Introduction
At present, a wide variety of drugs are commercially
available for the treatment of cancer. However, the majority of
these have serious adverse effects. Polysaccharides are rich in
sulfate radicals and have been regarded as one of the most
effective compounds for cancer prevention, constituting the major
natural resource of effective novel anti-cancer agents (1).
Undaria pinnatifida (U. pinnatifida),
also known as Qundaicai in China, has long been used as a
traditional Chinese medicine and functional food source in
countries including China, Korea, Australia, New Zealand and Japan.
It contains a variety of nutrients, including polysaccharides,
proteins and minerals (2).
Undaria pinnatifida polysaccharides (UPPS) exert multiple
biological functions, including immunomodulatory, anti-cancer,
anti-oxidation and anti-viral activities (3).
Previous studies have demonstrated that the
anti-tumor activities of Undaria pinnatifida are closely
associated with it being rich in sulfated polysaccharides (4). The sulfation of polysaccharides not
only enhances the water solubility of such compounds but also
improves certain biological activities (5), including anti-viral, immunostimulant,
anti-oxidant and anti-tumor effects (6). The chlorosulfonic acid-pyridine method
is widely used to produce highly active and functional
polysaccharide derivatives, producing high yields and a degree of
product substitution (7).
Few studies have characterized and determined the
monosaccharide composition of UPPS. In the present study, UPPS were
extracted, isolated, purified and modified, and the structural
differences between UPPS and sulfated (S)UPPS were subsequently
analyzed using DEAE-52 cellulose column chromatography, Sephadex
G-200 column chromatography and instrumental analyses. The
anti-tumor activity of UPPS and its sulfated derivatives were then
evaluated.
Materials and methods
Chemicals
The dried whole plant of U.
pinnatifida was purchased from a store (Dalian Longchang
Aquatic Products Co., Ltd.) in Dalian, China. The material was
identified by the corresponding author. DEAE-52 cellulose and
Sephadex G-200 were purchased from Whatman Co., Ltd. Monosaccharide
standards, T-series dextrans were purchased from Sigma-Aldrich
(Merck KGaA). All other reagents used were of analytical grade. The
murine sarcoma S180 cell line (original CCRF S-180 II) was obtained
from Cobioer Biosciences Co., Ltd. (Nanjing, China) and were
cultured in RPMI 1640 medium containing 10% FBS (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd.) and incubated at 37˚C
in an atmosphere containing 5% CO2. Kunming mice
(POO101009) were provided by the Animal Experiment Centre of
Heilongjiang University of Chinese Medicine. They were kept in a
temperature- and humidity-controlled environment under a 12 h
light/dark cycle and provided with food and water ad
libitum. The experimental protocol was approved by the
Institutional Animal Care and Use Committee of Heilongjiang
University of Chinese Medicine (Harbin, China).
Extraction and isolation of
polysaccharides
Dry U. pinnatifida was smashed using a
high-speed disintegrator. The resulting powder was refluxed with
95% ethanol at 80˚C for 2 h to remove any lipids and colored
materials. The residue was air-dried to obtain pre-treated powder.
The powder (100 g) was extracted using 5x103 ml
distilled water at 90˚C for 5 h. Following centrifugation at 1,157
x g (room temperature) for 10 min, the supernatant was collected.
The extracts were then concentrated using a vacuum rotary
evaporator and precipitated with ethanol to a ethanol concentration
of 80%. The mixture was stirred and kept overnight at 4˚C to
precipitate the polysaccharides and the resulting precipitate was
collected via vacuum filtration. The polysaccharides were then
sequentially washed with anhydrous ethanol, acetone and petroleum
ether. The precipitates were freeze-dried to obtain crude
polysaccharides.
Crude polysaccharide powder (5 g) was dissolved in
300 ml distilled water and the resulting polysaccharide solution
was subsequently deproteinized using the Papain method. The
deproteinized polysaccharide aqueous solution was adjusted to a pH
of 8-9 using ammonia solution, ~10% H2O2 was
added and the solution was incubated overnight at 4˚C. Samples were
then dialyzed against tap water for 72 h and distilled water for 24
h [dialysis bag; cut-off molecular weight (Mw), 8,000-14,000 Da].
The solution was concentrated using a vacuum rotary evaporator and
submitted to precipitate with four times the volume of pure
ethanol. The resultant mixture was stirred and kept overnight at
4˚C and subsequently collected via vacuum filtration.
Polysaccharides were then sequentially washed with anhydrous
ethanol and acetone, and freeze-dried to produce semi-pure
polysaccharides.
Purification of the major
polysaccharide
The crude polysaccharide mix (300 mg) was dissolved
in 60 ml distilled water and then injected into an anion exchange
chromatography column (2.6x55 cm) of DEAE-52 cellulose (140 g)
equilibrated with three column volumes with distilled water. After
sample loading, the column was sequentially eluted with distilled
water and NaCl aqueous solution (0.1, 0.2, 0.3, 0.4, 0.5 and 0.8 M)
at a flow rate of 1 ml/min (10 min/tube). A total of 3,000 ml NaCl
eluate was collected. The major polysaccharide fractions were
obtained using a fraction collector and identified with the
phenol-sulfuric acid method and then combined. Samples were then
dialyzed. The dialyzed solution was concentrated and freeze-dried
(8).
