Introduction
Preeclampsia (PE) is a pregnancy-specific disorder
that causes considerable maternal and perinatal morbidity and
mortality worldwide (1), occurring
in 2-8% of pregnancies, particularly in developing countries
(2,3). PE is associated with acute and
long-term complications, including enhanced platelet aggregation,
activation of the coagulation system, endothelial dysfunction and
vasospasm (4). It has been reported
that women who suffered from PE during their pregnancies had a
higher risk of heart failure, stroke and mortality as a result of
cardiovascular disease compared with that of healthy pregnant women
(5). Although the exact cause and
pathophysiology of PE is yet not fully understood, it is generally
considered that the placenta plays an important role in the
pathogenesis of PE, since removal of the placenta can eliminate the
clinical symptoms of women with PE (6). Furthermore, growing evidence indicates
that abnormally shallow placentation serves a major role in the
pathogenesis of PE (7). Inadequate
trophoblastic invasion of the uterine wall, excessive apoptosis of
trophoblast cells and impaired spiral artery transformation at the
maternal-fetal interface are major abnormalities in the development
of the placenta, which persistently increase resistance to blood
flow in the uteroplacental circulation (8,9). It has
recent been reported that the incidence of PE was decreased by
elective delivery, and antihypertensive drug therapy must be
applied to treat severe hypertension in women with PE (10).
Angiopoietin-like proteins (ANGPTLs) comprise a
class of secreted factors that are characterized by an N-terminal
coiled-coil domain and a C-terminal fibrinogen-like domain, and are
structurally similar to angiopoietin. However, unlike other
ANGPTLs, ANGPTL8 lacks the C-terminal fibrinogen-like domain
(11,12). A previous study has reported that
ANGPTL4 and ANGPTL8 are multifunctional factors associated with the
regulation of lipid metabolism, which can bind to lipoprotein
lipase (LPL) and antagonize its activity (13). Since LPL hydrolyzes triglycerides
(TGs) circulating in the capillaries of adipose tissue and the
muscles, resulting in increased TG concentrations in the plasma
(11,12,14,15). In
addition, enhanced levels of circulating ANGPTL8 have been reported
to be positively correlated with circulating low-density
lipoprotein-cholesterol levels and inversely correlated with
circulating high-density lipoprotein (HDL)-cholesterol levels in
the serum (13), suggesting that
circulating ANGPTL8 concentrations serve an important role in
cardiovascular disease development. A recent study has demonstrated
that ANGPTL4 mediates the protective role of activators of
peroxisome proliferator-activated receptor γ and is associated with
the pathogenesis of PE (16).
However, the expression and secretion of ANGPTL8 has not been
investigated in the onset of PE. Recently, it was revealed that
ANGPTL8 has a potential effect on cell proliferation (17). Thus, it is hypothesized that ANGPTL8
may have a potential effect on the pathogenesis of PE.
The present study aimed to investigate the effect of
ANGPTL8 and the molecular mechanisms underlying the pathogenesis of
PE in HTR8/SVneo cells. Furthermore, the serum expression of
ANGPTL8 and its potential correlation in patients with PE and
healthy subjects were explored. The current study results provided
evidence that ANGPTL8 affects the proliferation, invasion and
migration of trophoblast cells to promote PE.
Materials and methods
Study subjects and collection of blood
samples
A total of 30 patients with PE and 30 healthy
pregnant women who underwent cesarean deliveries and hospitalized
(July 2016 to April 2018) in Xuzhou Central Hospital (Xuzhou,
China) were recruited into the present study. The main
clinicopathological characteristics of the participants are
summarized in Table I. All
corresponding blood samples were collected after delivery (within 1
h) and used according to the protocol approved by the Ethics
Committee at Xuzhou Central Hospital. Pregnant women were excluded
from the present study if they presented any of the following:
<18 years of age without a legal guardian, was at >26 weeks
of gestation, had a multifetal gestation, suffered from a
cardiovascular disease (including chronic hypertension or valvular
heart disease) or pregestational diabetes, or had a history of
fenfluramine/phentermine use. All participants provided written
informed consent.
 | Table IBaseline characteristics of subjects
in the study population (n=30 per group). |
Table I
Baseline characteristics of subjects
in the study population (n=30 per group).
