Introduction
Keratoconus, also known as corneal ectasia or
corneal dilatation, is a non-inflammatory dilating disease of the
eyes that affects the cornea; the clinical manifestations are
progressive corneal thinning and protrusion leading to progressive
myopia, irregular astigmatism and corneal scarring (1). Keratoconus is a disease in which the
biomechanical properties of the cornea are gradually weakened by
changes in corneal collagen fiber structure and extracellular
matrix, as well as apoptosis of corneal cells (2). A recently developed technique known as
riboflavin/ultraviolet (UV) corneal collagen cross-linking (CXL)
may alleviate these pathological changes associated with
keratoconus, thereby effectively preventing the development of
secondary corneal dilation after keratoconus or refractive surgery
(3); however, the standard early
treatment plan is time-consuming. For patients with a thin cornea,
there is also a risk of damaging corneal endothelial cells; thus,
epithelial-off CXL should only be used in patients with a thick
cornea.
Accelerated transepithelial CXL (ATE-CXL) is a novel
and minimally invasive technique in which the use of
high-irradiation pulsed UV light significantly reduces the
treatment duration. In the present study, a pre- and post-control
study design was used to evaluate changes in clinical
characteristics after ATE-CXL, assess the safety and effectiveness
of this treatment and analyze the reproducibility and stability of
the long-term effects on keratoconus progression.
Materials and methods
Subjects and grouping
Patients diagnosed with advanced keratoconus who
underwent monocular or binocular ATE-CXL were enrolled between
January 2014 and December 2017 in the present prospective,
non-randomized, controlled clinical study. The inclusion criteria
were as follows: i) Primary keratoconus, corneal topography
indicating progressive enlargement of keratoconus lesions, maximal
curvature increased by ≥1 D or optometry astigmatism increased by
≥1 D, or equivalent spherical degree increased by ≥0.5 D; ii)
vision loss or corrected visual acuity (VA) <20/20; iii) at
least one of the following positive signs on slit lamp examination:
Thinning of the corneal stroma, tapered forward bulging, Fleischer
ring, Vogt line, or epithelial or subepithelial scar; iv) central
diopter of the corneal surface >47 D, difference between 3 mm
below the corneal center and the upper 3 diopters >3 D, and
difference between the central anterior surface of the cornea >1
D determined on corneal topographic examination. The exclusion
criteria were as follows: Thinnest corneal thickness (TCT) <340
µm; endothelial cell density (ECD) <2,000/mm2;
post-elastic membrane rupture matrix edema; acute keratoconus;
corneal trauma or secondary corneal ectasia after surgery; ocular
diseases other than keratoconus and refractive error; systemic
diseases affecting the eye, including collagen disease; and
pregnancy. Ultimately, 70 cases with 96 eyes (48 males and 22
females; age range, 13-30 years) with the time from disease onset
ranging from 0.52 to 120 months were included in the study. The
follow-up time was 3 years. According to the Amsier-Krumeich
grading standard and corneal thickness (4), different keratoconus types were divided
into the following four groups according to the surgical method
that was used: Group A, ATE-CXL central; group B, accelerated
epithelial-off (A)-CXL central; group C, ATE-CXL peripheral; and
group D, A-CXL peripheral. The treatment procedures were as
follows: Group A, ATE-CXL for central keratoconus [maximum corneal
curvature (Kmax)] <3 mm from the cornea; 340 µm
<TCT<400 µm; n=22 cases, 34 eyes); group B, A-CXL for central
keratoconus (Kmax <3 mm from the cornea; TCT >400
µm; n=17 cases, 26 eyes); group C, ATE-CXL for peripheral
keratoconus (Kmax in the central 3 mm of the cornea, 340
µm <TCT <400 µm; n=16 cases, 18 eyes; and group D, A-CXL for
peripheral keratoconus (Kmax outside the 3 mm corneal
area, TCT >400 µm; n=15 cases of 18 eyes).
Pre-operative examination
Follow-up examination included evaluation of
uncorrected distant (UD)VA, best-corrected distant (BD)VA, apparent
optometry, compensated intraocular pressure (IOPcc),
Kmax, mean corneal curvature (Km), TCT,
corneal astigmatism (CA), anterior corneal elevation (ACE) and
corneal ECD.
