Introduction
Rosacea is an inflammatory disorder associated with
symptoms such as flushing, erythema, telangiectasia, pustules,
papules and fibrosis affecting the central face (1). According to the National Rosacea
Society, there are four rosacea subtypes, namely,
erythematotelangiectatic rosacea (ETR), papulopustular rosacea
(PPR), phymatous rosacea (PhR) and ocular rosacea (OR) (2). ETR, characterized by transient flushing
and persistent centrofacial erythema with the presence or absence
of telangiectasia, is the most common subtype, followed by PPR,
which is characterized by central facial erythema, transient
papules and/or pustules, with or without telangiectases (2). PhR is characterized by thickened skin
with irregular surface nodularities, while OR is accompanied by
characteristic ophthalmic symptoms (3). Therapy should be based on the patients'
individual symptoms and subtypes. For ETR, brimonidine is approved
for symptomatic relief of the erythema and lasers and light devices
are effective in the treatment of telangiectasia and erythema
(4). PhR can be treated with
CO2 laser and sharp blade excision (5). The treatments of OR include
pharmaceutical agents, laser and light based therapies, and
surgical interventions (6).
Various treatments have been used for the management
of PPR, including oral doxycycline, minocycline, topical azelaic
acid and ivermectin, all of which are reported to have
anti-inflammatory properties (1,7,8). Combination therapy for rosacea often
yields better results than monotherapy. Oral
antimicrobials/isotretinoin combined with topical agents are the
mainstay of therapy for PPR (9).
However, the treatment of PPR is challenging due to its recurrence.
Twice monthly chemical peels with supramolecular salicylic acid
(SSA) 30%, the main ingredient of which is salicylic acid (SA), has
anti-inflammatory properties and whitening effects, and has been
widely used to treat acne, hyperpigmentation and other inflammatory
dermatosis (10). Therefore, in the
present study, a retrospective analysis of 19 cases of PPR was
conducted to assess the efficacy and safety of oral minocycline and
SSA 30% in the treatment of PPR.
Materials and methods
Patients
A retrospective study was conducted on 19 patients
(16 females and 3 males; 27-53 years old) with PPR who had been
treated with oral minocycline (100 mg/day) and SSA 30% chemical
peels (twice a month) between June 2018 and June 2019 in the
Department of Dermatology, West China Hospital, Sichuan University.
This study was conducted in accordance with the ethical guidelines
of the Declaration of Helsinki and approved by Medical Ethic and
Human Research Committee of West China Hospital (approval no.
2017.163). Written informed consent was obtained from each patient.
Patients were required to use moisturizer and avoid sunlight. The
inclusion criteria were as follows: i) Patients who were diagnosed
as PPR and developed papules or pustules with central facial
erythema; ii) patients who were treated with the above combination
treatment for 12 weeks and complete information about them was
available; and iii) patients who were followed up for an additional
8 weeks after the end of treatment. The exclusion criteria applied
to patients were as follows: i) Patients who had used any other
topical treatments or oral agents and/or chemical peel within the
previous 4 weeks; ii) patients who were pregnant or breastfeeding;
iii) patients who had a history of photo allergy, or tetracycline
or SA allergy; iv) patients who had active facial herpes simplex or
warts; v) patients with scar diathesis; and vi) patients with
severe defects of the heart, lung, liver or kidney.
Method of application of SSA 30%
SSA 30% was used twice a month. The chemical peel
was performed in the treatment room of a dermatological department.
Prior to treatment, each patient washed her/his face and then lay
on the treatment bed. According to the instructions of the
manufacturer, an appropriate amount of SSA (7 g/box) was added to a
small therapeutic bowl. SA was combined with Poloxamer 407 (a kind
of solubilizer) as an emulsifiable paste via supramolecular
technology (Broda, Shanghai Rui Zhi Medicine Technology Co., Ltd.;
patent numbers US8865143 and EP2689774), with an initial
concentration of 5-8%. After adding water (2 ml), SSA was released
from Poloxamer 407 and reached a concentration of 30%. After
stirring, the chemical substance was applied to the skin lesions.
Sites to avoid with SSA included the eyes, nasal cavities and lips.