| Characteristics | Preeclampsia
(n=30) | Healthy controls
(n=30) | P-value |
|---|
| Maternal age
(years)a | | | |
|
≤35 | 25 (83.3%) | 27 (90.0%) | 0.54 |
|
>35 | 5 (16.7%) | 3 (10.0%) | |
| Body mass index | 28.3±1.86
(22-32) | 25±1.12 (21-29) | <0.001 |
| Blood pressure | | | |
|
Systolic | 139±10
(118-160) | 115±10
(103-127) | <0.001 |
|
Diastolic | 95±5 (84-100) | 78.7±12.3
(65-93) | <0.001 |
| Proteinuria | 2.5±0.65
(0.5-4.5) | 0.13±0.05
(0-0.3) | <0.001 |
| Gestational age at
delivery (years) | 33.3±1.84
(31-35) | 33.2±2.53
(31-36) | 0.71 |
| Previous
pregnancya | 14 (46.7%) | 15 (50%) | 0.32 |
Cell culture
The immortalized human trophoblast cell line
HTR8/SVneo was purchased from the Type Culture Collection of the
Chinese Academy of Sciences. HTR/SVneo cells were cultured in RPMI
1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). The
cells were incubated in a humidified atmosphere containing 95% air
and 5% CO2 at 37̊C.
Small interfering RNA (siRNA)
transfection
siRNAs were synthesized by Santa Cruz Biotechnology,
Inc., and transfected into the HTR8/SVneo cells using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. The cells were divided into three groups,
as follows: Control group (without any treatment), si-negative
control (NC) group (transfected with an unrelated sequence), and
si-ANGPTL8 group (transfected with ANGPTL8-siRNA). At 48 h
post-transfection, the cells were harvested and subjected to
western blotting or reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) assay to detect the efficiency of
transfection. The sequences of si-ANGPTL8 used are as follows:
si-ANGPTL8-1: 5'-AAGCCCACCAAGAATTTGAGA-3'; si-ANGPTL8-2:
5'-TATGACAGAGCACTGGAATTC-3'.
Serum ANGPTL8 measurements
Serum was separated by centrifugation (900 x g; 5
min) of the blood samples at 4˚C and stored at -80̊C until further
assay. Subsequently, the serum concentration of ANGPTL8 was
quantified using an ELISA kit (cat. no. NBP2-68217; R&D
Systems) according to the manufacturer's protocol.
RT-qPCR assay
Cells were washed with ice-cold phosphate buffer,
and total RNA was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), and measured using a Qubit machine
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Next, RNA (1 µg) was reversed transcribed
using RT reagents (Takara Bio, Inc.) to synthesize complementary
DNA. qPCR was then conducted using a 7500 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
following PCR cycling protocol was used: 95˚C for 2 min and then 40
cycles of 94˚C for 15 sec, 60˚C for 20 sec, 72˚C for 20 sec,
followed by 72˚C for 7 min. The primer sequences for qPCR were as
follows: ANGPTL8 forward, 5'-ATTCCTGGGGACAGAAGTCA-3', and reverse,
5'-GCTTTACACCTTCGAGCTGA-3'; and GAPDH forward,
5'-CTCACCGGATGCACCAATGTT-3', and reverse,
5'-CGCGTTGCTCACAATGTTCAT-3'. GAPDH was used as an internal control
for mRNA expression, and the relative mRNA expression was
calculated by the 2-ΔΔCq method
(18).
Western blot analysis
After transfection for 24 h, cells were harvested,
and total protein was extracted using RIPA lysis buffer (Beyotime
Institute of Biotechnology). Protein concentration was then
measured with a BCA kit (Thermo Fisher Scientific, Inc.). A total
of 25 µg protein was separated by SDS-PAGE (10%), transferred to
PVDF membranes (EMD Millipore) and blocked in 5% non-fat milk for 2
h at room temperature. The membranes were incubated with the
primary antibodies overnight at 4˚C, followed by incubation with
HRP-conjugated goat anti-rabbit IgG (cat. no. R2004; 1:5,000;
Sigma-Aldrich; Merck KGaA) or goat anti-mouse IgG (cat. no. A6715;
1:5,000; Sigma-Aldrich; Merck KGaA) for 1 h at room temperature.
The antibodies used in the present study were as follows:
Anti-ANGPTL8 (1:1,000; cat. no. CSB-PA757793LA01HU), purchased from
Cusabio; anti-cyclin-dependent kinase 2 (CDK2; 1:1,000; cat. no.
2546), anti-p21 (1:1,000; cat. no. 2947), anti-proliferating cell
nuclear antigen (PCNA; 1:1,000; cat. no. 13110), anti-matrix
metalloproteinase (MMP)-2 (1:1,000; cat. no. 40994), anti-MMP-9
(1:1,000; cat. no. 13667), anti-tissue inhibitor of matrix
metalloproteinase (TIMP)-1 (1:1,000; cat. no. 8946) and anti-TIMP-2
(1:1,000; cat. no. 5738) antibodies, obtained from Cell Signaling
Technology, Inc. The proteins were visualized using a Tanon-5200
Chemiluminescence Imager (Tanon Science and Technology Co., Ltd.)
and an enhanced chemiluminescence western blotting substrate (EMD
Millipore).
