Introduction
Wet age-related macular degeneration (wAMD) is a
common cause of blindness in clinic. Molecular biological studies
have discovered that the imbalance between pro-angiogenic factors
and anti-angiogenic factors is the leading cause of wAMD, in which
vascular endothelial growth factor (VEGF) is highly expressed, and
choroidal neovascularization (CNV) is formed, resulting in visual
loss of the patients (1,2). Ranibizumab, a kind of monoclonal
antibody fragment, belongs to angiogenesis inhibitors and achieves
its therapeutic purposes by preventing vascular leakage and
blocking choroidal vessels (3).
Photodynamic therapy (PDT) is a new therapy for CNV
in recent years (4), which can
protect the peripheral normal tissues to the greatest extent while
blocking CNV. During PDT, the lesion site is irradiated by laser of
a specific wavelength, and the activated photosensitizing agent
produces photooxidation reaction to kill endothelial cells and
selectively destroy aberrant neovascularization, without damaging
surrounding normal retina (5).
Studies have demonstrated that ranibizumab combined with PDT has
preferable efficacy in treating wAMD (6-8).
In this study, the clinical data of 130 eyes of 130
patients with wAMD treated in Affiliated to Qingdao University
Yuhuangding Hospital of Yantai (Yantai, China) from May 2017 to May
2018 were retrospectively reviewed, and the efficacy and safety of
simple intravitreal ranibizumab (IVR) and IVR combined with PDT in
treating wAMD were comparative analyzed, with the aim to provide a
more scientific basis for formulating efficacious treatment
protocols.
Patients and methods
General data
A total of 130 eyes of 130 wAMD patients who were
treated in the hospital from May 2017 to May 2018 were selected as
the objects, which were divided into combination therapy group (IVR
combined with PDT, n=65) and ranibizumab group (simple intravitreal
injection of ranibizumab, n=65) using a random number table.
Inclusion criteria: patients aged ≥50 years, those with monocular
lesion and those clinically diagnosed with exudative AMD.
Diagnostic criteria: i) visual deterioration (or accompanied with
metamorphopsia), macular drusen, depigmentation or repigmentation
and typical CNV signs, ii) subretinal hemorrhage, exudate and
fibrotic membrane, iii) best corrected visual acuity (BCVA) ≥25
letters before treatment, and iv) basically clear refractive media,
without influences on fundus examination and fundus fluorescein
angiography (FFA). Exclusion criteria: i) patients whose CNV in the
trial eye was previously treated via intravitreal injection of
drugs, PDT, transpupillary thermotherapy (TTT), retinal laser
photocoagulation, macular grid photocoagulation or submacular
surgery; ii) those with ocular tumor, trauma, glaucoma, or eye
infection, iii) those with high myopia, iv) those complicated with
other types of retinal diseases (polypoidal choroidal vasculopathy
or dry AMD), v) those with severe cardiac, pulmonary hepatic or
renal dysfunction, or vi) those who had serious hypersensitivity
reactions to the medicines applied in this study. There were 70
males and 60 females aged 51-82 years, with a course of disease of
3-21 months. The differences in the age, sex, course of disease,
baseline visual acuity, central macular thickness (CMT) and CNV
type and location were not statistically significant between the
two groups (Table I). This study was
reviewed and approved by the Ethics Committee of Affiliated to
Qingdao University Yuhuangding Hospital of Yantai, and the patients
and their families were informed and signed the informed
consent.
 | Table IDemographics and general clinical data
of all studied patients. |
Table I
Demographics and general clinical data
of all studied patients.