The polysaccharide fractions (10 mg) were dissolved
in 2 ml distilled water and injected into a column (1.6x80 cm) of
Sephadex G-200 (10 g) equilibrated with distilled water. After
sample loading, the column was eluted with NaCl aqueous solution
(0.05 M) at 0.1 ml/min (20 min/tube) for a total of 160 ml. The
major polysaccharide fractions were collected using a fraction
collector and dialyzed. The dialyzed solution was concentrated and
freeze-dried.
Purity determination
The purity of the polysaccharides was determined via
high-performance liquid chromatography (HPLC; 2695 system; Waters)
with a gel-filtration chromatographic column containing
Ultrahydrogel™ Linear. A refractive index detector (RID) was also
utilized (2414 system; Waters). The purified polysaccharide (2 mg)
was dissolved in distilled water (10 ml) and passed through a
filter (pore size, 0.22 µm). The column was maintained at a
temperature of 40˚C, eluted with distilled water at a flow rate of
0.8 ml/min and detection was performed using RID.
Chemical analysis
The content of total sugar was measured using the
phenol-sulfuric acid method (9) with
D-glucose utilized as the standard. The content of total sugar was
calculated as follows: Polysaccharide content (%)=[X x volume x
dilution ratio/amount of polysaccharide (g)] x100, where X is the
absorbance calculated according to the regression equation of the
standard curve.
The content of uronic acid was measured via the
sulfuric acid-carbazole method, adopting D-glucuronic acid as the
standard (10).
The content of sulfate radicals was measured using
the gelatin-barium chloride method (11). The degree of sulfate substitution
(DS) was calculated as follows: DS=(1.62 x S%)/(32-1.02 x S%),
where S is the content of sulfur within the sample.
Sulfated modifications
Pyridine (60 ml) was added to a three-necked flask
equipped with a condenser and stirrer and cooled in an ice bath.
Chlorosulfonic acid (10 ml) was slowly dripped into the pyridine
solution for ~40 min. Samples were removed from the ice bath after
a large quantity of yellow solid product had appeared in the flask.
UPPS fraction B1 (UPPS-B1) (100 mg) was suspended in
dimethylformamide (10 ml) and agitated with a magnetic stirrer for
20 min, after which the mixture was added to the flask. The flask
was then immediately placed in a pre-heated water bath (85˚C). The
reaction was stirred for 3 h under 85˚C. The flask was then cooled
to room temperature and ice water was slowly added. The solution
was then neutralized with saturated NaOH and a triploid volume of
pure ethanol was added. The precipitate was collected via
centrifugation (1,157 x g for 10 min, room temperature), dissolved
in water and dialyzed with distilled water for 3 days. The solution
was concentrated and freeze-dried to obtain the sulfated
derivative, S-UPPS-B1.
Ultraviolet (UV) spectroscopy
UV spectroscopy is regularly used to detect whether
proteins or nucleic acids are present in polysaccharides (12). A total of 1 mg/ml UPPS-B1 and
S-UPPS-B1 were each prepared using deionized water. Subsequently, a
UV spectrum scan was performed using a DB-20 UV visible
spectrophotometer (Dynamica Pty Ltd.) in the range of 200-400
nm.
Fourier-transform infrared (IR)
spectroscopy
Fourier-transform IR spectroscopic analysis is
utilized to identify the fundamental groups of polysaccharide
structures (13). IR analysis was
performed using a Fourier transform IR spectrophotometer
(FTIR-8001; Shimadzu Corp.). A total of 2 mg of each polysaccharide
sample and 400 mg of KBr were mixed, ground for 5-10 min with an
agate mortar and pressed into a 1-mm tablet (14). IR spectra were recorded in the
frequency range of 4,000-400 cm-1.
Monosaccharide composition
analysis
It is widely recognized that the monosaccharide
composition of natural polysaccharides is closely associated with
its bioactivity (15). The
hydrolyzed and acetylated derivatives of the polysaccharides
assessed were loaded onto a gas chromatography-mass spectrometer
(GC-MS; model 6890/5973N-GC/MSD; Agilent Technologies, Inc.). Each
sample (10 mg) was hydrolyzed using 3.0 ml of 2 M trifluoroacetic
acid at 100˚C for 5 h in a sealed tube. The hydrolysate was dried
with a nitrogen blowing instrument and further dried in a
desiccator filled with P2O5 for 24 h. For
GC-MS analysis, the complete hydrolysate was mixed with 1.0 ml
pyridine and fully dissolved. A total of 0.4 ml
hexamethyldisilazane and 0.2 ml trimethylchlorosilane was then
added and the mixture was shaken at room temperature for 5 min.