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc.) was performed to detect cell
viability. Briefly, HTR8/SVneo cells were seeded into 96-well
plates (5x103 cells/well) and incubated at 37̊C with 5%
CO2. At the indicated time points (24, 48 and 72 h)
after transfection, 10 µl CCK-8 solution was added into each well
and incubated for 2 h at 37̊C in the dark. The absorbance of each
well was then measured with a microplate reader (ELx808; BioTek
Instruments, Inc.) at 450 nm.
Wound healing assay
A wound healing assay was performed to detect the
migration ability of HTR8/SVneo cells. Briefly, cells
(4x104) were cultured in six-well plates for 24 h. The
cells were transfected with si-NC or si-ANGPTL8-1 for 72 h.
HTR8/SVneo cells without treatment served as a control. The
monolayers were then scratched vertically using a 200-µl sterile
pipette tip, and any floating cells were washed off with PBS. Cells
were photographed at the indicated time points (0 and 24 h) using a
Leica DM IL LED microscope equipped with an Integrated 5.0
Mega-Pixel MC170 HD camera (Leica Microsystems GmbH).
Transwell invasion assay
A Transwell assay was performed to evaluate the
invasive capability of HTR8/SVneo cells with an 8-µm pore
polycarbonate membrane chamber insert in a 24-well plate (Corning,
Inc.). For the invasion assays, the chamber inserts were coated
with 50 µl Matrigel (200 mg/ml; BD Biosciences). Briefly, at 24 h
post-transfection, cells were resuspended in 200 µl serum-free
medium and seeded in the upper chamber of the transwell inserts. A
total of 600 µl RPMI 1640 medium containing 10% FBS was added to
the lower chambers. After 24 h of incubation, the non-invading
cells were gently removed with a cotton swab, and the invasive
