Introduction
Acute heart failure is a fatal condition
characterized by heterogeneous clinical manifestations, and its
high incidence and mortality rates reportedly increase economic
burden for many countries worldwide (1,2). Due to
the rapid onset of acute heart failure and the progressive
deterioration of its signs and symptoms, patients with acute heart
failure require immediate treatment or emergency hospital admission
(3). Currently, a number of
treatment options, including vasodilation, diuresis, heart
strengthening and symptomatic treatment are available for acute
heart failure; however, the efficacy of these treatments are not
satisfactory (4). Therefore, the
identification of more effective treatment methods for acute heart
failure remains vital for clinical research.
In recent years, with accumulating and detailed
studies on the pathological mechanism underlying heart failure,
neuroendocrine factors have drawn much attention (5,6).
Recombinant human brain natriuretic peptide (RHBNP), which is a
B-type natriuretic peptide, secretes an endogenous polypeptide,
which is usually distributed in heart tissues and can effectively
improve hemodynamic function (7). In
addition, as a local multifunctional drug with spatial structure
and biological activity similar to those of endogenous brain
natriuretic peptides, RHBNP can regulate diuresis, promote the
diuresis and relaxation of bladder smooth muscles, improve heart
and kidney functions after cardiopulmonary bypass and accelerate
healing following myocardial injury (7,8). Sodium
nitroprusside (SN) is considered as the drug of choice for cardiac
surgery as its short half-life enables high efficiency and rapid
titration during and after cardiac surgery (9). However, SN administration needs to be
carefully monitored as it can dilate venous and arterial vessels
and reduce blood pressure without limitation (10). RHBNP and SN can be used to treat
patients with acute heart failure and cardiac insufficiency, but
only few studies have reported the effects of the combinational use
of these drugs on the quality of life and cardiac function in
patients with acute heart failure. Therefore, the present study
investigated the effects of the combination treatment of RHBNP and
SN on acute heart failure based on improvements in clinical
symptoms, thus aiming to provide a clinical basis for RHBNP
applications in patients with acute heart failure.
Materials and methods
General characteristics
A total of 96 patients with acute heart failure
admitted to The First Affiliated Hospital of Yangtze University
(Hubei, China) from February 2018 to May 2019 were included in the
current study. Among them, 48 patients were treated with RHBNP
combined with SN (research group) and 48 patients were treated with
SN alone (control group). The research group comprised 21 males and
27 females, with a mean age of 65.43±5.87 years (range, 44-76
years) and mean heart failure duration of 17.2±1.6 years (range,
9-24 years). According to the New York Heart Association (NYHA)
classification, 15 patients belonged to Class II, 20 patients to
Class III and 13 patients to Class IV (11). The control group comprised 25 males
and 23 females, with a mean age of 66.31±6.12 years (range, 43-78
years) and mean heart failure duration of 17.4±1.8 years (range,
19-22 years). Based on NYHA classification, 13 patients belonged to
Class II, 25 patients to Class III and 10 patients to Class IV.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Diagnosis of
acute heart failure before treatment (12); either sex; complete general clinical
data available; age ≥18 years; expected survival time of ≥1 year
and no history of chemotherapy or radiotherapy until the initial
diagnosis. The exclusion criteria were as follows: Presence of
serious diseases such as infection, severe hepatic and kidney
dysfunction, malignant tutor, autoimmune disease, myocarditis,
severe valvular heart disease and mental disease; family history of
psychosis; inability to cooperate during treatment; history of a
major surgery within 15 days before the treatment; dropping out
from the study or allergies to RHBNP and SN.
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Yangtze University.
All study subjects and their family members were informed of the
experiment and signed informed consent forms were obtained.
Treatment methods
Both study groups were administered routine therapy
involving vital sign monitoring, oxygen inhalation, diuresis,
digitalis and angiotensin-converting enzyme inhibitor and β-blocker
administration. Angiotensin-converting enzyme inhibitors,
angiotensin receptor blockers, and β-blockers were administered to
patients according to current best practice. The use of diuretics
was determined by the attending physician. Digitalis was prohibited
in the initial 24 h of symptom onset. In addition to routine
medication, the control group was administered SN injections (cat.
no. H20058959; Youcare Pharmaceutical Group Co., Ltd.) at an
initial rate of 5 µg/min and then at timely increments of 5 µg
every 5 min based on the initial dose according to the patients'
response; the course of treatment was 3-7 days. In addition to
routine medication and SN injections as administered in the control
group, the research group was administered 0.5 mg freeze-dried
RHBNP at an initial pump speed of 5 ml/min, which was adjusted on a
timely basis according to the patients' blood pressure; the course
of treatment was a period of three days.
Observation indices
The following observation indices were determined:
i) Fluid intake and 24 h urine volumes following drug use; ii)
Pulmonary capillary wedge pressure (PCWP) and left ventricular
ejection fraction (LVEF) before and three days after treatment
detected using a non-invasive cardiac hemodynamic detector
[Shanghai Meike Medical Instrument Co., Ltd.; Item number: Country
feeds medical inspect machinery (quasi) word 2008 No. 2211255];
iii) Cardiac function before and three months after treatment based
on NYHA classification (13), where
a lower score indicated better treatment efficacy and iv) Levels of
biochemical indicators before and 24 h after treatment. To measure
levels of biochemical indicators, fasting venous blood samples (5
ml) were obtained from the research group 1 day before treatment
and 24 h after drug use. The samples were centrifuged at 1,500 x g
at 4°C for 10 min. Serum high-sensitivity C-reactive
protein (hs-CRP) (Wuhan Vector Science Technology Co. Ltd.; cat.
no. ELA-E0821r) levels were determined using rate nephelometry.
