Introduction
Acquired perforating dermatosis (APD) is a rare skin
disorder of unknown etiology and pathogenesis, which was first
identified by Rapini et al (1) in 1989. APD is characterized clinically
by umbilicated hyperkeratotic papules or nodules and histologically
by the transepidermal elimination (TEE) of basophilic collagen
bundles, as well as the onset of skin lesions in patients >18
years old (2,3). Although typical APD cases can be
diagnosed at a glance by the characteristic eruptions (4), a skin biopsy is mandatory for
definitive diagnosis (1,3,5,6). In some cases, the disease could
potentially be underdiagnosed or misdiagnosed as simple
excoriations (5,6). Some researchers suggest that APD is a
variant of prurigo nodularis, an umbilicated type of prurigo
(7). The substances eliminated
during the TEE process of APD include collagen, elastin and keratin
(1,8). When collagen is the only eliminated
substance, the disease is called acquired reactive perforating
collagenosis (ARPC), which was first proposed by Mehregan et
al in 1967 (5,8,9). There
has been some confusion regarding the terminology over the years
(1,10) since APD and ARPC are almost identical
with regard to their clinical manifestations, pathologies and
treatments, but display differences in their TEE substances
(5,6,8,10).
APD can occur at any age with no gender
predilection, but particularly occurs in the fourth to fifth decade
of adult life (9). The common
lesions are isolated papules with keratotic plugs, measuring
0.5-2.0 cm in diameter, which can occur on any part of the body but
primarily occur on the lower extremities and trunk (3,6). The
majority of patients with dermatosis suffer from pruritus with a
rare occurrence of pain (2-5,8).
In many situations, the lesions self-heal in six to eight weeks
without any r therapeutic treatment, however, there are cases where
the symptoms persist for >8 years (6). APD has been associated with systemic
diseases such as diabetes mellitus and renal failure (2). However, the cause of dermatosis remains
unknown and the prognosis undesirable, which poses challenges to
treatment of the disease.
In the present study, one case of ARPC and three
cases of APD were diagnosed in succession within two months. This
situation allowed close attention to be paid to the disease and
resulted in the discovery that all four patients experienced skin
lesions when their original systemic diseases were deteriorating.
The severity of the skin lesions and pruritus declined after the
patients received basic symptomatic treatment and had healed from
their systemic diseases. The present study describes four cases of
APD (including a case of ARPC) in patients with various systemic
diseases and the clinical characteristics and prognosis of
dermatosis are further summarized.
Case reports
The study protocol was approved by the Beijing
Friendship Hospital Ethics Committee for Human Research (approval
no. 2019-P2-178-01). Written informed consent was obtained from
each patient to have the case details and the accompanying images
published.
Case 1
In April 2017, a 54 year old woman was admitted to
the Beijing Friendship Hospital outpatient department for a 1 month
history of intensely pruritic skin eruptions, which began on the
arms and spread over the entire body without any specific cause.
