06,4.97] | 1.22
[0.10,14.84] | 0.86
[0.13,5.81] | - | 0.32
[0.02,6.23] | 0.64
[0.08,5.27] | 1.02
[0.10,10.69] | 1.24
[0.16,9.50] | 0.85
[0.11,6.67] | 0.64
[0.09,4.60] |
|
EVL+nadolol+ISMN | 3.39
[0.34,34.20] | 1.73
[0.14,21.63] | 3.82
[0.24,61.42] | 2.70
[0.28,26.39] | 3.14
[0.16,61.42] | - | 1.99
[0.17,23.05] | 3.20
[0.23,45.57] | 3.90
[0.45,34.12] | 2.67
[0.24,29.42] | 1.99
[0.19,20.50] |
|
EVL+propranolol | 1.70
[0.67,4.33] | 0.87
[0.21,3.59] | 1.91
[0.32,11.62] | 1.35
[0.54,3.37] | 1.57
[0.19,13.06] | 0.50
[0.04,5.80] | - | 1.61
[0.33,7.70] | 1.96
[0.63,6.08] | 1.34
[0.49,3.64] | 1.00
[0.47,2.12] |
|
EVL+propranolol+ISMN | 1.06
[0.26,4.39] | 0.54
[0.09,3.11] | 1.19
[0.15,9.71] | 0.84
[0.21,3.32] | 0.98
[0.09,10.28] | 0.31
[0.02,4.45] | 0.62
[0.13,2.99] | - | 1.22
[0.26,5.64] | 0.83
[0.22,3.16] | 0.62
[0.16,2.47] |
| Nadolol+ISMN | 0.87
[0.39,1.93] | 0.44
[0.12,1.62] | 0.98
[0.17,5.55] | 0.69
[0.34,1.40] | 0.80
[0.11,6.16] | 0.26
[0.03,2.24] | 0.51
[0.16,1.59] | 0.82
[0.18,3.80] | - | 0.68
[0.24,1.91] | 0.51
[0.22,1.20] |
|
Propranolol+ISMN | 1.27
[0.56,2.90] | 0.65
[0.17,2.47] | 1.43
[0.25,8.23] | 1.01
[0.47,2.19] | 1.18
[0.15,9.24] | 0.38
[0.03,4.14] | 0.75
[0.27,2.04] | 1.20
[0.32,4.55] | 1.46
[0.52,4.09] | - | 0.75
[0.39,1.45] |
| TIPS alone | 1.70
[0.97,2.97] | 0.87
[0.26,2.90] | 1.91
[0.37,9.87] | 1.35
[0.81,2.28] | 1.57
[0.22,11.39] | 0.50
[0.05,5.15] | 1.00
[0.47,2.11] | 1.61
[0.41,6.36] | 1.95
[0.83,4.58] | 1.34
[0.69,2.60] | - |
| D, Treatment
failure (Heterogeneity I2=29.4%, P=0.1739),
fixed-effects model |
| Group | ES alone | ES+EVL | EVL alone | EVL+nadolol |
EVL+nadolol+ISMN |
EVL+propranolol |
EVL+Propranolol+ISMN | Nadolol+ISMN |
Propranolol+ISMN | TIPS alone |
| ES alone | - | 3.72
[1.30,10.67] | 2.13
[1.31,3.45] | 8.65
[1.77,42.15] | 3.69
[1.65,8.27] | 9.09
[2.08,39.68] | 3.02
[1.22,7.53] | 2.78
[1.54,5.02] | 2.54
[1.06,6.12] | 8.24
[2.16,31.40] |
| ES+EVL | 0.27
[0.09,0.77] | - | 0.57
[0.22,1.48] | 2.32
[0.39,13.82] | 0.99
[0.31,3.21] | 2.44
[0.43,13.94] | 0.81
[0.24,2.77] | 0.75
[0.27,2.11] | 0.68
[0.21,2.27] | 2.21
[0.43,11.29] |
| EVL alone | 0.47
[0.29,0.76] | 1.75
[0.68,4.53] | - | 4.07
[0.90,18.39] | 1.74
[0.87,3.46] | 4.28
[0.99,18.49] | 1.42
[0.66,3.08] | 1.31
[0.86,1.98] | 1.20
[0.57,2.49] | 3.88
[1.03,14.61] |
| EVL+nadolol | 0.12 | 0.43 | 0.25 | - | 0.43 | 1.05 | 0.35 | 0.32 | 0.29 | 0.95 |
| EVL+nadolol | 0.12
[0.02,0.56] | 0.43
[0.07,2.56] | 0.25
[0.05,1.11] | - | 0.43
[0.08,2.24] | 1.05
[0.13,8.61] | 0.35
[0.06,1.91] | 0.32
[0.07,1.54] | 0.29
[0.05,1.58] | 0.95
[0.13,7.11] |
|
EVL+nadolol+ISMN | 0.27
[0.12,0.61] | 1.01
[0.31,3.26] | 0.58
[0.29,1.15] | 2.34
[0.45,12.30] | - | 2.46
[0.49,12.31] | 0.82
[0.29,2.31] | 0.75
[0.43,1.31] | 0.69
[0.25,1.89] | 2.23
[0.50,9.87] |
|
EVL+propranolol | 0.11
[0.03,0.48] | 0.41
[0.07,2.34] | 0.23
[0.05,1.01] | 0.95
[0.12,7.78] | 0.41
[0.08,2.03] | - | 0.33
[0.06,1.74] | 0.31
[0.07,1.39] | 0.28
[0.05,1.44] | 0.91
[0.49,1.68] |
|
EVL+propranolol+ISMN | 0.33
[0.13,0.82] | 1.23
[0.36,4.19] | 0.70
[0.32,1.52] | 2.86
[0.52,15.57] | 1.22
[0.43,3.44] | 3.01
[0.57,15.74] | - | 0.92
[0.38,2.21] | 0.84
[0.37,1.93] | 2.72
[0.59,12.65] |
| Nadolol+ISMN | 0.36
[0.20,0.65] | 1.34
[0.47,3.77] | 0.76
[0.50,1.16] | 3.11
[0.65,14.86] | 1.33