Plasma N-terminal pro-brain/B-type natriuretic peptide (NT-proBNP)
(Shanghai Xiyuan Biotechnology Co. Ltd., cat. no. XY-RD191486200R)
and cardiac troponin I (cTnI) (Guangzhou Wondfo Biotech Co. Ltd.,
cat. no. A217) levels were determined using chemiluminescence ELISA
with magnetic bead isolation.
Efficacy assessment
Based on previously reported criteria (14), efficacy was assessed as a i) Marked
effect, defined as complete resolution of clinical symptoms,
improvement in cardiac function by at least two classes and
recovery of vital signs to normal; ii) Effect, defined as
alleviation of clinical symptoms, improvement in cardiac function
by at least one class and recovery of vital signs to normal and
iii) No effect, defined as the absence of improvement in vital
signs, clinical symptoms, and cardiac function. The total effective
rate was calculated using the following formula: (Number of
patients with marked effect + number of patients with effect)/total
number of patients x100%.
Follow-up
Following discharge, all patients were routinely
followed up and heart failure was treated with rest, blood glucose
control and basic medication such as angiotensin-converting enzyme
inhibitors, diuretics, oral nitrates and aspirin. After a period of
1 month, all patients were followed up via telephone interviews and
were inquired whether they developed recurrence of heart failure
and if so, those patients were re-admitted for treatment.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used to evaluate the data
and GraphPad Prism 5.0 (GraphPad Software, Inc.) was used to
generate figures. Intragroup enumeration data were expressed as
number and percentage [n (%)]. Intergroup comparison was analysed
using the χ2 test and variables with a theoretical
frequency of <5 in the χ2 test were analysed using
the continuity correction χ2 test. Measured data were
expressed as the mean ± standard deviation. Measured data between
research and control groups were compared using Student's t-test.
Measured data before and after treatment were compared within the
groups using a paired Student's t-test. Kaplan-Meier method was
used to plot the overall survival of patients with acute heart
failure in one month. P<0.05 was used to indicate a
statistically significant difference.
Results
General characteristics
No significant difference was observed between the
two groups regarding baseline demographic data, including sex, age,
heart failure duration, weight, residence place, nationality,
educational background, history of smoking and alcohol consumption,