The severity of the pruritus and lesions increased over time. The
patient received topical corticosteroid treatment, which proved
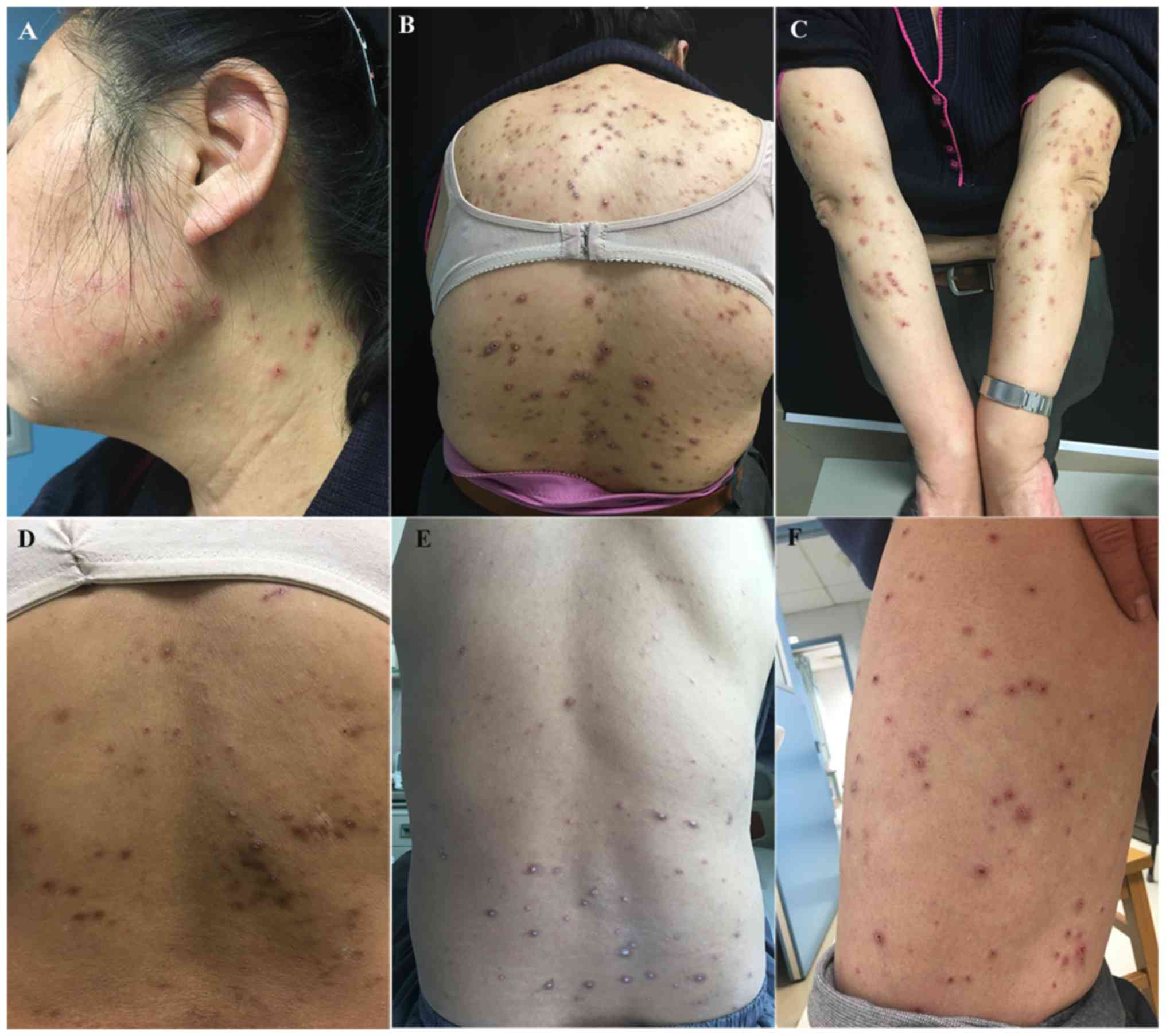
ineffective. A cutaneous examination revealed the diffuse
distribution of several well-defined circular umbilicated papules
with central keratotic plugs on the trunk and bilateral upper
limbs, as well as a few papules on the face and lower limbs
(Fig. 1A). A number of papules were
identified in a linear arrangement on the back and upper right
limb, which were suspected to be Koebner phenomenon (Fig. 1B and C). The patient had a history of type 2
diabetes, hypertension, atrial premature beat, cataracts and
hypothyroidism. Laboratory tests confirmed that the patient had
albuminuria without creatinine abnormalities two months before the
onset of the dermatosis. The patient's laboratory results were as