TCT was measured by optical coherence tomography
(Heidelberg Engineering Ltd.) using an Scheimpflug imaging corneal
topographer (Pentacam HR software v.6.07r12; Oculus Optikgeräte
GmbH). Corneal topography (Fig. 1)
and corneal ECD were measured using a corneal endothelial cell
counter (SP-2000P; Topcon Corporation).
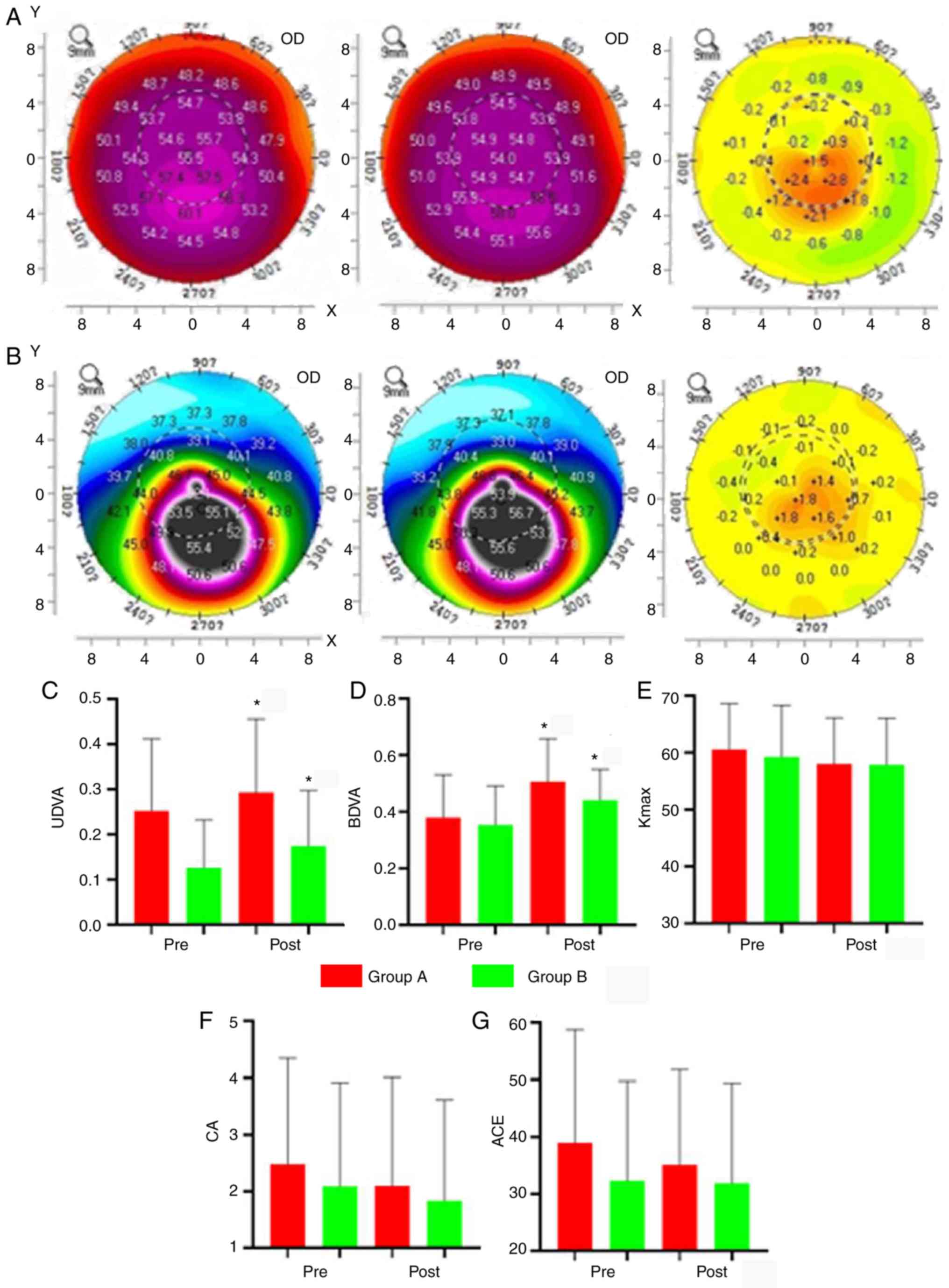 | Figure 1Pre- and post-operative results of
groups A and B. (A) Left: Pre-operative topographic map of group A,
indicating a steep lower central region with high curvature.