diabetes, hypertension, disease type and NYHA classification. These
findings are summarized in Table
I.
 | Table IGeneral clinical data of patients in
research and control groups. |
Table I
General clinical data of patients in
research and control groups.
| Classification | Research group
(n=48) | Control group
(n=48) | t/χ2
value | P-value |
|---|
| Sex | | | 0.668 | 0.414 |
|
Male | 21 (43.75) | 25 (52.08) | | |
|
Female | 27 (56.25) | 23 (47.92) | | |
| Age (years) | 65.43±5.87 | 66.31±6.12 | 0.719 | 0.474 |
| Course (years) | 17.2±1.6 | 17.4±1.8 | 0.575 | 0.566 |
| Weight
(kg/cm2) | 25.9±3.9 | 26.3±4.2 | 0.484 | 0.629 |
| Residence place | | | 1.503 | 0.220 |
|
Urban
area | 22 (45.83) | 28 (58.33) | | |
|
Rural
area | 26 (54.17) | 20 (41.67) | | |
| Nationality | | | 0.178 | 0.673 |
|
Han | 29 (60.42) | 31 (64.58) | | |
|
Minority | 19 (39.58) | 17 (35.42) | | |
| Educational
background | | | 0.168 | 0.682 |
|
≥Senior high
school | 23 (47.92) | 21 (43.75) | | |
|
<Senior
high school | 25 (52.08) | 27 (56.25) | | |
| Smoking history | | | 0.464 | 0.496 |
|
Yes | 33 (68.75) | 36 (75.00) | | |
|
No | 15 (31.25) | 12 (25.00) | | |
| Alcohol consumption
history | | | 0.168 | 0.682 |
|
Yes | 25 (52.08) | 27 (56.25) | | |
|
No | 23 (47.92) | 21 (43.75) | | |
| Diabetes
history | | | 1.191 | 0.275 |
|
Yes | 35 (72.92) | 30 (62.50) | | |
|
No | 13 (27.08) | 18 (37.50) | | |
| Hypertension
history | | | 0.405 | 0.525 |
|
Yes | 29 (60.42) | 32 (66.67) | | |
|
No | 19 (39.58) | 16 (33.33) | | |
| Etiology of heart
failure | | | 0.749 | 0.945 |
|
After acute
myocardial infarction | 11 (22.92) | 12 (25.00) | | |
|
Ischemic
cardiomyopathy | 9 (18.75) | 7 (14.58) | | |
|
Valvular
heart disease | 8 (16.67) | 10 (20.83) | | |
|
Dilated
cardiomyopathy | 13 (27.08) | 11 (22.92) | | |
|
Perinatal
cardiomyopathy | 7 (14.58) | 8 (16.67) | | |
| NYHA
classification | | | 1.090 | 0.579 |
|
Class
II | 15 (31.25) | 13 (27.08) | | |
|
Class
III | 20 (41.67) | 25 (52.08) | | |
|
Class
IV | 13 (27.08) | 10 (20.83) | | |
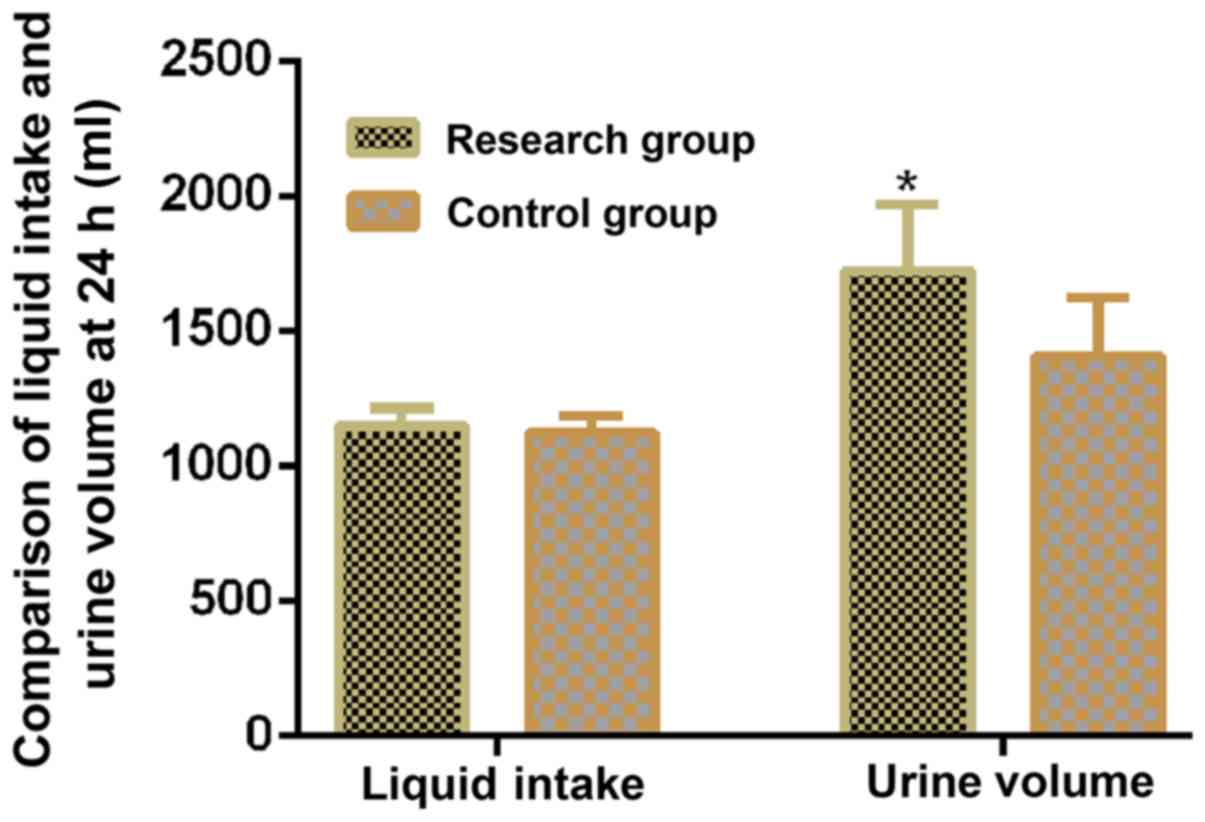
Comparison between research and
control groups in terms of fluid intake and 24 h urine volume after
drug use
The fluid intake and 24 h urine volume following
drug use were higher in the research group compared with the
control group (P<0.05; Table II;
Fig. 1).
 | Table IIComparison of the fluid intake and 24
h urine volumes after drug use between research and control
groups. |
Table II
Comparison of the fluid intake and 24
h urine volumes after drug use between research and control
groups.