follows: 8.9% glycated hemoglobin level, 0.8 g quantitative 24 h
urinary protein and >1,300 U/ml quantitative determination of
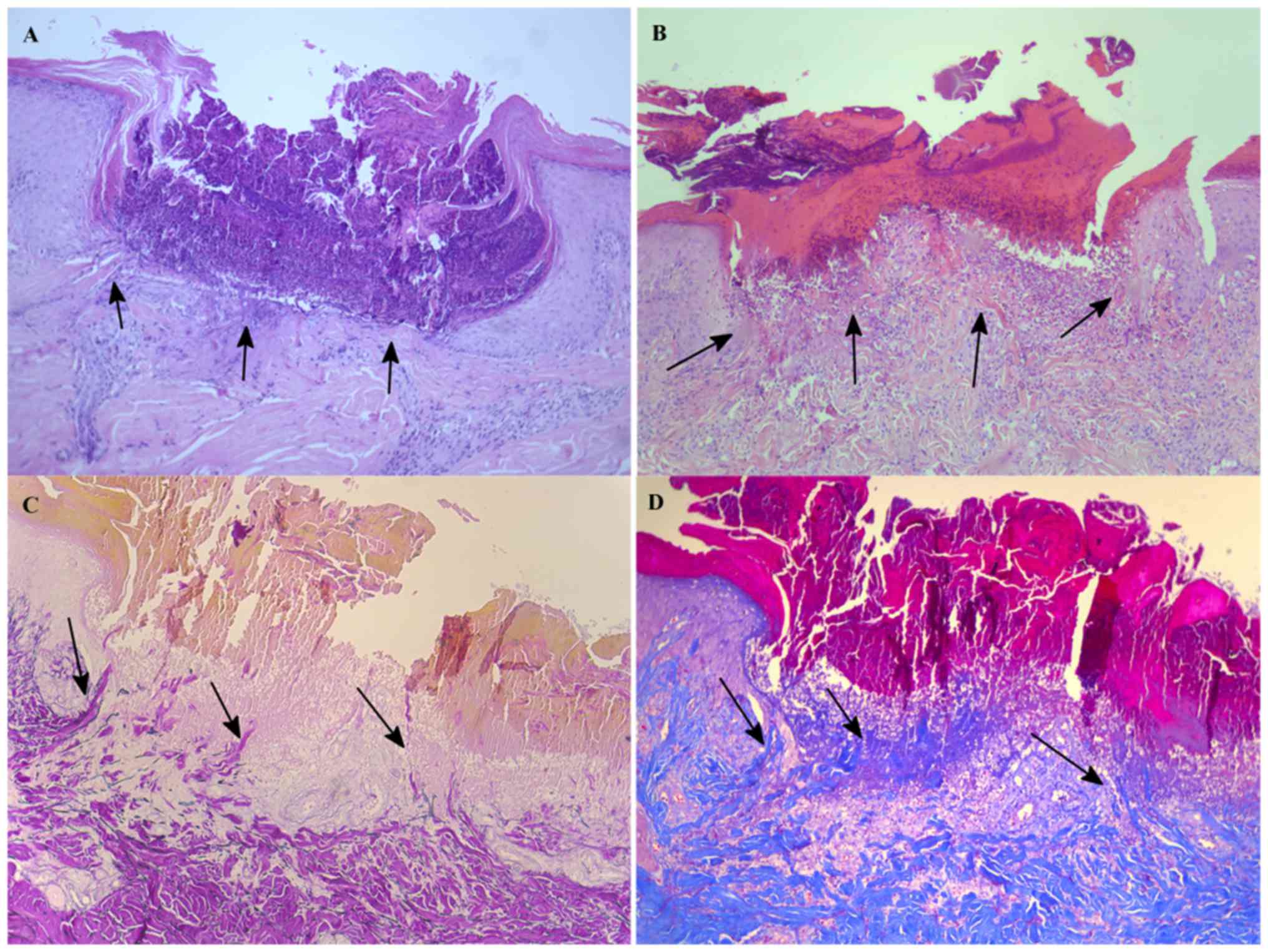
thyroid peroxidase antibody. A skin biopsy showed a cup-shaped
invagination of the epidermis forming a short channel (Fig. 2A), within which there were densely
packed degenerated basophilic-staining materials and lymph cells
that had accumulated as a result of lymphocytic infiltration, as
well as altered collagen bundles. These degenerated fiber bundles
were identified as collagen fibers using an elastic fiber stain as
Verhoeff-Van Gieson and Masson's trichrome staining. The patient
was treated with oral antihistamines and topical corticosteroids,
which led to some improvement at the patient's second visit, one
month later. However, the improvement was lost by the subsequent
follow-up after three months.