[0.77,2.30] | 3.27
[0.72,14.83] | 1.09
[0.45,2.61] | - | 0.91
[0.39,2.12] | 2.96
[0.74,11.78] |
|
Propranolol+ISMN | 0.39
[0.16,0.95] | 1.46
[0.44,4.86] | 0.84
[0.40,1.74] | 3.40
[0.63,18.20] | 1.45
[0.53,3.98] | 3.57
[0.70,18.38] | 1.19
[0.52,2.73] | 1.09
[0.47,2.54] | - | 3.24
[0.71,14.76] |
| TIPS alone | 0.12
[0.03,0.46] | 0.45
[0.09,2.30] | 0.26
[0.07,0.97] | 1.05
[0.14,7.83] | 0.45
[0.10,1.98] | 1.10
[0.60,2.05] | 0.37
[0.08,1.71] | 0.34
[0.08,1.34] | 0.31
[0.07,1.41] | - |
| E, Bleeding from
gastroesophageal ulcer (Heterogeneity I2=0%, P=0.8354),
fixed-effects model |
| Group | ES alone | ES+EVL | EVL alone | EVL+nadolol |
EVL+nadolol+ISMN |
EVL+propranolol |
EVL+Propranolol+ISMN | Nadolol+ISMN |
Propranolol+ISMN | TIPS alone |
| ES alone | - | 0.55
[0.04,6.88] | 1.16
[0.48,2.82] | 4.05
[0.37,44.27] | 1.07
[0.04,28.38] | 2.71
[0.22,32.94] | 1.64
[0.24,11.51] | 9.41
[2.07,42.79] | 7.51
[0.35,160.14] | 8.02
[1.50,42.92] |
| ES+EVL | 1.81
[0.15,22.50] | - | 2.10
[0.20,22.27] | [0.29,187.21] | 1.94
[0.03,109.86] | 4.89
[0.14,170.35] | 2.97
[0.16,55.53] | 17.01
[1.03,280.81] | 13.58
[0.32,583.96] | 14.51
[0.70,299.70] |
| EVL alone | 0.86
[0.35,2.09] | 0.48
[0.04,5.04] | - | 3.49
[0.38,32.10] | 0.92
[0.03,24.40] | 2.33
[0.16,33.00] | 1.41
[0.25,7.99] | 8.09
[1.78,36.77] | 6.46
[0.35,120.78] | 6.90
[1.04,46.00] |
| EVL+nadolol | 0.25
[0.02,2.69] | 0.14
[0.01,3.48] | 0.29
[0.03,2.64] | - | 0.26
[0.01,13.83] | 0.67
[0.02,21.21] | 0.41
[0.02,6.78] | 2.32
[0.16,34.08] | 1.85
[0.05,73.08] | 1.98
[0.11,36.71] |
|
EVL+nadolol+ISMN | 0.93
[0.04,24.71] | 0.52
[0.01,29.25] | 1.09
[0.04,28.72] | 3.78
[0.07,197.91] | - | 2.52
[0.04,155.57] | 1.53
[0.04,62.41] | 8.78
[0.48,160.33] | 7.01
[0.09,567.49] | 7.49
[0.19,297.05] |
|
EVL+propranolol | 0.37
[0.03,4.50] | 0.20
[0.01,7.11] | 0.43
[0.03,6.09] | 1.50
[0.05,47.60] | 0.40
[0.01,24.40] | - | 0.61
[0.03,14.43] | 3.48
[0.19,64.62] | 2.78
[0.05,144.26] | 2.97
[0.46,18.92] |
|
EVL+propranolol+ISMN | 0.61
[0.09,4.26] | 0.34
[0.02,6.28] | 0.71
[0.13,4.00] | 2.47
[0.15,41.18] | 0.65
[0.02,26.51] | 1.65
[0.07,39.07] | - | 5.72
[0.57,57.07] | 4.57
[0.23,91.89] | 4.88
[0.37,63.66] |
| Nadolol+ISMN | 0.11
[0.02,0.48] | 0.06
[0.00,0.97] | 0.12
[0.03,0.56] | 0.43
[0.03,6.33] | 0.11
[0.01,2.08] | 0.29
[0.02,5.35] | 0.17
[0.02,1.74] | - | 0.80
[0.03,21.58] | 0.85
[0.09,8.18] |
|
Propranolol+ISMN | 0.13
[0.01,2.84] | 0.07
[0.00,3.17] | 0.15
[0.01,2.89] | 0.54
[0.01,21.28] | 0.14
[0.00,11.55] | 0.36
[0.01,18.72] | 0.22
[0.01,4.40] | 1.25
[0.05,33.83] | - | 1.07
[0.03,34.99] |
| TIPS alone | 0.12
[0.02,0.67] | 0.07
[0.00,1.42] | 0.14
[0.02,0.97] | 0.51
[0.03,9.37] | 0.13
[0.00,5.30] | 0.34
[0.05,2.15] | 0.20
[0.02,2.67] | 1.17
[0.12,11.23] | 0.94
[0.03,30.66] | - |
Mortality due to rebleeding
A total of 27 trials with 2,447 patients
investigated all 11 treatments and reported death due to
rebleeding. The evidence network presented in Fig. S1B connects all of the treatments.
Cochran's Q test did not identify any statistical heterogeneity
among the selected trials for this outcome measure (Heterogeneity
I2=0, P=0.9963, Table
III). Compared with TIPS alone, ES plus propranolol increased
the risk of mortality due to rebleeding (OR=10.39, 95% CI:
2.24-48.26, Fig. 2B; P-score=0.0842;
Table II).