After 20 min, the chemical substance was washed with water.
Patients were required to apply moisturizer and sun protection.
Clinical assessment
Rosacea lesions were imaged by VISIA (Canfield
Imaging Systems) monthly. Investigator Severity Assessment (ISA) of
rosacea severity was scored using a 5-point scale (11): 0, clear (no erythema and no
papules/pustules); 1, almost clear (very mild erythema and very few
small papules/pustules); 2, mild (mild erythema and few small
papules/pustules); 3, moderate (moderate erythema and several small
or large papules/pustules); 4, severe (severe erythema and numerous
small and/or large papules/pustules) by three blinded
dermatologists to evaluate the clinical outcomes. If the assessment
results of three physicians varied, another two physicians were
enrolled to re-evaluate the outcome. Meanwhile, Investigator Global
Assessment (IGA) of efficacy was scored using a 6-point scale
(11): -1, worsening; 0, no
response; 1, mild response (<50% improvement); 2, moderate
(50-80% improvement); 3, excellent (>80% improvement); 4,
complete response/clear. Patients were asked to carry out
self-assessment for rosacea symptoms after the last treatment,
grading the improvement of rosacea as 1, good; 2, fair; 3, poor;
and 4, even worse.
Statistical analysis
All statistical analyses were conducted using Graph
Prism 7. The data measurements are presented as mean ± standard
deviation. Statistical analyses were carried out by repeated
measures one-way analysis of variance (ANOVA) test with post hoc
Tukey's tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cases and patient characteristics
A total of 19 patients (16 females and 3 males)
diagnosed with PPR were enrolled in the study, and received the
therapy for 12 weeks. All 19 patients showed persistent erythema
and transient papules/pustules, and presented ≥1 subjective
symptom. The characteristics of the patients were in accord with
the typical characteristics of patients with PPR (Table I).