Following centrifugation at 9,391 g (4˚C) for 30 min, the
supernatant was analyzed via GC-MS. The quantity of the
monosaccharide component was determined via the peak area. The
total injection volume was 1 µl. GC conditions included the use of
an HP-5MS quartz capillary column (0.25 mm x30 m; Agilent
Technologies, Inc), an inlet temperature of 270˚C and a temperature
program of 150˚C for 1 min, a temperature increase by 10˚C/min to
250˚C and a hold at 7 min. The MS conditions were an interface
temperature of 230˚C, an electron ionization+ ion
source, energy of 70 ev, detector voltage of 280 ev and mass
spectrometry detection range of 43.00-550.00.
Mw determination
Mws were calculated using a calibration curve
obtained from various standard dextrans of different Mws (including
Dextran Blue, Dextran T10, T40, T70, T110, T500 and glucose)
(16).
UPPS-B1 (2 mg) or S-UPPS-B1 (2 mg) was dissolved in
distilled water (1 ml) and passed through a filter (pore size, 0.22
µm). Polysaccharides were then added to a gel-filtration
chromatographic column [the same as aforementioned (HPLC; purity
determination)] that was maintained at 40˚C, eluted with distilled
water at a flow rate of 0.8 ml/min and detected via RID. The sample
retention time was recorded.
Anti-tumor experiment in tumor-bearing
mice
Mice (4 weeks; 20±2 g; females and males) were used
for experimentation and maintained under standard laboratory
conditions. The ascites of S180 tumor-bearing mice that had been
inoculated with ascites for 7 days and had grown well were
extracted under aseptic conditions, and diluted to a cell
suspension with a concentration of 1x107 cells/ml with
normal saline. A tumor cell suspension of 0.2 ml was subcutaneously
injected into the right forelimb armpit of each mouse to create a
solid tumor model.
After 24 h, the mice were randomly divided into five
groups of 10 mice. The negative control group was treated with an
equal volume of 0.9% normal saline. U. pinnatifida and
Astragalus are traditional Chinese medicines and their active
components are polysaccharides. Furthermore, astragalus
polysaccharide (APS) is a clinically effective anti-tumor drug, so
it was selected as the positive control in the animal experiment.
The positive control group was treated with APS [intraperitoneal
(i.p.), 100 mg/kg per day]. Treatment groups received UPPS-B1 (25,
50 or 100 mg/kg; i.p.) and S-UPPS-B1 (25, 50 or 100 mg/kg; i.p.), a
maximum tolerated dose experiment was performed beforehand (data
not shown). In tumor-bearing mice, if weight loss of >20%, tumor
diameter of >1.5 cm or tumor showed ulcers and necrosis
occurred, the experiment was suspended and the animals were
euthanized. Pentobarbital sodium (45 mg/kg; i.p.) was administered
prior to cervical dislocation and the percentage of animals lost
due to these issues was <5%. On the 8th day following
administration, mice were weighed and sacrificed via cervical
dislocation. Subsequently, tumor masses were removed and weighed.
Tumor inhibition rates were calculated using the following
formula:
Tumor inhibition rate=[(average tumor weight of the
control group-average tumor weight of the treatment group)/average
tumor weight of the control group] x100%.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Differences between groups were assessed by one-way
analysis of variance followed by Dunnett's post-hoc test. SPSS16.0
software (SPSS, Inc.) was used for all statistical analyses.
P<0.05 was considered to indicate statistical significance.
Results
Chemical characterization of the
homogeneous polysaccharides
The UPPS crude polysaccharide was obtained from
U. pinnatifida dry powder (100 g), which was formed
following water extraction, ethanol precipitation, deproteinization
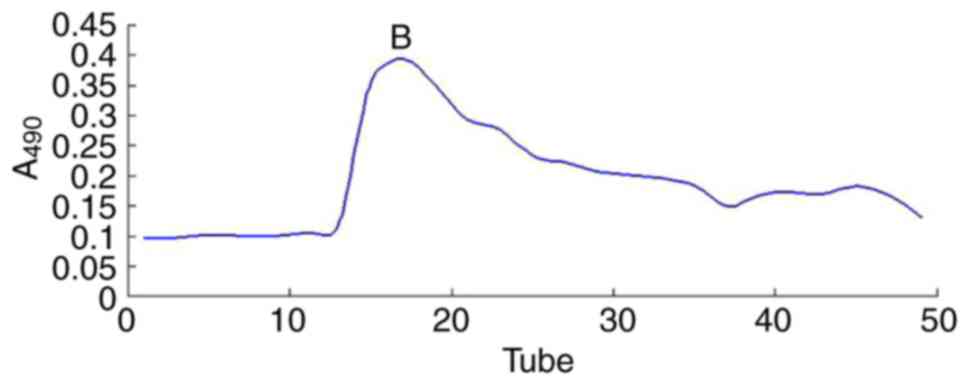
and further separated via DEAE-52 ion-exchange chromatography
(Fig. 1) and Sephadex G-200
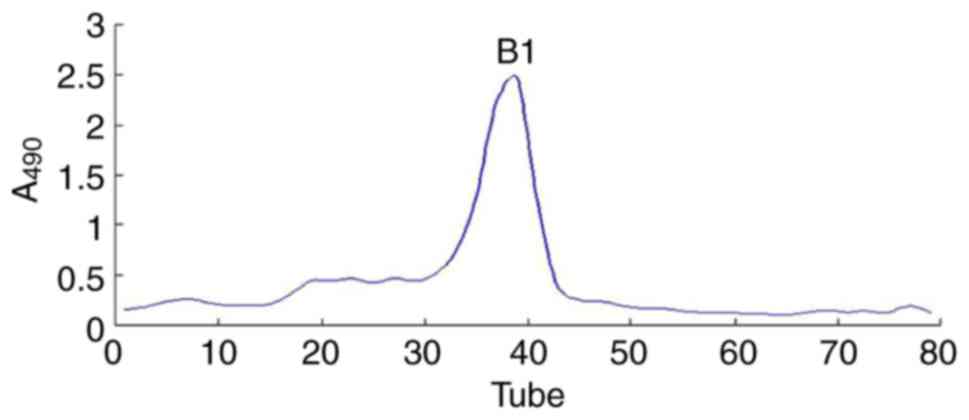
size-exclusion chromatography (Fig.