cells attached to the lower surface of the chamber membranes were
fixed with polyoxymethylene and stained with 0.1% crystal violet
solution. Finally, the number of cells in five random fields was
counted, and images were captured under an inverted microscope
(Olympus Corporation).
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Differences among the data were analyzed using
Student's t-test (unpaired) or analysis of variance followed by
Bonferroni correction, as appropriate. Statistical data were
analyzed using SPSS software, version 13.0 (SPSS, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
ANGPTL8 expression and secretion are increased in
PE. As shown in Table I, there
was no significant difference in the age or ratio of previous
pregnancy between the pregnant women with (n=30) or without PE
(n=30) enrolled in the present study. However, the systolic and
diastolic pressure values of pregnant women with PE were higher
compared with those exhibited by healthy pregnant women. Next, the
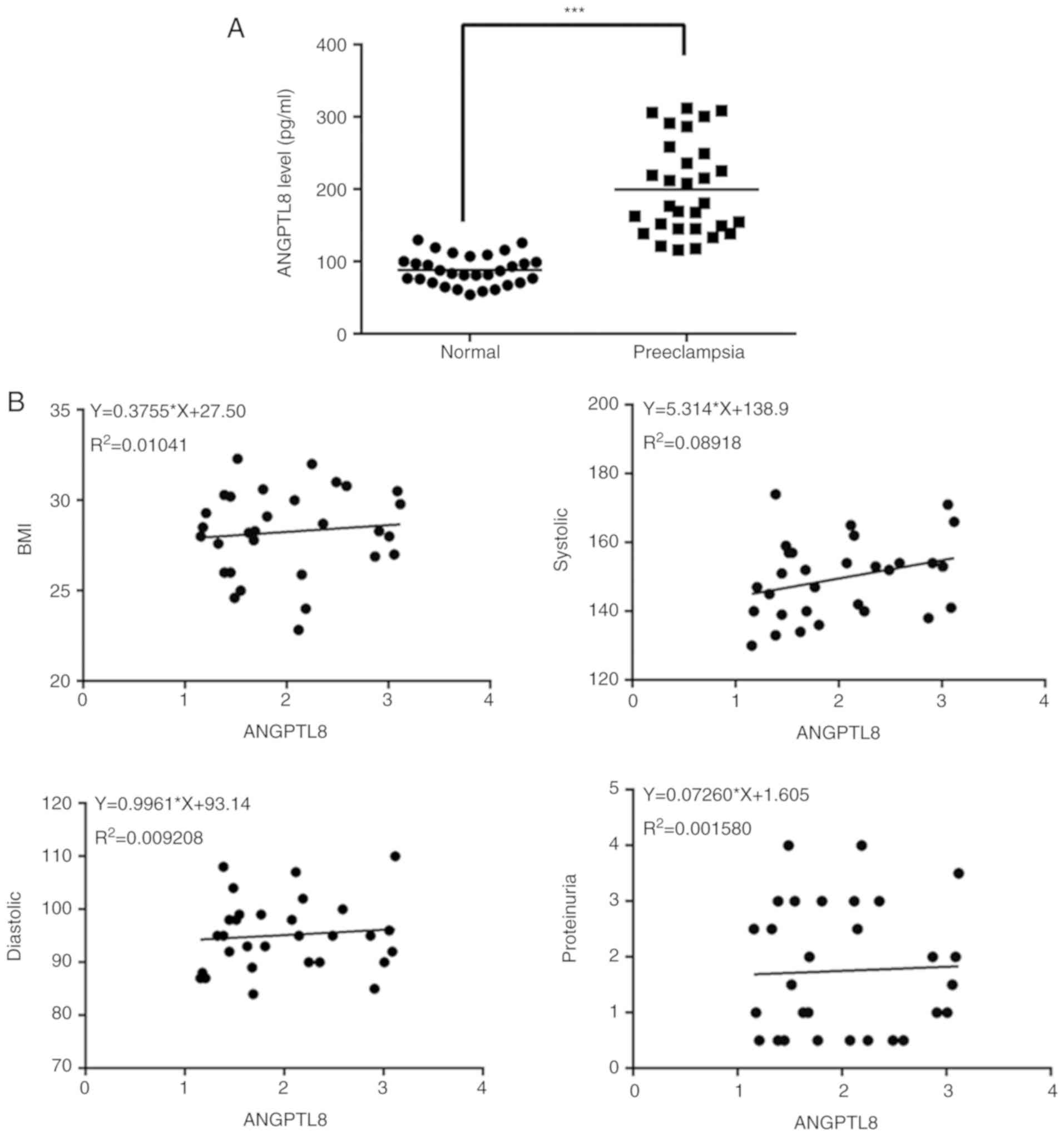
ANGPTL8 levels in the serum of the subjects were detected by ELISA.
The results revealed that the expression levels of ANGPTL8 were
notably increased in pregnant women with PE compared with those
observed in the normal controls (Fig.
1A). Correlation analysis further revealed a positive
association between the expression level of ANGPTL8 and the body
mass index, systolic pressure, diastolic pressure and proteinuria.
Based on the results shown in Fig.
1, it can be hypothesized that ANGPTL8 has a potential effect
on the pathogenesis of PE.
Downregulation of ANGPTL8 promotes
trophoblast cell proliferation
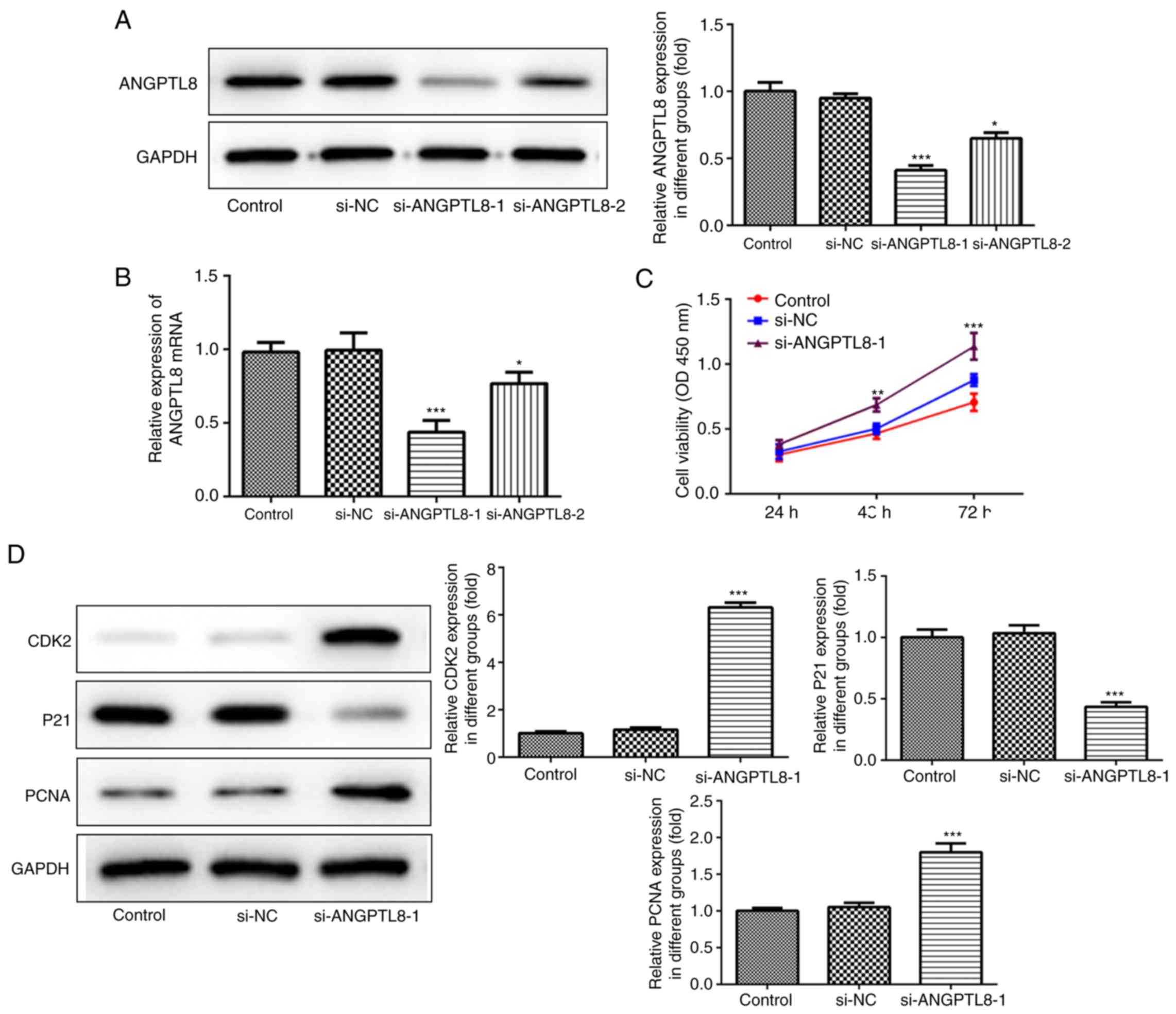
Successful interference on ANGPTL8 expression in
