Introduction
Limbic encephalitis (LE) is characterized by
autoimmune inflammation of structures of the limbic system. In the
clinic, patients with LE present with mesial temporal lobe
epilepsy, memory disturbance and neuropsychiatric symptoms
(1-3).
LE occurs in paraneoplastic and non-paraneoplastic settings
(2). Furthermore, LE with
autoantibodies against synaptic antigens includes leucine-rich
glioma inactivated protein 1 (LGI1),
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptor, metabotropic glutamate receptor 5 and γ-aminobutyric acid
B (GABAB) receptors (1-3).
Among these autoantibodies associated with LE, the autoantibody to
GABAB receptor was first described by Lancaster et al
(4).
Anti-GABAB receptor encephalitis is a relatively
rare disease: Only 100 cases have been reported in the literature
since 2010 (4-9).
In addition, ~1/2 of patients with antibodies against GABAB
receptor encephalitis have small cell lung cancer (SCLC). The
majority of patients exhibit neurological improvement after
immunotherapy and tumor therapy (4-9).
To date, only a small number of cases of positivity
for antibodies against GABAB have been reported in the Asian
population (7-9).
Therefore, the present study investigated the clinical
manifestations, therapy and outcomes in Chinese patients with GABAB
receptor antibodies.
Materials and methods
Patients and methods
In total, 12 patients diagnosed with anti-GABAB
receptor encephalitis at Qilu Hospital of Shandong University
(Jinan, China) between August 2015 and December 2018 were included
in the study. This study was approved by the Ethics Committee of
Qilu Hospital of Shandong University (Jinan, China; no.
KYLL-2017-550). Written informed consent was obtained from each
patient or a relative serving as a legal representative. The
diagnostic criteria were based on characteristic neurological
syndromes suspected to be autoimmune-associated and the detection
of specific GABAB receptor antibodies, as previously reported
(10,11). All neurological syndromes, including
LE and other neuropsychiatric manifestations, including ataxia,
opsoclonus-myoclonus syndrome and brainstem encephalitis, were
considered during patient selection. Detailed information,
including clinical symptoms and results of laboratory examinations,
cerebrospinal fluid (CSF) assay, electroencephalogram (EEG),
radiologic examination (CT and MRI), as well as therapies and
outcome information, were obtained.
Detection of autoimmune
antibodies
Cell-based indirect immunofluorescence tests were
used to detect the following autoantibodies:
Anti-N-methyl-D-aspartate receptor, anti-GABAB receptor, anti-AMPA
receptor, anti-LGI1 and anti-contactin-associated protein-like 2,
and paraneoplastic antibodies anti-Yo (anti-Purkinje cell
antibody), anti-Hu (anti-neuronal nuclear antibody 1), anti-Ri
(anti-neuronal nuclear antibody 2), anti-CV2 (collapsin response
mediator protein 5), anti-amphiphysin in serum and CSF samples
(Euroimmun AG; cat. nos. FA 112d-1, FA 1111-1). Diluted patient
samples were reacted with 293 cells (Euroimmun AG) transfected with
plasmids containing human target gene sequences, and FITC-labeled
anti-human immunoglobulin (Ig)G (cat. no. ab97224; 1:500 dilution;
Abcam) was used as the secondary antibody (8). Positive and negative reactions were
determined based on the intensity of cytoplasmic immunofluorescence
compared with positive and negative controls under a fluorescence
microscope(Olympus IX-70; Olympus Corporation).
Immunohistochemical staining
Anti-GABAB receptor in the tumor tissues were
evaluated by immunohistochemical staining with specific antibodies.