2). The fractions were collected and dialyzed, and impurities
were removed, including pigments and free proteins. Samples were
then freeze-dried. As a result, one major polysaccharide fraction
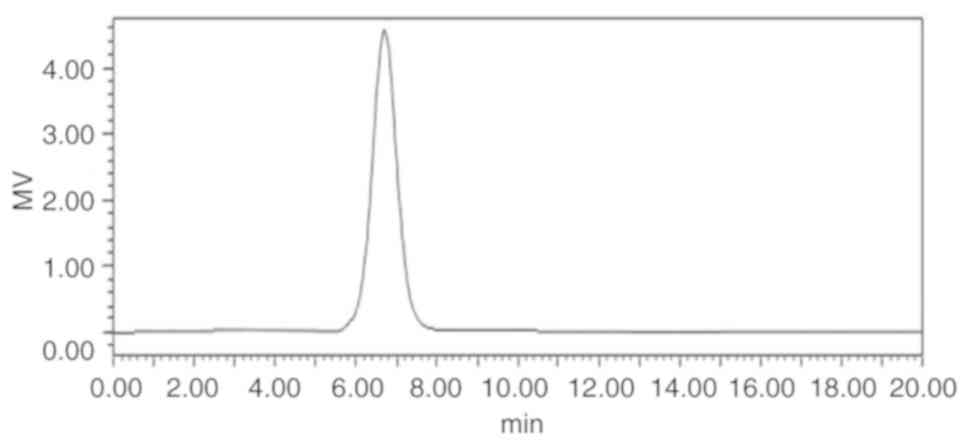
(UPPS-B1) was isolated with a yield of 79.78%. HPLC profiles
indicated that the polysaccharides were homogeneous, as each
polysaccharide exhibited a symmetrical peak (Fig. 3).
Preliminary characterization of
UPPS
The linear regression equation of the glucose
standard curve was A=0.0371C (µg/ml) -0.005 (R2=0.9986)
and the content of glucose exhibited a positive linear correlation
in the range of 0-25 µg/ml. The linear regression equation of the
uronic acid standard curve was A=0.0049C-0.005
(R2=0.9996) and the content of D-glucuronic acid
demonstrated a positive linear correlation in the range of 0-100
µg/ml. In addition, the linear regression equation of the sulfate
standard curve was A=0.0173C-0.0025 (R2=0.9994) and the
content of SO42- exhibited a positive linear
correlation in the range of 0-24 µg/ml.
The results of the analysis indicated that the
content of total sugar, sulfate radicals and uronic acid in UPPS-B1
were 79.78, 8.53 and 9.29%, respectively. In UPPS-B1, acidic
polysaccharides were identified. The results of the analysis
indicated that the content of total sugar, sulfate radicals and
uronic acid in S-UPPS-B1 was 77.28, 29.12 and 7.98%, respectively.
The substitution degree was 0.67.