trophoblast cells by siRNA transfection was confirmed by western
blotting and RT-qPCR. The results demonstrated that the silencing
effect of si-ANGPTL8-1 was better than that of si-ANGPTL8-2; thus,
si-ANGPTL8-1 was selected for use in subsequent experiments
(Fig. 2A and B). Next, a CCK-8 assay was performed to
elucidate the function of ANGPTL8 in trophoblast cells, and the
results revealed that reduced expression of ANGPTL8 increased the
viability and proliferation of HTR8/SVneo cells (Fig. 2C). Western blotting was also
performed to detect the levels of proteins involved in cell
proliferation, including CDK2, p21 and PCNA. As shown in Fig. 2D, downregulation of ANGPTL8 decreased
p21 expression, and increased the CDK2 and PCNA expression levels
in HTR8/SVneo cells compared with the expression levels in the
control and si-NC groups.
Downregulation of ANGPTL8 promotes
trophoblast cell migration and invasion
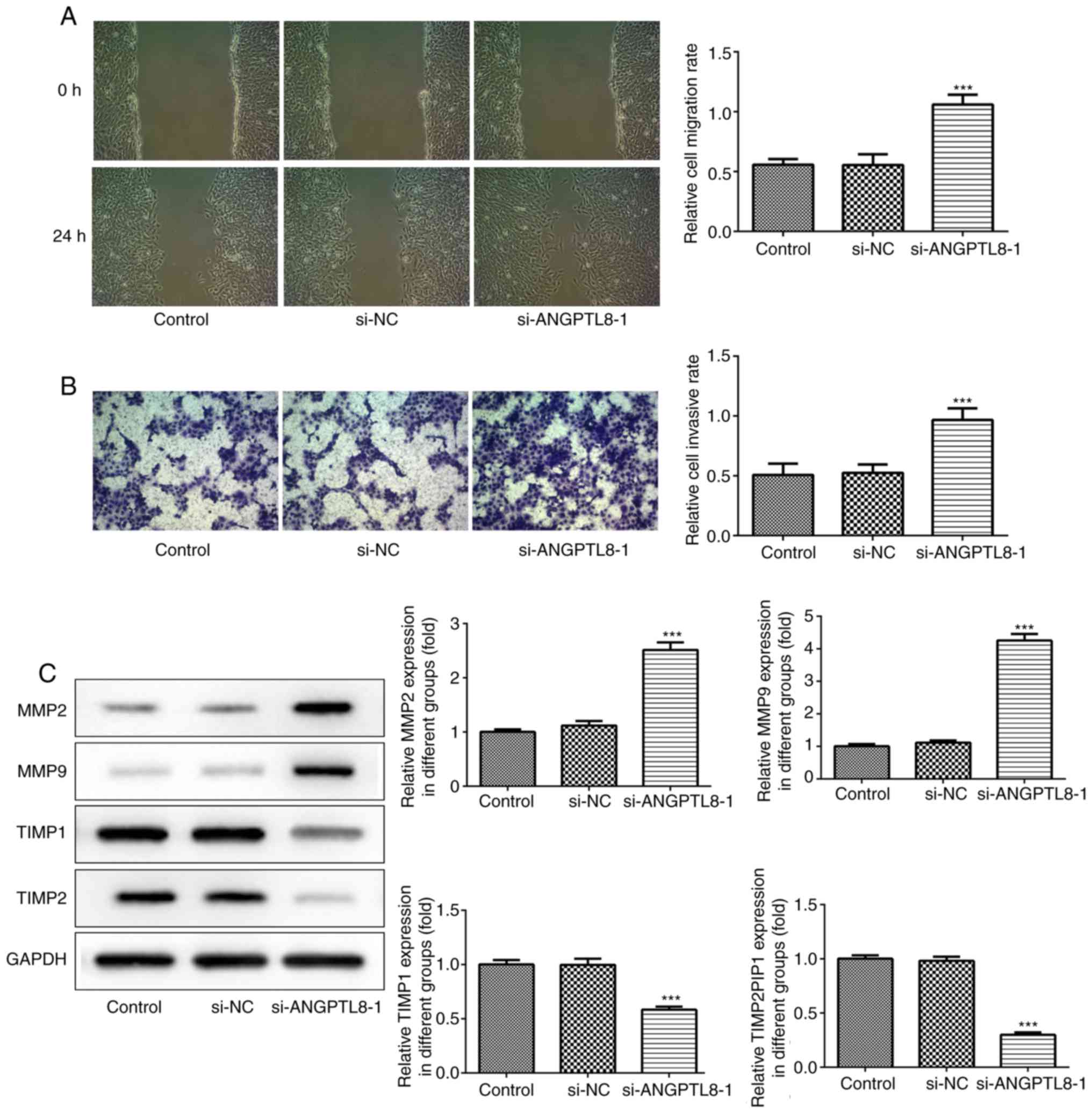
Wound healing and transwell assays were performed to
investigate the effect of ANGPTL8 on trophoblast cell migration and
invasion, respectively. The results demonstrated that silencing of
ANGPTL8 led to a significant acceleration in the migratory and
invasive capacities of trophoblast cells (Fig. 3A and B). Next, western blotting was used to
evaluate the levels of proteins associated with migration and
invasion, including MMP-2, MMP-9, TIMP-1 and TIMP-2. The data
revealed that downregulation of ANGPTL8 markedly enhanced the
levels of MMP-2 and MMP-9, while the protein expression levels of
TIMP-1 and TIMP-2 were significantly decreased in the si-ANGPTL8-1
group as compared with those in the control and si-NC groups
(Fig. 3C).
Discussion
PE is a systemic disorder that occurs during
pregnancy and is characterized by various manifestations of organ