| Group | Number of
cases | Fluid intake volume
(ml) | Urine volume
(ml) |
|---|
| Research group | 48 |
1,148.69±65.24a |
1,721.47±246.24a |
| Control group | 48 | 1,121.23±62.57 |
1,405.69±216.74 |
| t-value | | 2.105 | 6.669 |
| P-value | | 0.038 | <0.001 |
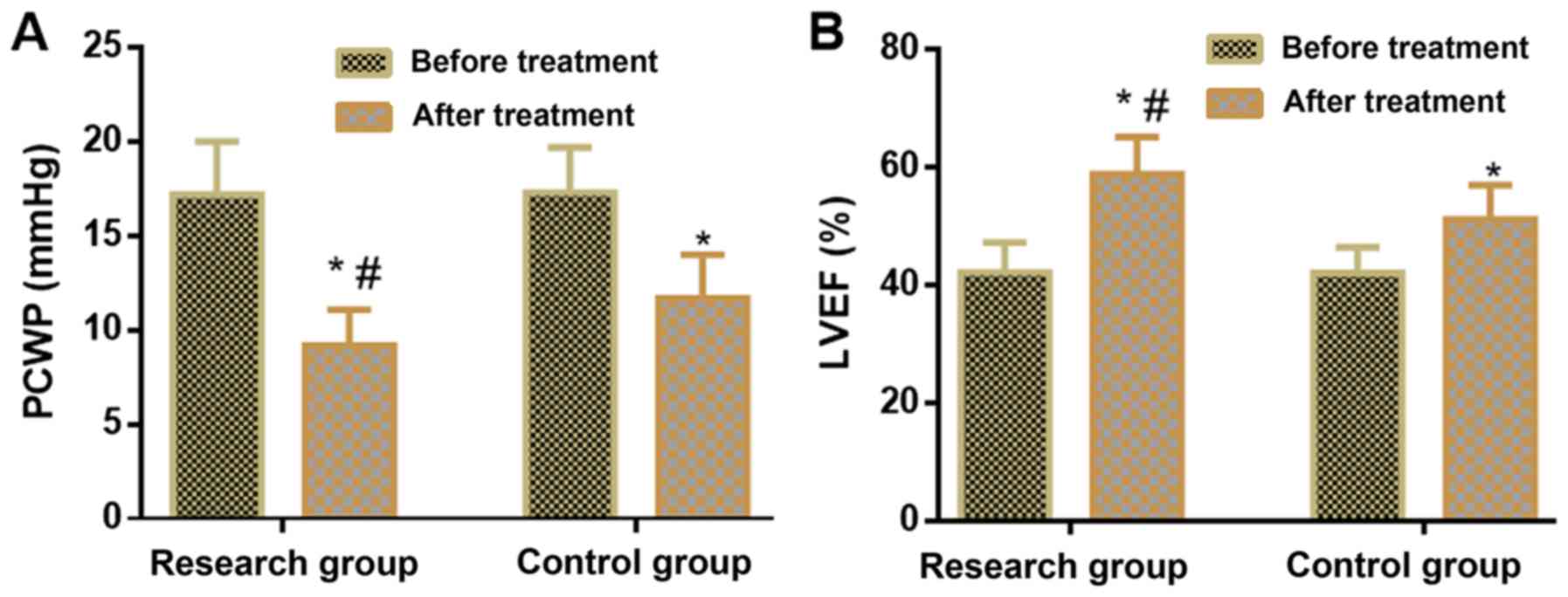
Comparison between research and
control groups in terms of cardiac function indices before and
after treatment
No significant difference was observed between the
two groups in terms of cardiac function indices before treatment.
Following treatment, PCWP levels were lower and LVEF levels were
higher in the research group compared with the control group
(P<0.05; Table III; Fig. 2).
 | Table IIIComparison of cardiac function
indices before and after treatment between research and control
groups. |
Table III
Comparison of cardiac function
indices before and after treatment between research and control
groups.
| | PCWP (mmHg) | LVEF (%) |
|---|
| Group | Number of
cases | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| Research group | 48 | 17.2±2.8 |
9.2±1.9a,b | 42.2±5.1 |
58.9±6.3a,b |
| Control group | 48 | 17.3±2.4 |
11.7±2.3a | 42.1±4.4 |
51.2±5.8a |
| t-value | | 0.188 | 5.806 | 0.103 | 6.230 |
| P-value | | 0.851 | <0.001 | 0.918 | <0.001 |
Comparison between research and
control groups in terms of cardiac function classification after
treatment
No significant difference was observed between the
two groups in terms of cardiac function classification before
treatment. Following treatment, compared with the control group,
the research group had more patients belonging to NYHA Class I and
Class II and less patients belonging to NYHA Class III and Class IV
(both, P<0.05; Table IV).
 | Table IVComparison of cardiac function
classification before and after treatment between research and
control groups. |
Table IV
Comparison of cardiac function
classification before and after treatment between research and
control groups.