Case 2
In April 2017, a 54 year old woman was admitted to
the Beijing Friendship Hospital outpatient department for a 5 year
history of recurrent rash with severe pruritus. Two years prior to
admission, the patient sought medical advice for a severe pruritic
eruption with a central keratin-filled crater that appeared over
her entire body. Despite diagnostic uncertainties, the patient was
treated with conservative measures that led to symptomatic relief.
At the most recent admission, significant clinical improvements
were observed, with a general disappearance of itching and an
obvious reduction in skin lesions. For the last 15 years, the
patient had been undergoing hemodialysis for chronic renal
insufficiency. The patient complained of generalized malaise, body
edema and severe itching due to repeated adjustments to
hemodialysis parameters during the treatment period. After
receiving a subtotal parathyroidectomy to treat parathyroid
carcinoma, the patient had a history of hypertension,
hyperlipidemia and hyperparathyroidism. Laboratory tests
highlighted a sharp rise in urea (19.55 mmol/l) and serum
creatinine (805.2 µmol/l). The patient's fasting blood sugar level
was 11.57 mmol/l and serum phosphate levels were elevated to 2.52
mmol/l. During the present study, hyperpigmented macular patches
were identified on the flexor and extensor surface of the legs,
while a few rice-sized keratotic papules with central keratinous
plugs were found on the back (Fig.
1D). Histopathological examination of a characteristic lesion
detected infiltrating inflammatory cells in the cup-shaped
invagination of the epidermis, as well as collagen and elastic
fibers in the superficial dermis, with a striking pattern of TEE
through the epidermis and into the stratum corneum (Fig. 2B). At the time of the study, the
patient was satisfied with the efficacy of hemodialysis. After
treatment with topical corticosteroids, the skin lesions improved
and other symptoms such as malaise and body edema were
relieved.
Case 3
In May 2017, a 51 year old male patient who had been
hospitalized in the Department of Nephrology (Beijing Friendship
Hospital) was referred to the Department of Dermatology for
consultation due to a pruritic skin eruption on the back that had
continued for one month. The patient had end-stage renal disease
(ESRD) and had been undergoing peritoneal dialysis over the
previous year. The patient was hospitalized for further adjustment
to his therapeutic plan following the development of symptoms such
as fatigue, oliguria, lower limb edema and a gradual increase in
blood creatinine and urea nitrogen levels. The patient also had a
history of metabolic disorders such as hypertension, diabetes
mellitus, hyperlipidemia, hyperuricemia and coronary heart disease,
as well as other diseases, including hyperparathyroidism and
diabetic retinopathy. The examination conducted in the present
study revealed a scattered distribution of some well-defined
keratotic papules on the back (Fig.
1E). Laboratory tests showed the following: 789.68 µmol/l serum
creatinine, 51.55 mmol/l blood urea nitrogen, 3.29 mmol/l blood
potassium, 1.88 mmol/l blood calcium and 2.47 mmol/l blood
phosphorus. A skin biopsy was performed due to clinical suspicion
of a TEE disorder. The histopathology examination detected a
cup-shaped invagination of the epidermis filled with perforating
collagen and elastic fibers (demonstrated using the Verhoeff-Van
Gieson staining), as well as some necrotic debris (Fig. 2C). At one month after the
concentration and capacity of the peritoneal dialysis fluid had
been adjusted, the dermatosis significantly improved, with a
disappearance of the rash and an obvious relief of itching, without
any dermatological management.
Case 4
In August 2017, a 61 year old male patient was
admitted to the Beijing Friendship Hospital outpatient department
for a 5 year history of frequent pruritic rash that had been
aggravated and spread all over the body over the previous three
months. The patient had a 40-year history of hypertension and one
month before the present visit was diagnosed with hepatocirrhosis
secondary to chronic hepatitis C due to a blood transfusion that
occurred 27 years ago. The results of a hepatitis C virus RNA
quantification test, conducted a month prior to his visit,
identified the replicative state of the virus in the body and thus
confirmed hepatocirrhosis. Subsequently, the patient received
treatment of subcutaneous injections of pegylated interferon α-2a
(180 µg/week) in combination with daily oral intake of ribavirin
(900 mg). The cutaneous examination conducted in the present study
found a scattered distribution of papules and nodules and a central
keratin-filled crater on the trunk and lower limbs of the patient
(Fig. 1F). A number of papules on
the lower limbs were distributed in a linear arrangement, which was
identified as the Koebner phenomenon. A punch biopsy showed
cup-shaped plugs in the excavated epidermis, filled with keratin,
collagen and cell debris. The collagen fibers vertically aligned
near the epidermis, demonstrated by Masson's trichrome staining
(Fig. 2D). The symptoms improved
after receiving NB-UVB phototherapy 2-3 times a week for 1 month.