| Parameters | Combination group
n=65 | Ranibizumab group
n=65 | P-value |
|---|
| Sex
(male/female) | 37/28 | 33/32 | 0.598 |
| Age (years) | 58.53±10.38 | 59.74±10.76 | 0.515 |
| Course of the disease
(months) | 11.8±7.3 | 13.5±8.1 | 0.211 |
| BCVA (logMAR) | 0.88±0.44 | 0.91±0.39 | 0.412 |
| CMT (µm) | 403.6±56.7 | 393.1±74.8 | 0.369 |
| IOP (mmHg) | 14.6±3.7 | 15.0±3.4 | 0.522 |
| Type | | | 0.602 |
|
Predominantly
classic CNV | 31 (47.7%) | 28 (43.1%) | |
|
Minimally
classic CNV | 20 (30.8%) | 18 (27.7%) | |
|
Occult
CNV | 14 (21.5%) | 19 (29.2%) | |
| Location | | | 0.474 |
|
Subfoveal | 38 (58.5%) | 43 (66.2%) | |
|
Juxtafoveal | 19 (29.2%) | 13 (20.0%) | |
|
Extrafoveal | 8 (12.3%) | 9 (13.8%) | |
Therapeutic methods
IVR: The eyes were instilled with ofloxacin eye
drops (Tarivid, Santen Pharmaceutical Co., Ltd.) 3 days before
operation (4 times/day). The operation was performed under sterile
conditions. The patients were in the supine position, underwent
routine disinfection and draping and received oxybuprocaine
hydrochloride eye drops (Benoxil, Pharmaceutical Co., Ltd.) for
topical anesthesia of the eyeball, followed by flushing of the
conjunctival sac with 5% povidone iodine and normal saline. Then a
30 G needle was vertically inserted into the vitreous cavity
through the pars plana of ciliary at 3.5 mm away from the
corneoscleral limbus toward 10 o'clock, and 0.5 mg (0.05 ml) of
ranibizumab (Lucentis, Novartis AG) was injected slowly. After
operation, the eye was packed with Tarivid eye ointment. The
intraocular pressure was closely monitored at 2 h after operation,
and the eye was given Tarivid eye drops 4 times/day for 1
consecutive week to prevent postoperative infection. Course of
treatment: once a month for 3 consecutive months.
IVR combined with PDT: The photosensitizer
verteporfin (Visudyne, Novartis AG) (6 mg/m2) was
diluted to 30 ml using 5% glucose and intravenously injected in a
dark room within 10 min. Fifteen minutes later, the macular
degeneration was persistently irradiated by laser at the wavelength
of 689 nm for 83 sec, with an intensity of 600 mW/cm2
and a density of 50 J/cm2. The eye was protected against
light within 5 days after treatment. At 3 days after PDT,
ranibizumab was intravitreally injected once according to the same
mode of injection and course of treatment.
Observation indexes
The FFA, BCVA examination, optical coherence
tomography (OCT) and color fundus photography were performed for
the two groups of patients before treatment and at 1, 3 and 6
months after treatment. As for BCVA examination, the standard
logarithmic visual acuity chart was employed, and the results were
converted to logarithm of the minimal angle resolution (logMAR)
visual acuity during statistics. Kowa nonmyd α-DIII non-mydriatic
fundus camera was used for color fundus photography, and Spectralis
HRA was utilized for FFA. Spectralis OCT instrument was adopted for
OCT, and horizontal linear scanning was performed centered on the
central fovea of macula (scanning depth, 1.9 mm; scanning area, 6
mm x 6 mm; lateral resolution, 14 µm; axial resolution, 7 µm; and
scanning mode, 512x496). The distance from the inner limiting
membrane of retinal neurosensory layer to the lateral high
reflection band of the retinal pigment epithelium layer was
measured using the built-in locating software of the instrument,
which was regarded as the CMT. The measurement was completed by two
experienced technicians and repeated 3 times to calculate the
average value. The FFA combined with ICGA was carried out at 3 and
6 months after treatment to determine the area of macular
degeneration, so as to reflect the CNV leakage. The degree of CNV
leakage was classified as progressive (the lesion area is increased
compared with that before treatment), stable (the lesion area is
slightly increased or decreased compared with that before
treatment), improved (the lesion area is decreased by <1/2) and
ameliorated (the lesion area is decreased by >1/2), of which
stable, improved and ameliorated grades are all considered as
effective treatment.
Criteria of IVR retreatment: The retinal thickness
in the macular region is increased by >100 µm, with subretinal
fluid and retinal edema, and active CNV is detected by FFA when
there is new retinal hemorrhage or short-term diminution of visual
acuity by 5 letters.
The BCVA, change in mean CMT, change in intraocular
pressure, CNV leakage and occurrence of complications were recorded
before treatment and at 1, 3 and 6 months after treatment.
Moreover, 5 ml of fasting venous blood was drawn from every patient
in the two groups before treatment and at 3 months after treatment
separately to detect and compare the levels of VEGF and
transforming growth factor-β1 (TGF-β1) via enzyme-linked
immunosorbent assay.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
22.0 (IBM) was adopted for statistical analysis. The enumeration
data were presented as ratio (%), and χ2 test was
conducted for inter-group comparison. The measurement data were
expressed as mean ± standard deviation (mean ± SD), and repeated
measures analysis of variance was applied to compare the BCVA, CMT
and intraocular pressure of the patients at different time points,
independent-samples t-test was performed for comparison between
groups, and least significant difference (LSD) t-test was conducted
for comparison of differences at different time points within
groups. P<0.05 indicates that the difference is statistically
significant.