 | Table ICharacteristics of the 19
patients. |
Table I
Characteristics of the 19
patients.
| Characteristics | Value, n (%) |
|---|
| Skin phototype | |
|
III | 13 (68.42) |
|
IV | 6 (31.58) |
| Affected areas | |
|
Nose and
paranasal area | 19(10) |
|
Cheek | 14 (73.68) |
|
Forehead | 4 (21.05) |
| Manifestation | |
|
Flushing | 13 (68.42) |
|
Erythema | 19(100) |
|
Papules and
pustules | 19(100) |
|
Telangiectasia | 8 (42.11) |
| Subjective
symptoms | |
|
Burning | 17 (89.47) |
|
Itching | 6 (31.58) |
Clinical assessment
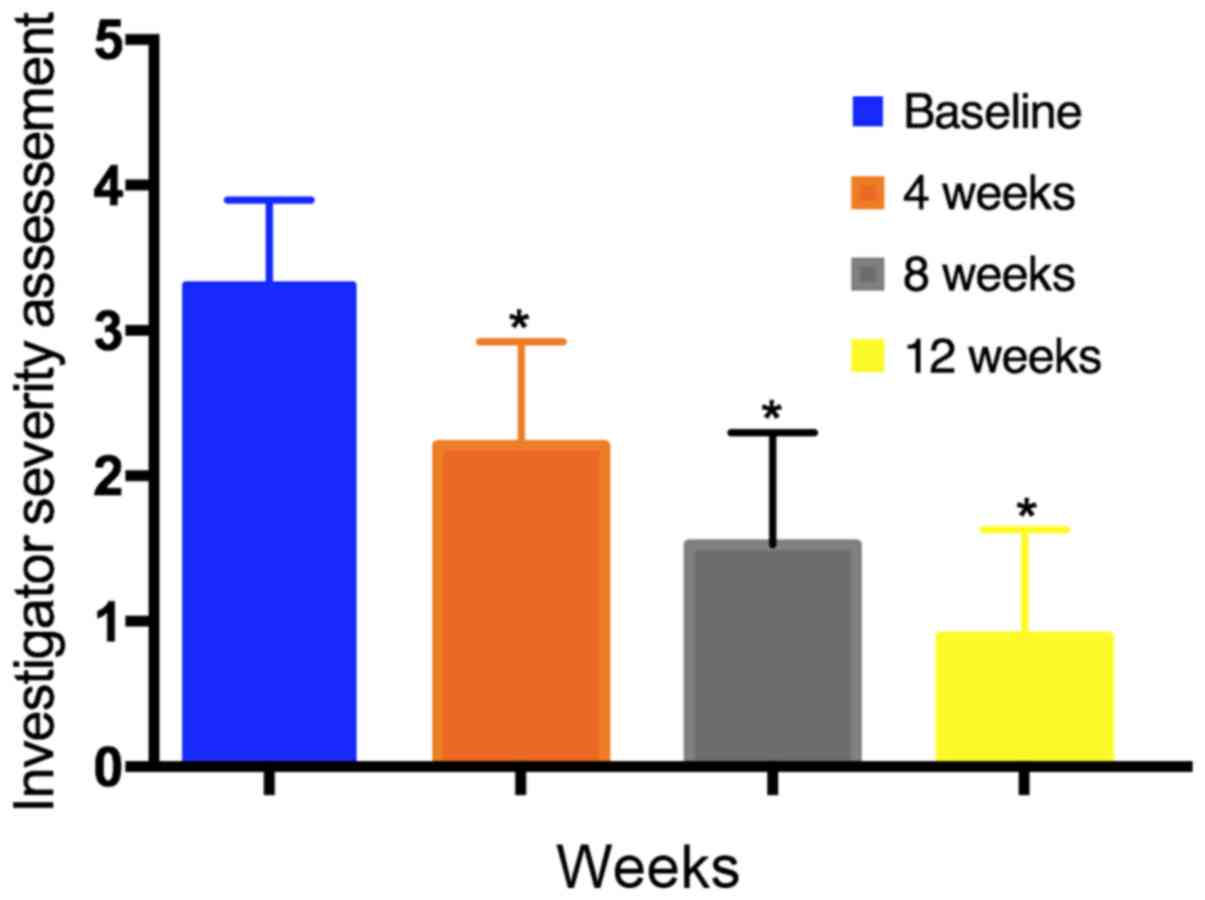
At 4, 8 and 12 weeks, a significant reduction in
rosacea severity was observed in the ISA; the mean score reduced
from 3.32±0.6 at baseline to 2.21±0.7 at 4 weeks, 1.53±0.8 at 8
weeks, and 0.89±0.7 at 12 weeks (P<0.05; Fig. 1). In the IGA assessment, after 4
weeks, 10 patients (52.63%) exhibited a ‘moderate’ ‘response’, and
after 8 weeks, 18 patients (94.74%) showed at least a ‘moderate’