Pairwise comparisons indicated that ES plus EVL, EVL
alone, EVL combined with nadolol plus ISMN, Nadolol plus ISMN,
Propranolol plus ISMN and TIPS alone were significantly more
effective than ES alone in reducing mortality due to rebleeding
(OR=0.23, 95% CI: 0.08-0.69; OR=0.37, 95% CI: 0.21-0.63; OR=0.18,
95% CI: 0.04-0.70; OR=0.29, 95% CI: 0.12-0.69; OR=0.17, 95% CI:
0.04-0.77; OR=0.12, 95% CI: 0.04-0.35, respectively, Table III).
Mortality due to liver failure
A total of 24 trials with 2,258 patients
investigating all 11 treatments reported on death due to liver
failure. The evidence network presented in Fig. S1C connects all of the treatments. No
statistical heterogeneity was observed (Heterogeneity
I2=0%, P=0.8985; Table
III). The results of the fixed-effects model analysis comparing
with TIPS alone indicated that none of the other treatments were
superior, though nadolol plus ISMN may be the next best option
(OR=0.51, 95% CI: 0.22-1.20, Fig.
2C; P-score=0.7536; Table II).
Furthermore, EVL combined with nadolol plus ISMN had the lowest
P-score (P-score=0.2167, Table II),
indicating the highest probability to increase mortality due to
liver failure. Results of pairwise comparisons indicated no
statistically significant differences when comparing with
treatments other than TIPS (Table
III).
Treatment failure
In total, 14 trials with a total of 1,445 patients
reported on treatment failure. No data of this outcome were
available for the treatment regimen ES plus propranolol. The
evidence network in Fig. S1B
connects the other 10 treatments for assessment of this outcome.
Mild heterogeneity was identified for treatment failure
(Heterogeneity I2=29.4%, P=0.1739; Table III). A fixed-effects model analysis
was performed for comparing with TIPS alone. Differences were not
statistically significant (Fig. 2D).
The evidence network presented in Fig.
S1D connects all of the treatments. EVL plus propranolol had
the highest efficacy (P-score=0.8071), closely followed by TIPS
(P-score=0.7938) and EVL plus nadolol (P-score=0.7932). ES alone
ranked last (P-score=0.0199), suggesting that it was most likely to
have the highest rate of treatment failure. Rankings are presented
in Table II.
Pooled ORs suggested that ES alone was
disadvantageous compared with the other 9 treatments with regard to
treatment failure (OR=3.72, 95% CI: 1.30-10.67 compared with ES
plus EVL; OR=2.13, 95% CI: 1.31-3.45 compared with EVL alone;
OR=8.65, 95% CI: 1.77-42.15 compared with EVL plus nadolol;
OR=3.69, 95% CI: 1.65-8.27 compared with EVL plus nadolol plus
ISMN; OR=9.09, 95% CI: 2.08-39.68 compared with EVL plus
propranolol; OR=3.02, 95% CI: 1.22-7.53 compared with EVL plus
propranolol plus ISMN; OR=2.78, 95% CI: 1.54-5.02 compared with
nadolol plus ISMN; OR=2.54, 95% CI: 1.06-6.12 compared with
propranolol plus ISMN; OR=8.24, 95% CI: 2.16-31.40 compared with
TIPS alone; Table III).
Bleeding from gastroesophageal
ulcer
A total of 24 trials with a total of 2,258 patients
investigated all 11 treatments and reported death due to bleeding
from gastroesophageal ulcer. The evidence network is presented in
Fig. S1E. There was no
statistically significant heterogeneity for this outcome measure
(Heterogeneity I2=0, P=0.8354; Table III). Results of the fixed-effects
model analysis performed in comparison with TIPS alone indicated
that none of the other 10 treatments were superior, but nadolol
plus ISMN appeared to be the best among the compared treatments
(OR=0.85, 95% CI: 0.09-8.18, Fig.
2E; P-score=0.7536, Table
II).
Nadolol plus ISMN had the highest P-score
(P-score=0.7536), indicating that it had the highest probability of
reducing mortality due to rebleeding, followed closely by ES alone
(P-score=0.6964) and ES plus propranolol (P-score=0.6651). The
lowest P-score was obtained for EVL plus nadolol and ISMN
(P-score=0.2167), indicating the lowest probability to reduce
bleeding from gastroesophageal ulcer (Table II).
Pairwise comparisons among the treatments indicated
that nadolol plus ISMN was associated with a relatively lower risk
of causing bleeding from gastroesophageal ulcer when compared with
ES alone (OR=0.11, 95% CI: 0.02-0.48), ES plus EVL (OR=0.06, 95%
CI=0.00-0.97) or EVL alone (OR=0.12, 95% CI: 0.03-0.56) in Table III.
