Introduction
Metabolic syndrome is an association of several risk
factors for cardiovascular disease and other diseases (1,2). Many
criteria have been proposed for the diagnosis of metabolic
syndrome, all of them included the presence of high blood pressure,
high fasting glycaemia, dyslipidaemia and obesity; however, the
cut-off values for these variables differed depending on the
organisations. A meeting of several major organisations including
International Diabetes Federation and American Heart Association
attempted to give unifying criteria for the definition of metabolic
syndrome. For the diagnosis of metabolic syndrome 3 of the 5
following criteria must be present: increased waist circumference,
in the case of Caucasian patients ≥94 cm for men and ≥80 cm for
women; elevated triglycerides ≥150 mg/dl; elevated blood pressure,
systolic blood pressure ≥130 mmHg and/or diastolic blood pressure
≥85 mg/dl; reduced high-density lipoprotein (HDL) cholesterol
<40 mg/dl for men and ≤50 mg/dl for women (3).
The prevalence of metabolic syndrome began to
increase worldwide because of obesity epidemics. The observation is
supported by the findings of National health and nutrition
examination survey (NHANES) where the prevalence of metabolic
syndrome was 5% among individuals with normal body mass index (BMI)
and 60% among individuals with obesity (4). The importance of metabolic syndrome
consists in the high burden of complications it generates,
epidemiological studies proving that subjects affected by metabolic
syndrome have a 3-4 times greater risk of myocardial infarction and
a 2-4 times greater risk of stroke, as well as therapeutic
implications (5,6).
Lifestyle changes have triggered the obesity
epidemic; obesity is the main cause for metabolic syndrome because
obesity generates insulin resistance and insulin resistance is
associated with all the components of metabolic syndrome (7). Adipocytes are metabolically active
cells generating different metabolites called adipokines that act
at different levels, some of them increasing the risk of diabetes
and cardiovascular disease such as C-reactive protein (CRP), tumour
necrosis factor α (TNFα) and interleukin-6 (IL-6), plasminogen
activator inhibitor-1 (PAI-1), some of them decreasing, at least
theoretically, the risk of diabetes and atherosclerosis such as
adiponectin (8).
CRP is an inflammatory and endothelial dysfunction
biomarker generally considered accurate in predicting
cardiovascular disease. Correlations were established between
highly sensitive C-reactive protein (hsCRP) levels, coronary events
and different metabolic risk factors especially BMI and insulin
resistance (9). It appears that CRP
interferes with the insulin signalling pathway and therefore
generates insulin resistance (10).
Despite a variety of studies, the role of hsCRP in the pathogenesis
of metabolic syndrome and cardiovascular disease remains
controversial. Risk factors, such as smoking, age, BMI, blood
pressure, triglycerides have been associated with elevated hsCRP
levels (11).
In addition, because of the role of hsCRP levels in
identifying prognosis and recurrent events in patients with stroke
and peripheral arterial disease, it may be useful in assessing
cardiovascular risks and in identifying high-risk populations as
target for prevention (12). Racial
and ethnic differences have been demonstrated in the hsCRP levels
and sex difference (women having higher hsCRP levels than men). One
of the possible explanations is relatively higher degree of
adiposity in women so the body fat quantity and distribution appear
to influence hsCRP levels to a greater extent in women than men
(13).
Adiponectin or adipocyte complement-related protein
is the most abundant peptide secreted by adipocytes that negatively
correlates with obesity and type 2 diabetes because it acts by
increasing insulin sensitivity; it is probably the only adipokine
with higher levels correspond to lower BMI and decreased
cardiovascular risk because of its anti-inflammatory, and
antiatherogenic effects (14). These
are reasons to be proposed as novel therapeutic target for diabetes
and metabolic syndrome.
Human adiponectin is encoded by the Adipo Q
gene, which spans 17 kb on chromosome locus 3q27. This chromosome
has been identified as carrying a gene susceptible for type 2
diabetes and metabolic syndrome (15). Adiponectin is also involved in energy
homeostasis by action in hypothalamus, therefore the name
‘starvation gene’ has been proposed.
AdipoR1 and AdipoR2, two structurally related seven
transmembrane receptors, have been identified to function as
adiponectin receptors, structurally and functionally distinct from
classical G-protein coupled receptors. AdipoR1 is expressed
ubiquitously, but most abundantly, in skeletal muscle while AdipoR2
is predominantly expressed in the liver (16). It was demonstrated that adiponectin
improves the utilization of glucose at the level of the skeletal
muscle, protects against atherosclerosis plaque formation,
decreases liver glucose production and decreases visceral adiposity
(17). Insulin and adiponectin
interact with their receptors, fact that generates a cascade of
metabolic actions such as increased protein synthesis, lipogenesis,
reduces plasma glucose levels (increase glucose uptake and
utilization), glycogen synthesis, lipolysis and gluconeogenesis
(18).
Leptin is a polypeptide hormone produced by
adipocytes in proportion to their triglyceride content. It is
involved in the central control of energy balance. Its action
consists in binding to and activating the long form of its receptor
in the brain and the result is decreasing food intake while
increasing energy expenditure. Plasma leptin concentration
increases in proportion to body fat content, regulate food intake
and energy expenditure to maintain body fat stores. Circulating
leptin is secreted into the blood and after crossing the
brain-blood and cerebrospinal fluid barrier, it acts in the
hypothalamus, where leptin inhibits neuropeptide Y (NPY) neurons
and causes anorexia. The arcuate nucleus has been proposed as an
important site of leptin action. The central administration of
leptin increases glucose turn-over and glucose uptake in peripheral
tissues (heart, skeletal muscle, adipose tissue), stimulates
hepatic gluconeogenesis and hepatic insulin sensitivity via the
hepatic branch of the vagus nerve.