After deparaffinization in xylene and graded alcohol
concentrations, endogenous peroxidase was blocked in 0.3% hydrogen
peroxide. Non-specific binding was blocked by incubation in 10%
bovine serum albumin (Sigma Aldrich; Merck KGaA). Sections were
incubated with primary polyclonal antibody against human GABAB
receptor (cat. no. sc-393270; 1:200 dilution; Santa Cruz
Biotechnology, Inc.). A horseradish peroxidase-labeled secondary
antibody (cat. no. sc-2005; 1:500 dilution; Santa Cruz
Biotechnology, Inc.) was then added. The slides were stained with
diaminobenzidine and then counterstained with hematoxylin. The
stained slides were dehydrated and observed under a microscope. In
total, lung cancer tissues from three patients were stained. The
lung cancer tissues from one other patient who had SCLC without the
manifestations of anti-GABAB receptor encephalitis was also stained
and used as a control.
Treatment and follow-up
Patients received antiepileptic drug therapy,
immunotherapy and tumor therapy when required. The therapeutic
effects were assessed using the modified Rankin Scale (mRS)
(12).
Results
Clinical manifestations
In total, nine patients were male (75%) and three
were female (25%). The age of symptom onset ranged from 54 to 74
years (median, 65.1 years). The time of symptom onset to diagnosis
was from 1 to 36 weeks (median, 6.9 weeks). Seizures occurred in
all 12 patients and nine (75%) patients presented with seizures as
the initial symptoms. Furthermore, three patients developed status
epilepticus prior to the treatments. No seizure was recorded during
EEG exams. The seizure frequency prior to treatment ranged from 2
to 15 times per week (median, 4.2 times per week). Furthermore,
memory deficits and psychiatric symptoms (including behavioral,
mood and personality changes) were documented in 11 (91.6%) and
seven (58.3%) patients, respectively. Awareness impairment was
observed in four patients (33.3%) (Table
I). However, none of the patients had the clinical
manifestations of ataxia, opsoclonus-myoclonus syndrome and
brainstem encephalitis.
 | Table IClinical manifestations of
encephalitis patients with anti-GABABR antibody. |
Table I
Clinical manifestations of
encephalitis patients with anti-GABABR antibody.
| | Case1 | Case2 | Case3 | Case4 | Case5 | Case6 | Case7 | Case8 | Case9 | Case10 | Case11 | Case12 |
|---|
| Sex | M | M | M | M | M | F | F | M | M | M | M | F |
| Age (years) | 62 | 70 | 64 | 54 | 66 | 62 | 69 | 74 | 67 | 65 | 69 | 60 |
| TOSD (weeks) | 8 | 3 | 2 | 2 | 7 | 2 | 8 | 9 | 2 | 3 | 1 | 36 |
| Psychiatric
symptoms | - | + | + | + | + | - | + | + | - | - | + | - |
| Memory deficits | + | + | + | + | + | + | - | + | + | + | + | + |
| Awareness
impairment | + | + | + | - | - | - | - | - | - | - | - | + |
| Seizures | + | + | + | + | + | + | + | + | + | + | + | + |
| Status
epilepticus | + | - | - | + | - | - | - | - | - | - | + | - |
| Seizure frequency