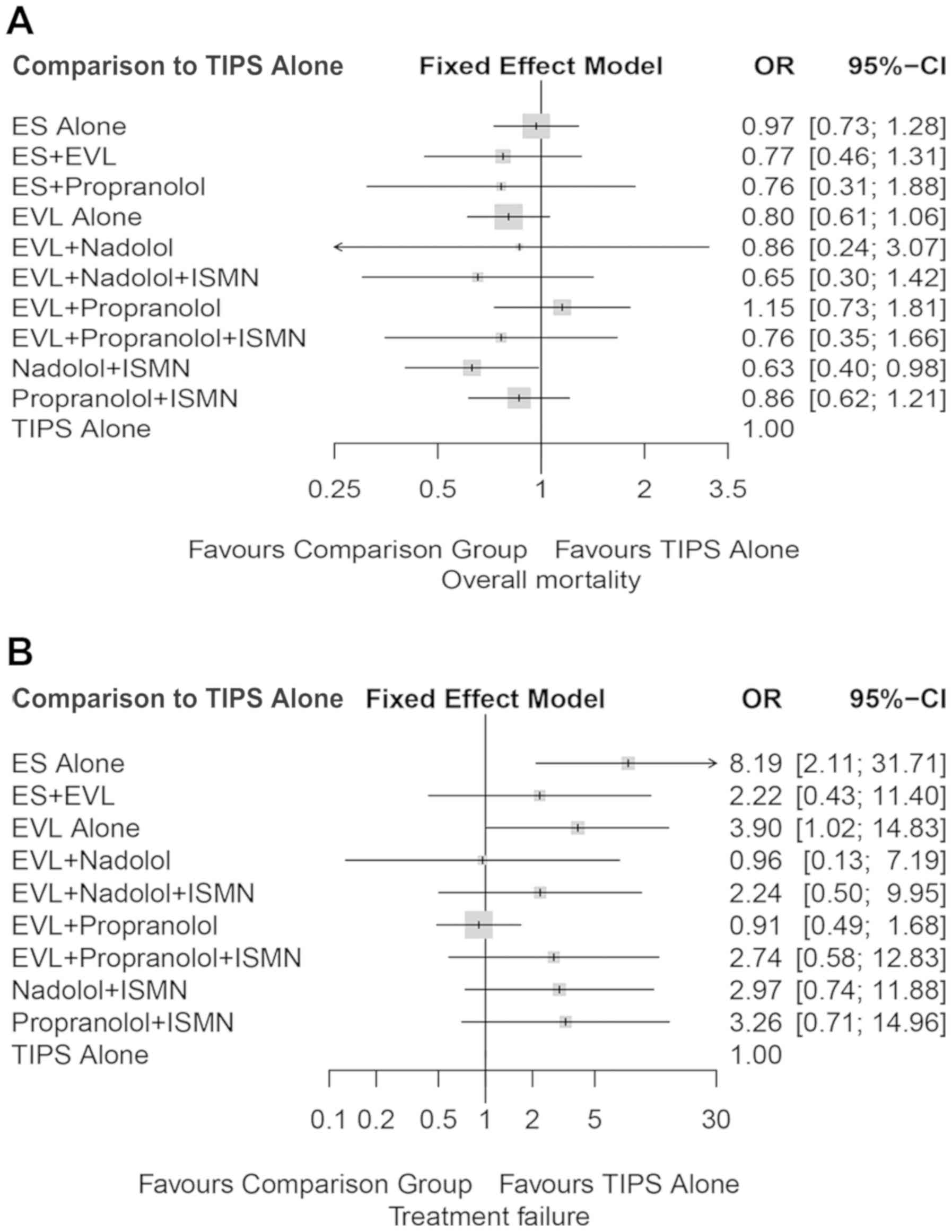
Sensitivity analysis
A sensitivity analysis was performed by removing
several studies. The only criterion for removal was a mean
follow-up time of <6 months, based on which 5 trials (43,47,55,60,61) were
removed. The results were consistent with those of the primary
meta-analysis (Table IV). Nadolol
plus ISMN was still superior to TIPS with regard to overall
mortality (OR=0.63, 95% CI: 0.40-0.98, Fig. 3A; Heterogeneity I2=0,
P=0.9249, Table IV), while no
significant differences were obtained for treatment failure
(OR=2.97, 95% CI: 0.74-11.88, Fig.
3B; Heterogeneity I2=37.3%, P=0.1206, Table IV).
 | Table IVHeterogeneity test results. |
Table IV
Heterogeneity test results.
| Item | I2
(%) | Q | DF | P-value |
|---|
| Overall
mortality | 0 | 19.34 | 32 | 0.9618 |
| Overall mortality
(sensitivity analysis) | 0 | 18.05 | 28 | 0.9249 |
| Mortality due to
rebleeding | 0 | 5.97 | 18 | 0.9963 |
| Treatment
failure | 29.4 | 12.76 | 9 | 0.1739 |
| Treatment failure
(sensitivity analysis) | 37.3 | 12.75 | 8 | 0.1206 |
| Bleeding from
gastroesophageal ulcer | 0 | 4.23 | 8 | 0.8354 |
| Mortality due to
liver failure | 0 | 8.58 | 15 | 0.8985 |
Discussion
The present network meta-analysis included 43
randomized controlled trials to compare the treatment effectiveness
of 11 mainstay secondary prophylaxes in patients with cirrhosis in
terms of mortality, treatment failure and bleeding from
gastroesophageal ulcers. The results suggested that nadolol plus
ISMN was most likely to reduce the risk of overall mortality,
mortality due to liver failure and bleeding from gastroesophageal
ulcers, and was superior to ES and TIPS alone for reducing overall
mortality. ES was inferior to 9 treatments for reducing treatment
failure. The combination of endoscopic therapy and NSBB with or
without ISMN was not significantly more effective than EVL or the
drug combination alone.
The present study included 4 trials that
investigated nadolol plus ISMN (26,36,51,57) with
a total of 250 randomized patients. Nadolol plus ISMN was indicated
to be more effective than TIPS alone in reducing overall mortality.
The present results are consistent with those of Villanueva et
al (28), which concluded that
combination therapy was more effective than endoscopic ligation for
the prevention of recurrent bleeding and was associated with lower
rates of major complications. As a vasoconstrictor, nadolol is able
to reduce portal pressure and blood flow in the porto-collateral
system. The vasodilator ISMN has been demonstrated to decrease
portal pressure in patients with cirrhosis by reducing
intra-hepatic resistance (29).
Despite adverse drug-associated effects, including hypotension,
asthenia and headaches, proper dosage of this combination is most
likely to reduce mortality and other complications of bleeding from
ulcers.
In the newly published AASLD and UK guidelines, TIPS
is recommended as a treatment option when endoscopic and
pharmacologic treatments have failed (5). In the present study, a total of 16
trials provided data for TIPS in 590 patients with cirrhosis
(20,21,25-27,29,30,32-35,38-40,42,43).
The mean follow-up time for TIPS groups was 748.5 days [data for
one trial (43) were not available].
Although TIPS is known to have the potential to increase hepatic
encephalopathy (20,27), the present study demonstrated that it
may reduce the risk of death due to rebleeding. The trials were
contradictory regarding whether TIPS is superior to endoscopic or
combination therapies. In one previous trial (26), TIPS alone was superior to EVL plus
propranolol in the prevention of rebleeding, but this superiority
did not result in improved survival. In this previous study
(26), liver failure, hepatobiliary
cancer and sepsis were the predominant causes of death. Clinically,
it may be difficult to attribute death to rebleeding or to any one
cause. Zheng et al (62)
performed a meta-analysis of 12 RCTs to compare TIPS with
endoscopic therapy, and the results suggested that TIPS reduced
variceal rebleeding, but was associated with an increased risk of
encephalopathy, although no differences in survival were observed.