dysfunction. PE causes gestational hypertension and proteinuria,
which is a sign of renal dysfunction (19). The clinical data reported in the
present study indicated that the systolic and diastolic pressure
values of pregnant women with PE were higher compared with those of
healthy pregnant women, as shown in Table I. PE is considered as a two-stage
disorder, since the pathogenesis and mechanisms of PE are
considered to involve antiangiogenic and angiogenic factors and/or
placental debris, leading to trophoblast cell apoptosis and
abnormal development of the placenta (20-22).
A previous study reported that PE is an ischemic placental disease,
associated with trophoblastic metabolic disorders (23). Defective trophoblast invasion of the
uterus has been reported to be one of the crucial mechanisms of PE.
Furthermore, increased oxidative stress and reduced uteroplacental
blood flow and oxygen availability were closely associated with
reduced trophoblast invasion (24).
ANGPTL8 is a newly identified hormone with the
ability to regulate the glucose and lipid metabolic pathways.
Abnormal expression of ANGPTL8 has been reported in nonalcoholic
fatty liver disease, insulin resistance and diabetes mellitus
(25). In addition, ANGPTL8 exerts a
protective effect on atherosclerosis through the inhibition of
HDL-mediated cholesterol efflux capacity (26). A previous study demonstrated that
ANGPTL8 levels were increased in obesity, impaired glycometabolism
and dyslipidemia (13). In the
present study, the results revealed that ANGPTL8 expression was
also increased in pregnant women with PE as compared with that in
the normal controls (Fig. 2).
Therefore, it was hypothesized that ANGPTL8 is involved in the
pathogenesis of PE.