This study evaluated the correlation between the
adipocyte biomarkers: adiponectin, leptin, HOMA-IR and inflammation
biomarker, hs-CRP, and metabolic syndrome and its components.
Patients and methods
Patients
The study included 160 individuals, 80 with normal
body weight defined as BMI ≥18.5 kg/m2 and <25
kg/m2 and 80 with obesity defined as BMI ≥30
kg/m2; all of them were selected from the list of
patients of a primary care physician from Oradea, Romania. The
inclusion criteria were as follows: subjects aged between 18 and 65
years, subjects that gave their written consent for the
participation in the study. For the reference group, the additional
criteria were BMI ≥18.5 kg/m2 and <25
kg/m2; for the control group the additional criteria was
BMI ≥30 kg/m2. Since the study addresses to the general
population, to the clinically healthy individuals (with the
exception of obesity), the idea was to include individuals without
significant chronic comorbidities therefore the exclusion criteria
were: patients with diabetes mellitus, patients with stage II or
III hypertension, patients with proved coronary artery disease or
cerebrovascular disease (history of myocardial infarction or
stroke), patients with other chronic diseases (cirrhosis, chronic
obstructive pulmonary disease, chronic kidney disease, cancer,
endocrine, haematological, psychiatric and neurological diseases),
patients that were taking medications that can influence blood
pressure or glycaemia. The exclusion criteria were similar for the
reference and the control group. The research was performed
according the WMA Declaration of Ethics, Helsinki - Medical
Research Involving Human Principles for Subjects; the study was
also approved by the Ethics Commission of the Council of Clinical
County Emergency Hospital of Oradea (Romania). All subjects gave
their written consent for the participation in the study and the
medical practitioner gave written consent for the selection of the
subjects from the patient lists.
Method
The method for selection of subjects was the
following: in an interval of three months (1st June 2019-1st
September 2019), the individuals that addressed the primary care
physician for administrative reasons (requirement of medical
certificate that attests the general health condition) were
considered for inclusion in the study. When presented to the
primary care physician, the subject was evaluated regarding the
fulfilment of inclusion criteria and absence of exclusion criteria.
A total of 80 obese subjects (reference group) and 80 normal weight
subjects (control group) met the criteria and were further selected
for clinical and biochemical evaluation. When selected, the subject
was instructed to return the following day for physical examination
that included measurement of height, weight, BMI calculation, waist
circumference measurement and hip circumference measurement,
determination of blood pressure, and collection of venous blood
samples. Special determinations included serum insulin,
adiponectin, leptin and hsCRP protein. hsCRP was determined using
the immunoturbidimetric method, adiponectin and leptin were
determined using ELISA method, insulin was determined by the
chemiluminescence immunoassay. Quality control was conducted before
testing samples. Normal values were: for adiponectin 12-25 ng/ml,
for leptin (14.1-37.0) ng/ml in women and (3.3-14.3) ng/ml in men,
for hsCRP <0.3 mg/dl and for insulin 2.5-25 µU/ml.
Determinations were performed in the Laboratory Department of
Clinical County Hospital of Oradea (Oradea, Romania).
Statistical analysis
Statistical analysis was performed using Biostat
software. P<0.05 was considered statistically significant. The
comparison of variables between the two groups was performed using
t-test for variables with normal distribution and Mann-Whitney U
test for variables with skewed distribution.
Results
Increased BMI is associated with statistically
significant higher age, waist circumference, hip circumference,
systolic blood pressure, diastolic blood pressure, fasting
glycaemia, hemoglobin A1c (HbA1c), triglycerides and lower
HDL-cholesterol as shown in Table I.
The adipocyte biomarkers of the two groups are presented in
Table II.
 | Table ICharacteristics of the normal weight
and obese subjects. |
Table I
Characteristics of the normal weight
and obese subjects.
| Variable | Normal weight
(n=80) | Standard
deviation | Obese (n=80) | Standard
deviation | P-value |
|---|
| Sex (women/men)
% | 60/40 | - | 57.5/42.5 | - | 0.74 |
| Age (years) | 43.05 | 10.93 | 46.74 | 10.37 | 0.01 |
| Weight (kg) | 62.35 | 8.21 | 97.68 | 14.71 | <0.01 |
| Height (m) | 1.67 | 0.08 | 1.68 | 0.09 | 0.29 |
| BMI
(kg/m2) | 22.32 | 1.81 | 34.68 | 3.82 | <0.01 |
| Waist circumference
(cm) | 78.19 | 7.36 | 108.42 | 14.61 | <0.01 |
| Hip circumference
(cm) | 94.29 | 6.43 | 117.08 | 10.98 | <0.01 |
| Waist/hip
circumference | 0.83 | 0.08 | 0.93 | 0.08 | <0.01 |
| SBP (mmHg) | 116.8 | 11.41 | 127.51 | 14.27 | <0.01 |
| DBP (mmHg) | 72.24 | 7.49 | 80.28 | 9.61 | <0.01 |
| Glycaemia
(mg/dl) | 88.11 | 8.6 | 92.9 | 9.76 | <0.01 |
| HbA1c (%) | 5.2 | 0.26 | 5.46 | 0.39 | <0.01 |
| Triglycerides
(mg/dl) | 80.94 | (60, 91.5) | 114.08 | (73.25, 135.5) | <0.01 |
| Cholesterol
(mg/dl) | 181.69 | 36.72 | 186.06 | 37.59 | 0.46 |
| LDL-cholesterol
(mg/dl) | 106.65 | 31.8 | 114.94 | 34.3 | 0.11 |
| HDL-cholesterol
(mg/dl) | 58.95 | 13.94 | 49.01 | 13.22 | <0.01 |
 | Table IIInflammation and adipocyte biomarkers
in the two groups (P<0.01). |
Table II
Inflammation and adipocyte biomarkers
in the two groups (P<0.01).