The present study indicated that TIPS was superior to ES alone, ES
plus propranolol and EVL alone with a tendency for reduced
mortality due to rebleeding. In clinical practice, TIPS has certain
advantages in reducing portal pressure and reducing the risk of
rebleeding. However, compared with endoscopic treatment, TIPS is
more costly and technically more difficult.
Data from 4 previous studies (21,26,27,38) that
used covered stents in their trials provided similar results among
TIPS, EVL, EVL plus propranolol and propranolol plus ISMN in terms
of overall mortality, although TIPS appeared to cause less
mortality due to rebleeding than EVL plus propranolol (21).
Although ES was not recommended by the Baveno VI
Consensus Workshop as a first-line treatment (15), it is still commonly used in China
(61). Furthermore, the guidelines
of the Chinese Society of Gastroenterology, Chinese Medical
Association and Chinese Society of Endoscopy suggest that
physicians choose EVL or ES for secondary prophylaxis based on
their experience and the patients' clinical conditions (62). Therefore, RCTs on ES were not
excluded from the present study.
Results of pooled ORs demonstrated inferiority of ES
regarding treatment failure over the other 9 treatments. ES plus
propranolol may increase the risk of mortality due to rebleeding.
No statistically significant benefit of ES alone or ES plus drugs
was identified in the present study. The results of the present
study are consistent with those of previous studies (7-12,15)
and support the most recent UK and AASLD guidelines, which do not
recommend ES for secondary prevention of variceal bleeding in
patients with cirrhosis (5).
EVL has been accepted as the preferred endoscopic
treatment for the prevention of variceal rebleeding (37). Although EVL plus NSBB is now the
first-line treatment, a review by Cotoras et al (64) reported that addition of β-blockers to
EVL does not lead to a difference in mortality. In line with this,
in the present study, the combination of EVL and NSBB with or
without ISMN was not more effective than EVL alone or the drug
combination of NSBB and ISMN.
The present study identified a tendency of EVL plus
propranolol to increase mortality, which may be attributed to the
data that were extracted from the included trials for this outcome
measure. In a previous trial whose patients all had grade II-IV
portal vein thrombosis (PVT), Luo et al (21) determined that the ability of EVL plus
propranolol to reduce variceal bleeding may be counteracted by
deteriorated PVT. Additional evidence from high-quality RCTs is
required to address this issue.
The present study has several limitations. Therapy
using drugs alone or using drugs other than NSBB or ISMN were
beyond the scope of the present study, as regimens of single drugs
are now seldom used in clinical practice for secondary prophylaxis.
Although the included trials provided a solid foundation based on
collected data, more data on serious adverse effects, the frequency
and severity of drug-associated adverse effects,
procedure-associated complications and consequent hospitalization
may be helpful for further comparison. The quality of the present
study depends on the RCTs that were included. A total of 4 RCTs
focusing on acute variceal bleeding (37,38,47,54) were
included, as they also assessed the outcomes of rebleeding and
overall mortality. The included references were published between
1992 and 2015, which is a long period of time; there may be
technical differences in the early stages; however, with the
continuous standardization and maturity of operation technology,
the differences are gradually narrowing. Therefore, the clinical
value of the present results may be limited.
In conclusion, the present network meta-analysis
suggested that for prophylaxis of variceal rebleeding in patients
with cirrhosis, nadolol plus ISMN may be the preferred choice to
decrease mortality and ES may be associated with a relatively
higher risk of unfavourable treatment outcomes, particularly
treatment failure.
Supplementary Material
Evidence network with regard to (A)
overall mortality, (B) mortality due to rebleeding, (C) mortality
due to liver failure, (D) treatment failure and (E) bleeding from
gastroesophageal ulcer.
Risk of bias assessment in included
randomized controlled trials.
Acknowledgements
The authors would like to thank Dr Sam Zhong and Dr
Michelle Liu (Shanghai KNOWLANDS MedPharm Consulting Co.,Ltd.) for
their support with data analyses in the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the present
study are availabled from the correspoding author on reasonable
request.
Authors' contributions
YK was responsible for the study design, data
collection and analysis and manuscript review. LS reviewed the data
collection and analysis and drafted the manuscript. Both authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bresci G: Portal hypertension: The
management of esophageal/gastric varices, portal hypertensive
gastropathy or hypertensive colopathy. Therapy. 4:91–96. 2007.
|
|
2
|
Mansour L, El-Kalla F, EL-Bassat H,
Abd-Elsalam S, El-Bedewy M, Kobtan A, Badawi R and Elhendawy M:
Randomized controlled trial of scleroligation versus band ligation
alone for eradication of gastroesophageal varices. Gastrointest
Endosc. 86:307–315. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sarin SK, Lahoti D, Saxena SP, Murthy NS
and Makwana UK: Prevalence, classification and natural history of
gastric varices: A long-term follow-up study in 568 portal
hypertension patients. Hepatology. 16:1343–1349. 1992.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Trudeau W and Prindiville T: Endoscopic
injection sclerosis in bleeding gastric varices. Gastrointest
Endosc. 32:264–268. 1986.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tripathi D, Stanley AJ, Hayes PC, Patch D,
Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M,
et al: U.K. guidelines on the management of variceal haemorrhage in
cirrhotic patients. Gut. 64:1680–1704. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bosch J and García-Pagán JC: Prevention of
variceal rebleeding. Lancet. 361:952–954. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brunner F, Berzigotti A and Bosch J:
Prevention and treatment of variceal haemorrhage in 2017. Liver
Int. 37 (Suppl 1):S104–S115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gonzalez R, Zamora J, Gomez-Camarero J,
Molinero LM, Bañares R and Albillos A: Meta-analysis: Combination
endoscopic and drug therapy to prevent variceal rebleeding in
cirrhosis. Ann Intern Med. 149:109–122. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ravipati M, Katragadda S, Swaminathan PD,
Molnar J and Zarling E: Pharmacotherapy plus endoscopic
intervention is more effective than pharmacotherapy or endoscopy
alone in the secondary prevention of esophageal variceal bleeding:
A meta-analysis of randomized, controlled trials. Gastrointest
Endosc. 70:658–664.e5. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Garcia-Tsao G, Abraldes J, Berzigotti A
and Bosch J: Portal Hypertensive Bleeding in Cirrhosis: Risk
Stratification, Diagnosis, and Management: 2016 practice guidance
by the American association for the study of liver diseases.