prior to therapy (times/week) | 3 | 5 | 4 | 15 | 3 | 3 | 2 | 3 | 4 | 2 | 3 | 3 |
| Anti-GABABR antibody
(grading), serum/CSF | +/+++ | ++/+++ | +++/+++ | ++/++ | ++/++ | ++/+ | +++/++ | ++/+ | ++/++ | ++/+ | ++/+ | +++/++ |
| Anti-GABABR antibody
(grading), lung cancer tissue | + | ND | ND | ND | ND | + | ND | ND | ND | ND | ND | ND |
| Other positive
autoimmune antibodies | TG;TPO | - | - | - | - | - | TG;TPO | - | - | - | Hu | Hu; TG; TPO |
| Serum sodium
(mmol/l) | 144 | 138 | 123 | 140 | 146 | 142 | 141 | 142 | 147 | 139 | 135 | 140 |
| CSF WC
(/mm3; RR:0-6) | 2 | 130 | 4 | 62 | 2 | 6 | 4 | 1 | 34 | 1 | 6 | 4 |
| CSF protein (g/l;
RR:0-0.45) | 0.74 | 0.84 | 0.26 | 0.24 | 0.33 | 0.35 | 0.70 | 0.45 | 0.42 | 0.44 | 0.62 | 0.42 |
| CSF OB | + | - | - | - | ND | - | - | ND | - | + | - | + |
| Lung tumor (CT
scan) | + | + | - | + | + | + | - | - | - | + | - | + |
| Tumor tissue
pathology | Aden | ND | - | ND | ND | SCLC | - | ND | ND | - | ND | SCLC |
| Brain MRI limbic
lobes abnormality | + | - | + | - | + | + | - | - | - | - | - | - |
| Cortical
atrophy | + | - | - | - | - | + | - | - | - | - | - | - |
| EEG generalized
slow waves | + | + | + | ND | + | - | + | + | + | + | + | + |
| Epileptic
waves | - | - | + | ND | + | - | - | + | + | - | + | + |
| Immunotherapy
drugs | IVIg+Dex | Mpd | IVIg+Dex | IVIg+Dex | Mpd | IVIg | IVIg+Dex | IVIg+Mpd | IVIg+Mpd | IVIg | IVIg+Dex | IVIg |
| Anti-epileptic
drugs | LEV | LEV | LEV+VPA | LEV | OXA+LEV | LEV | LEV | LEV+VPA | LEV+VPA | VPA | LEV+VAP | LEV+TOP |
| Tumor treatment
mRS | Yes | No | ND | No | No | Yes | ND | ND | ND | No | No | No |
| (before/after
treatments) | 4/2 | 4/4 | 4/2 | 3/3 | 3/3 | 3/2 | 3/1 | 3/2 | 3/1 | 3/3 | 3/2 | 4/4 |
| Follow-up duration
(Months) | 30 | 6 | 18 | 4 | 5 | 8 | 18 | 6 | 20 | 10 | 8 | 3 |
| Follow-up
results | No relapse | Died | No relapse | Died | Died | Died | No relapse | No relapse | No relapse | Died | Died | Died |
Diagnostic examinations
No changes in the routine hematological and
biochemical examinations among patients were identified. Only two
patients (16.7%) presented with mild hyponatremia (<137 mmol/l).
Antibodies against the GABAB receptor were detected in serum and
CSF samples of all patients and titers ranged from 1:1 to 1:100. In
addition, patients were screened for paraneoplastic antibodies,
revealing that two patients (16.7%) had positive Hu antibodies in
their serum and CSF. Furthermore, anti-thyroglobulin and
anti-thyroperoxidase antibodies were detected in the serum samples
of three patients (25%).
All patients underwent CSF analysis. It was
demonstrated that white blood cells were elevated (>6
cells/mm3) in three cases (25%; range,
1-130/mm3). Furthermore, the protein concentration of
CSF was elevated in four patients (33.3%; >0.45 g/l; range,
0.24-0.84 g/l). In total, three out of the 10 patients tested for
CSF oligoclonal bands had positive results (30%; Table I).