| Parameter | Normal weight
(n=80) | Standard
deviation | Obese (n=80) | Standard
deviation |
|---|
| Insulin
(µU/ml) | 6.17 | (3.8, 7.82) | 13.31 | (7.77, 15.75) |
| HOMA-IR | 1.36 | (0.79, 1.69) | 3.06 | (1.78, 3.55) |
| hsCRP (mg/dl) | 0.22 | (0.08, 0.21) | 0.58 | (0.17, 0.73) |
| Leptin (ng/ml) | 7.21 | (2.02, 10.75) | 25.7 | (12, 33.79) |
| Adiponectin
(ng/ml) | 16.84 | (14.34, 17.69) | 7.52 | (6.16, 8.55) |
|
Adiponectin/HOMA-IR | 17.11 | (9.62, 19.53) | 3.41 | (1.73, 4.51) |
|
Leptin/adiponectin | 0.42 | (0.13, 0.61) | 3.47 | (1.77, 4.50) |
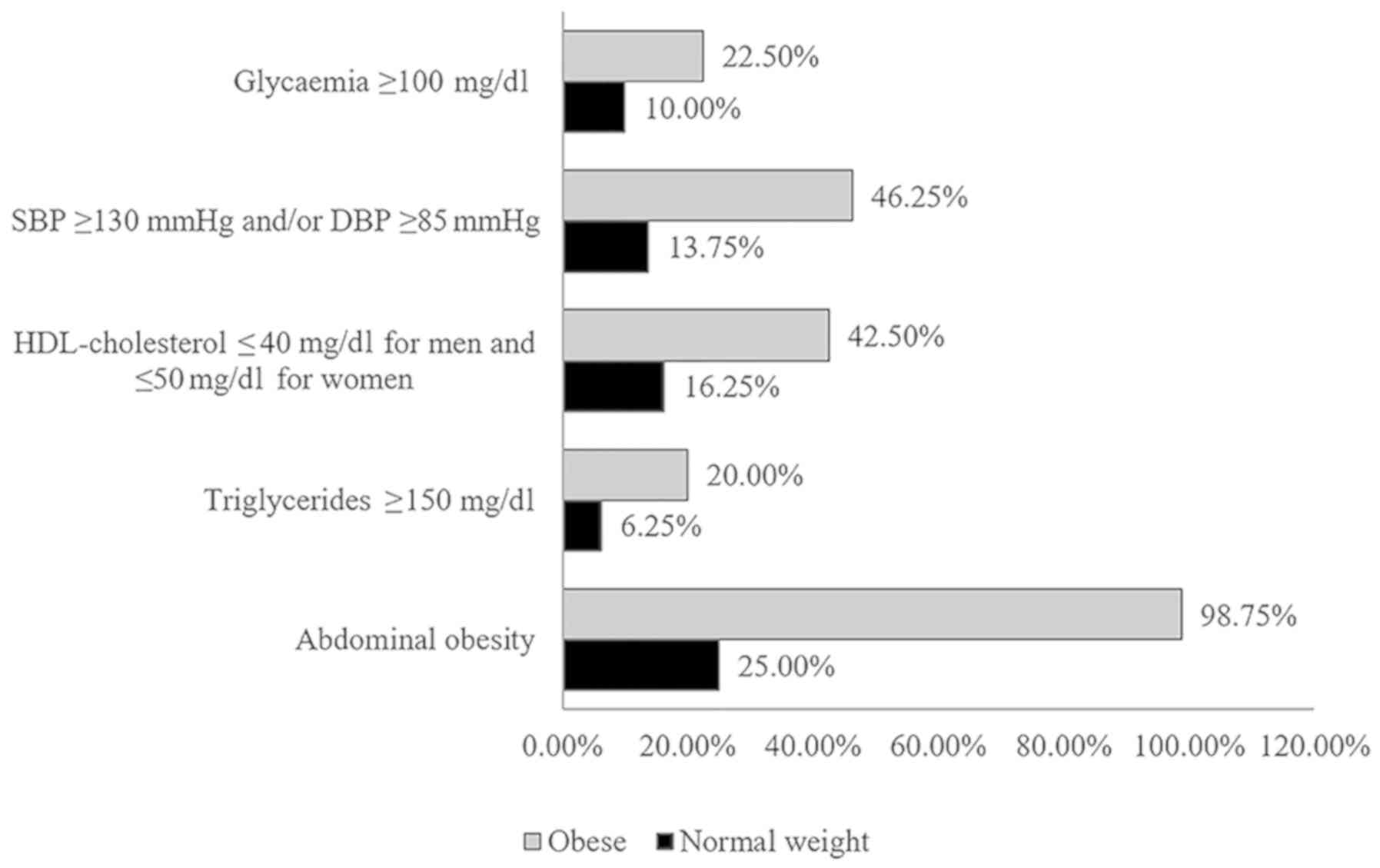
The prevalence of metabolic syndrome was
statistically significantly higher (35%) in obese subjects than in
normal weight subjects (5%) (P<0.01). The prevalence of
metabolic syndrome components was statistically significantly
higher (P<0.01) in obese subjects than in normal weight subjects
(Fig. 1).