response. After 12 weeks of treatment, all patients exhibited at
least a ‘moderate’ response, and there were 17 patients (89.47%)
with ‘excellent’ improvement (Table
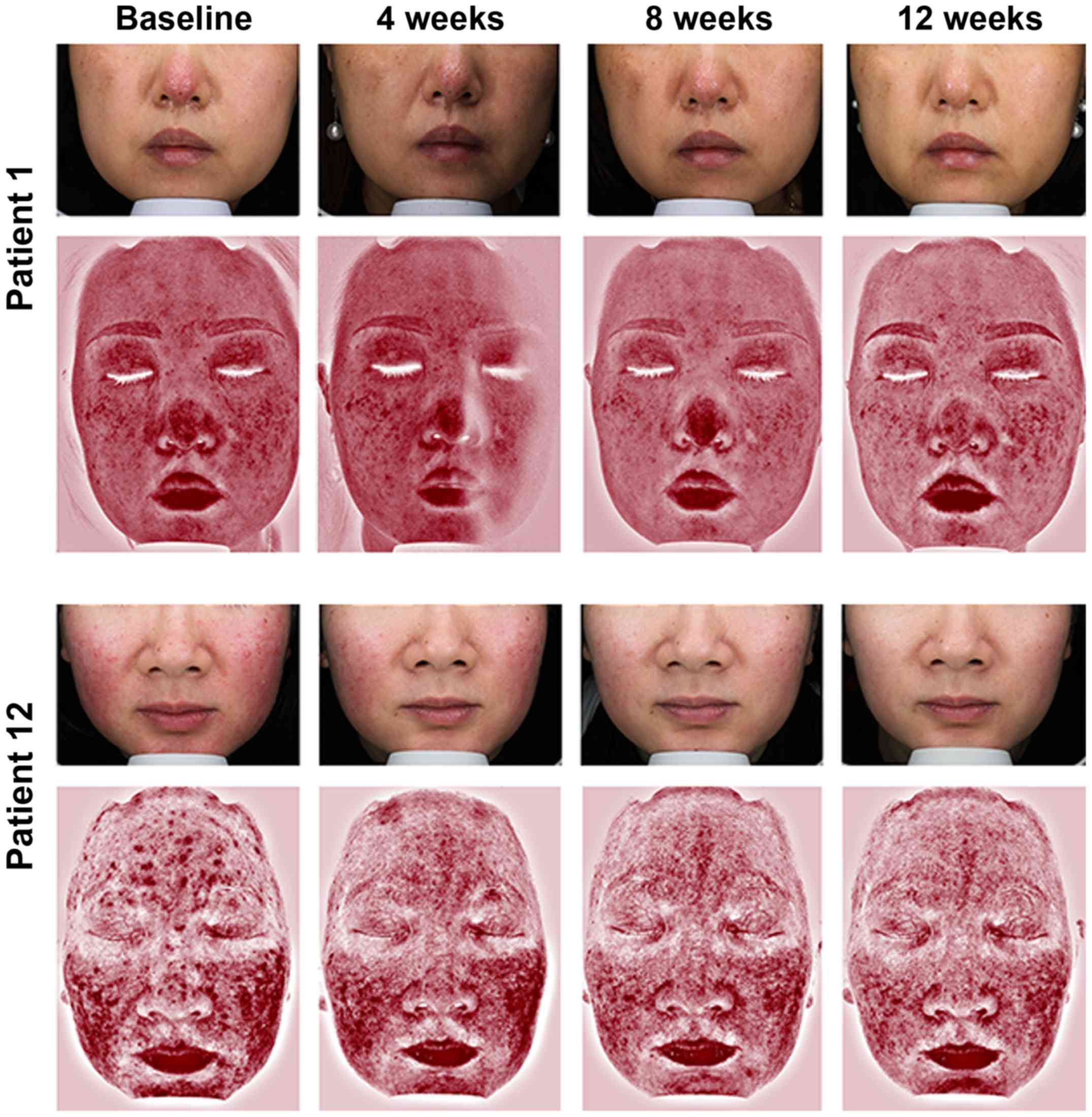
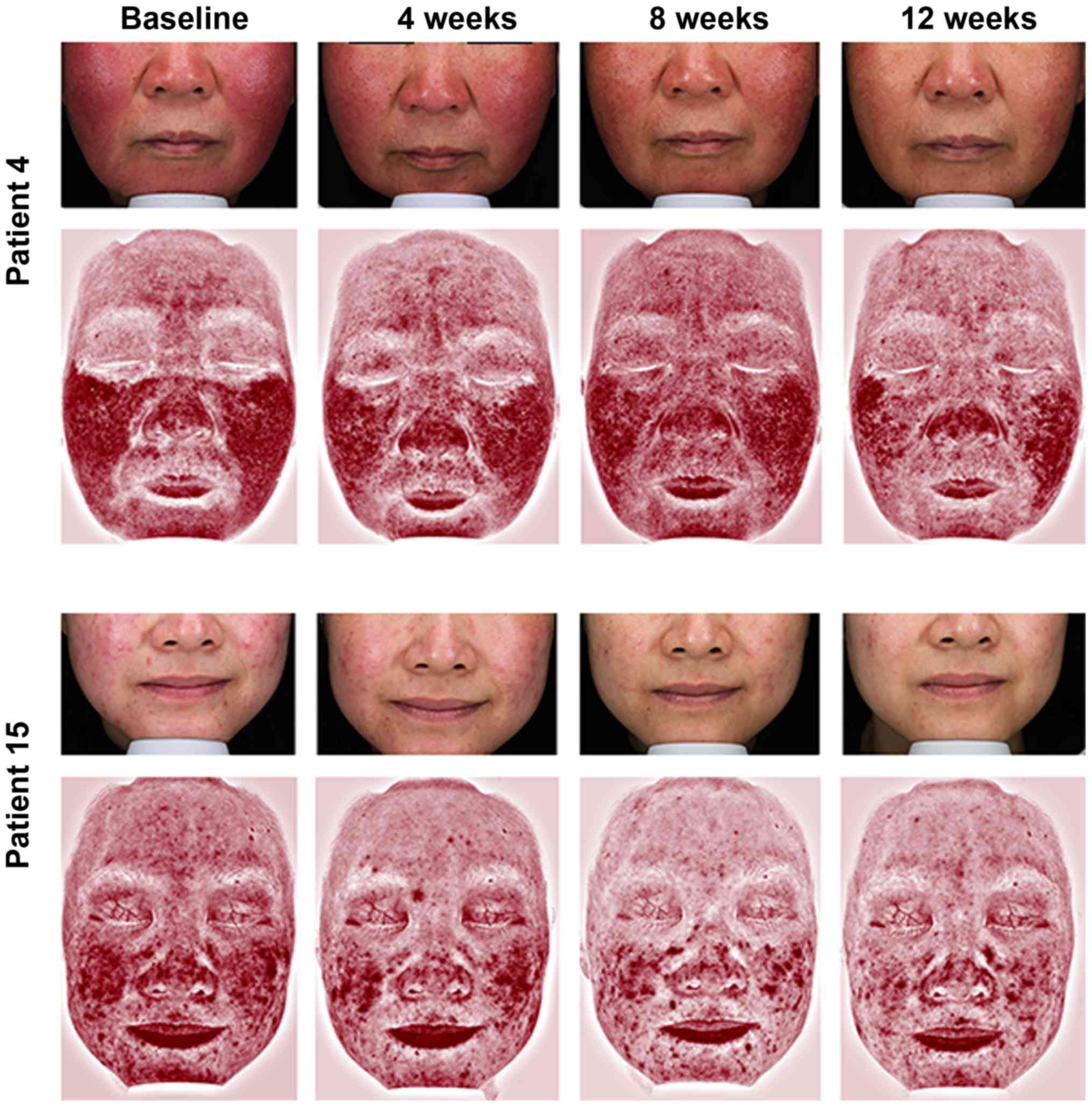
II). Representative photographs and redness area on VISIA of
four patients are shown in Figs. 2
and 3. None of the patients were
scored as ‘no response’ or ‘worsening’. After the last treatment,
17 patients (89.47%) reported the improvement of rosacea as ‘good’,
2 patients reported ‘fair’, and no patients reported ‘poor’ or
‘even worse’. There were 3 patients who reported mild burning
sensation during the first SA chemical peel, while no adverse
reactions were observed for other patients during the visit. At 4-
and 8-week follow-up visits, no patients reported a relapse or
worsening.
 | Table IIInvestigator Global Assessment of
improvement of the 19 patients. |
Table II
Investigator Global Assessment of
improvement of the 19 patients.