When the patient returned for a follow-up two months later, the
rash had mostly disappeared.
The clinical features, associated systemic diseases
and abnormal laboratory results of the four patients are
illustrated in Table I. All of the
four patients' skin specimen was stained with three dying methods,
including hematoxylin and eosin, Verhoeff-Van Gieson and Masson's
trichrome staining. The specimen was firstly stained using
hematoxylin and eosin staining, which was fixed by 10% neutral
buffered formalin for 4-12 h at room temperature, and dyed using
hematoxylin solution for 3-5 min and eosin solution for 30-60
seconds at room temperature. The specimen was secondly stained via
Verhoeff-Van Gieson staining, which was fixed with 10% neutral
buffered formalin for 2-4 h at room temperature, dyed with elastin
for 8-24 h and Van Gieson for 1 min at room temperature. The
specimen was thirdly stained via Masson's trichrome staining, which
was fixed with 10% neutral buffered formalin for 2-4 h at room
temperature, stained with Weigert hematoxylin for 5-10 min, acid
Fuchsin for 5-10 min and aniline blue for 3-5 min at room
temperature. All of the above reagents were supplied by Baso
Diagnostics Inc. The thickness of pathological section of skin was
4 µm, which was observed under ordinary light microscope and
analyzed using Leica camera software (LAS V4.5; Leica Microsystems
Ltd).
 | Table IClinical features, associated systemic
diseases and abnormal laboratory results of the four patients with
APD. |
Table I
Clinical features, associated systemic
diseases and abnormal laboratory results of the four patients with
APD.
| Case no. | Sex | Age | APD duration
(months) | Pruritus
severity | Koebner
phenomenon | Distribution | TEE material | Systemic
disorders/number of years with the disorder | Abnormal laboratory
results (reference range) |
|---|
| 1 | F | 54 | 1 | Very severe | Positive | Mainly trunk and | Collagen fibers | Hypertension/2,
proteinuria with normal bilateral upper limbs, few on face and
lower limbs renal function/1, hypothyroidism/1, NIDDM/2,
cataract/5, atrial premature beat/2, multiple uterine myoma/5,
albuminuria/0.17 | HbAIc 8.9%
(4.27-6.07), 24-h urinary protein quantitative 0.8 g (0-0.15), ATPO
>1,300 U/ml (0-60) |
| 2 | F | 54 | >24 | Severe | Negative | Back and extensor
surface of legs | Collagen and elastic
fibers | Hypertension/20, ESRD
requiring hemodialysis/15, hyperparathyroidism and subtotal
parathyroidectomy because of parathyroid carcinoma/3,
hyperlipidemia/20, generalized malaise and body edema need to
adjust hemodialysis parameters | Urea 19.55 mmol/l
(2.60-7.50), serum creatinine 805.2 µmol/l (53.0-115.0), GLU 11.57
mmol/l (3.92-6.16), serum phosphate 2.52 mmol/l (0.85-1.51) |
| 3 | M | 51 | 1 | Severe | Negative | Back | Collagen and elastic
fibers | Hypertension/10, ESRD
requiring peritoneal dialysis/1, hyperparathyroidism/4, NIDDM/2
diabetic retinopathy/2, hyperlipidemia/4, hyperuricemia/4, coronary
heart disease/4, increase of blood creatinine and urea nitrogen
levels for adjustment of the peritoneal dialysis | Serum creatinine
789.68 µmol/l (53-115), blood urea nitrogen 51.55 mmol/l (3.6-9.5),
blood potassium 3.29 mmol/l (3.5-5.3), blood calcium 1.88 mmol/l
(2.11-2.52), blood phosphorus 2.47 mmol/l (0.85-1.51) |
| 4 | M | 61 | 3 or maybe 60 | Severe | Positive | Trunk and lower
limbs | Collagen and elastic
fibers | Hypertension/40,
hepatocirrhosis secondary to chronic hepatitis C/27,
hepatocirrhosis and replicative state of the hepatitis C
virus/0.08 | Quantitative analysis
of hepatitis C virus nucleic acid 2.407x103 IU/ml
(<1.0x10-2) |
Discussion
Diagnosis of APD is based on the patient's medical
history, the clinical appearance of lesions and the results of