Subjects with metabolic syndrome had statistically
significantly higher weight, BMI, waist circumference, hip
circumference, systolic blood pressure, diastolic blood pressure,
glycaemia, HbA1c, triglycerides and lower HDL-cholesterol compared
with subjects without metabolic syndrome (Table III).
 | Table IIICharacteristics of the subjects with
and without metabolic syndrome. |
Table III
Characteristics of the subjects with
and without metabolic syndrome.
| Parameter | Without metabolic
syndrome (n=128) | Standard
deviation | With metabolic
syndrome (n=32) | Standard
deviation | P-value |
|---|
| Sex (women/men)
% | 64/36 | - | 43.75/56.25 | - | 0.05 |
| Age (years) | 44.07 | 11.19 | 48.15 | 8.39 | 0.05 |
| Weight (kg) | 75.95 | 19.93 | 96.27 | 18.82 | <0.01 |
| Height (m) | 1.67 | 0.08 | 1.7 | 0.08 | 0.03 |
| BMI
(kg/m2) | 27.32 | 6.79 | 33.2 | 4.86 | <0.01 |
| Waist circumference
(cm) | 89.67 | 17.18 | 107.83 | 14.72 | <0.01 |
| Hip circumference
(cm) | 103.16 | 13.48 | 115.78 | 14.11 | <0.01 |
| Waist/hip
circumference | 0.86 | 0.1 | 0.93 | 0.06 | <0.01 |
| SBP (mmHg) | 119.57 | 13.08 | 132.5 | 12.67 | <0.01 |
| DBP (mmHg) | 74.59 | 8.9 | 82.94 | 8.9 | <0.01 |
| Glycaemia
(mg/dl) | 88.6 | 8.51 | 98.28 | 9.29 | <0.01 |
| HbA1c (%) | 5.29 | 0.33 | 5.49 | 0.41 | <0.01 |
| Triglycerides
(mg/dl) | 85.31 | (60, 104) | 95.25 | (84.25, 189.5) | <0.01 |
| Cholesterol
(mg/dl) | 182.34 | 37.11 | 190 | 37.05 | 0.3 |
| LDL-cholesterol
(mg/dl) | 108.87 | 32.36 | 118.5 | 35.96 | 0.14 |
| HDL-cholesterol
(mg/dl) | 56.84 | 14.16 | 42.56 | 9.01 | <0.01 |
The presence of the metabolic syndrome was
associated with statistically significant higher values of serum
insulin, HOMA-IR, leptin and leptin/adiponectin ratio and
statistically significant lower values of adiponectin and
adiponectin/HOMA-IR ratio (Table
IV).
 | Table IVInflammation and adipocyte biomarkers
in the subjects with/without metabolic syndrome. |
Table IV
Inflammation and adipocyte biomarkers
in the subjects with/without metabolic syndrome.
| Parameter | Without metabolic
syndrome (=128) | Standard
deviation | With metabolic
syndrome (n=32) | Standard
deviation | P-value |
|---|
| Insulin
(µU/ml) | 8.95 | (5.32, 11.7) | 12.89 | (6.62, 14.97) | <0.01 |
| HOMA-IR | 1.97 | (1.15, 2.69) | 3.16 | (1.61, 3.82) | <0.01 |
| hsCRP (mg/dl) | 0.4 | (0.09, 0.46) | 0.41 | (0.13, 0.46) | 0.28 |
| Leptin (ng/ml) | 15.22 | (4.76, 21.98) | 21.4 | (5.30, 28.36) | 0.14 |
| Adiponectin
(ng/ml) | 13.27 | (8.49, 16.74) | 7.79 | (6.03, 8.01) | <0.01 |
|
Adiponectin/HOMA-IR | 11.77 | (3.81, 15.61) | 4.23 | (1.50, 5.13) | <0.01 |
|
Leptin/adiponectin | 1.64 | (0.27, 2.57) | 3.18 | (0.57, 3.87) | <0.01 |
The parameters that correlated statistically
significantly with metabolic syndrome were HOMA-IR, adiponectin,
adiponectin/HOMA-IR ratio and leptin/adiponectin ratio. Adiponectin
and adiponectin/HOMA-IR ratio correlated with all the components of
the metabolic syndrome. In multivariate regression analysis of
adiponectin was the only biochemical marker that correlated with
metabolic syndrome. Also, adiponectin correlated with abdominal
obesity, low HDL-cholesterol and raised blood pressure. HOMA-IR
correlated with low HDL-cholesterol and raised glycaemia (Table V).
 | Table VCorrelation between inflammation and
adipocyte biomarkers and metabolic syndrome and its components. |
Table V
Correlation between inflammation and
adipocyte biomarkers and metabolic syndrome and its components.