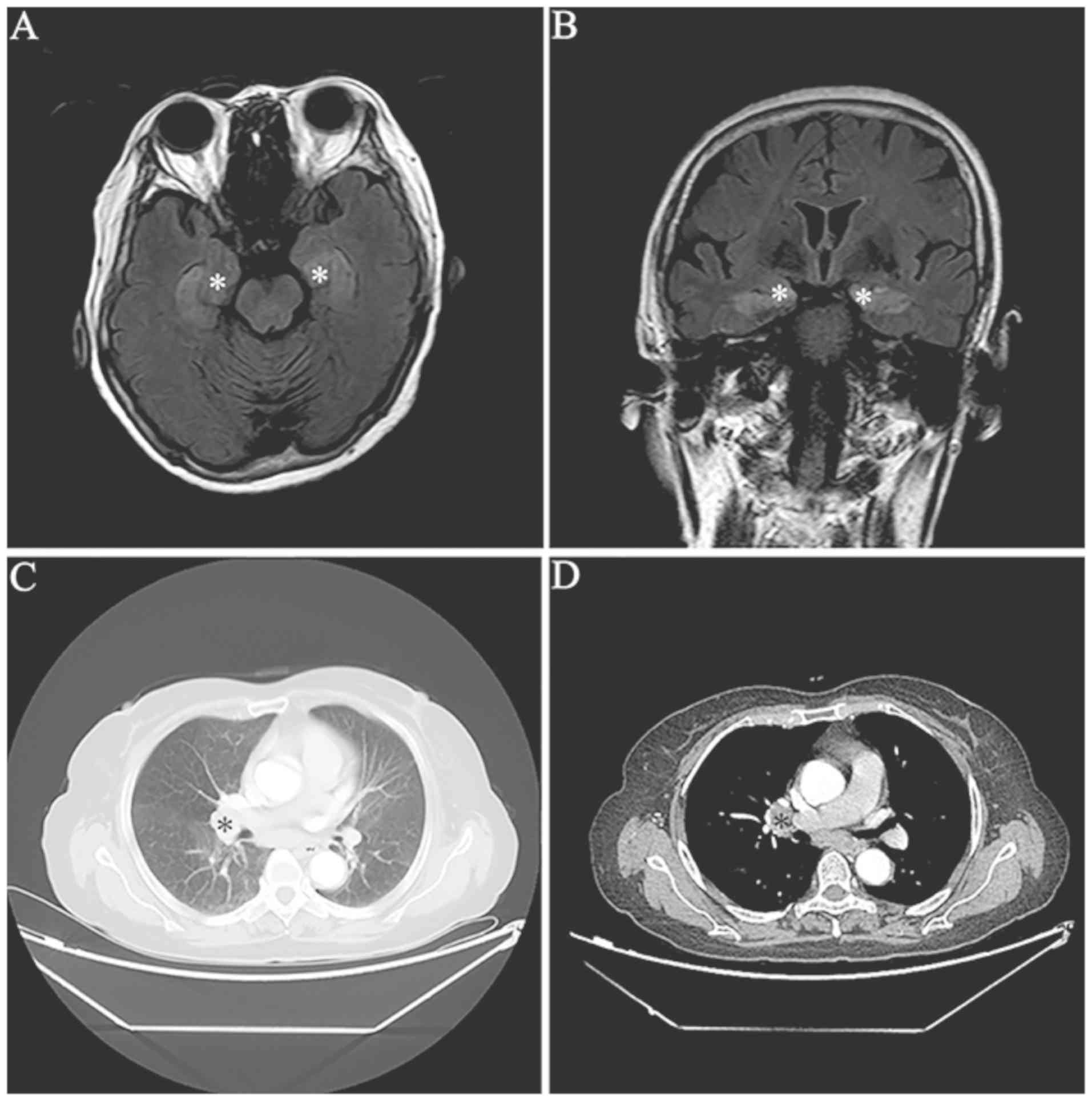
MRI scans demonstrated abnormalities in mesial
temporal regions on T2-weighted and fluid-attenuated inversion
recovery MRI sequences in four patients (33.3%) (Fig. 1). Furthermore, three patients
exhibited bilateral abnormalities and one patient had unilateral
abnormalities. It was identified that two patients (16.7%) had
diffused cortical atrophy. In addition, EEG examination results
were available for 11 patients. It was demonstrated that there were
temporal lobe epileptic activities in six patients (6/11; 54.5%)
and general slow waves in 10 patients (10/11; 90.9%; Table I).
All patients received tumor screening by CT scans.