Centre: After 3 years, the cornea had an eccentric oval shape with
a steep area. Right: Differences between the pre- and
post-operative topographic maps, which indicates that the high
pre-operative curvature of the central region was decreased and
flattened after surgery, whereas the low pre-operative curvature of
the surrounding region was increased and became steeper. (B) Left:
Pre-operative topographic map of group B, indicating high curvature
in the lower region, lower curvature in the peripheral region and
the lowest curvature in the central symmetric region. Centre: After
3 years, the cornea had an eccentric irregular shape with a steep
region at the center and high curvature. Right: The differences
between the pre- and post-operative topographic maps, which
indicates that the curvature of the region with high pre-operative
curvature was reduced and flattened, and that of the region with
low pre-operative curvature was increased and became steeper. (C-G)
Comparisons of parameters post-surgery with the baseline. (C) UDVA,
and (D) BDVA were significantly decreased. (E) Kmax, (F)
CA and (G) ACE in groups A and B appeared decreased after the
operation, though this was not significant. *P<0.05.
Groups: A, accelerated transepithelial-CXL central; B, accelerated
epithelial-off CXL central. CXL, corneal collagen crosslinking;
Pre, prior to surgery; Post, following surgery; OD, optical
density; UDVA, uncorrected distant visual acuity; ACE, anterior
corneal elevation; BDVA, best-corrected distant visual acuity; CA,
corneal astigmatism; ECD, corneal endothelial cell density;
Kmax, maximum corneal curvature. |
ATE-CXL
The septum was opened and surface anesthetic was
applied to the cornea, which was infiltrated with ParaCel solution
(Avedro, Inc.) every 90 sec for a total induction time of 4 min.
The corneal surface was rinsed and then infiltrated with VibeXXtra
solution (0.25% riboflavin isotonic saline solution; Avedro, Inc.)
every 90 sec for a total induction time of 6-10 min. After washing
with balanced salt solution (BSS), the cornea was subjected to
ATE-CXL with UV radiation cross-linking reinforcement. The
irradiation intensity was 45 mW/cm2, the irradiation
spot diameter was 9 mm and a pulse irradiation mode was used
(interval: 1 sec on/1 sec off). The total irradiation time was 320
sec and the total irradiation energy was 7.2 J/cm2.
During irradiation, 5% keratin solution (Shanghai Xianding
Biotechnology Co., Ltd.) was applied to keep the corneal surface
moist. The cornea was rinsed with BSS and eye drops were applied
once. A sterile corneal bandage lens and an eye mask were applied
to complete the procedure. There was no difference between the
peripheral and central keratoconus operations and the eye position
was not adjusted.
A-CXL
The septum was opened and after soaking the cornea
with 20% alcohol for 15 sec under topical anesthesia, a 9-mm
diameter corneal epithelial ring saw was used to remove the
epithelium in the central region of the cornea. The cornea was
covered with 0.1% riboflavin solution and the process was repeated
every 90 sec for 10 min. The cornea was rinsed with BSS and A-CXL
was performed by UVA radiation cross-linking reinforcement using an
Avedro corneal remodeling platform (Avedro, Inc.) with the
following settings: Spot diameter, 9 mm; pulse irradiation mode
with pulse irradiation interval of 1 sec on/1 sec off; total
energy, 7.2 J/cm2; power intensity, 30
mW/cm2; induction time, 10 min, UV pulse time, 8 min;
and actual action time, 4 min. During irradiation, keratin solution
was used to keep the corneal surface moist and afterwards, the
cornea was rinsed with BSS. Eye drops were applied, followed by a
sterile soft corneal bandage lens along with an eye mask. There was
no difference between the peripheral and central keratoconus
operations and the eye position was not adjusted.
Post-operative treatment and
examination
At 1 day after surgery, antibiotics [levofloxacin
(Clonidine) eye drops] were applied 4 times a day, 1 drop each time
along with artificial tears (polyvinyl alcohol eye drops; Ruizhu
PVA 20; Hubei Yuanda Tiantianming Pharmaceutical Co., Ltd.) once
daily, 1 drop each time. When the epithelium had completely healed,
the contact lens was removed and tobramycin dexamethasone eye drops
were applied 4 times a day for 1 week. Timolol eye drops (Wuhan
Wujing Pharmaceutical Co., Ltd) were applied twice per day and
tobramycin dexamethasone eye drops (Hangzhou Minsheng
Pharmaceutical Co., Ltd) were used once a week for 4 weeks until
inflammation of the ocular surface subsided. During this period,
patients were regularly examined and the cornea and IOP were
closely monitored. UDVA, BDVA, apparent optometry, IOPcc,
Kmax, Km, CA, TCT, ACE and ECD were recorded
3 years after the procedure.