| | Univariate
regression |
|---|
| Parameter | Metabolic
syndrome | Abdominal
obesity | Raised
triglycerides | Low HDL | Increased blood
pressure | Increased
glycaemia |
|---|
| HOMA-IR | 0.0002 | 0.0001 | 0.09 | 0.001 | 0.004 | 0.0001 |
| Leptin (ng/ml) | 0.05 | 0.0001 | 0.91 | 0.17 | 0.039 | 0.01 |
| Adiponectin
(ng/ml) | 0.0001 | 0.0001 | 0.005 | 0.0001 | 0.0001 | 0.01 |
| hsCRP (mg/dl) | 0.87 | 0.0002 | 0.79 | 0.05 | 0.26 | 0.72 |
|
Adiponectin/HOMA-IR | 0.0005 | 0.0001 | 0.03 | 0.0005 | 0.03 | 0.006 |
|
Leptin/adiponectin | 0.0007 | 0.0001 | 0.41 | 0.01 | 0.0001 | 0.03 |
| Multivariate
regression adjusted by age and sex |
| Sex | 0.5187 | 0.0238 | 0.0975 | 0.9951 | 0.3345 | 0.0645 |
| Age | 0.1250 | 0.0039 | 0.2134 | 0.2032 | 0.0074 | 0.6219 |
| hsCRP (mg/dl) | 0.1756 | 0.7135 | 0.9193 | 0.2979 | 0.7130 | 0.5728 |
| HOMA-IR | 0.1229 | 0.6734 | 0.4094 | 0.0133 | 0.5291 | 0.0102 |
| Leptin (ng/ml) | 0.8549 | 0.0815 | 0.6002 | 0.1475 | 0.2036 | 0.8684 |
| Adiponectin
(ng/ml) | 0.0003 | 0.0000 | 0.1290 | 0.0037 | 0.0343 | 0.1203 |
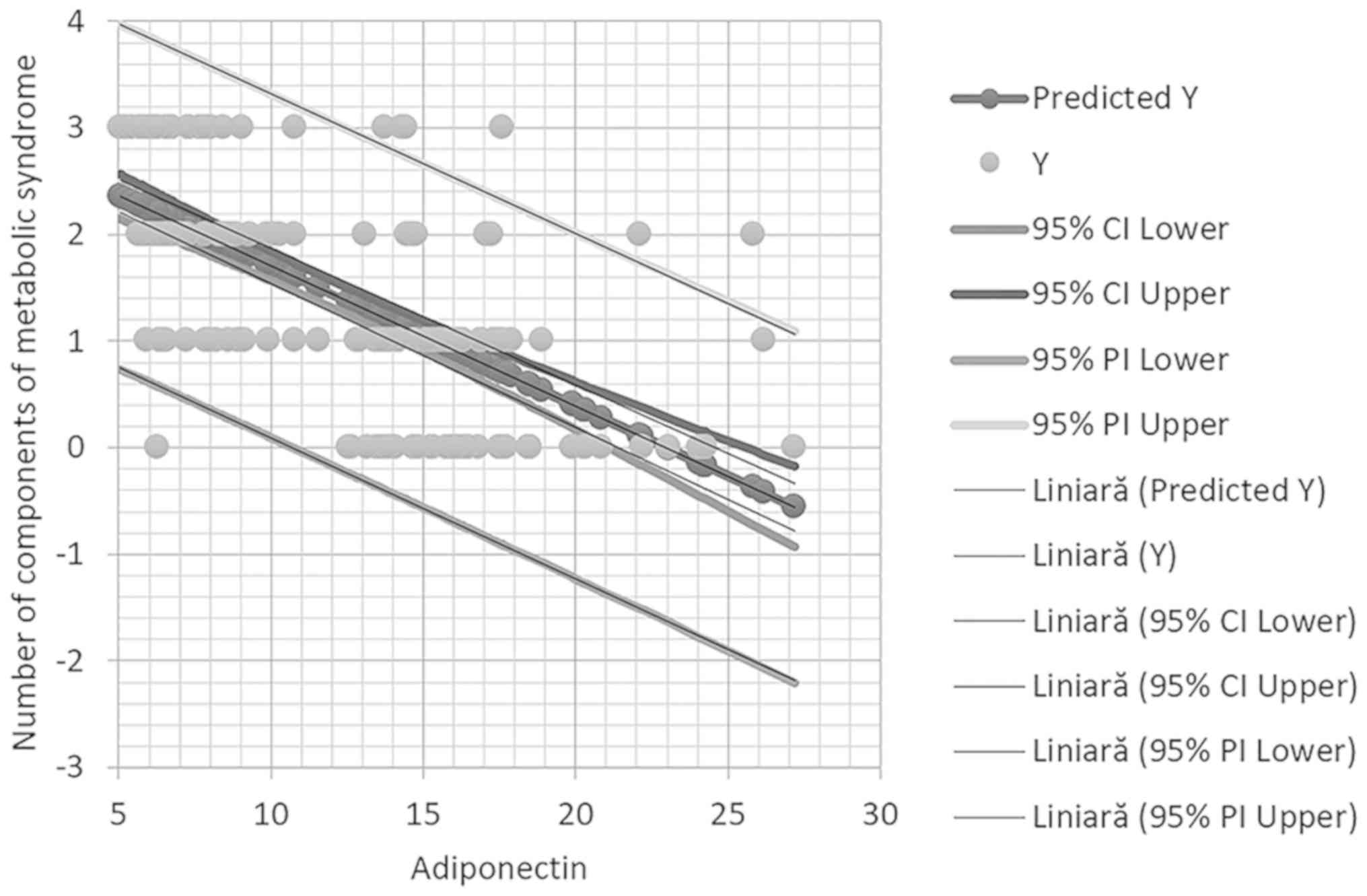
It should be indicated that in multivariate
regression analysis adiponectin remained statistically
significantly associated with high blood pressure (Fig. 2).
Discussion
Increased prevalence worldwide predisposes to
insulin resistance, which is the central key of metabolic syndrome.
The aetiology of the metabolic syndrome is complex, being involved
in genetic mechanisms and environmental factors that predispose to
it (19-21).
Therapeutic intervention may be a preventive measure, at first
disease should be recorded to register and monitor the chronic
patients in order to assess their needs and improve their care
(22-24).
The present study confirmed that lower adiponectin
levels are negatively associated with the presence of obesity and
metabolic syndrome, a finding reported by previous studies
(25). Adiponectin was associated
with the presence of all the components of metabolic syndrome:
abdominal obesity, hypertriglyceridemia, low HDL-cholesterol, high
blood pressure and high glycaemia. Studies report that adiponectin
levels are lower with increasing number of metabolic syndrome
components (25). A study performed
on a large population including 2,471 men and 3,463 women of Korean
origin confirmed that adiponectin levels are associated with
metabolic syndrome phenotype and all its components (26); similarly to the present study, all
these individuals were persons not suffering of diabetes mellitus.