UV analysis
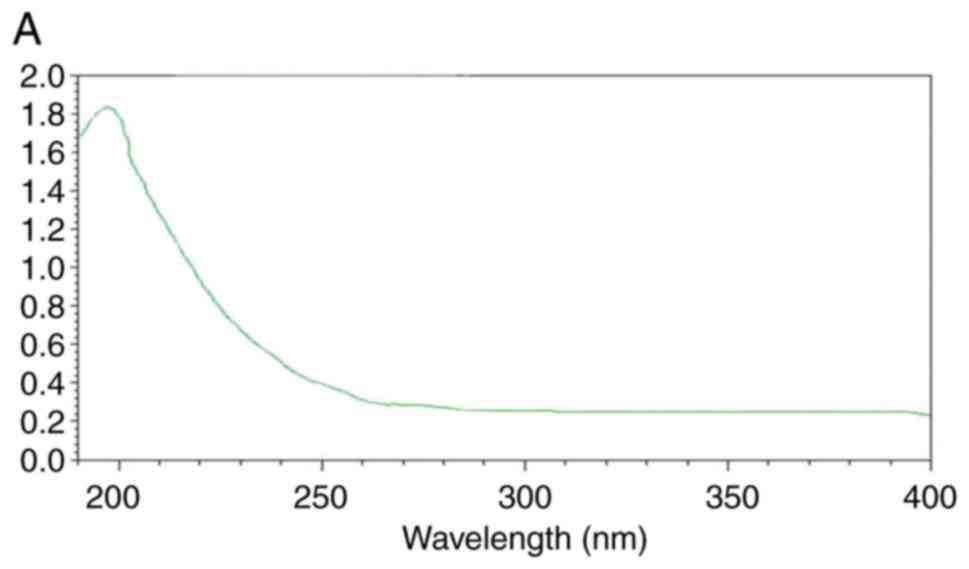
The UV spectra are presented in Fig. 4. There was no absorption peak at
260-280 nm, indicating that the UPPS-B1 and S-UPPS-B1 did not
include any nucleic acid or protein (17).
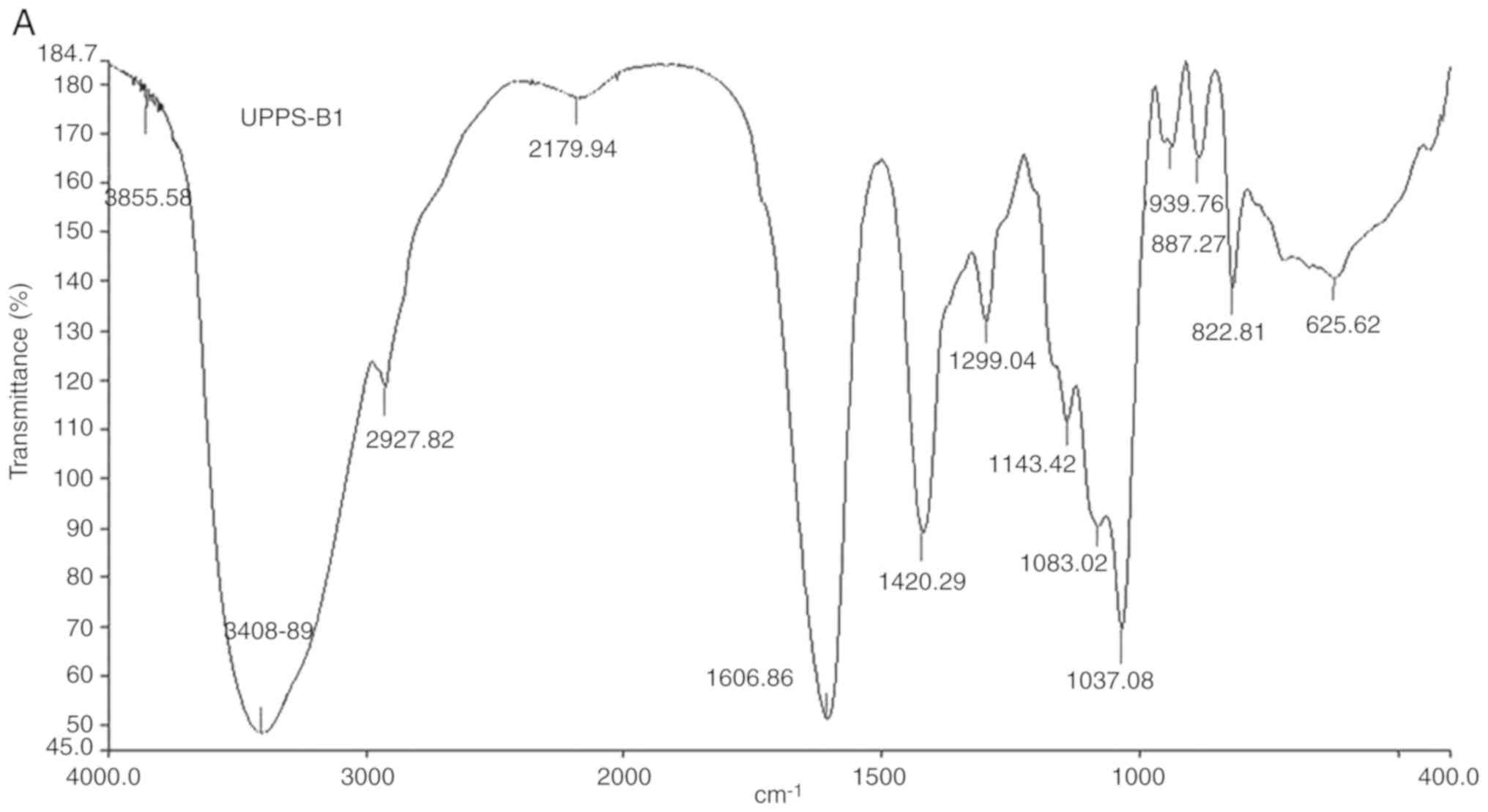
Fourier-transform IR analysis
The Fourier-transform IR spectra and analysis of the
peaks are presented in Fig. 5 and
Table I, respectively. The IR
spectrum of UPPS-B1 and S-UPPS-B1 respectively exhibited a broad,
stretched and intense characteristic band at ~3,408.89 and 3,442.03
cm-1 for the O-H group. Furthermore, a weak C-H bond was
represented by the bands at ~2,927.82 and 2,926.84 cm-1,
respectively. The O-H and C-H absorption bands were typical
absorptions of polysaccharides (18). The characteristic absorption peaks of
-O-SO3 were present at ~1,238 and 822 cm-1.