| | Class I | Class II | Class III | Class IV |
|---|
| Group | n | Before
treatment | After
treatment | Before
treatment | After
treatment | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| Research group | 48 | 0 | 25
(52.08)a | 0 | 18
(37.50)a | 29 (60.42) | 4
(8.33)a | 19 (39.58) | 1
(2.08)a |
| Control group | 48 | 0 | 15 (31.25) | 0 | 9 (18.75) | 31 (64.58) | 18 (37.50) | 17 (35.42) | 6 (12.50) |
| t-value | | - | 4.286 | - | 4.174 | 0.178 | 11.561 | 0.178 | 3.852 |
| P-value | | - | 0.038 | - | 0.041 | 0.673 | 0.001 | 0.673 | 0.049 |
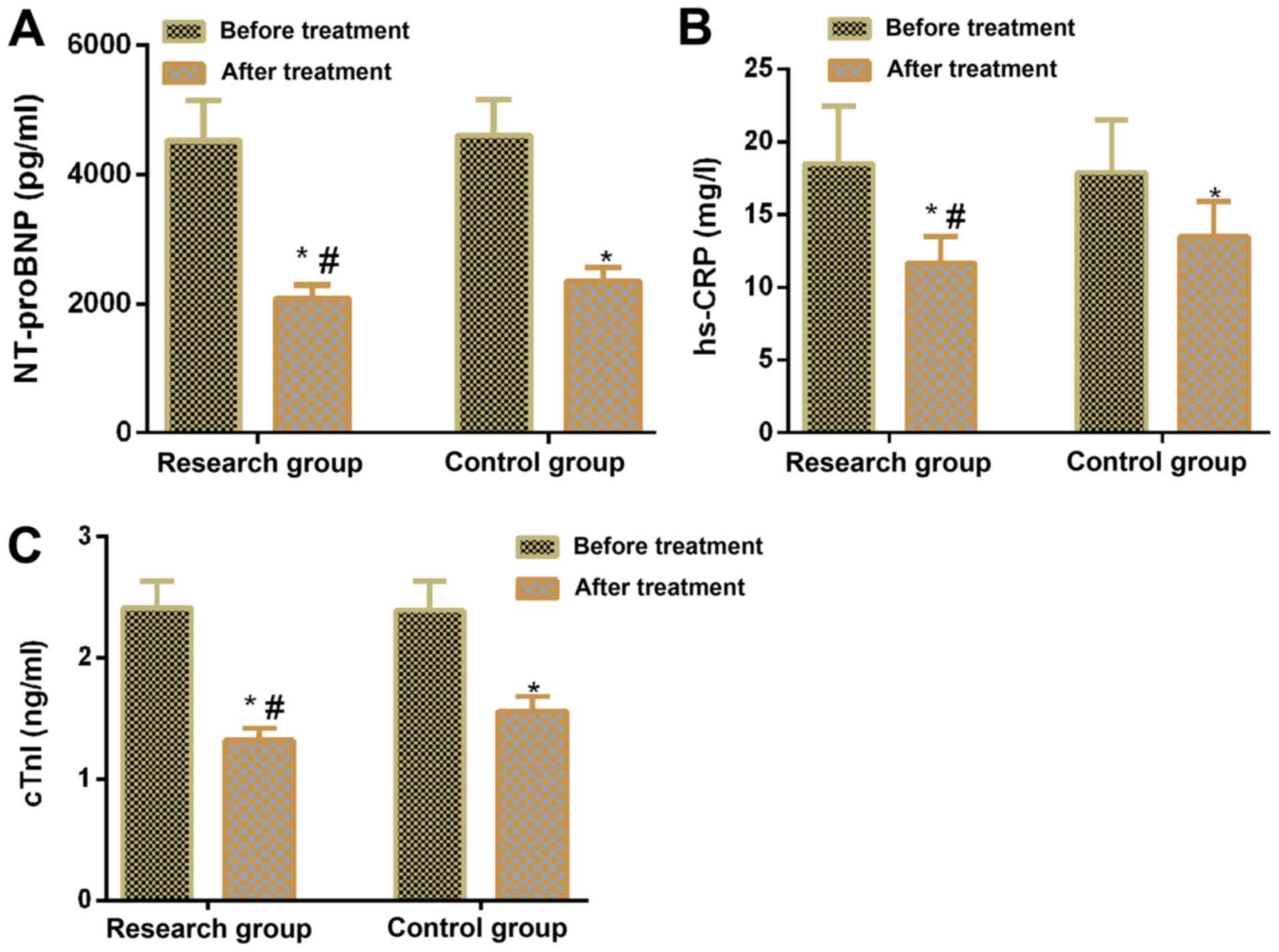
Comparison between research and
control groups in terms of biochemical indicator levels before and
after treatment
No significant difference was observed between the
two groups in terms of NT-proBNP, hs-CRP and cTnI levels before
treatment. However, following treatment, these levels decreased in
both groups compared with before treatment (P<0.05) and
NT-proBNP, hs-CRP, and cTnI levels were lower in the research group
compared with the control group (P<0.05; Table V; Fig.
3).
 | Table VComparison of biochemical indicator
levels before and after treatment between research and control
groups. |
Table V
Comparison of biochemical indicator
levels before and after treatment between research and control
groups.
| | NT-proBNP
(pg/ml) | hs-CRP (mg/l) | cTnI (ng/ml) |
|---|
| Group | n | Before
treatment | After
treatment | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| Research group | 48 | 4,521±625 |
2,083±204a,b | 18.51±3.93 |
11.65±1.82a,b | 2.41±0.22 |
1.32±0.10a,b |
| Control group | 48 | 4,606±549 | 2,348±
217a | 17.8± 3.61 |
13.47±2.43a | 2.39±0.24 |
1.56±0.12a |
| t-value | | 0.708 | 6.164 | 0.818 | 4.153 | 0.426 | 10.640 |
| P-value | | 0.481 | <0.001 | 0.416 | <0.001 | 0.671 | <0.001 |
Comparison between research and
control groups in terms of treatment efficacy
The research group showed a total effective rate of
97.92%, with marked effects in 30 patients (62.50%), effects in 17
patients (35.42%) and no effect in 1 patient (2.08%). The control
group showed a total effective rate of 81.25%, with marked effects
in 20 patients (41.67%), effects in 19 patients (39.58%) and no
effects in 9 patients (18.75%). The total effective rate of
treatment was higher in the research group compared with the
control group (P<0.05; Table
VI).
 | Table VIComparison of adverse reactions after
treatment between research and control groups. |
Table VI
Comparison of adverse reactions after
treatment between research and control groups.