histopathological examinations. In the present study, the patients
involved were middle-aged, without a family history of related
diseases. All patients displayed lesions with severe pruritus,
primarily on their trunk. One of the patients had lesions on the
face, while two demonstrated the Koebner phenomenon. In addition to
these clinical features, the skin lesions of the four patients were
in the form of isolated round or oval papules that were uniform in
size and shape, with keratotic plugs that were difficult to scratch
off. In all four cases, the histological features of the dermatosis
were cup-shaped invaginations of the epidermis, which formed a
short channel. The channel was densely packed with degenerated
basophilic staining materials, neutrophils, infiltrating lymph
cells and altered connective tissue. These typical pathological
findings are one of the important diagnostic parameters for APD
(1,3,5,8). The substances in the cup-shaped plugs
may, to some extent, affect the clinical manifestation of the
disease. For instance, neutrophils and infiltrating lymphocytic
cells were identified in the patient in case 1, who had advanced
stage dermatosis, while few infiltrating inflammatory cells were
observed in the epidermal invagination of the patient in case 2,
who was in the recovery stage.
It is widely recognized that this dermatosis occurs
most commonly among those with systemic disorders, especially
diabetes mellitus and renal failure (1-12).
It is worth noting that despite its rarity, a 2-11% incidence of
APD onset has been reported in patients receiving maintenance
dialysis for end stage renal disease (13). Some diseases, such as hypertension,
hepatitis, hypothyroidism and chronic obstructive pulmonary
disease, are frequently mentioned as being related to the
dermatosis (10,14,15).
Similarly, certain malignancies, including lymphoma and thyroid,
prostate and breast carcinoma, were found in 9.1% of patients with
APD (16-18).
In addition, the possibility of APD onset in the presence of
certain autoimmune diseases, such as systemic lupus erythematosus,
vasculitis, dermatomyositis and Mikulicz disease, has been
confirmed (19,20). Moreover, APD has also been identified
among patients with various infections, such as scabies, herpes
zoster and insect stings (8).
However, few cases of APD were reported in patients using
biologics, such as infliximab, natalizumab and gefitinib (6). Although the pathogenesis of the
dermatosis remains unclear, some reports suggest that trauma and
microvasculopathy may trigger TEE and degeneration of the collagen
fibers (8,18).
In the present study, three patients with APD had
various systemic disorders, whilst the dermatosis in the fourth
patient was associated with hepatocirrhosis secondary to hepatitis
C virus infection. The patient in case 1 had seven different types
of systemic disorder, including diabetes mellitus, hypothyroidism,
hypertension, atrial premature beat, proteinuria, cataracts and
multiple uterine myoma. The patient in case 2 had four systemic
diseases including ESRD, parathyroid carcinoma, hypertension and
hyperlipidemia. The patient in case 3 had eight systemic diseases,
including ESRD, diabetes mellitus, diabetic retinopathy, coronary
heart disease, hyperparathyroidism, hypertension, hyperlipidemia
and hyperuricemia. Two of the patients had been undergoing dialysis
for kidney problems, such as ESRD, while one had been receiving
hemodialysis for 15 years and another had been receiving peritoneal
dialysis for one year. In the available literature, few cases
reported as many concomitant diseases in one patient as was found
in the patients in the present study (1,5,6,10,16-22).