In addition, -COOH was represented by bands at ~1,606 and 1,420
cm-1. A strong absorption was also observed in the
region of 1,000-1,200 cm-1, which was attributed to
C-O-C and C-OH bands. Other absorption peaks of pyranose were
present at ~1,037.08 and 1,046.08 cm-1. A peak at 887.27
cm-1 indicated that the glycosidic bonds in UPPS-B1 were
of the β-type. A peak at 856.54 cm-1 indicated that the
glycosidic bonds in S-UPPS-B1 were of the α-type.
 | Table IFunctional groups of UPPS-B1 and
S-UPPS-B1 in the infrared spectrum. |
Table I
Functional groups of UPPS-B1 and
S-UPPS-B1 in the infrared spectrum.
| | Absorption peak
(cm-1) |
|---|
| Vibration mode | UPPS-B1 | S-UPPS-B1 |
|---|
| Stretching of
O-H | 3408.89 | 3442.03 |
| Stretching of
C-H | 2927.82 | 2926.84 |
| Asymmetric stretching
of C=O | 1606.86 | 1638.62 |
| Symmetric stretching
of C=O | 1420.29 | 1401.57 |
| Stretching of
S=O | - | 1238.60 |
| Stretching of
C-O-S | 822.81 | 822.29 |
Analysis of the monosaccharide
composition of polysaccharides
The monosaccharide composition of each
polysaccharide was determined using GC-MS (Table II). The results revealed that
UPPS-B1 and S-UPPS-B1 exhibited a different monosaccharide
composition, indicating that their individual features may be
relevant to their biological activities.
 | Table IIMonosaccharide composition of
polysaccharides isolated from UPPS. |
Table II
Monosaccharide composition of
polysaccharides isolated from UPPS.
| Substance | Xylose (%) | Mannose (%) | Glucose (%) | Galactose (%) |
|---|
| UPPS-B1 | 7.946 | 8.719 | 11.551 | 9.773 |
| S-UPPS-B1 | 1.000 | 9.700 | 6.400 | 1.600 |
Mw
The linear regression equation of the Mw standard
curve was calculated as lgMw=-6.7881TR (retention time)
+8.7134 (r2=0.9984). Furthermore, the average Mws of
UPPS-B1 and S-UPPS-B1 were calculated as 37 and 110 kDa,
respectively.
Tumor growth inhibition
The results of the tumor growth inhibition
experiment are presented in Table
III. Compared with the control, tumor inhibition rates in the
high, medium and low-dose UPPS-B1-treated mice were 54.77, 36.96
and 20.83%, respectively. Furthermore, the tumor inhibition rates
in the high, middle and low-dose S-UPPS-B1-treated groups were
63.05, 50.41 and 39.52%, respectively. The tumor inhibition rate of
APS was 54.01%. The S-UPPS-B1 group demonstrated a significant
reduction in tumor weight compared with the control group.
 | Table IIIInhibitory effect of UPPS-B1 and
S-UPPS-B1 on solid S180 tumors in mice. |
Table III
Inhibitory effect of UPPS-B1 and
S-UPPS-B1 on solid S180 tumors in mice.
| Group | Dose
[mg/(kg·day)] | Tumor weight
(g) |
|---|
| Control | - | 1.0755±0.1075 |
| APS | 100 |
0.4946±0.0609a |
| UPPS-B1 | 25 |
0.8515±0.0215a |
| | 50 |
0.6780±0.0640a |
| | 100 |
0.4864±0.0479a |
| S-UPPS-B1 | 25 |
0.6505±0.0714a |
| | 50 |
0.5333±0.1354a |
| | 100 |
0.3974±0.0465a |
Discussion
The major polysaccharide fraction from U.
Pinnatifida, UPPS-B1, was purified by DEAE-52 and Sephadex
G-200 column chromatography. The chlorosulfonic acid-pyridine
method was applied for sulfation modification. Subsequently,
UPPS-B1 and the sulfated derivative, S-UPPS-B1, were characterized
using chemical analysis, UV-visible spectroscopy, FT-IR
spectroscopy, gas chromatography and HPLC. The Mw and
monosaccharide composition of polysaccharides was determined. The
in vivo study then indicated that UPPS-B1 and S-UPPS-B1
exhibited marked anti-tumor activity.