Lung cancer was detected in seven patients (58.3%) (Fig. 1). Furthermore, tissue pathology exams
indicated that two patients had SCLC and one patient had
neuroendocrine adenocarcinoma (Table
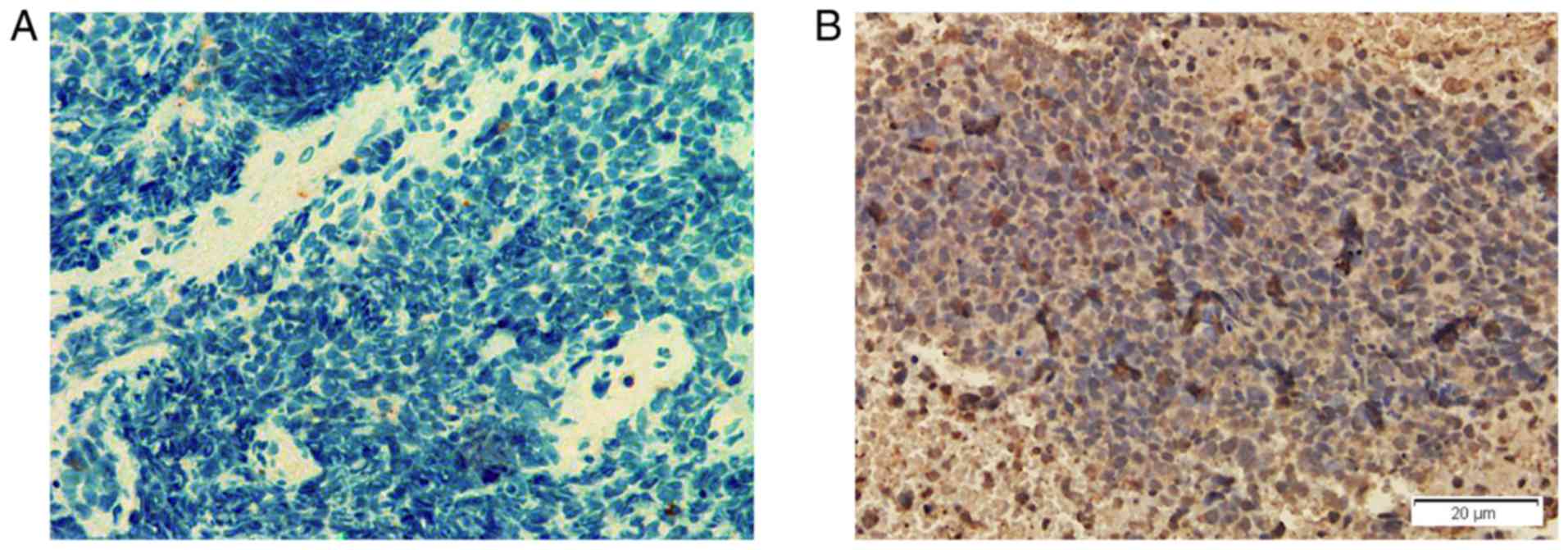
I). It was revealed that the lung cancer tissues of these three
patients were positively stained for anti-GABAB receptors by
immunohistochemistry (Fig. 2).
However, the other four patients with tumors refused further
pathological examinations and surgical treatments.
Treatment and outcome assessment
All patients received antiepileptic drug treatments,
including oxcarbazepine, sodium valproate, topiramate and
levetiracetam. In addition, all patients received immunotherapy,
which included intravenous immunoglobulin and/or the steroid
hormones methylprednisolone or dexamethasone. In total, three
patients with lung cancer received tumor resection and
chemotherapy. The neurological function scores evaluated by mRS and
the scores of the patients were 3.37±0.52 (range, 3-4) prior to
therapy and 2.38±0.92 (range, 1-4) after therapy. The mean
follow-up duration was 11.3 months (range, 3-30 months). Mortality
occurred in seven patients at follow-up. Furthermore, after
therapy, patients without tumors exhibited neurological
improvement, including seizure control, and had no relapse at
follow-up (range, 3-24 months; Table
I).