Statistical analysis
Data were analyzed using SPSS v.20.0 software (IBM
Corp.) and values are expressed as the mean ± standard deviation.
Pre- and post-operative changes in the same group were analyzed
with the paired-samples t-test, while one-way analysis of variance
and Student-Newman-Keuls were was used for inter-group comparisons.
The sex ratio was compared by χ2 analysis, and t-test
and binary logistic regression analysis were used to evaluate
associations between UDVA, BDVA, Kmax, CA, ACE, TCT and
ECD. P<0.05 was considered to indicate statistical
significance.
Results
Comparative analysis of general data
between groups A and B and groups C and D
Regarding the clinical data, there were no
statistically significant differences in terms of age, sex, onset,
Kmax position or corneal thickness between groups A and
B and between groups C and D (P>0.05; Table I).
 | Table IBasic information of the patients at
baseline. |
Table I
Basic information of the patients at
baseline.
| Group | Patients
(eyes) | Age (years) | Sex
(male/female) | Onset (months) | Kmax
position (mm) | Corneal thickness
(µm) |
|---|
| Total | 70(96) | 20.5±4.1 | 48/22 | 20.5±25.5 | 2.6±1.7 | 430.68±56.33 |
| A | 22(34) | 19.6±3.2 | 12/10 | 20.4±27.8 |
2.1±0.8b |
423.43±63.88b |
| B | 17(26) | 21.5±2.4 | 16/1 | 21.4±26.8 |
1.8±0.7a |
467.75±41.83a |
| C | 16(18) | 18.5±2.3 | 13/3 | 20.5±22.6 |
4.6±1.3a,b |
418.22±45.77b |
| D | 15(18) | 19.5±5.1 | 7/8 | 22.5±23.4 |
4.3±1.5a,b |
463.55±48.22a |
Post-operative outcomes of ATE-CXL and
A-CXL for patients with central keratoconus
In group A, UDVA and BDVA differed significantly
pre- vs. post-surgery (P<0.05; Table