Results
Changes in BCVA before and after
treatment in the two groups of patients
There were statistically significant changes in the
BCVA before and after treatment in the two groups of patients
(Fgroup = 17.96, Ftime = 11.89,
Ftime x groupe = 16.34, P<0.01). The BCVA
at 1, 3, 6 and 12 months after treatment was superior to that
before treatment in both groups (P<0.05). As for comparisons
between the two groups at 1, 3, 6 and 12 months after treatment,
combination therapy group had remarkably better BCVA than
ranibizumab group (P=0.041, P=0.007, P<0.001, P=0.004) (Table II).
 | Table IIComparison of pretreatment and
posttreatment BCVA (logMAR) and CMT (µm) of the studied patients in
two different groups. |
Table II
Comparison of pretreatment and
posttreatment BCVA (logMAR) and CMT (µm) of the studied patients in
two different groups.
| | Combination group
n=65 | Ranibizumab group
n=65 |
|---|
| BCVA (logMAR) |
|
Before
treatment | 0.88±0.44 | 0.91±0.39 |
|
1 month
after treatment | 0.80±0.15 | 0.86±0.18 |
|
3 months
after treatment | 0.71±0.17 | 0.80±0.20 |
|
6 months
after treatment | 0.61±0.14 | 0.72±0.19 |
|
12 months
after treatment | 0.57±0.22 | 0.67±0.17 |
| CMT (µm) |
|
Before
treatment | 403.6±56.7 | 393.1±74.8 |
|
1 month
after treatment | 343.2±41.4 | 360.6±40.1 |
|
3 months
after treatment | 305.8±35.8 | 329.9±36.5 |
|
6 months
after treatment | 281.1±31.9 | 297.4±33.6 |
|
12 months
after treatment | 260.2±29.3 | 278.2±28.8 |
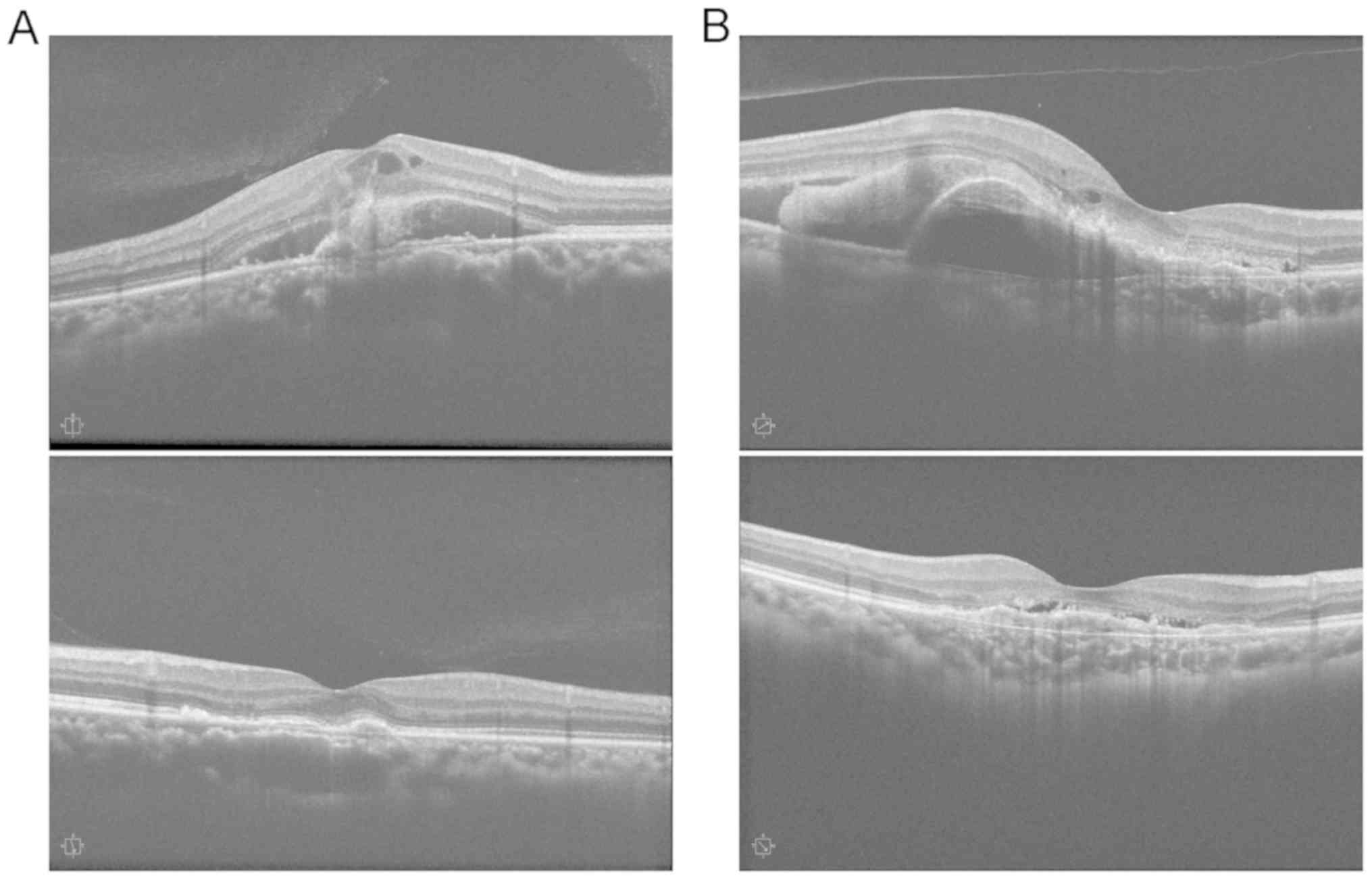
Changes in CMT before and after
treatment in the two groups of patients
The differences in CMT were statistically