Hepatology. 65:310–335. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thiele M, Krag A, Rohde U and Gluud LL:
Meta-analysis: Banding ligation and medical interventions for the
prevention of rebleeding from oesophageal varices. Aliment
Pharmacol Ther. 35:1155–1165. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Puente A, Hernández-Gea V, Graupera I,
Roque M, Colomo A, Poca M, Aracil C, Gich I, Guarner C and
Villanueva C: Drugs plus ligation to prevent rebleeding in
cirrhosis: An updated systematic review. Liver Int. 34:823–833.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Funakoshi N, Ségalas-Largey F, Duny Y,
Oberti F, Valats JC, Bismuth M, Daurès JP and Blanc P: Benefit of
combination β-blocker and endoscopic treatment to prevent variceal
rebleeding: A meta-analysis. World J Gastroenterol. 16:5982–5992.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Garcia-Tsao G, Abraldes JG, Berzigotti A
and Bosch J: Portal hypertensive bleeding in cirrhosis: Risk
stratification, diagnosis, and management: 2016 practice guidance
by the American Association for the study of liver diseases.
Hepatology. 65:310–335. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
de Franchis R: BavenoV Faculty: Expanding
consensus in portal hypertension: Report of the Baveno VI Consensus
Workshop: stratifying risk and individualizing care for portal
hypertension. J Hepatol. 63:743–752. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen J, Zeng XQ, Ma LL, Huang XQ, Tseng
YJ, Wang J, Luo TC and Chen SY: Long-term efficacy of endoscopic
ligation plus cyanoacrylate injection with or without sclerotherapy
for variceal bleeding. J Dig Dis. 17:252–259. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rücker G and Schwarzer G: Ranking
treatments in frequentist network meta-analysis works without
resampling methods. BMC Med Res Methodol. 15(58)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schwarzer G, Carpenter J and Rücker G:
Netmeta: Network meta-analysis with R (2016). R Package Version
0.9-2. netmeta, Cited February 22, 2016. Available from http://CRAN.R-project.org/package=netmeta.
|
|
19
|
Gupta V, Rawat R and Shalimar Saraya A:
Carvedilol versus propranolol effect on hepatic venous pressure
gradient at 1 month in patients with index variceal bleed: RCT.
Hepatol Int. 11:181–187. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sauerbruch T, Mengel M, Dollinger M,
Zipprich A, Rössle M, Panther E, Wiest R, Caca K, Hoffmeister A,
Lutz H, et al: Prevention of rebleeding from esophageal varices in
patients with cirrhosis receiving small-diameter stents versus
hemodynamically controlled medical therapy. Gastroenterology.
149:p. 660–688.e1. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Luo X, Wang Z, Tsauo J, Zhou B, Zhang H
and Li X: Advanced cirrhosis combined with portal vein thrombosis:
A randomized trial of TIPS versus endoscopic band ligation plus
propranolol for the prevention of recurrent esophageal variceal
bleeding. Radiology. 276:286–293. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kumar P, Saxena KN, Misra SP and Dwivedi
M: Secondary prophylaxis of variceal hemorrhage: A comparative
study of band ligation, carvedilol and propranolol plus isosorbide
mononitrate. Ind J Gastroenterology. 34(A54)2015.
|
|
23
|
Kong DR, Wang JG, Chen C, Yu FF, Wu Q and
Xu JM: Effect of intravariceal sclerotherapy combined with
esophageal mucosal sclerotherapy using small-volume sclerosant for
cirrhotic patients with high variceal pressure. World J
Gastroenterol. 21:2800–2806. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kumar A, Jha SK, Sharma P, Dubey S, Tyagi
P, Sharma BC and Sarin SK: Addition of propranolol and isosorbide
mononitrate to endoscopic variceal ligation does not reduce
variceal rebleeding incidence. Gastroenterology. 137:892–901.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sauer P, Hansmann J, Richter GM, Stremmel
W and Stiehl A: Endoscopic variceal ligation plus propranolol vs.
transjugular intrahepatic portosystemic stent shunt: A long-term
randomized trial. Endoscopy. 34:690–697. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gülberg V, Schepke M, Geigenberger G, Holl
J, Brensing KA, Waggershauser T, Reiser M, Schild HH, Sauerbruch T
and Gerbes AL: Transjugular intrahepatic portosystemic shunting is
not superior to endoscopic variceal band ligation for prevention of
variceal rebleeding in cirrhotic patients: A randomized, controlled
trial. Scand J Gastroenterol. 37:338–343. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Escorsell A, Bañares R, García-Pagán JC,
Gilabert R, Moitinho E, Piqueras B, Bru C, Echenagusia A, Granados
A and Bosch J: TIPS versus drug therapy in preventing variceal
rebleeding in advanced cirrhosis: A randomized controlled trial.