| Treatment
duration | Clear | Excellent | Moderate | Mild | No response | Worsening |
|---|
| 4 weeks | 0 (0.00) | 1 (5.26) | 10 (52.63) | 8 (42.11) | 0 (0.00) | 0 (0.00) |
| 8 weeks | 0 (0.00) | 6 (31.58) | 12 (63.16) | 1 (5.26) | 0 (0.00) | 0 (0.00) |
| 12 weeks | 8 (42.11) | 9 (47.37) | 2 (10.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
Discussion
The precise pathogenesis of rosacea is complex and
remains unclear, although it is currently thought to have a genetic
background, with neurovascular dysfunction, disorganized innate and
acquired immunity (12). Karpouzis
et al (13) observed a
statistically significant predominance of tachykinin receptor 3
rs3733631 G allele in PPR, indicating it may predispose the
evolution of the disease. Chang et al (14) suggested a role for antigen
presentation by class II HLA in the etiology of rosacea by
genome-wide association study (GWAS). Similarly, a GWAS by Aponte
et al (15) reported that
rosacea is associated with gene regions that are involved in an
inflammatory component. In the development of PPR, innate immune
mediators, including Toll-like receptor (TLR) and
nucleotide-binding oligomerization domain (NOD)-like receptor, can
be activated by extracellular pathogen- or damage-associated
molecular patterns and are required for innate immune peptide
cathelicidin and inflammasome activation, which causes inflammatory
infiltration and induces the activation of adaptive immune cells,
facilitating the development of inflammatory papules and pustules
(12). Symptoms of PPR are
bothersome to patients and have a negative effect on their quality
of life (16). Triggers include
microbes, ultraviolet radiation and stress, which can stimulate
receptors such as TLR and NOD-like receptor. Dysregulated receptors
induce the activation of neurogenic inflammation and innate immune
pathways, such as the inflammasome activation and NF-kB pathways,
resulting in abnormally sensitive skin and the production of
distinct cytokines, chemokines, matrix metalloproteinases (MMPs)
and prostanoids, which may facilitate the clinical manifestation of
erythema, papules and pustules (12). However, treatment of PPR is
difficult, particularly in patients with underlying persistent
erythema (17). According to these
mechanisms, the current therapeutics for PPR mainly include trigger
avoidance, oral antimicrobials and topical agents to control
inflammatory reactions (9). The
present study reviewed the combination treatment of minocycline and
SSA 30% chemical peel for PPR in Department of Dermatology, West
China Hospital, Sichuan University. The results of present study
showed a gradual decrease in erythema and inflammatory lesions in
19 patients with PPR after receiving this treatment. Objective and
subjective symptoms improved significantly without any relapse
during the 8-week follow-up visit.
Despite the approval of doxycycline for the
treatment of rosacea in 2006 by the USA Food and Drug
Administration, subsequent studies have demonstrated that
minocycline is effective and could be a good alternative treatment
for those patients who, for any reason, are unable or unwilling to
receive doxycycline (8,18). A study by van der Linden et al
(8) suggested that the efficacy of
minocycline is comparable with that of doxycycline in treating PPR,
and patients treated with minocycline group possessed a better
quality of life compared with those treated with doxycycline.
Minocycline, a type of broad-spectrum tetracycline, inhibits
various pathways of inflammation. Its anti-inflammatory effect is
attributed to the inhibition of MMPs, bacterial products that
stimulate inflammation and phospholipase A2, and the suppression of
neutrophil migration and chemotaxis, which play important roles in
the pathogenesis of PPR (8,12,19).
Furthermore, minocycline can increase the hydration of the stratum
corneum, which may facilitate the repair of the epidermal barrier
function. Although uncommon, tetracycline can cause
hyperpigmentation as a side effect, and there is an association
between the duration of minocycline intake and the duration of
pigmentation, with a median duration of 17 months (20). Therefore, when using minocycline,
patients are required to avoid sunlight and long-term use.
SA at a 30% concentration is a naturally active
ingredient with anti-inflammatory and keratin-exfoliating
properties, which has been widely used in skin diseases,
particularly in acne vulgaris (21).
SA decreases sebum secretion through downregulation of the
adenosine monophosphate-activated protein kinase/sterol regulatory
element-binding protein-1 signaling pathway, and antagonizes the
inflammatory response through inhibition of the NF-κB signaling
pathway (22). In addition, SA at
20-30% concentration can disrupt intercorneocyte cohesion, causing
a rapid differentiation of keratinocytes and peeling the entire
epidermis, resulting in the reorganization of the epidermis and the
removal of excess melanin (23,24). Due
to its antioxidant properties and inhibition of tyrosinase
expression, SA can be used to treat pigmented dermatosis (25). However, SA is insoluble in water due
to its lipophilic characteristics, and requires the addition of an
alcoholic solution to be completely dissolved. With reversible and
non-covalent bonding to form a water-soluble SSA complex, SSA 30%
is characterized by slow release upon application, and achieves its
maximum efficacy at low pH, reducing skin irritation (10).