The current study further employed HTR8/SVneo cells
to investigate the aberrant trophoblastic invasion in the
pathogenesis of PE in vitro. The HTR8/SVneo cells were
transfected with ANGPTL8-siRNA to downregulate ANGPTL8 expression
in order to investigate the molecular mechanism by which ANGPTL8
regulates trophoblast invasion during PE development. It is widely
accepted that excessive apoptosis and poor invasion of trophoblast
cells serve major roles in the pathogenesis of PE. Wang et
al reported that regulation of the microRNA-210/NOTCH1
signaling pathway attenuated the impaired HTR-8/SVneo cell
proliferation, migration and invasion, which contributed to
trophoblast dysfunction in the placenta of patients with PE
(27). This is consistent with the
data of the present study, which indicated that downregulation of
ANGPTL8 increased the viability and proliferation of HTR8/SVneo
cells (Fig. 2C), as well as
corrected for the expression levels of proteins involved in cell
proliferation, including CDK2, p21 and PCNA. Furthermore, silencing
of ANGPTL8 promoted trophoblast cell migration and invasion, and
altered the expression levels of proteins associated with migration
and invasion, including MMP-2, MMP-9, TIMP-1 and TIMP-2 (Fig. 3). Wu et al (28) previously demonstrated that
downregulation of hypoxia-inducible factor 1α antisense RNA-2 may
be involved in the pathogenesis and progression of PE by decreasing
trophoblast cell proliferation, migration and invasion.
Furthermore, Ebegboni et al (29) reported that a dietary intake of food
that is rich in quercetin or hesperidin during early pregnancy was
able to significantly improve trophoblast (placenta) health and
function against oxidative stress by enhancing spheroid stem-like
cell generation in HTR8/SVneo cells, thus aiding their invasion
(29). These previous findings,
along with the results of the present study, provide an insight
into the potential role of ANGPTL8 in the process of PE.
In conclusion, the present study demonstrated that
ANGPTL8 serves an important role in the pathogenesis of PE, and
that the downregulation of ANGPTL8 significantly promoted
trophoblast cell proliferation, invasion and migration.
Collectively, these data support the potential application of
ANGPTL8 as a target for clinical diagnosis and treatment of PE.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and XL designed the experiments and drafted the
manuscript. DJ mainly performed the experiments and analyzed the
data. WW performed the western blot and RT-qPCR experiments. XL
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xuzhou Central Hospital (Xuzhou, China). All
participants provided written informed consent.
Patient consent for publication
All participants provided written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bilano VL, Ota E, Ganchimeg T, Mori R and
Souza JP: Risk factors of pre-eclampsia/eclampsia and its adverse
outcomes in low- and middle-income countries: A WHO secondary
analysis. PLoS One. 9(e91198)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ospina-Prieto S, Chaiwangyen W, Herrmann
J, Groten T, Schleussner E, Markert UR and Morales-Prieto DM:
MicroRNA-141 is upregulated in preeclamptic placentae and regulates
trophoblast invasion and intercellular communication. Transl Res.
172:61–72. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lenfant C: Working group report on high
blood pressure in pregnancy. J Clin Hyper (Greenwich). 3:75–88.
2001.PubMed/NCBI
|
|
5
|
Wu P, Haththotuwa R, Kwok CS, Babu A,
Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA
and Mamas MA: Preeclampsia and future cardiovascular health: A
systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes.
10(e003497)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
He P, Chen Z, Sun Q, Li Y, Gu H and Ni X:
Reduced expression of 11β-hydroxysteroid dehydrogenase type 2 in
preeclamptic placentas is associateβd with decreased PPARγ but
increased PPARα expression. Endocrinology. 155:299–309.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu L, Li D, Liao QP, Yang HX, Cao B, Fu G,
Ye G, Bai Y, Wang H, Cui N, et al: High levels of activin A
detected in preeclamptic placenta induce trophoblast cell apoptosis
by promoting nodal signaling. J Clin Endocrinol Metab.