Hepatology. 35:385–392. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Villanueva C, Miñana J, Ortiz J, Gallego
A, Soriano G, Torras X, Sáinz S, Boadas J, Cussó X, Guarner C and
Balanzó J: Endoscopic ligation compared with combined treatment
with nadolol and isosorbide mononitrate to prevent recurrent
variceal bleeding. N Engl J Med. 345:647–655. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pomier-Layrargues G, Villeneuve JP,
Deschênes M, Bui B, Perreault P, Fenyves D, Willems B, Marleau D,
Bilodeau M, Lafortune M and Dufresne MP: Transjugular intrahepatic
portosystemic shunt (TIPS) versus endoscopic variceal ligation in
the prevention of variceal rebleeding in patients with cirrhosis: A
randomised trial. Gut. 48:390–396. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Narahara Y, Kanazawa H, Kawamata H, Tada
N, Saitoh H, Matsuzaka S, Osada Y, Mamiya Y, Nakatsuka K, Yoshimoto
H, et al: A randomized clinical trial comparing transjugular
intrahepatic portosystemic shunt with endoscopic sclerotherapy in
the long-term management of patients with cirrhosis after recent
variceal hemorrhage. Hepatol Res. 21:189–198. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hou MC, Chen WC, Lin HC, Lee FY, Chang FY
and Lee SD: A new ‘sandwich’ method of combined endoscopic variceal
ligation and sclerotherapy versus ligation alone in the treatment
of esophageal variceal bleeding: A randomized trial. Gastrointest
Endosc. 53:572–578. 2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Meddi P, Merli M, Lionetti R, De Santis A,
Valeriano V, Masini A, Rossi P, Salvatori F, Salerno F, de Franchis
R, et al: Cost analysis for the prevention of variceal rebleeding:
A comparison between transjugular intrahepatic portosystemic shunt
and endoscopic sclerotherapy in a selected group of Italian
cirrhotic patients. Hepatology. 29:1074–1077. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
García-Villarreal L, Martínez-Lagares F,
Sierra A, Guevara C, Marrero JM, Jiménez E, Monescillo A,
Hernández-Cabrero T, Alonso JM and Fuentes R: Transjugular
intrahepatic portosystemic shunt versus endoscopic sclerotherapy
for the prevention of variceal rebleeding after recent variceal
hemorrhage. Hepatology. 29:27–32. 1999.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sauer P, Theilmann L, Stremmel W, Benz C,
Richter G and Stiehl A: Transjugular intrahepatic portosystemic
stent shunt versus sclerotherapy plus propranolol for variceal
rebleeding. Gastroenterology. 113:1623–1631. 1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sanyal AJ, Freedman AM, Luketic VA, Purdum
PP III, Shiffman ML, Cole PE, Tisnado J and Simmons S: Transjugular
intrahepatic portosystemic shunts compared with endoscopic
sclerotherapy for the prevention of recurrent variceal hemorrhage.
a randomized, controlled trial. Ann Intern Med. 126:849–857.
1997.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Villanueva C, Balanzó J, Novella MT,
Soriano G, Sáinz S, Torras X, Cussó X, Guarner C and Vilardell F:
Nadolol plus isosorbide mononitrate compared with sclerotherapy for
the prevention of variceal rebleeding. N Engl J Med. 334:1624–1629.
1996.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li P, Kong RD, Xie HJ, Sun B and Xu JM:
Efficacy and safety of endoscopic variceal ligation and endoscopic
injection sclerotherapy in patients with cirrhosis and esophageal
varices: A prospective study. World Chinese J Digestology.
18:3791–3795. 2010.
|
|
38
|
Holster IL, Tjwa E, Moelker A, Wils A,
Hansen BE, Vermeijden JR, Scholten P, van Hoek B, Nicolai JJ,
Kuipers EJ, et al: Covered transjugular intrahepatic portosystemic
shunt versus endoscopic therapy+β-blocker for prevention of
variceal rebleeding. Hepatology. 63:581–589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
van Buuren HR, Groeneweg M, Vleggaar FP,
Lesterrhuis W, Van Tillburg AJP, Hop WCJ, Peiterman H, Schalam SW,
van Hout BA and Lameris JS: The results of a randomized controlled
trial evaluating TIPS and endoscopic therapy in cirrhotic patients
with gastro-esophageal variceal bleeding. J Hepatol.
32(71)2000.
|
|
40
|
Cabrera J, Maynar M, Granados R, Gorriz E,
Reyes R, Pulido-Duque JM, Rodriguez SanRoman JL, Guerra C and
Kravetz D: Transjugular intrahepatic portosystemic shunt versus
sclerotherapy in the elective treatment of variceal hemorrhage.
Gastroenterology. 110:832–839. 1996.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dobrucali A, Bal K, Tuncer M, Ilkova F,
Çelik A, Uzunismail H, Yurdakul I and Oktay E: Endoscopic banding
ligation compared with sclerotherapy for the treatment of
esophageal varies. Turk J Gastroenterol. 9:340–344. 1998.
|
|
42
|
Holster IL, Moelker A, Tjwa ET, et al:
Early transjugular intrahepatic portosystemic shunt (TIPS) as
compared to endoscopic treatment reduces rebleeding but not
mortality in cirrhotic patients with a 1st or 2nd episode of
variceal bleeding: A multicentre randomized controlled trial.
United European Gastroenterology Journal. 1(A84)2013.
|
|
43
|
Merli M, Riggio O, Capocaccia L, et al:
TIPS vs sclerotherapy in preventing variceal rebleeding in
cirrhotic patients: Preliminary results of a randomised controlled
trial (EASL abstract). Journal of hepatology. 21(S41)1994.
|
|
44
|
Ahmad I, Khan AA, Alam A, Butt AK, Shafqat
F and Sarwar S: Propranolol, isosorbide mononitrate and endoscopic
band ligation-alone or in varying combinations for the prevention
of esophageal variceal rebleeding. J Coll Physicians Surg Pak.
19:283–286. 2009.PubMed/NCBI
|
|
45
|
Argonz J, Kravetz D, Suarez A, Romero G,
Bildozola M, Passamonti M, Valero J and Terg R: Variceal band
ligation and variceal band ligation plus sclerotherapy in the
prevention of recurrent variceal bleeding in cirrhotic patients: A
randomized, prospective and controlled trial. Gastrointest Endosc.
51:157–163. 2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Avgerinos A, Armonis A, Manolakopoulos S,
Poulianos G, Rekoumis G, Sgourou A, Gouma P and Raptis S:
Endoscopic sclerotherapy versus variceal ligation in the long-term
management of patients with cirrhosis after variceal bleeding. A
prospective randomized study. J Hepatol. 26:1034–1341.