significant before and after treatment in the two groups of
patients (Fgroupe = 10.81, Ftimee = 19.29,
Ftime x groupe = 22.79, P<0.01). The CMT
was decreased markedly at 1, 3, 6 and 12 months after treatment in
both groups compared with that before treatment (P<0.05).
Combination therapy group exhibited remarkably smaller CMT than
ranibizumab group at 1, 3, 6 and 12 months after treatment
(P=0.016, P<0.001, P=0.005, P<0.001) (Table II and Fig. 1).
Changes in intraocular pressure before
and after treatment in the two groups of patients
After treatment, 1 case of intraocular pressure was
>21 mmHg in the combination therapy group. In the ranibizumab
group, there were 2 cases with intraocular pressure >21 mmHg.
None of the three patients were treated with ocular hypotensive
drugs, but they returned to normal after 3 days. The average
intraocular pressure in the two groups was 14.9±4.3 and 15.2±4.6
mmHg, 14.7±4.1 and 14.8±3.9 mmHg, 14.6±4.2 and 14.9±4.5 mmHg, and
14.5±4.4 and 14.7±3.8 mmHg at 1, 3, 6 and 12 months after
treatment, respectively. No statistically significant difference in
the intraocular pressure was observed between the two groups before
and after treatment (P>0.05).
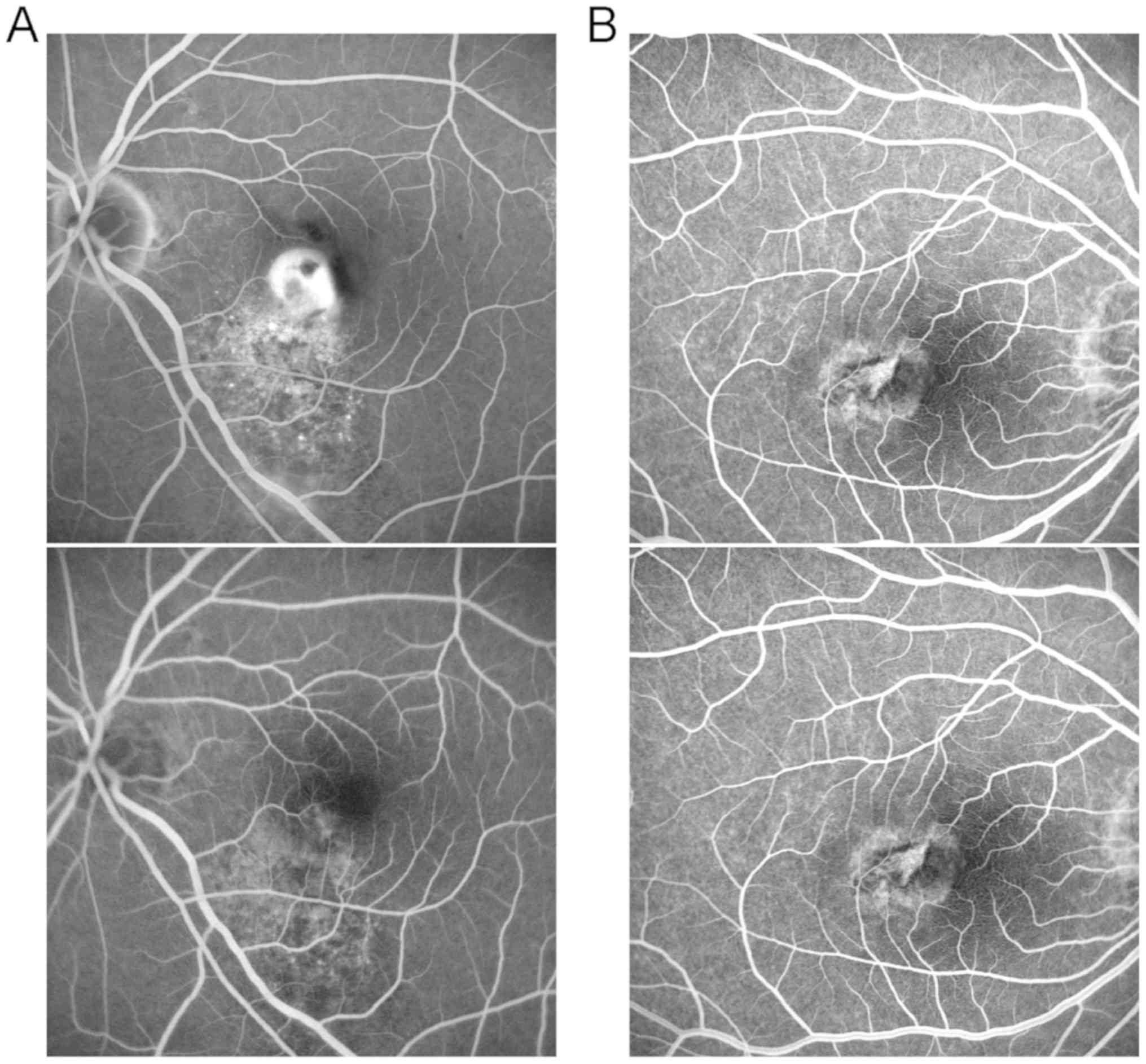
Comparison of the area of macular
degeneration and CNV leakage before and after treatment between the
two groups of patients
FFA showed that the area of macular degeneration
before treatment was 3.6±0.6 and 3.5±0.7 mm2 (P=0.384).
After treatment, the area of macular degeneration was reduced
notably in both groups, and repeated measures analysis of variance