97:E1370–E1379. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wallace AE, Fraser R, Gurung S, Goulwara
SS, Whitley GS, Johnstone AP and Cartwright JE: Increased
angiogenic factor secretion by decidual natural killer cells from
pregnancies with high uterine artery resistance alters trophoblast
function. Hum Reprod. 29:652–660. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wallace AE, Whitley GS, Thilaganathan B
and Cartwright JE: Decidual natural killer cell receptor expression
is altered in pregnancies with impaired vascular remodeling and a
higher risk of pre-eclampsia. J Leukoc Biol. 97:79–86.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Hao M, Sampson S and Xia J:
Elective delivery versus expectant management for pre-eclampsia: A
meta-analysis of RCTs. Arch Gynecol Obstet. 295:607–622.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kersten S: Physiological regulation of
lipoprotein lipase. Biochim Biophys Acta. 1841:919–933.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Quagliarini F, Wang Y, Kozlitina J,
Grishin NV, Hyde R, Boerwinkle E, Valenzuela DM, Murphy AJ, Cohen
JC and Hobbs HH: Atypical angiopoietin-like protein that regulates
ANGPTL3. Proc Natl Acad Sci USA. 109:19751–19756. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morinaga J, Zhao J, Endo M, Kadomatsu T,
Miyata K, Sugizaki T, Okadome Y, Tian Z, Horiguchi H, Miyashita K,
et al: Association of circulating ANGPTL 3, 4, and 8 levels with
medical status in a population undergoing routine medical checkups:
A cross-sectional study. PLoS One. 13(e0193731)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee EC, Desai U, Gololobov G, Hong S, Feng
X, Yu XC, Gay J, Wilganowski N, Gao C, Du LL, et al: Identification
of a new functional domain in angiopoietin-like 3 (ANGPTL3) and
angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of
lipoprotein lipase (LPL). J Biol Chem. 284:13735–13745.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yoshida K, Shimizugawa T, Ono M and
Furukawa H: Angiopoietin-like protein 4 is a potent
hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein
lipase. J Lipid Res. 43:1770–1772. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu L, Zhuang X, Jiang M, Guan F, Fu Q and
Lin J: ANGPTL4 mediates the protective role of PPARgamma activators
in the pathogenesis of preeclampsia. Cell Death Dis.
8(e3054)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Luo M and Peng D: ANGPTL8: An important
regulator in metabolic disorders. Front Endocrinol (Lausanne).
9(169)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brown MA, Magee LA, Kenny LC, Karumanchi
SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G and Ishaku S:
International Society for the Study of Hypertension in Pregnancy
(ISSHP): The hypertensive disorders of pregnancy: ISSHP
classification, diagnosis & management recommendations for
international practice. Pregnancy Hypertens. 13:291–310.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chelbi ST and Vaiman D: Genetic and
epigenetic factors contribute to the onset of preeclampsia. Mol
Cell Endocrinol. 282:120–129. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seki H: Balance of antiangiogenic and
angiogenic factors in the context of the etiology of preeclampsia.
Acta Obstet Gynecol Scand. 93:959–964. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shah DA and Khalil RA: Bioactive factors
in uteroplacental and systemic circulation link placental ischemia
to generalized vascular dysfunction in hypertensive pregnancy and
preeclampsia. Biochem Pharmacol. 95:211–226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Makris A, Yeung KR, Lim SM, Sunderland N,
Heffernan S, Thompson JF, Iliopoulos J, Killingsworth MC, Yong J,
Xu B, et al: Placental growth factor reduces blood pressure in a
uteroplacental ischemia model of preeclampsia in nonhuman primates.
Hypertension. 67:1263–1272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gutiérrez JA, Gómez I, Chiarello DI,
Salsoso R, Klein AD, Guzmán-Gutiérrez E, Toledo F and Sobrevia L:
Role of proteases in dysfunctional placental vascular remodelling
in preeclampsia. Biochim Biophys Acta Mol Basis Dis.
1866(165448)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Siddiqa A, Cirillo E, Tareen SHK, Ali A,
Kutmon M, Eijssen LMT, Ahmad J, Evelo CT and Coort SL: Biological
pathways leading from ANGPTL8 to diabetes mellitus-A co-expression
network based analysis. Front Physiol. 9(1841)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Luo M, Zhang Z, Peng Y, Wang S and Peng D:
The negative effect of ANGPTL8 on HDL-mediated cholesterol efflux
capacity. Cardiovasc Diabetol. 17(142)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang R, Liu W, Liu X, Liu X, Tao H, Wu D,
Zhao Y and Zou L: MicroRNA-210 regulates human trophoblast cell
line HTR-8/SVneo function by attenuating Notch1 expression:
Implications for the role of microRNA-210 in pre-eclampsia. Mol
Reprod Dev. 86:896–907. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu D, Yang N, Xu Y, Wang S, Zhang Y,
Sagnelli M, Hui B, Huang Z and Sun L: lncRNA HIF1A antisense RNA 2
modulates trophoblast cell invasion and proliferation through
upregulating PHLDA1 expression. Mol Ther Nucleic Acids. 16:605–615.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ebegboni VJ, Dickenson JM and
Sivasubramaniam SD: Antioxidative effects of flavonoids and their
metabolites against hypoxia/reoxygenation-induced oxidative stress
in a human first trimester trophoblast cell line. Food Chem.
272:117–125. 2019.PubMed/NCBI View Article : Google Scholar
|