Despite previous studies illustrating the treatment
of rosacea with minocycline combined with other methods, such as
pulsed dye laser and tranexamic acid, to the best of the authors'
knowledge, there have been few studies written in English on the
effectiveness of oral minocycline and chemical peel in rosacea or
PPR (18,26). Based on the advantages of minocycline
and SSA, this combination modality was applied to treat PPR in the
present study. Patients experienced a marked improvement in
inflammatory lesions with regard to their clinical manifestations
and redness area on VISIA. After 8 weeks, the majority of patients
exhibited a moderate response. After the last treatment and
follow-up for an additional 8 weeks, all patients experienced
improvement and reported no obvious side effects. Although the
exact mechanism by which SA acts in PPR is not clear, two factors
are hypothesized to support its functional mechanism. First, SSA
30% can inhibit inflammation via the NF-κB signaling pathway and
inhibit interleukin-1β and tumor necrosis factor involved in
rosacea (12,22). Second, the chemical exfoliation and
whitening effect of SSA 30% facilitate the reorganization of the
epidermis and prevent pigmentation.
There are certain limitations to the present study.
First, the number of patients was limited. Second, it was a
retrospective analysis with no control group; strict comparison
between single and combination regimens should be performed for
PPR. Third, further high-quality, prospective, blinded, controlled
clinical trials are required to evaluate the efficacy of this
retrospective study.
In conclusion, the present study demonstrates that
combination treatment with minocycline and SSA 30% is effective for
PPR. This retrospective study is of significance in guiding
clinical practice, and provides a new combination therapy that may
be safe and effective in treating PPR.
Acknowledgements
The authors are grateful to Xiao-qin Xie (bachelor's
degree) and Dan HAO (PhD) from Department of Dermatology, West
China Hospital, Sichuan University for their comments on this paper
and to Shanghai Rui Zhi Medicine Technology Co., Ltd., Shanghai,
China for its technical support.
Funding
This work was financially supported by the
transverse project of West China hospital (grant nos. HX-H1704048
and HX-H1902027).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW, XW and XJ conceived and designed the study. LW,
XHL, XXL, YL and XJ performed the experiments. LW and XW analyzed
the data. DD assisted in the study design and discussed and
interpreted the data. LW, XHL, and XJ wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
ethical guidelines of the Declaration of Helsinki and approved by
Medical Ethics and Human Research Committee of West China Hospital
(no. 2017.163).
Patient consent for publication
Written informed consent was obtained from each
patient. All patients agreed to the publication of their
photographs and their clinical data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rainer BM, Kang S and Chien AL: Rosacea:
Epidemiology, pathogenesis, and treatment. Dermatoendocrinol.
9(e1361574)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wilkin J, Dahl M, Detmar M, Drake L,
Feinstein A, Odom R and Powell F: Standard classification of
rosacea: Report of the national rosacea society expert committee on
the classification and staging of rosacea. J Am Acad Dermatol.
46:584–587. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Two AM, Wu W, Gallo RL and Hata TR:
Rosacea: Part I. Introduction, categorization, histology,
pathogenesis, and risk factors. J Am Acad Dermatol. 72:749–760.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Piwnica D, Rosignoli C, de Menonville ST,
Alvarez T, Schuppli Nollet M, Roye O, Jomard A and Aubert J:
Vasoconstriction and anti-inflammatory properties of the selective
α-adrenergic receptor agonist brimonidine. J Dermatol Sci.