Discussion
The present study assessed a number of Chinese
patients with anti-GABAB receptor encephalitis. This rare disease
primarily affects middle-aged and aged males who have a high risk
of receptor encephalitis, usually manifesting as LE, and has
symptoms including seizures, memory deficits, psychosis and altered
consciousness (4-9).
Furthermore, seizures are frequently the initial and most prominent
symptom, which are usually refractory to anti-epileptic drugs but
exhibit a response to immunotherapy (4-9,13).
In the present study, all patients had seizures as the major
symptom. Consistent with previous studies, manifestations including
memory deficits, psychiatric changes and confusion were observed in
the present study (4-9).
The GABAB receptor is a G protein-coupled receptor for the
inhibitory neurotransmitter GABA. The GABAB receptor is able to
mediate pre-synaptic and post-synaptic GABAergic inhibition and
suppress high activity states. Autoantibodies binding to GABAB
receptor may promote synaptic activity states with excessive
synchronization in neuronal networks, which leads to epileptic
seizures (1-3,14).
It has been previously demonstrated that mice with GABAB receptor
dysfunction developed seizures and learning difficulties (15).
Hyponatremia was detected in two patients. One
patient (Case 3) with obvious hyponatremia (123 mmol/l) had a
symptom of vomiting. No malignant tumor was detected in this
patient during the follow-up. CSF cytology of patients with
anti-GABAB receptor encephalitis has no specific features compared
with that of other types of autoimmune or viral encephalitis.
Consistent with previous studies, certain patients in the present
study had lymphocytic pleocytosis and a mildly elevated protein
concentration (4-9).
Furthermore, in the majority of patients, EEG exam results
indicated slow or epileptic activity in the temporal lobes. In
addition, MRI scans identified that 1/3 of patients had
hyperintense signals in the mesial temporal lobes, which was
consistent with the results of previous studies (4-9).
It has been reported that brain MRI scans may exhibit dynamic
changes in volume and signal intensity in the amygdala and
hippocampus, which indicates considerable inflammation and
subsequent degeneration (4-9).
Furthermore, brain 18-fluoro-deoxyglucose positron emission
tomography hypermetabolism has been identified in certain patients
(7). In addition, MRI changes in
patients cannot provide specific information for the diagnosis of
anti-GABAB receptor encephalitis. Therefore, negative brain MRI
scan results may not exclude the diagnosis of this disease.
Antibodies against GABAB receptors are mainly from
the IgG1 subclass, which may induce neuronal damage directly via
complement activation and antibody-dependent cell-mediated
cytotoxicity (2). In the central
nervous system, the GABAB receptor is primarily expressed in the
hippocampus, amygdala, thalamus and cerebellum (1-3).
Furthermore, ~1/2 of patients with anti-GABAB receptor encephalitis
have a paraneoplastic etiology, which is usually SCLC and is
frequently identified after the development of neurologic symptoms
(4-9).
Thymus carcinoid, melanoma and gastric adenocarcinoma are also
reported in patients with anti-GABAB receptor encephalitis and
patients with SCLC usually have a poor prognosis after
immunotherapy (4-9,16,17).
Similarly, at the follow-up for the present study, high mortality
was reported in patients with lung cancer. In addition, the present
immunohistochemistry results indicated that GABAB receptor was
expressed in lung cancer tissues; to the best of our knowledge,
this has not been previously reported. Pulmonary neuroendocrine
cells may produce GABA and GABAB receptors are expressed in airway
epithelium (18). Therefore, the
present results supported the hypothesis that the ectopic
expression of neuronal proteins by the tumor reduces immune
tolerance for these proteins, which then contributes to the
development of the autoimmune encephalitis (19). The GABAB receptors become autoimmune
antigens, which leads to extensive infiltration of cytotoxic T
cells and neuronal degeneration. This effect also triggers B-cell
immune responses, thus leading to the production of autoantibodies
with neuronal functional alterations (1-3).