Malignant tumors are increasingly posing a threat to
human health. The search for highly efficient anti-neoplastic drugs
with low toxicity have therefore become the focus of cancer
treatment. Natural compounds are effective therapeutic agents and
numerous polysaccharides exert anti-tumor effects. The biological
activity of polysaccharides is closely associated with their
structure and physicochemical properties. The steric and
electrostatic repulsion effects of sulfate groups may alter the
spatial structure of polysaccharides and the flexion of the sugar
chain, thus increasing water solubility and resulting in changes to
their biological activity (19,20).
After sulfated modification, the contents of xylose,
glucose and galactose were markedly decreased in the sulfated
derivatives examined in the present study, while there was a slight
increase in the content of mannose. These results were consistent
with those of a previous study (16), in which sulfated modifications caused
alterations in the monosaccharide composition of polysaccharides.
However, in the present study, the application of sulfated
modification did not result in the destruction of the major chain
of the sulfated derivatives.
The Mw of sulfated polysaccharides is an important
parameter that affects the biological activity of polysaccharides
(21). In general, the degradation
of polysaccharides is accomplished during the sulfation reaction
via the chlorosulfonic acid method (22). However, the results of the present
study revealed that S-UPPS-B1 exhibited an increase in Mw compared
with that of UPPS-B1, indicating that these sulfated derivatives
were successfully produced in the present study without
degradation.
Nuclear magnetic resonance (NMR) may provide a vast
amount of information on the monosaccharide composition of
polysaccharides, the sequence of monosaccharide residues, the
position of monosaccharide residues in glycoside bonds, the type of
ring structure and the configuration of glycoside bonds. Therefore,
in order to obtain comprehensive information on the structure of
polysaccharides, further research by our group will endeavor to use
NMR technology to assign 1H and 13C signals
of each sugar residue in UPPS-B1 and S-UPPS-B1.
The results of the anti-tumor experiment revealed
that, after sulfated modification, the anti-tumor activity of
S-UPPS-B1 was greater than that of UPPS-B1 at the same dose. MTT
assay has been done by other group as a preliminary experiment.
This may be due to the sulfated modification altering the molecular
structure and sulfate content of the polysaccharide, leading to
changes in biological activity.
The crude polysaccharide obtained from U.
pinnatifida in the present study predominantly contained the
water-extractable polysaccharide UPPS-B1, which was purified via
DEAE-52 and Sephadex G-200 chromatography. S-UPPS-B1, with a
substitution degree of 0.67, was synthesized using the
chlorosulfonic acid-pyridine method. The HPLC results indicated
that UPPS-B1 and S-UPPS-B1 were homogeneous and the compounds had
an average Mw of 37 and 110 kDa, respectively. The GC-MS indicated
that UPPS-B1 and S-UPPS-B1 were primarily composed of xylose,
mannose, glucose, galactose and glucuronic acid. The results
indicated that sulfation of polysaccharides may improve solubility
and result in high anti-tumor activity. S-UPPS that was synthesized
in the present study exhibited enhanced anti-tumor activity when
compared with that of UPPS. However, the structural properties and
degree of substitution, as well as the underlying anti-tumor
mechanisms, require further study. The present study provides a
theoretical basis for the structure-activity association of
polysaccharides, which may lead to pharmaceutical of the U.
pinnatifida polysaccharides.
Acknowledgements
In the present study, separation and purification of
polysaccharides from U. Pinnatifida polysaccharides was
performed using the alcohol precipitation method, sulfation was
performed using the chlorsulfonate-pyridine method and UV and IR
spectrum analyses were used for verification. The subsequent in
vivo study indicated that UPPS and S-UPPS exhibited anti-tumor
activity. This is a previously published conference abstract
(23).
Funding
This work was supported by the Harbin Municipal
Science and Technology Bureau Project (grant nos. 2016RQQXJ124 and
2016RAXXJ064), the Innovation Talent Project of the Education
Department of Heilongjiang Province (grant no. UNPYSCT-2016181) and
the Science Foundation Project of Harbin University of Commerce
(grant no. 18XN067).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YBJ designed the study, FLW performed the research
and wrote the manuscript. YBJ, FLW and BY analyzed the data and
revised the manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Institutional Animal Care and Use Committee of Heilongjiang
University of Chinese Medicine (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Costa LS, Fidelis GP, Cordeiro SL,
Oliveira RM, Sabry DA, Câmara RB, Nobre LT, Costa MS, Almeida-Lima
J, Farias EH, et al: Biological activities of sulfated
polysaccharides from tropical seaweeds. Biomed Pharmacother.