II); however, there were no significant changes in
Kmax, CA, TCT, ECD, IOP and ACE values (P>0.05). In
group B, UDVA and BDVA differed significantly pre- vs. post-surgery
surgery (P<0.05), but there were no changes in the values of
Kmax, CA, ACE, TCT or ECD (P>0.05; Table II). On the other hand, there was a
significant correlation between the change in IOP and changes in
BDVA and TCT after surgery as compared to the pre-operative values
in group B. IOP was not significantly different between the post-
and pre-operative stages in groups A and B (Fig. 2A and B).
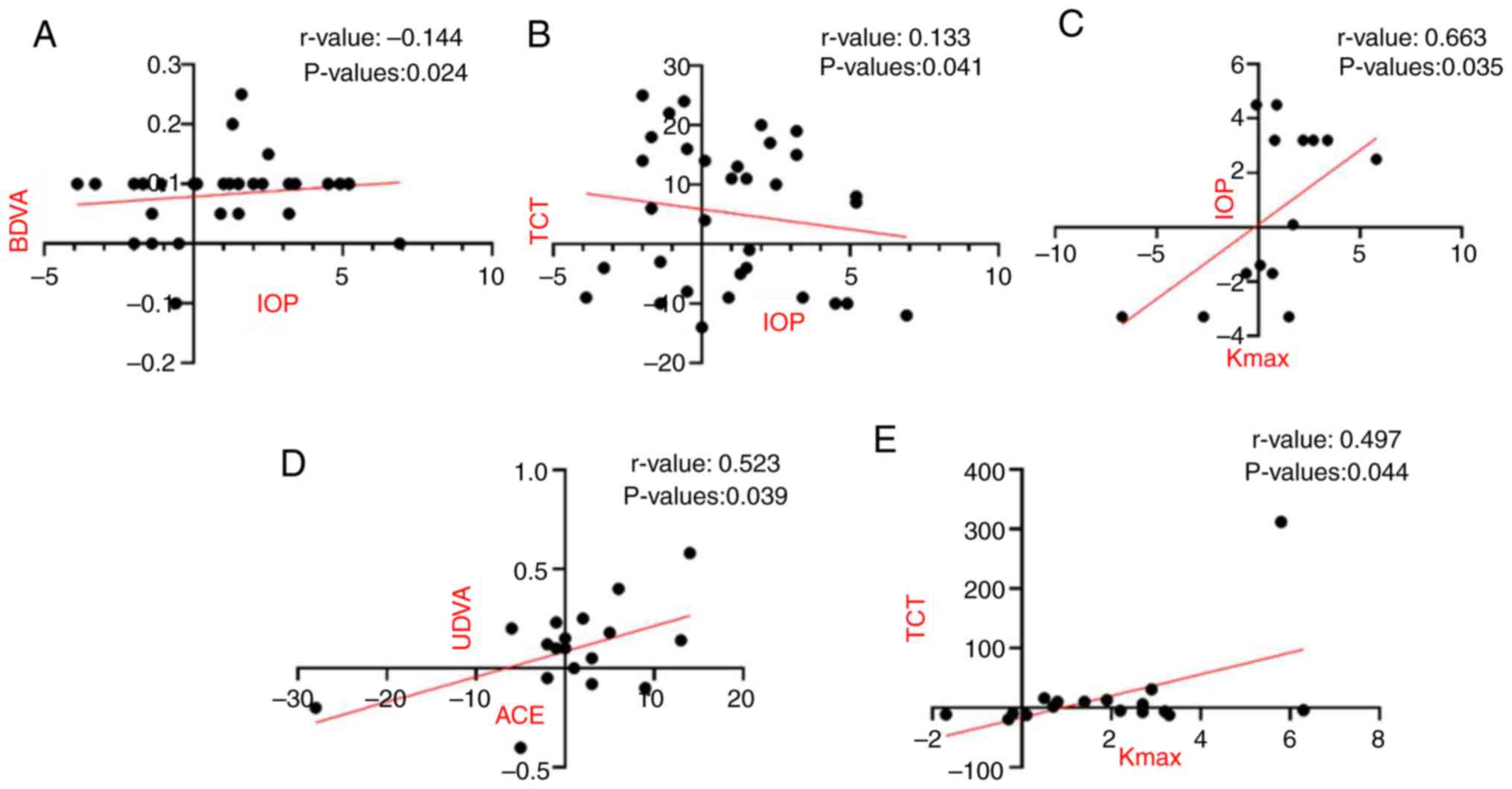 | Figure 2Correlation analysis. (A and B) In
group A, the difference between pre- and post-operative ∆IOP values
(A) decreased with increasing BDVA and (B) increased as a function
of TCT. (C) In group C, the pre-operative Kmax increased
with greater changes in IOP. (D) In group D, the difference between
pre- and post-operative ∆ACE increased with the change in UDVA,
while (E) the change in ∆Kmax increased with greater
change in TCT; these values were significantly correlated. Groups:
A, ATE-CXL central; B, A-CXL central; C, ATE-CXL peripheral; D,
A-CXL peripheral. ATE-CXL, accelerated transepithelial CXL; A-CXL,
accelerated epithelial-off CXL; CXL, corneal collagen crosslinking;
ACE, anterior corneal elevation; BDVA, best-corrected distant
visual acuity; CA, corneal astigmatism; Kmax, maximum
corneal curvature; TCT, thinnest corneal thickness; UDVA,
uncorrected distant visual acuity; IOP, intraocular pressure. |
 | Table IIDifferences (post- vs.
pre-operatively) in four groups. |
Table II
Differences (post- vs.
pre-operatively) in four groups.