| Efficacy | Research group
(n=48) | Control group
(n=48) | χ2
value | P-value |
|---|
| Marked effect | 30 (62.50) | 20 (41.67) | - | - |
| Effect | 17 (35.42) | 19 (39.58) | - | - |
| No effect | 1 (2.08) | 9 (18.75) | - | - |
| Total effective
rate | 47 (97.92) | 39 (81.25) | 7.144 | 0.001 |
Comparison between research and
control groups in terms of adverse reactions following
treatment
Adverse reactions after treatment are listed in
Table VII. The total incidence of
adverse reactions in the research group was 10.42%, including
headache in 2 patients (4.17%), hypotension in 1 patient (2.08%)
and rashes in 2 patients (4.17%); bradycardia was not observed in
any patient. The total incidence of adverse reactions in the
control group was 27.08%, including headache in 4 patients (8.33%),
hypotension in 5 patients (10.42%), rashes in 3 patients (6.25%)
and bradycardia in 1 patient (2.08%), indicating that the total
incidence of adverse reactions was lower in the research group
compared with the control group (P<0.05; Table VII).
 | Table VIIComparison of the incidence of
adverse reactions after treatment between research and control
groups. |
Table VII
Comparison of the incidence of
adverse reactions after treatment between research and control
groups.
| Category | Research group
(n=48) | Control group
(n=48) | χ2
value | P-value |
|---|
| Headache | 2 (4.17) | 4 (8.33) | 0.711 | 0.399 |
| Hypotension | 1 (2.08) | 5 (10.42) | 2.844 | 0.092 |
| Rash | 2 (4.17) | 3 (6.25) | 0.211 | 0.646 |
| Bradycardia | 0 (0.00) | 1 (2.08) | 1.011 | 0.315 |
| Total incidence of
adverse reactions | 5 (10.42) | 13 (27.08) | 4.376 | 0.036 |
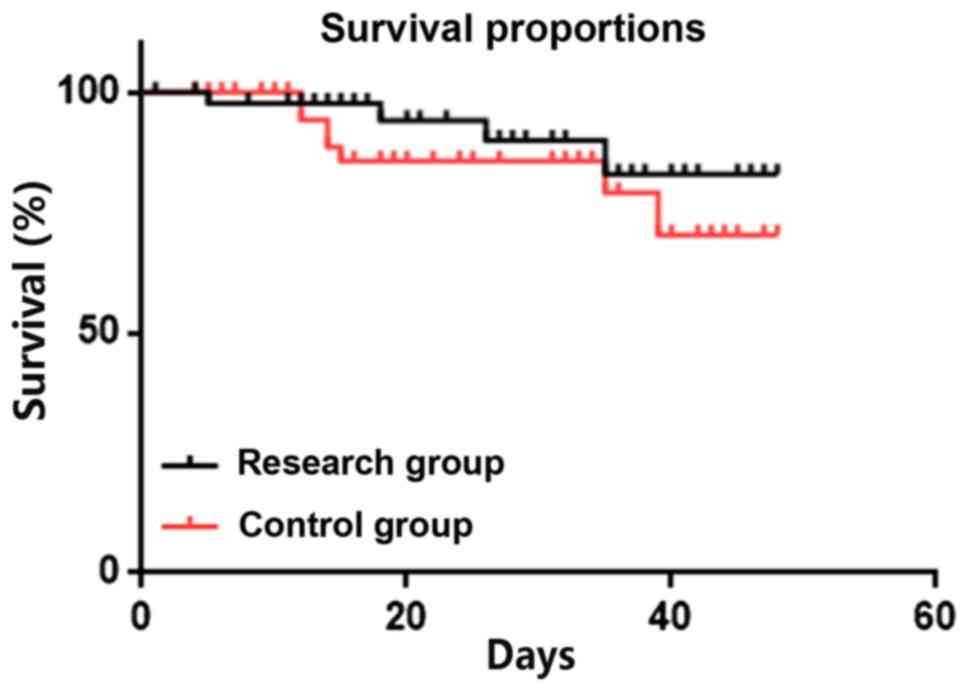
Comparison between research and
control groups in terms of mortality within 1 month of
treatment
No significant difference was observed between the
two groups in terms of mortality within 1 month of treatment:
Research group, 4 deaths (8.33%) and control group, 9 deaths
(18.75%; Fig. 4).
Discussion
Heart failure is the leading cause of morbidity and
mortality in developed countries, with increasing incidence
(15). As one of the common causes
of hospitalization, acute heart failure is a symptom, which
indicates acute or subacute cardiac function deterioration owing to
several possible underlying heart diseases and stimulating factors
(16). Primary clinical symptoms of
acute heart failure include hyperemia, cardiogenic shock or
peripheral hypoperfusion, subsequently causing organ injury and
failure (17,18). Therefore, clinicians are facing
increasing pressure regarding the treatment of acute heart failure,
shortening hospital stays and preventing urgent treatment,
re-hospitalization and short-term death after discharge (19). Therefore, a novel and safe treatment
is required to alleviate acute heart failure.