1997.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Avgerinos A, Armonis A, Stefanidis G,
Mathou N, Vlachogiannakos J, Kougioumtzian A, Triantos C,
Papaxoinis C, Manolakopoulos S, Panani A and Raptis SA: Sustained
rise of portal pressure after sclerotherapy, but not band ligation,
in acute variceal bleeding in cirrhosis. Hepatology. 39:1623–1630.
2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Avgerinos A, Rekoumis G, Klonis C,
Papadimitriou N, Gouma P, Pournaras S and Raptis S: Propranolol in
the prevention of recurrent upper gastrointestinal bleeding in
patients with cirrhosis undergoing endoscopic sclerotherapy. A
randomized controlled trial. J Hepatol. 19:301–311. 1993.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Baroncini D, Milandri GL, Borioni D,
Piemontese A, Cennamo V, Billi P, Dal Monte PP and D'Imperio N: A
prospective randomized trial of sclerotherapy versus ligation in
the elective treatment of bleeding esophageal varices. Endoscopy.
29:235–240. 1997.PubMed/NCBI View Article : Google Scholar
|
|
50
|
García-Pagán JC, Villanueva C, Albillos A,
Bañares R, Morillas R, Abraldes JG and Bosch J: Spanish Variceal
Bleeding Study Group: Nadolol plus isosorbide mononitrate alone or
associated with band ligation in the prevention of recurrent
bleeding: A multicentre randomised controlled trial. Gut.
58:1144–1150. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hou MC, Lin HC, Kuo BI, Chen CH, Lee FY
and Lee SD: Comparison of endoscopic variceal injection
sclerotherapy and ligation for the treatment of esophageal variceal
hemorrhage: A prospective randomized trial. Hepatology.
21:1517–1522. 1995.PubMed/NCBI
|
|
52
|
Hou MC, Lin HC, Lee FY, Chang FY and Lee
SD: Recurrence of esophageal varices following endoscopic reagent
and impact on rebleeding: Comparison of sclerotherapy and ligation.
J Hepatology. 32:202–208. 2000.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lo GH, Lai KH, Cheng JS, Hwu JH, Chang CF,
Chen SM and Chiang HT: A prospective, randomized trial of
sclerotherapy versus ligation in the management of bleeding
esophageal varices. Hepatology. 22:466–4671. 1995.PubMed/NCBI
|
|
54
|
Lo GH, Lai KH, Cheng JS, Lin CK, Huang JS,
Hsu PI and Chiang HT: Emergency banding ligation versus
sclerotherapy for the control of active bleeding from esophageal
varices. Hepatology. 25:1101–1104. 1997.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Peña J, Brullet E, Sanchez-Hernández E,
Rivero M, Vergara M, Martin-Lorente JL and Garcia Suárez C:
Variceal ligation plus nadolol compared with ligation for
prophylaxis of variceal rebleeding: A multicenter trial.
Hepatology. 41:572–578. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
de la Peña J, Rivero M, Sanchez E, Fábrega
E, Crespo J and Pons-Romero F: Variceal ligation compared with
endoscopic sclerotherapy for variceal hemorrhage: Prospective
randomized trial. Gastrointest Endosc. 49:417–423. 1999.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Romero G, Kravetz D, Argonz J, Vulcano C,
Suarez A, Fassio E, Dominguez N, Bosco A, Muñoz A, Salgado P and
Terg R: Comparative study between nadolol and 5-isosorbide
mononitrate vs. Endoscopic band ligation plus sclerotherapy in the
prevention of variceal rebleeding in cirrhotic patients: A
randomized controlled trial. Aliment Pharmacol Ther. 24:601–611.
2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Stiegmann GV, Goff JS, Michaletz-Onody PA,
Korula J, Lieberman D, Saeed ZA, Reveille RM, Sun JH and Lowenstein
SR: Endoscopic sclerotherapy as compared with endoscopic ligation
for bleeding esophageal varices. N Engl J Med. 326:1527–1532.
1992.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Umehara M, Onda M, Tajiri T, Toba M,
Yoshida H and Yamashita K: Sclerotherapy plus ligation versus
ligation for the treatment of esophageal varices: A prospective
randomized study. Gastrointest Endosc. 50:7–12. 1999.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Viazis N, Armonis A, Vlachogiannakos J,
Rekoumis G, Stefanidis G, Papadimitriou N, Manolakopoulos S and
Avgerinos A: Effects of endoscopic variceal treatment on
oesophageal function: a prospective, randomized study. Eur J
Gastroenterol Hepatol. 14:263–269. 2002.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Vinel JP, Lamouliatte H, Cales P, Combis
JM, Roux D, Desmorat H, Pradere B, Barjonet G and Quinton A:
Propranolol reduces the rebleeding rate during endoscopic
sclerotherapy before variceal obliteration. Gastroenterology.
102:1760–173. 1992.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zheng M, Chen Y, Bai J, Zeng Q, You J, Jin
R, Zhou X, Shen H, Zheng Y and Du Z: Transjugular intrahepatic
portosystemic shunt versus endoscopic therapy in the secondary
prophylaxis of variceal rebleeding in cirrhotic patients:
meta-analysis update. J Clin Gastroenterol. 42:507–516.
2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chinese Society of Gastroenterology,
Chinese Medical Association, Chinese Society of Endoscopy:
Guidelines for the diagnosis and treatment of esophageal and
gastric variceal bleeding in cirrhotic portal hypertension. Chinese
Society of Hepatology, Chinese Medical Association. Chinese Medial
Association. J Clin Hepatol 32: 203-219, 2016 (In Chinese).
|
|
64
|
Cotoras P, Faundez J and Candia R: Should
we add beta-blockers to band ligation for secondary prophylaxis of
variceal bleeding? Medwave. 17 (Suppl 1)(e6847)2017.PubMed/NCBI View Article : Google Scholar
|