At the same time, the patients in the present study did not pay
enough attention to the dermatosis. The prevalence of this
condition may be underestimated because the patients with multiple
systemic disorders were not aware of the severity of the skin rash
and were reluctant to undergo a pathological examination.
In the four cases described in the present study,
the dermatosis occurred with the progression of systemic disease,
three of which were kidney problems and one of which was liver
cirrhosis. The patient in case 1 was diagnosed with albuminuria
with no creatinine abnormalities two months before the onset of the
skin disease. The eruption of the rash in case 2 may have been
associated with the repeated adjustments of the hemodialysis that
the patient had been receiving, however, the symptoms were relieved
without specific treatment by a dermatologist. The patient in case
3 was hospitalized for the adjustment of peritoneal dialysis, which
he had been undergoing to alleviate the discomfort of the symptoms
inflicted by the dialysis before the onset of APD. Likewise, the
dermatosis symptoms subsided without any dermatological
interventions. The patient in case 4 was diagnosed with
hepatocirrhosis before the skin pruritus was exacerbated. After
systematic antiviral therapy and NB-UVB phototherapy for about one
month, the skin symptoms disappeared almost entirely. These four
cases demonstrated that the occurrence and remission of APD
symptoms were synchronized with the occurrence and remission of the
patients' systemic diseases. In other words, APD associated with
systemic diseases occurred when systemic disorders worsened or had
not been well-controlled for a long period, while its symptoms may
have lessened with the successful treatment of the associated
systemic diseases (21). As such,
the occurrence or exacerbation of the systemic diseases that a
patient has should be taken into consideration regarding APD
diagnosis.
Currently, there is no definitive or efficient
treatment for APD (5,8,22,23).
Topical steroids and oral antihistamines are the most commonly
prescribed drugs, which primarily aim to control the symptoms
(5,8,22,23).
Other effective therapies include topical agents such as
emollients, keratolytics, retinoids, steroids, doxycycline,
imiquimod, maxacalcitol and allopurinol; oral agents such as
retinoids, systemic antibiotics and amitriptyline, and medical
treatments such as cryotherapy, curettage, electrocautery, excision
and phototherapy such as NB-UVB and 308 nm excimer laser (6,8,19,20). In
the present study, clinical symptoms of APD improved gradually even
though the treatment was primarily aimed at relieving the symptoms
of pruritus. The effective control of the associated systemic
diseases was essential for the successful therapeutic result of the
dermatological treatment.
In conclusion, APD skin lesions are characterized by
isolated circular papules with uniformity in size and shape.
Accompanied by severe pruritus, APD is often associated with a
variety of systemic disease (3,5,14). The substances generated via TEE may
include collagen, elastin and keratin, whilst the materials in the
cup-shaped plugs are related to the clinical manifestation of the
dermatosis. There is a high association between APD and the
systemic disorders in regard to their occurrence and development.
Desirable prognoses were obtained in the four APD cases described
in the present study and the management of the associated systemic
diseases was important for the APD treatment outcomes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MFW was responsible for the clinical management of
patient, the evaluation and analysis of data, and was a major
contributor in writing the manuscript. XLM and LW was responsible
for the preparation of biopsy, analyzed and interpreted the patient
data regarding the hematological disease. LFL was involved in
making substantial contributions to conception and design, drafting
and revising the manuscript for important intellectual content, and
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethics approval was obtained from the Beijing
Friendship Hospital Ethics Committee for Human Research (approval
no. 2019-P2-178-01). Written informed consent was obtained from
each patient to have the case details and the accompanying images
published.
Patient consent for publication
Written informed consent of the patient were
obtained for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rapini RP, Herbert AA and Drucker CR:
Acquired perforating dermatosis. Evidence for combined
transepidermal elimination of both collagen and elastic fibers.