indicated that there were statistically significant differences in
the area of macular degeneration at different time points
(Ftime=8.21, P<0.001) and between the two groups
(Fgroup=12.42, P<0.001). Moreover, the area of
macular degeneration in combination therapy group was evidently
smaller than that in ranibizumab group at 1, 3 and 6 months after
treatment (P=0.047, P=0.025, P=0.027), while it manifested no
statistically significant difference between the two groups at 12
months after treatment (P=0.083) (Table III and Fig. 2).
 | Table IIIComparison of pretreatment and
posttreatment maculopathy area (mm2) of the studied
patients in two groups. |
Table III
Comparison of pretreatment and
posttreatment maculopathy area (mm2) of the studied
patients in two groups.
| | Combination group
n=65 | Ranibizumab group
n=65 |
|---|
| Maculopathy area
(mm2) |
|
Before
treatment | 3.6±0.6 | 3.5±0.7 |
|
1 month
after treatment | 2.3±0.8 | 2.6±0.9 |
|
3 months
after treatment | 2.2±0.7 | 2.5±0.8 |
|
6 months
after treatment | 2.0±0.6 | 2.3±0.9 |
|
12 months
after treatment | 2.1±0.6 | 2.3±0.7 |
According to the statistics of FFA results, there
were 31 cases of stable, 14 cases of improved, 16 cases of
ameliorated and 4 cases of progressive CNV leakage at 12 months
after treatment in combination therapy group, while the numbers of
cases were 25, 18, 11 and 11, respectively, in ranibizumab group.
The effective rate was 93.8% in combination therapy group and 83.1%
in ranibizumab group. The difference in the CNV leakage at 12
months after treatment was not statistically significant between
the two groups (P=0.097).