RHBNP can promote hemangiectasis, diuresis and
excretion, reduce cardiac load and preload, retrieve dynamic
disturbance in patients with heart failure, inhibit neuroendocrine
activation and improve ventricular remodelling (20). The perioperative administration of
RHBNP can improve prognosis, shorten hospitalization time and
decrease postoperative mortality in patients who have undergone
cardiac surgery (21). SN is used to
treat hypertensive emergency cases due to its ability to rapidly
release nitric oxide and reduce blood pressure (22). As a vasodilator, it has the
advantages of being cost-effective, useful, rapid and safe in
pulmonary hypertension (23). Wei
et al (24) demonstrated the
advantages of RHBNP over SN in terms of short-term treatment
efficacy for acute heart failure and reported that RHBNP can
improve hemodynamics and cardiac function, decrease inflammatory
cytokine levels and upregulate inflammatory cytokines. In another
study, Guiha et al (25)
reported that SN could effectively treat refractory heart failure
by reducing the impedance to left ventricular ejection.
Furthermore, Mullens et al (26) demonstrated that RHBNP combined with
SN could effectively optimize inflammatory cytokine levels and
improve cardiac function and hemodynamics. Inflammation is a
well-known feature of heart failure (27), and studies have shown that the
increase of serum hs-CRP, NT-proBNP and cTnI in patients can
increase the risk of heart failure (28). The results of the present study
indicated that compared with SN alone, the combination of RHBNP and
SN can improve the urine volume of the patients, which is more
effective for diuresis, such that it reduces pulmonary artery
pressure, decreases cardiac preload and afterload, improves cardiac
function classification, promotes the recovery of patients,
inhibits the expression of inflammatory cytokines, exerts
anti-inflammatory effects, accelerates healing after myocardial
injury, rapidly improves dyspnea, and subsequently relieves
clinical symptoms. In the present study, the combination treatment
had a significantly lower incidence rate of adverse reactions and
similar mortality rate within 1 month of treatment. Notably, the
similar baseline clinical data between the study groups confirmed
the rigor and reliability of the current study.
In the present study, the clinical indicators,
adverse reactions and short-term follow-up of the two groups of
patients after treatment were compared, which confirmed that RHBNP
combined with SN was more effective than SN alone in the treatment
of acute heart failure. The novelty of the present study was to
observe changes in fluid intake volume at 24 h after treatment and
to follow up the one-month survival rate. The changed records of
fluid intake volume of patients at 24 h confirmed that the
combination of the two methods could improve the urine volume and
exhibited an improved diuretic effect. However, the present study
did not evaluate quality of life of the patients. This needs to be
assessed in future studies to further corroborate the results of
the present study.
In conclusion, compared with SN alone, RHBNP
combined with SN is more effective in the treatment of acute heart
failure, such that it can effectively promote urination, reduce
inflammatory response and rapidly improve clinical symptoms without
significant adverse reactions. This may be due to the synergistic
effects of RHBNP and SN, indicating its potential use in further
clinical applications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP drafted the manuscript. HW reviewed the
manuscript. YP and HW designed the study, collected the data and
performed statistical analysis. YP and HW also read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Yangtze University.
All research subjects and their family members were informed of the
experiment and signed informed consent forms were obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mebazaa A, Yilmaz MB, Levy P, Ponikowski
P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP,
et al: Recommendations on pre-hospital & early hospital
management of acute heart failure: A consensus paper from the Heart
Failure Association of the European Society of Cardiology, the
European Society of Emergency Medicine and the Society of Academic
Emergency Medicine. Eur J Heart Fail. 17:544–558. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Matsue Y, Damman K, Voors AA, Kagiyama N,
Yamaguchi T, Kuroda S, Okumura T, Kida K, Mizuno A, Oishi S, et al:
Time-to-Furosemide treatment and mortality in patients hospitalized
with acute heart failure. J Am Coll Cardiol. 69:3042–3051.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chioncel O, Mebazaa A, Harjola VP, Coats
AJ, Piepoli MF, Crespo-Leiro MG, Laroche C, Seferovic PM, Anker SD,
Ferrari R, et al: Clinical phenotypes and outcome of patients
hospitalized for acute heart failure: The ESC Heart Failure
Long-term registry. Eur J Heart Fail. 19:1242–1254. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Maaten JMT, Valente MAE, Damman K, Hillege
HL, Navis G and Voors AA: Diuretic response in acute heart
failure[mdash]pathophysiology, evaluation, and therapy. Nat Rev
Cardiol. 12(184)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu H, Wang B, Meng Q, Li J, Sun W, Xin L
and Chen L: Effectiveness and safety of recombinant human brain
natriuretic peptide in the treatment of acute myocardial infarction
in elderly in combination with cardiac failure. Pak J Med Sci.
33:540–544. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yu L, Shi X, Han C, Rao C and Wang J: A
rapid reporter assay for recombinant human brain natriuretic
peptide (rhBNP) by GloSensor technology. J Pharm Anal. 8:297–301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang S and Wang Z: Effect of recombinant
human brain natriuretic peptide (rhBNP) versus nitroglycerin in
patients with heart failure: A systematic review and meta-analysis.