64:21–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhou AY, Robertson J, Hamid N, Ma Q and Lu
J: Changes in total nitrogen and amino acid composition of New
Zealand Undaria pinnatifida with growth, location and plant
parts. Food Chem. 186:319–325. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim KJ, Yoon KY and Lee BY: Low molecular
weight fucoidan from the sporophyll of Undaria pinnatifida
suppresses inflammation by promoting the inhibition of
mitogen-activated protein kinases and oxidative stress in RAW264.7
cells. Fitoterapia. 83:1628–1635. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vishchuk OS, Ermakova SP and Zwaqintseva
TN: The fucoidans from brown algae of far-eastern seas: Anti-tumor
activity and structure-function relationship. Food Chem.
141:1211–1217. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang JL, Guo HY, Zhang J, Wang XF, Zhao B,
Yao J and Wang Y: Sulfated modification, characterization and
structure-antioxidant relationships of Artemisia
sphaerocephala polysaccharides. Carbohydr Polym. 81:897–905.
2010.
|
|
6
|
Sun ZW, He YL, Liang ZH, Zhou WW and Niu
TG: Sulfation of (1→3)-β-D-glucan from the fruiting bodies of
Russula virescens and antitumor activities of the modifiers.
Carbohydr Polym. 77:628–633. 2009.
|
|
7
|
Wang J, Hu Y, Wang D, Liu J, Zhang J,
Abula S, Zhao B and Ruan S: Sulfated modification can enhance the
immune-enhancing activity of lycium barbarum polysaccharides. Cell
Immunol. 263:219–223. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Hu X, Han J, Ni L, Tang X, Hu Y
and Chen T: Integrated method of thermosensitive triblock
copolymer-salt aqueous two phase extraction and dialysis membrane
separation for purification of lyceum barbarum polysaccharide. Food
Chem. 194:257–624. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang ZJ, Luo DH and Ena C: Optimization of
polysaccharides extraction from gynostemma pentaphyllum makino
using uniform design. Carbohydr Polym. 69:311–317. 2007.
|
|
10
|
Blumenkrantz N and Asboe-Hansen G: New
method for quantitative determination of uronic acids. Anal
Biochem. 54:484–489. 1973.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ji CF, Ji YB and Meng DY: Sulfated
modification and anti-tumor activity of laminarin. Exp Ther Med.
6:1259–1264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yin X, You Q, Jiang Z and Zhou X:
Optimization for ultrasonic-microwave synergistic extraction of
polysaccharides from cornus officinalis and characterization of
polysaccharides. Int J Biol Macromol. 83:226–232. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lü H, Gao Y, Shan H and Lin Y: Preparation
and antibacterial activity studies of degraded polysaccharide
selenide from Enteromorpha prolifera. Carbohydr Polym.
107:98–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang TT, Lu CL, Jiang JG, Wang M, Wang DM
and Zhu W: Bioactivities and extraction optimization of crude
polysaccharides from the fruits and leaves of Rubus chingii
hu. Carbohydr Polym. 130:307–315. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ji X, Peng Q, Yuan Y, Shen J, Xie X and
Wang M: Isolation, structures and bioactivities of the
polysaccharides from jujube fruit (Ziziphus jujube Mill.): A
review. Food Chem. 227:349–357. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang ZJ, Luo DH and Liang ZY: Structure of
polysaccharides from the fruiting body of Hericium erinaceus
Pers. Carbohydr Polym. 57:241–247. 2004.
|
|
17
|
Yu XH, Liu Y, Wu XL, Liu LZ, Fu W and Song
DD: Isolation, purification, characterization and immunostimulatory
activity of polysaccharides derived from American ginseng.
Carbohydr Polym. 156:9–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yi P, Li N, Wan JB, Zhang D, Li M and Yan
C: Structural characterization and antioxidant activity of a
heteropolysaccharide from ganoderma capense. Carbohydr Polym.
121:183–189. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bao H, Choi WS and You S: Effect of
sulfated modification on the molecular characteristics and
biological activities of polysaccharides from Hypsizigus
marmoreus. Biosci Biotechnol Biochem. 74:1408–1414.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Y, Lu X, Zhang Y, Qin L and Zhang J:
Sulfated modification and immunomodulatory activity of
water-soluble polysaccharides derived from fresh Chinese persimmon
fruit. Int J Biol Macromol. 46:67–71. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xie JH, Wang ZJ, Shen MY, Nie SP, Gong B,
Li HS, Zhao Q, Li WJ and Xie MY: Sulfated modification,
characterization and antioxidant activities of polysaccharide from
Cyclocarya paliurus. Food Hydrocolloids. 53:7–15. 2016.
|
|
22
|
Chen Y, Zhang H, Wang Y, Nie S, Li C and
Xie M: Sulfated modification of the polysaccharides from
Ganoderma atrum, and their antioxidant and immunomodulating
activities. Food Chem. 186:231–238. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang FL, Yang B and Ji YB: Purification of
polysaccharides from Undaria pinnatifida and antitumor
effect of sulfated before and after modification[C]. Kunming: The
10th National Conference on Medicinal Plants and
Phytomedicines, 113, 2011.
|