Incidence of treatment-related
complications in the two groups of patients
Among the 65 patients receiving PDT, 2 had transient
back pain that disappeared within several minutes after the
termination of drug injection. A minority of patients had temporary
blurred vision, which was improved 1 week later, without severe
hypopsia. There was 1 patient in ranibizumab group who had a small
amount of macular hemorrhage, which was absorbed in a short
term.
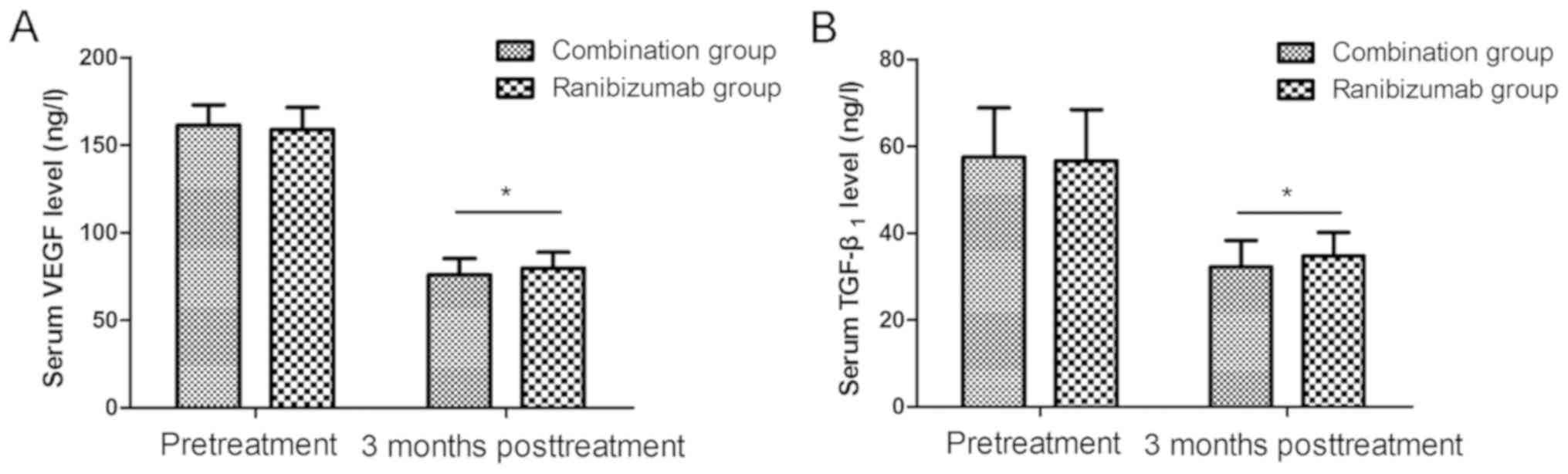
Comparison of serum VEGF and TGF-β1
concentrations between the two groups of patients
The levels of serum VEGF and TGF-β1 displayed no
statistically significant differences before treatment between the
two groups (P=0.241, P=0.658). At 3 months after treatment, the
level of serum VEGF declined from 161.3±11.6 to 75.9±9.3 ng/l and
from 158.8±12.6 to 79.6±9.1 ng/l, and that of TGF-β1 was reduced
from 57.5±11.3 to 32.2±6.1 ng/l and from 56.6±11.8 to 34.7±5.4 ng/l
in the two groups. The levels were distinctly lower after treatment
than those before treatment (P<0.05), and they were prominently
decreased in combination therapy group in comparison with those in
ranibizumab group (P=0.024, P=0.015) (Fig. 3).
Discussion
Currently, the commonly applied therapeutic methods
for exudative AMD include traditional laser photocoagulation, PDT,
TTT, anti-VEGF drug therapy, glucocorticoid drug therapy, surgical
treatment, radiotherapy and gene therapy, but none of them can
completely cure CNV, and repeated treatments are necessary, thereby
increasing the treatment costs and complications (9). The disturbed balance between
pro-angiogenic and anti-angiogenic factors is the main cause of
CNV, and anti-VEGF drugs have become the preferred therapy
(10). As a kind of high-affinity
recombinant humanized monoclonal antibody-binding fragment (Fab),
ranibizumab has relatively strong affinity to all VEGF-A subunits,
and it can inhibit neovascularization, block secondary vascular
effects, effectively prevent vascular leakage and control disease
progression through competitively binding to VEGF (11).