Arch Dermatol. 125:1074–1078. 1989.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schreml S, Hafner C, Eder F, Landthaler M,
Burgdorf W and Babilas P: Kyrle disease and acquired perforating
collagenosis secondary to chronic renal failure and diabetes
mellitus. Case Rep Dermatol. 3:209–211. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Faver IR, Daoud MS and Su WP: Acquired
reactive perforating collagenosis. Report of six cases and review
of the literature. J Am Acad Dermatol. 30:575–580. 1994.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kosumi H, Iwata H, Tsujiwaki M and Shimizu
H: Diagnosis at a glance: Acquired perforating dermatosis. Diabetes
Care. 41:911–912. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Karpouzis A, Giatromanolaki A, Sivridis E
and Kouskoukis C: Acquired reactive perforating collagenosis:
Current status. J Dermatol. 37:585–592. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
García-Malinis AJ, Del Valle Sánchez E,
Sánchez-Salas MP, Del Prado E, Coscojuela C and Gilaberte Y:
Acquired perforating dermatosis: Clinicopathological study of 31
cases, emphasizing pathogenesis and treatment. J Eur Acad Dermatol
Venereol. 31:1757–1763. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kestner RI, Ständer S, Osada N, Ziegler D
and Metze D: Acquired reactive perforating dermatosis is a variant
of prurigo nodularis. Acta Derm Venereol. 97:249–254.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wagner G and Sachse MM: Acquired reactive
perforating dermatosis. J Dtsch Dermatol Ges. 11:723–730.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mehregan AH, Schwartz OD and Livingood CS:
Reactive perforating collagenosis. Arch Dermatol. 96:277–282.
1967.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Metterle L, Magro CM and Zang JB: Giant
variant of acquired perforating dermatosis in a renal dialysis
patient. JAAD Case Rep. 3:42–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsuboi H and Katsuoka K: Characteristics
of acquired reactive perforating collagenosis. J Dermatol.
34:640–644. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Matsui A, Nakano H, Aizu T and Sawamura D:
Treatment of acquired reactive perforating collagenosis with 308 nm
excimer laser. Clin Exp Dermatol. 41:820–821. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Blaha T, Nigwekar S, Combs S, Kaw U,
Krishnappa V and Raina R: Dermatologic manifestations in end stage
renal disease. Hemodial Int. 23:3–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim SW, Kim MS, Lee JH, Son SJ, Park KY,
Li K, Seo SJ and Han TY: A clinicopathologic study of thirty cases
of acquired perforating dermatosis in Korea. Ann Dermatol.
26:162–171. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Saray Y, Seçkin D and Bilezikçi B:
Acquired perforating dermatosis: Clinicopathological features in
twenty-two cases. J Eur Acad Dermatol Venereol. 20:679–688.
2016.
|
|
16
|
Yazdi S, Saadat P, Young S, Hamidi R and
Vadmal MS: Acquired reactive perforating collagenosis associated
with papillary thyroid carcinoma: A paraneoplastic phenomenon? Clin
Exp Dermatol. 35:152–155. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim RH, Kwa M, Adams S, Meehan SA and
Stein JA: Giant acquired reactive perforating collagenosis in a
patient with diabetes mellitus and metastatic breast carcinoma.
JAAD Case Rep. 2:22–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kawahara S, Mitom C, Murai M and Furue M:
Acquired perforating collagenosis in a non-diabetic patient with
advanced prostate carcinoma: A review of perforating dermatosis
associated with malignancy. J Dermatol. 45:e219–e220.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eriyagama S, Wee JS, Ho B and Natkunarajah
J: Acquired reactive perforating collagenosis associated with
urticarial vasculitis in pregnancy. Clin Exp Dermatol. 39:81–83.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fei C, Wang Y, Gong Y, Xu H, Yu Q and Shi
Y: Acquired reactive perforating collagenosis: A report of a
typical case. Medicine (Baltimore). 95(e4305)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Razmi TM, Chatterjee D and Parsad D: Giant
variant of acquired reactive perforating collagenosis in diabetic
nephropathy. Postgrad Med J. 95:52–53. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Villela-Segura U, Miranda-Aguirre AI and
Estrada-Aguilar L: Crateriform plaques in a patient with end-stage
renal disease. The case of an acquired reactive perforating
collagenosis. Nefrologia. 19:30114–30116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lukács J, Schliemann S and Elsner P:
Treatment of acquired reactive perforating dermatosis-A systematic
review. J Dtsch Dermatol Ges. 16:825–842. 2018.PubMed/NCBI View Article : Google Scholar
|