Medicine (Baltimore). 95(e4757)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Su Z, Wei G, Wei L, Liu J and Li X:
Effects of rhBNP on myocardial fibrosis after myocardial infarction
in rats. Int J Clin Exp Pathol. 8:6407–6415. 2015.PubMed/NCBI
|
|
9
|
Cruz JE, Thomas Z, Lee D, Moskowitz DM and
Nemeth J: Therapeutic interchange of clevidipine for sodium
nitroprusside in cardiac surgery. P T. 41:635–639. 2016.PubMed/NCBI
|
|
10
|
Olesen ND, Fischer M and Secher NH: Sodium
nitroprusside dilates cerebral vessels and enhances internal
carotid artery flow in young men. J Physiol. 596:3967–3976.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Fisher JD: New York Heart Association
classification. Arch Intern Med. 129(836)1972.PubMed/NCBI
|
|
12
|
Chiem AT, Chan CH, Ander DS, Kobylivker AN
and Manson WC: Comparison of expert and novice sonographers'
performance in focused lung ultrasonography in dyspnea (FLUID) to
diagnose patients with acute heart failure syndrome. Acad Emerg
Med. 22:564–573. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bredy C, Ministeri M, Kempny A,
Alonso-Gonzalez R, Swan L, Uebing A, Diller GP, Gatzoulis MA and
Dimopoulos K: New York Heart Association (NYHA) classification in
adults with congenital heart disease: Relation to objective
measures of exercise and outcome. Eur Heart J Qual Care Clin
Outcomes. 4:51–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kaski JC: Cardiac Syndrome X and
Microvascular Angina, 1999.
|
|
15
|
Ziaeian B and Fonarow GC: Epidemiology and
aetiology of heart failure. Nat Rev Cardiol. 13:368–378.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sinnenberg L and Givertz MM: Acute heart
failure. Trends Cardiovasc Med. 30:104–112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Harjola VP, Mullens W, Banaszewski M,
Bauersachs J, Brunner-La Rocca HP, Chioncel O, Collins SP, Doehner
W, Filippatos GS, Flammer AJ, et al: Organ dysfunction, injury and
failure in acute heart failure: From pathophysiology to diagnosis
and management. A review on behalf of the Acute Heart Failure
Committee of the Heart Failure Association (HFA) of the European
Society of Cardiology (ESC). Eur J Heart Fail. 19:821–836.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Kastl SP, Krychtiuk KA, Lenz M,
Distelmaier K, Goliasch G, Huber K, Wojta J, Heinz G and Speidl WS:
Intestinal fatty acid binding protein is associated with mortality
in patients with acute heart failure or cardiogenic shock. Shock.
51:410–415. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Harjola VP, Parissis J, Brunner-La Rocca
HP, Čelutkienė J, Chioncel O, Collins SP, De Backer D, Filippatos
GS, Gayat E, Hill L, et al: Comprehensive in-hospital monitoring in
acute heart failure: Applications for clinical practice and future
directions for research. A statement from the Acute Heart Failure
Committee of the Heart Failure Association (HFA) of the European
Society of Cardiology (ESC). Eur J Heart Fail. 20:1081–1099.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He XM, Chen L, Luo JB, Feng XX, Zhang YB,
Chen QJ, Ji XL and Wang TS: Effects of rhBNP after PCI on
non-invasive hemodynamic in acute myocardial infarction patients
with left heart failure. Asian Pac J Trop Med. 9:791–795.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hua P, Liu J, Tao J, Lin X, Zou R, Zhang D
and Yang S: Safety and efficacy of the perioperative administration
of recombinant human brain natriuretic peptide (rhBNP): A
systematic review and meta-analysis. Ther Clin Risk Manag.
14:313–321. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Farooq A, Whitehead D and Azzawi M:
Attenuation of endothelial-dependent vasodilator responses, induced
by dye-encapsulated silica nanoparticles, in aortic vessels.
Nanomedicine (Lond). 9:413–425. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fida TT, Voordouw J, Ataeian M, Kleiner M,
Okpala G, Mand J and Voordouw G: Synergy of sodium nitroprusside
and nitrate in inhibiting the activity of sulfate reducing bacteria
in Oil-containing bioreactors. Front Microbiol.
9(981)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wei X, Yan T, Dai H and Wei Z: Clinical
efficacy of rhBNP in treating AHF and its effect on hemodynamics
and inflammatory factors. Chongqing Med. 27:2017.(In Chinese).
|
|
25
|
Guiha NH, Cohn JN, Mikulic E, Franciosa JA
and Limas CJ: Treatment of refractory heart failure with infusion
of nitroprusside. N Engl J Med. 291:587–592. 1974.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mullens W, Abrahams Z, Francis GS, Skouri
HN, Starling RC, Young JB, Taylor DO and Tang WH: Sodium
nitroprusside for advanced low-output heart failure. J Am Coll
Cardiol. 52:200–207. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Strassheim D, Dempsey EC, Gerasimovskaya
E, Stenmark K and Karoor V: Role of inflammatory cell subtypes in
heart failure. J Immunol Res. 2019:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Apple FS, Steffen LM, Pearce LA, Murakami
MM and Luepker RV: Increased cardiac troponin I as measured by a
high-sensitivity assay is associated with high odds of
cardiovascular death: The Minnesota Heart Survey. Clin Chem.
58:930–935. 2012.PubMed/NCBI View Article : Google Scholar
|