PDT, a selective treatment of CNV, is the major
clinical treatment method for wAMD at present, which selectively
acts on neovascularization by virtue of photosensitizer and
produces photochemical reactions via laser irradiation to shrink
the neovascularization, thus achieving the therapeutic purpose. PDT
possesses clinical features such as good tissue selectivity,
superficial action, strong damage effects on microvascular tissues,
topical treatment and few systemic side effects (12,13). The
study of Spielberg et al (14) indicated that PDT combined with IVR is
safe and efficacious in treating macular CNV. Nakamura et al
(15) treated 38 eyes of 38 AMD
patients with ranibizumab combined with PDT (intravitreal injection
of 0.5 mg of ranibizumab, execution of PDT of standard dose within
1 week and the second intravitreal injection 1 month later). The
follow-up results manifested that the majority of patients (94.8%)
had remarkably ameliorated visual acuity and central foveal
thickness as well as a low recurrence rate. Saviano et al
(16) proved that PDT combined with
intravitreal injection of bevacizumab is superior to monotherapy in
treating macular CNV. A randomized, two-way clinical trial of 36
patients with exudative AMD was performed by Potter et al
(17). The first group received
bevacizumab in combination with V-PDT (25 J/cm2), the
second group received bevacizumab in combination with reduced PDT
(12 J/cm2), and the third group received only
bevacizumab. Based on the OCT results of the monthly follow-up
after the initial treatment, it was decided whether to re-inject
bevacizumab or re-administer the combination for 6 months. The test
results showed that the patients need 2.8, 2.5 and 5.1 times of
bevacizumab injection on average in group I, group II and group
III, respectively, so it can be seen that the frequency of
injection in group I and group II is notably lower than that in
group III (P<0.01) (17). Kim
et al (18) also confirmed
that V-PDT combined with 3 successive times of bevacizumab
injection can distinctly improve the visual acuity and reduce
CMT.
In this study, it was revealed that ranibizumab
combined with PDT was able to prominently enhance the BCVA and
decrease CMT of the AMD patients in comparison with ranibizumab
monotherapy. The area of macular degeneration was reduced after
treatment, and it was evidently smaller in combination therapy
group than that in ranibizumab group, suggesting that the
combination therapy can strengthen the improvement effect on
macular morphology more favorably. In terms of improving CNV
leakage, combination therapy group was more effective than
ranibizumab group, but the difference was not statistically
significant, which may be associated with the smaller sample
included in this study.
As the most potent pro-angiogenic factor recognized
internationally so far, VEGF can enhance the microvascular
permeability, increase the release of tissue factors and proteases
and stimulate division of endothelial cells. TGF-β1 directly
regulates VEGF expression and participates in CNV (19-21).
According to the results in this study, obviously reduced
concentrations of serum VEGF and TGF-β1 were observed after
combination therapy, implying that the disease is controlled
efficiently, and the visual acuity is improved. PDT can block the
existing CNV, and ranibizumab is capable of resisting VEGF and
repressing CNV, so the clinical efficacy is strengthened apparently
by combining the two methods.
In conclusion, the IVR combined with PDT can
effectively improve the visual acuity, decrease CMT and prominently
reduce the area of macular degeneration of wAMD patients, and its
therapeutic effects are long-standing and tolerable for the
patients, so it is worthy of clinical popularization.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LC, BW and SF designed the study and performed the
experiments, LC and WC collected the data, BW and WC analyzed the
data, LC, BW and SF prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Affiliated to Qingdao University Yuhuangding Hospital of Yantai
(Yantai, China). Signed informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Mehta S: Age-related macular degeneration.
Prim Care. 42:377–391. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I,
Wong TY and Cheung CMG: Age-related macular degeneration and
polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res.
53:107–139. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Waldstein SM, Simader C, Staurenghi G,
Chong NV, Mitchell P, Jaffe GJ, Lu C, Katz TA and Schmidt-Erfurth
U: Morphology and visual acuity in aflibercept and ranibizumab
therapy for neovascular age-related macular degeneration in the
VIEW trials. Ophthalmology. 123:1521–1529. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cruess AF, Zlateva G, Pleil AM and
Wirostko B: Photodynamic therapy with verteporfin in age-related
macular degeneration: A systematic review of efficacy, safety,
treatment modifications and pharmacoeconomic properties. Acta
Ophthalmol. 87:118–132. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Teper SJ, Nowinska A, Pilat J and Wylegala
E: Photodynamic therapy in VEGF inhibition non-responders -
Pharmacogenetic study in age-related macular degeneration assessed
with swept-source optical coherence tomography. Photodiagn Photodyn
Ther. 13:108–113. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saviano S, Leon PE, Mangogna A and
Tognetto D: Combined therapy (intravitreal bevacizumab plus
verteporfin photodynamic therapy) versus intravitreal bevacizumab
monotherapy for choroidal neovascularization due to age-related
macular degeneration: A 1-year follow-up study. Digit J Ophthalmol.