Also, increased levels of serum adiponectin were associated with
higher number of metabolic syndrome components.
The usefulness of these findings consists in the
metabolic action of adiponectin, it is important to mention that
low levels of adiponectin are not only a consequence of increased
adiposity, but also adiponectin has substantial beneficial roles in
many metabolic pathways and therefore obese individuals do not
benefit of the favourable effects of adiponectin. Firstly,
adiponectin activated APPL1 signalling pathway. APPL1 has many
roles, among them is activating the insulin receptor substrate
proteins, therefore adiponectin stimulates the activity of insulin
pathway and reduces insulin resistance (27). On the other hand, adiponectin
activates AMPK signalling pathway increasing fat oxidation and
energy expenditure (28,29). An increase of adiponectin can be
obtained with the help of pharmacotherapy, by administration of
thiazolidinediones, or by aerobic exercise (17). There is also a negative relationship
between adiponectin levels and hypertension. Experimentally, in
mice with reduced levels of adiponectin, the existence of
endothelial dysfunction was found with alteration of vasodilatation
and increased transformation of macrophages into foam cells,
therefore adiponectin is associated with decreased atherosclerosis
(16). In the present study
adiponectin was negatively associated with increased blood
pressure, systolic blood pressure ≥130 mmHg and/or diastolic blood
pressure ≥85 mmHg.
Adiponectin/HOMA-IR ratio and leptin/adiponectin
ratio were statistically significantly associated with metabolic
syndrome in the present study. Similar findings have been reported
by Ding et al (30), who
demonstrated that adiponectin/HOMA-IR is a better predictor of
metabolic syndrome than adiponectin or HOMA-IR alone. In the
present study the degree of correlation in univariate regression
between adiponectin, HOMA-IR, adiponectin/HOMA-IR and
leptin/adiponectin ratio and the presence of metabolic syndrome was
comparable.
No relationship between hs-CRP and metabolic
syndrome was found in this study. Also, except for obesity, hs-CRP
did not correlate with any components of metabolic syndrome.
Results from literature are contradictory regarding the association
between hs-CRP and metabolic syndrome and its components. There are
studies that reported that patients with metabolic syndrome had
values of hs-CRP 4 times higher than patients without metabolic
syndrome and that hs-CRP was associated with metabolic syndrome and
all its components (31). However,
other scientists reported that hs-CRP has limited capacity to
predict metabolic syndrome (32).
Leptin/adiponectin ratio was a strong predictor of
metabolic syndrome revealed in the present study. Leptin has higher
levels in patients with obesity mainly because of leptin
resistance. Also, it was demonstrated that high levels of leptin
are associated with increased insulin secretion which further
exacerbates obesity and increases leptin levels (33). Leptin increases insulin resistance
and has proinflammatory effects, adiponectin increases insulin
sensitivity and decreases inflammatory response, therefore because
of their antagonistic actions the leptin/adiponectin ratio is a
good predictor of diabetes risk and of metabolic syndrome (34).
Although individuals included in the study were not
suffering of diabetes mellitus type 2, the metabolic syndrome group
are at high risk for progression towards this disease, showing
statistically significant higher levels of HOMA-IR index, leptin
and lower adiponectin. Adiponectin is genetically linked with
diabetes mellitus type 2, it was proven that one of the loci of
diabetes mellitus susceptibility is 3q27, in this region the gene
responsible for adiponectin synthesis is also located (35).
Given these findings, adiponectin emerges as a
target molecule for reducing the risk of metabolic syndrome, type 2
diabetes mellitus and atherosclerotic disease. Research is
currently conducted to determine the measures, pharmacological or
non-pharmacological, that may increase the circulating levels of
adiponectin (19,36-38).
Physical effort appears to be associated with an increase in serum
adiponectin levels; one week of aerobic exercise was reported to
lead to a significant increase in adiponectin in obese men
(39). Complex experiments involving
administration of adiponectin using adenoviruses as vectors in
obese mice, with low blood adiponectin levels, demonstrated that
adiponectin reduces obesity related hypertension (40).
Increased leptin/adiponectin ratio is strongly
influenced by radical measures such as bariatric surgery. Severely
obese type 2 diabetes mellitus patients that underwent Roux-en-Y
gastric bypass had significantly lower leptin levels and
significantly higher adiponectin levels compared to baseline values
(41), these modifications of
adipokines could contribute to the remission of glycaemic
misbalance frequently observed in this category of patients.
In conclusion, adiponectin appears to be the
hallmark molecule negatively associated with metabolic syndrome and
its components. The derived variables such as leptin/adiponectin
ratio or adiponectin/HOMA-IR gain statistical significance mainly
because of the markedly decreased levels of adiponectin in
metabolic syndrome. HsCRP is associated only with obesity, not with
the metabolic syndrome. Therefore, assessment of adiponectin in
population could help identify patients with high risk of diabetes
mellitus and cardiovascular disease.
Acknowledgements
Not applicable.
Funding
This study was supported by the project ‘SmartDoct -
High quality programs for PhD students and postdoctoral researchers
of the University of Oradea for increasing the relevance of
research and innovation in the context of the regional economy’,
ID/Project code: 123008, co-financed by the European Social Fund
through the Human Capital Operational Program 2014-2020.
Availability of data and materials
At the private medical offices where the data were
collected.