75:49–54. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Serowka KL, Saedi N, Dover JS and Zachary
CB: Fractionated ablative carbon dioxide laser for the treatment of
rhinophyma. Lasers Surg Med. 46:8–12. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wladis EJ and Adam AP: Treatment of ocular
rosacea. Surv Ophthalmol. 63:340–346. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schaller M, Almeida LM, Bewley A, Cribier
B, Dlova NC, Kautz G, Mannis M, Oon HH, Rajagopalan M, Steinhoff M,
et al: Rosacea treatment update: Recommendations from the global
ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 176:465–471.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van der Linden MMD, van Ratingen AR, van
Rappard DC, Nieuwenburg SA and Spuls PI: DOMINO, doxycycline 40 mg
vs. minocycline 100 mg in the treatment of rosacea: A randomized,
single-blinded, noninferiority trial, comparing efficacy and
safety. Br J Dermatol. 176:1465–1474. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McGregor SP, Alinia H, Snyder A, Tuchayi
SM, Fleischer A Jr and Feldman SR: A review of the current
modalities for the treatment of papulopustular rosacea. Dermatol
Clin. 36:135–150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng Y, Yin S, Xia Y, Chen J, Ye C, Zeng
Q and Lai W: Efficacy and safety of 2% supramolecular salicylic
acid compared with 5% benzoyl peroxide/0.1% adapalene in the acne
treatment: A randomized, split-face, open-label, single-center
study. Cutan Ocul Toxicol. 38:48–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dall'Oglio F, Lacarrubba F, Luca M,
Boscaglia S and Micali G: Clinical and erythema-directed imaging
evaluation of papulo-pustular rosacea with topical ivermectin: A 32
weeks duration study. J Dermatolog Treat. 30:703–707.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Holmes AD and Steinhoff M: Integrative
concepts of rosacea pathophysiology, clinical presentation and new
therapeutics. Exp Dermatol. 26:659–667. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karpouzis A, Avgeridis P, Tripsianis G,
Gatzidou E, Kourmouli N and Veletza S: Assessment of tachykinin
receptor 3' gene polymorphism rs3733631 in rosacea. Int Sch Res
Notices. 2015(469402)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chang ALS, Raber I, Xu J, Li R, Spitale R,
Chen J, Kiefer AK, Tian C, Eriksson NK, Hinds DA and Tung JY:
Assessment of the genetic basis of rosacea by genome-wide
association study. J Invest Dermatol. 135:1548–1555.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aponte JL, Chiano MN, Yerges-Armstrong LM,
Hinds DA, Tian C, Gupta A, Guo C, Fraser DJ, Freudenberg JM, Rajpal
DK, et al: Assessment of rosacea symptom severity by genome-wide
association study and expression analysis highlights
immuno-inflammatory and skin pigmentation genes. Hum Mol Genet.
27:2762–2772. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu Y, Fu C, Zhang W, Li C and Zhang J: The
dermatology life quality index (DLQI) and the hospital anxiety and
depression (HADS) in Chinese rosacea patients. Psychol Health Med.
23:369–374. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee WJ, Lee YJ, Lee MH, Won CH, Chang SE,
Choi JH and Lee MW: Prognosis of 234 rosacea patients according to
clinical subtype: The significance of central facial erythema in
the prognosis of rosacea. J Dermatol. 43:526–531. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kwon HJ, Suh JH, Ko EJ and Kim BJ:
Combination treatment of propranolol, minocycline, and tranexamic
acid for effective control of rosacea. Dermatol Ther 30, 2017.
|
|
19
|
Perret LJ and Tait CP: Non-antibiotic
properties of tetracyclines and their clinical application in
dermatology. Australas J Dermatol. 55:111–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS
and Allam BF: Skin pigmentation due to minocycline treatment of
facial dermatoses. Br J Dermatol. 129:158–162. 1993.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thuangtong R, Tangjaturonrusamee C,
Rattanaumpawan P and Ditre CM: Comparison of salicylic acid 30%
peel and pneumatic broadband light in the treatment of mild to
moderately severe facial acne vulgaris. Cutis. 100:43–48.
2017.PubMed/NCBI
|
|
22
|
Lu J, Cong T, Wen X, Li X, Du D, He G and
Jiang X: Salicylic acid treats acne vulgaris by suppressing
AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 28:786–794.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sgontzou T, Armyra K, Kouris A, Bokotas C
and Kontochristopoulos G: Repeated salicylic acid peels for the
treatment of hyperplastic sebaceous glands in hypohidrotic
ectodermal dysplasia. J Cosmet Laser Ther. 16:293–295.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Monheit GD and Chastain MA: Chemical
peels. Facial Plast Surg Clin North Am. 9:239–255, viii.
2001.PubMed/NCBI
|
|
25
|
Amann R and Peskar BA: Anti-inflammatory
effects of aspirin and sodium salicylate. Eur J Pharmacol. 447:1–9.
2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ko HS, Suh YJ, Byun JW, Choi GS and Shin
J: Pulsed dye laser treatment combined with oral minocycline
reduces recurrence rate of rosacea. Ann Dermatol. 29:543–547.
2017.PubMed/NCBI View Article : Google Scholar
|