22:46–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Su Y, Wu J and Gu Y: Photodynamic therapy
in combination with ranibizumab versus ranibizumab monotherapy for
wet age-related macular degeneration: A systematic review and
meta-analysis. Photodiagn Photodyn Ther. 22:263–273.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Datseris I, Kontadakis GA, Diamanti R,
Datseris I, Pallikaris IG, Theodossiadis P and Tsilimbaris MK:
Prospective comparison of low-fluence photodynamic therapy combined
with intravitreal bevacizumab versus bevacizumab monotherapy for
choroidal neovascularization in age-related macular degeneration.
Semin Ophthalmol. 30:112–117. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al-Zamil WM and Yassin SA: Recent
developments in age-related macular degeneration: A review. Clin
Interv Aging. 12:1313–1330. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mitchell P, Liew G, Gopinath B and Wong
TY: Age-related macular degeneration. Lancet. 392:1147–1159.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rufai SR, Almuhtaseb H, Paul RM, Stuart
BL, Kendrick T, Lee H and Lotery AJ: A systematic review to assess
the 'treat-and-extend’ dosing regimen for neovascular age-related
macular degeneration using ranibizumab. Eye (Lond). 31:1337–1344.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Singh CN and Saperstein DA: Combination
treatment with reduced-fluence photodynamic therapy and
intravitreal injection of triamcinolone for subfoveal choroidal
neovascularization in macular degeneration. Retina. 28:789–793.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kang EC, Seo JG, Kim BR and Koh HJ:
Clinical outcomes of Iintravitreal bevacizumab versus photodynamic
therapy with or without bevacizumab for myopic choroidal
neovascularization: A 7-year follow-up study. Retina. 37:1775–1783.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Spielberg L and Leys A: Treatment of
neovascular age-related macular degeneration with a variable
ranibizumab dosing regimen and one-time reduced-fluence
photodynamic therapy: The TORPEDO trial at 2 years. Graefes Arch
Clin Exp Ophthalmol. 248:943–956. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nakamura T, Miyakoshi A, Fujita K, Yunoki
T, Mitarai K, Yanagisawa S, Fuchizawa C and Hayashi A: One-year
results of photodynamic therapy combined with intravitreal
ranibizumab for exudative age-related macular degeneration. J
Ophthalmol. 2012(154659)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Saviano S, Piermarocchi R, Leon PE,
Mangogna A, Zanei A, Cavarzeran Sc F and Tognetto D: Combined
therapy with bevacizumab and photodynamic therapy for myopic
choroidal neovascularization: A one-year follow-up controlled
study. Int J Ophthalmol. 7:335–339. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Potter MJ, Claudio CC and Szabo SM: A
randomised trial of bevacizumab and reduced light dose photodynamic
therapy in age-related macular degeneration: The VIA study. Br J
Ophthalmol. 94:174–179. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim HW, Kim JL, Lee MH, Yoo HG, Chung IY
and Lee JE: Combined treatment of photodynamic therapy and
bevacizumab for choroidal neovascularization secondary to
age-related macular degeneration. Korean J Ophthalmol. 25:231–237.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cheung GCM, Lai TYY, Gomi F, Ruamviboonsuk
P, Koh A and Lee WK: Anti-VEGF therapy for neovascular AMD and
polypoidal choroidal vasculopathy. Asia Pac J Ophthalmol (Phila).
6:527–534. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tosi GM, Caldi E, Neri G, Nuti E,
Marigliani D, Baiocchi S, Traversi C, Cevenini G, Tarantello A,
Fusco F, et al: HTRA1 and TGF-β1 concentrations in the aqueous
humor of patients with neovascular age-related macular
degeneration. Invest Ophthalmol Vis Sci. 58:162–167.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cabral T, Mello LGM, Lima LH, Polido J,
Regatieri CV, Belfort R Jr and Mahajan VB: Retinal and choroidal
angiogenesis: A review of new targets. Int J Retina Vitreous.
3(31)2017.PubMed/NCBI View Article : Google Scholar
|