Autoantibodies recognizing the extracellular domain
of the GABAB receptor may be detected in serum and CSF of patients
with GABAB receptor encephalitis (1). These patients may also have other
autoantibodies, including anti-Hu, anti-voltage-gated calcium
channel and anti-thyroid antibodies (4-9).
Furthermore, co-existence of anti-GABAB receptor antibodies and
onconeuronal antibodies in patients with SCLC are frequently
associated with poor prognosis (4-9).
In the present study, two patients were also determined to have
anti-Hu antibodies. As the diagnosis of tumors is established after
the diagnosis of anti-GABAB receptor encephalitis, screening for
cancer is important once the clinical diagnosis is confirmed.
Furthermore, it has been suggested that tumor screening should be
performed after the encephalitis diagnosis (20).
For the treatment of GABAB receptor encephalitis
with malignancy, immunotherapy and tumor treatment are necessary
(4-9).
The first line of immunotherapy includes corticosteroids, Igs and
plasmapheresis, either alone or in combination (11). Furthermore, it is strongly
recommended that the therapy should be started once the anti-GABAB
receptor encephalitis is diagnosed. Seizures caused by anti-GABAB
receptor encephalitis are frequently refractory to any
antiepileptic drugs, but respond well to immunotherapy (21). In line with this, the present results
suggested that patients without cancer also responded well to
immunotherapy.
In conclusion, it was indicated that seizures and
memory deficits are the major manifestations of anti-GABAB receptor
encephalitis in Chinese patients. Therefore, testing for anti-GABAB
receptor antibodies may be used for elderly patients with LE or
new-onset refractory seizures. Most patients with anti-GABAB
receptor encephalitis without cancer responded well to
immunotherapy. However, patients with underlying lung cancer had a
relatively poor prognosis.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Natural
Science Foundation of China (grant no. 81873786), the Natural
Science Foundation of Shandong Province (grant no. ZR2017MH082),
Innovative Research Project of Resident Standardization Training of
Qilu Hospital, Shandong University (grant no. ZPZX2019A04) and
Undergraduate Teaching Reform and Research Project of Cheeloo
College of Medicine, Shandong University (grant no.
qlyxjy-201917).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ was responsible for the analysis of the data and
the drafting of the manuscript. XY was responsible for the
autoimmune antibody detection experiments and immunohistochemistry
staining. XL was responsible for the analysis of the radiology data
and the revision of the manuscript. SW was responsible for the
design, data analysis, critical revision and final approval of the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Qilu Hospital of Shandong University (Jinan, China; no.
KYLL-2017-550). Written informed consent was obtained from each
patient or a relative serving as a legal representative.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lancaster E and Dalmau J: Neuronal
autoantigens--pathogenesis, associated disorders and antibody
testing. Nat Rev Neurol. 8:380–390. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Melzer N, Meuth SG and Wiendl H:
Paraneoplastic and nonparaneoplastic autoimmunity to neurons in the
central nervous system. J Neurol. 260:1215–1233. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dalmau J, Geis C and Graus F:
Autoantibodies to synaptic receptors and neuronal cell surface
proteins in autoimmune diseases of the central nervous system.
Physiol Rev. 97:839–887. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lancaster E, Lai M, Peng X, Hughes E,
Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura
A, et al: Antibodies to the GABA(B) receptor in limbic encephalitis
with seizures: Case series and characterisation of the antigen.
Lancet Neurol. 9:67–76. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Höftberger R, Titulaer MJ, Sabater L, Dome
B, Rózsás A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L,
et al: Encephalitis and GABAB receptor antibodies: Novel findings
in a new case series of 20 patients. Neurology. 81:1500–1506.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dogan Onugoren M, Deuretzbacher D, Haensch
CA, Hagedorn HJ, Halve S, Isenmann S, Kramme C, Lohner H, Melzer N,
Monotti R, et al: Limbic encephalitis due to GABAB and AMPA
receptor antibodies: A case series. J Neurol Neurosurg Psychiatry.