Authors' contributions
DCZ, CV, DU, OF and CP selected the patients,
analyzed and interpreted the patient data regarding BMI and
biochemical markers. OB, DMT, CCD and SB made substantial
contributions to the conception of the work and interpretation of
data; also, they drafted the manuscript and were major contributors
in writing the manuscript. All authors read and approved the final
manuscript to be published. All the authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The research was performed according the WMA
Declaration of Ethics, Helsinki - Medical Research Involving Human
Principles for Subjects; the study was also approved by the Ethics
Commission of the Council of Clinical County Emergency Hospital of
Oradea (Romania). All subjects gave their written consent for the
participation in the study and the medical practitioner gave
written consent for the selection of the subjects from the patient
lists.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saklayen MG: The global epidemic of the
metabolic syndrome. Curr Hypertens Rep. 20(12)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adambekov S, Yi Y, Fabio A, Miljkovic I,
Edwards RP, Lopa S and Linkov F: Metabolic syndrome in endometrial
cancer patients: Systematic review. Metab Syndr Relat Disord.
17:241–249. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alberti KG, Eckel RH, Grundy SM, Zimmet
PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith
SC Jr, et al: Harmonizing the metabolic syndrome: A joint interim
statement of the International Diabetes Federation Task Force on
Epidemiology and Prevention; National Heart, Lung, and Blood
Institute; American Heart Association; World Heart Federation;
International Atherosclerosis Society; and International
Association for the Study of Obesity. Circulation. 120:1640–1645.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Park Y-W, Zhu S, Palaniappan L, Heshka S,
Carnethon MR and Heymsfield SB: The metabolic syndrome: Prevalence
and associated risk factor findings in the US population from the
Third National Health and Nutrition Examination Survey, 1988-1994.
Arch Intern Med. 163:427–436. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alberti KG, Zimmet P and Shaw J: IDF
Epidemiology Task Force Consensus Group. The metabolic syndrome - a
new worldwide definition. Lancet. 366:1059–1062. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rajput R, Rajput M, Mishra S and Ahlawat
P: Prevalence of metabolic syndrome in prediabetes. Metab Syndr
Relat Disord. 17:406–410. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Popa AR, Bungau S, Vesa CM, Bondar AC,
Pantis C, Maghiar O, Dimulescu (Nica) IA, Nistor Cseppento DC and
Rus M: Evaluating the efficacy of the treatment with benfotiamine
and alpha-lipoic acid in distal symmetric painful diabetic
polyneuropathy. Rev Chim. 70:3108–3114. 2019.
|
|
8
|
Xu H, Li X, Adams H, Kubena K and Guo S:
Etiology of metabolic syndrome and dietary intervention. Int J Mol
Sci. 20(E128)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lau DC, Dhillon B, Yan H, Szmitko PE and
Verma S: Adipokines: Molecular links between obesity and
atheroslcerosis. Am J Physiol Heart Circ Physiol. 288:H2031–H2041.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Festa A, D'Agostino R Jr, Howard G,
Mykkänen L, Tracy RP and Haffner SM: Chronic subclinical
inflammation as part of the insulin resistance syndrome: The
Insulin Resistance Atherosclerosis Study (IRAS). Circulation.
102:42–47. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Saito M, Ishimitsu T, Minami J, Ono H,
Ohrui M and Matsuoka H: Relations of plasma high-sensitivity
C-reactive protein to traditional cardiovascular risk factors.
Atherosclerosis. 167:73–79. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Suzuki T, Voeks J, Zakai NA, Jenny NS,
Brown TM, Safford MM, LeWinter M, Howard G and Cushman M: Metabolic
syndrome, C-reactive protein, and mortality in U.S. Blacks and
Whites: The reasons for geographic and racial differences in stroke
(REGARDS) study. Diabetes Care. 37:2284–2290. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Khera A, Vega GL, Das SR, Ayers C, McGuire
DK, Grundy SM and de Lemos JA: Sex differences in the relationship
between C-reactive protein and body fat. J Clin Endocrinol Metab.
94:3251–3258. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Devaraj S, Singh U and Jialal I: Human
C-reactive protein and the metabolic syndrome. Curr Opin Lipidol.
20:182–189. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mori Y, Otabe S, Dina C, Yasuda K,
Populaire C, Lecoeur C, Vatin V, Durand E, Hara K, Okada T, et al:
Genome-wide search for type 2 diabetes in Japanese affected
sib-pairs confirms susceptibility genes on 3q, 15q, and 20q and
identifies two new candidate loci on 7p and 11p. Diabetes.
51:1247–1255. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yamauchi T, Kamon J, Ito Y, Tsuchida A,
Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et
al: Cloning of adiponectin receptors that mediate antidiabetic
metabolic effects. Nature. 423:762–769. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sanjari M, Khodashahi M, Gholamhoseinian A
and Shokoohi M: Association of adiponectin and metabolic syndrome
in women. J Res Med Sci. 16:1532–1540. 2011.PubMed/NCBI
|
|
18
|
Yamauchi T, Kamon J, Minokoshi Y, Ito Y,
Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al:
Adiponectin stimulates glucose utilization and fatty-acid oxidation
by activating AMP-activated protein kinase. Nat Med. 8:1288–1295.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Achari AE and Jain SK: Adiponectin, a
therapeutic target for obesity, diabetes, and endothelial
dysfunction. Int J Mol Sci. 18(E1321)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Abdel-Daim MM, El-Tawil OS, Bungau SG and
Atanasaov AG: Applications of antioxidants in metabolic disorders
and degenerative diseases: Mechanistic approach. Oxid Med Cell
Longev. 2019(4179676)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Abdel-Daim MM, Zakhary NI, Aleya L, Bungau
SG, Bohara RA and Siddiqi NJ: Aging, metabolic, and degenerative
disorders: Biomedical value of antioxidants. Oxid Med Cell Longev.