86:965–972. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim TJ, Lee ST, Shin JW, Moon J, Lim JA,
Byun JI, Shin YW, Lee KJ, Jung KH, Kim YS, et al: Clinical
manifestations and outcomes of the treatment of patients with GABAB
encephalitis. J Neuroimmunol. 270:45–50. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guan HZ, Ren HT, Yang XZ, Lu Q, Peng B,
Zhu YC, Shao XQ, Hu YQ, Zhou D and Cui LY: Limbic encephalitis
associated with Anti-γ-aminobutyric Acid B receptor antibodies: A
case series from china. Chin Med J (Engl). 128:3023–3028.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qiao S, Zhang YX, Zhang BJ, Lu RY, Lai QL,
Chen LH and Wu J: Clinical, imaging, and follow-up observations of
patients with anti-GABAB receptor encephalitis. Int J
Neurosci. 127:379–385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zuliani L, Graus F, Giometto B, Bien C and
Vincent A: Central nervous system neuronal surface antibody
associated syndromes: Review and guidelines for recognition. J
Neurol Neurosurg Psychiatry. 83:638–645. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Graus F, Titulaer MJ, Balu R, Benseler S,
Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M,
et al: A clinical approach to diagnosis of autoimmune encephalitis.
Lancet Neurol. 15:391–404. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van Swieten JC, Koudstaal PJ, Visser MC,
Schouten HJ and van Gijn J: Interobserver agreement for the
assessment of handicap in stroke patients. Stroke. 19:604–607.
1988.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hainsworth JB, Shishido A, Theeler BJ,
Carroll CG and Fasano RE: Treatment responsive GABA(B)-receptor
limbic encephalitis presenting as new-onset super refractory status
epilepticus (NORSE) in a deployed U.S. soldier. Epileptic Disord.
16:486–493. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Emson PC: GABA(B) receptors: Structure and
function. Prog Brain Res. 160:43–57. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Prosser HM, Gill CH, Hirst WD, Grau E,
Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, et
al: Epileptogenesis and enhanced prepulse inhibition in
GABA(B1)-deficient mice. Mol Cell Neurosci. 17:1059–1070.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boronat A, Sabater L, Saiz A, Dalmau J and
Graus F: GABA(B) receptor antibodies in limbic encephalitis and
anti-GAD associated neurologic disorders. Neurology. 76:795–800.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jia XT, Pan Y, Di Z, Gu N, Liu Z and Kang
YM: Anti-GABAB receptor encephalitis in a patient with gastric
adenocarcinoma. Neurol Sci. 39:1981–1984. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mizuta K, Osawa Y, Mizuta F, Xu D and
Emala CW: Functional expression of GABAB receptors in airway
epithelium. Am J Respir Cell Mol Biol. 39:1981–1984. 2018.
|
|
19
|
DeLuca I, Blachère NE, Santomasso B and
Darnell RB: Tolerance to the neuron-specific paraneoplastic HuD
antigen. PLoS One. 4(e5739)2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Titulaer MJ, Soffietti R, Dalmau J, Gilhus
NE, Giometto B, Graus F, Grisold W, Honnorat J, Sillevis Smitt PA,
Tanasescu R, et al: Screening for tumours in paraneoplastic
syndromes: Report of an EFNS task force. Eur J Neurol. 18:e19–e3.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dubey D, Samudra N, Gupta P, Agostini M,
Ding K, Van Ness PC, Vernino S and Hays R: Retrospective case
series of the clinical features, management and outcomes of
patients with autoimmune epilepsy. Seizure. 29:143–147.
2015.PubMed/NCBI View Article : Google Scholar
|