2018(2098123)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Popa AR, Vesa CM, Uivarosan D, Jurca CM,
Isvoranu G, Socea B, Stanescu AMA, Iancu MA, Scarneciu I and Zaha
DC: Cross-sectional study regarding the association between
sweetened beverages intake, fast-food products, body mass index,
fasting blood glucose and blood pressure in the young adults from
North-western Romania. Rev Chim. 70:156–160. 2019.
|
|
23
|
Grigore O, Mihailescu AI, Solomon I, Boda
D and Caruntu C: Role of stress in modulation of skin neurogenic
inflammation. Exp Ther Med. 17:997–1003. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ghita MA, Caruntu C, Lixandru D, Pitea A,
Batani A and Boda D: The quest for novel biomarkers in early
diagnosis of diabetic neuropathy. Curr Proteomics. 14:86–99.
2017.
|
|
25
|
Gabor-Harosa FM, Stan OP, Daina L and
Mocean F: Proposed model for a Romanian register of chronic
diseases in children. Comput Methods Programs Biomed. 130:198–204.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Koh SB, Yoon J, Kim JY, Yoo BS, Lee SH,
Park JK and Choe KH: Relationships between serum adiponectin with
metabolic syndrome and components of metabolic syndrome in
non-diabetic Koreans: ARIRANG study. Yonsei Med J. 52:234–241.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
von Frankenberg AD, do Nascimento FV,
Gatelli LE, Nedel BL, Garcia SP, de Oliveira CSV, Saddi-Rosa P,
Reis AF, Canani LH and Gerchman F: Major components of metabolic
syndrome and adiponectin levels: A cross-sectional study. Diabetol
Metab Syndr. 6(26)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deepa SS, Zhou L, Ryu J, Wang C, Mao X, Li
C, Zhang N, Musi N, DeFronzo RA, Liu F, et al: APPL1 mediates
adiponectin-induced LKB1 cytosolic localization through the
PP2A-PKCzeta signaling pathway. Mol Endocrinol. 25:1773–1785.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nigro E, Scudiero O, Monaco ML, Palmieri
A, Mazzarella G, Costagliola C, Bianco A and Daniele A: New insight
into adiponectin role in obesity and obesity-related diseases.
Biomed Res Int. 2014(658913)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ding YS, Guo SX, Ma RL, Li SG, Guo H,
Zhang JY, Zhang M, Liu JM, He J, Yan YZ, et al: Association of
metabolic syndrome with the adiponectin to homeostasis model
assessment of insulin resistance ratio. Mediators Inflamm.
2015(607364)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huffman FG, Gomez GP and Zarini GG:
Metabolic syndrome and high-sensitivity C-reactive protein in
Cubans. Ethn Dis. 19:115–120. 2009.PubMed/NCBI
|
|
32
|
den Engelsen C, Koekkoek PS, Gorter KJ,
van den Donk M, Salomé PL and Rutten GE: High-sensitivity
C-reactive protein to detect metabolic syndrome in a centrally
obese population: A cross-sectional analysis. Cardiovasc Diabetol.
11(25)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Abdella NA, Mojiminiyi OA, Moussa MA, Zaki
M, Al Mohammedi H, Al Ozairi ES and Al Jebely S: Plasma leptin
concentration in patients with type 2 diabetes: Relationship to
cardiovascular disease risk factors and insulin resistance. Diabet
Med. 22:278–285. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
López-Jaramillo P, Gómez-Arbeláez D,
López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A
and Triana-Cubillos S: The role of leptin/adiponectin ratio in
metabolic syndrome and diabetes. Horm Mol Biol Clin Investig.
18:37–45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Takahashi M, Arita Y, Yamagata K,
Matsukawa Y, Okutomi K, Horie M, Shimomura I, Hotta K, Kuriyama H,
Kihara S, et al: Genomic structure and mutations in
adipose-specific gene, adiponectin. Int J Obes Relat Metab Disord.
24:861–868. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ilie MA, Caruntu C, Tampa M, Georgescu SR,
Matei C, Negrei C, Ion RM, Constantin C, Neagu M and Boda D:
Capsaicin: Physicochemical properties, cutaneous reactions and
potential applications in painful and inflammatory conditions. Exp
Ther Med. 18:916–925. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ghiţă MA, Căruntu C, Rosca AE, Căruntu A,
Moraru L, Constantin C, Neagu M and Boda D: Real-time investigation
of skin blood flow changes induced by topical capsaicin. Acta
Dermatovenerol Croat. 25:223–227. 2017.PubMed/NCBI
|
|
38
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinol (Copenh). 10:545–558.
2014.
|
|
39
|
Kriketos AD, Gan SK, Poynten AM, Furler
SM, Chisholm DJ and Campbell LV: Exercise increases adiponectin
levels and insulin sensitivity in humans. Diabetes Care.
27:629–630. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ohashi K, Kihara S, Ouchi N, Kumada M,
Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, et al:
Adiponectin replenishment ameliorates obesity-related hypertension.
Hypertension. 47:1108–1116. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Unamuno X, Izaguirre M, Gómez-Ambrosi J,
Rodríguez A, Ramírez B, Becerril S, Valentí V, Moncada R, Silva C,
Salvador J, et al: Increase of the Adiponectin/leptin ratio in
patients with obesity and type 2 diabetes after Roux-en-Y gastric
bypass. Nutrients. 11(2069)2019.PubMed/NCBI View Article : Google Scholar
|