| A, Group A
(n=34) |
|---|
| Parameter |
Pre-operatively |
Post-operatively | t | P-value |
|---|
| UDVA | 0.25±0.16 | 0.28±0.17 | -0.446 | 0.032 |
| BDVA | 0.38±0.18 | 0.50±0.19 | -5.831 | 0.003 |
|
Kmax | 64.70±14.00 | 59.30±9.30 | 3.539 | 0.373 |
| CA | 4.80±3.10 | 3.60±2.00 | 2.700 | 0.061 |
| TCT | 423.43±63.88 | 410.35±59.87 | 2.511 | 0.304 |
| ACE | 38.80±20.30 | 33.70±14.50 | 1.693 | 0.063 |
| ECD |
2,376.38±132.71 |
2,224.15±174.22 | 2.888 | 0.054 |
| IOP | 15.13±1.50 | 16.13±1.40 | 2.228 | 0.079 |
| B, Group B
(n=26) |
| Parameter |
Pre-operatively |
Post-operatively | t | P-value |
| UDVA | 0.12±0.09 | 0.15±0.11 | -4.550 | 0.015 |
| BDVA | 0.38±0.12 | 0.44±0.16 | -5.660 | 0.003 |
|
Kmax | 63.80±10.60 | 59.40±9.40 | 5.829 | 0.334 |
| CA | 4.50±3.00 | 3.50±2.30 | 2.189 | 0.069 |
| TCT | 467.75±41.83 | 452.75±38.48 | 1.190 | 0.270 |
| ACE | 35.40±16.20 | 33.80±15.40 | 0.248 | 0.232 |
| ECD |
2,637.48±141.57 |
2,589.52±153.32 | 1.095 | 0.723 |
| IOP | 14.98±1.90 | 16.03±1.70 | 1.838 | 0.072 |
| C, Group C
(n=18) |
| Parameter |
Pre-operatively |
Post-operatively | t | P-value |
| UDVA | 0.22±0.14 | 0.25±0.14 | -2.825 | 0.023 |
| BDVA | 0.28±0.19 | 0.41±0.18 | -6.970 | 0.244 |
|
Kmax | 51.20±5.40 | 45.80±3.50 | 0.743 | 0.032 |
| CA | 2.30±1.80 | 2.10±1.50 | 6.050 | 0.570 |
| TCT | 418.22±45.77 | 402.33±43.42 | 1.056 | 0.837 |
| ACE | 36.60±14.70 | 30.80±12.30 | 2.749 | 0.035 |
| ECD |
2,508.26±252.63 |
2,343.16±110.05 | 6.088 | 0.099 |
| IOP | 16.07±0.42 | 16.49±0.54 | 0.772 | 0.445 |
| D, Group D
(n=18) |
| Parameter |
Pre-operatively |
Post-operatively | t | P-value |
| UDVA | 0.16±0.12 | 0.24±0.15 | -4.484 | 0.031 |
| BDVA | 0.41±0.20 | 0.57±0.15 | -7.198 | 0.064 |
|
Kmax | 56.8±9.60 | 51.6±8.20 | 3.947 | 0.048 |
| CA | 2.40±2.00 | 2.00±1.90 | 2.281 | 0.032 |
| TCT | 463.55±48.22 | 442.36±38.44 | 11.039 | 0.110 |
| ACE | 34.6±13.80 | 32.5±17.20 | 0.288 | 0.042 |
| ECD |
2,496.45±162.38 |
2,324.43±165.67 | 2.154 | 0.131 |
| IOP | 14.34±1.36 | 15.71±0.54 | 2.493 | 0.177 |
Groups A and B did not differ in terms of change in
UDVA value (post- vs. pre-operative; P>0.05); the changes in
Kmax in groups A and B were -5.4±8.0 and -4.4±7.0 D,
respectively, and there was no significant difference between the
two groups (P>0.05). Similarly, the two groups exhibited no
difference in terms of the change in CA (-1.4±2.0 and -1.5±1.0 D,
respectively; P>0.05). The change in ACE in groups A and B was
-4.2±1.6 and -6.3±2.8 µm, respectively, and there was no
statistically significant difference between the two groups either
for ACE (P>0.05) or TCT (P=0.20) (Table II and Fig. 1).
Table II indicates
that there was no statistical difference between group A and group
B before treatment. After treatment, there was remission in both
groups, but group A had a statistically significant improvement in
BDVA (P=0.032) when compared with group B.
Post-operative outcomes of ATE-CXL and
A-CXL in patients with peripheral keratoconus
The differences in UDVA, Kmax and ACE
prior to vs. after surgery in group C were statistically
significant (P<0.01); however, there were no significant
differences in BDVA, CA, TCT or ECD (P>0.05; Table II and Fig. 3). There was a significant correlation
between changes in IOP and Kmax after as compared to
prior to treatment in group C (Fig.
2).
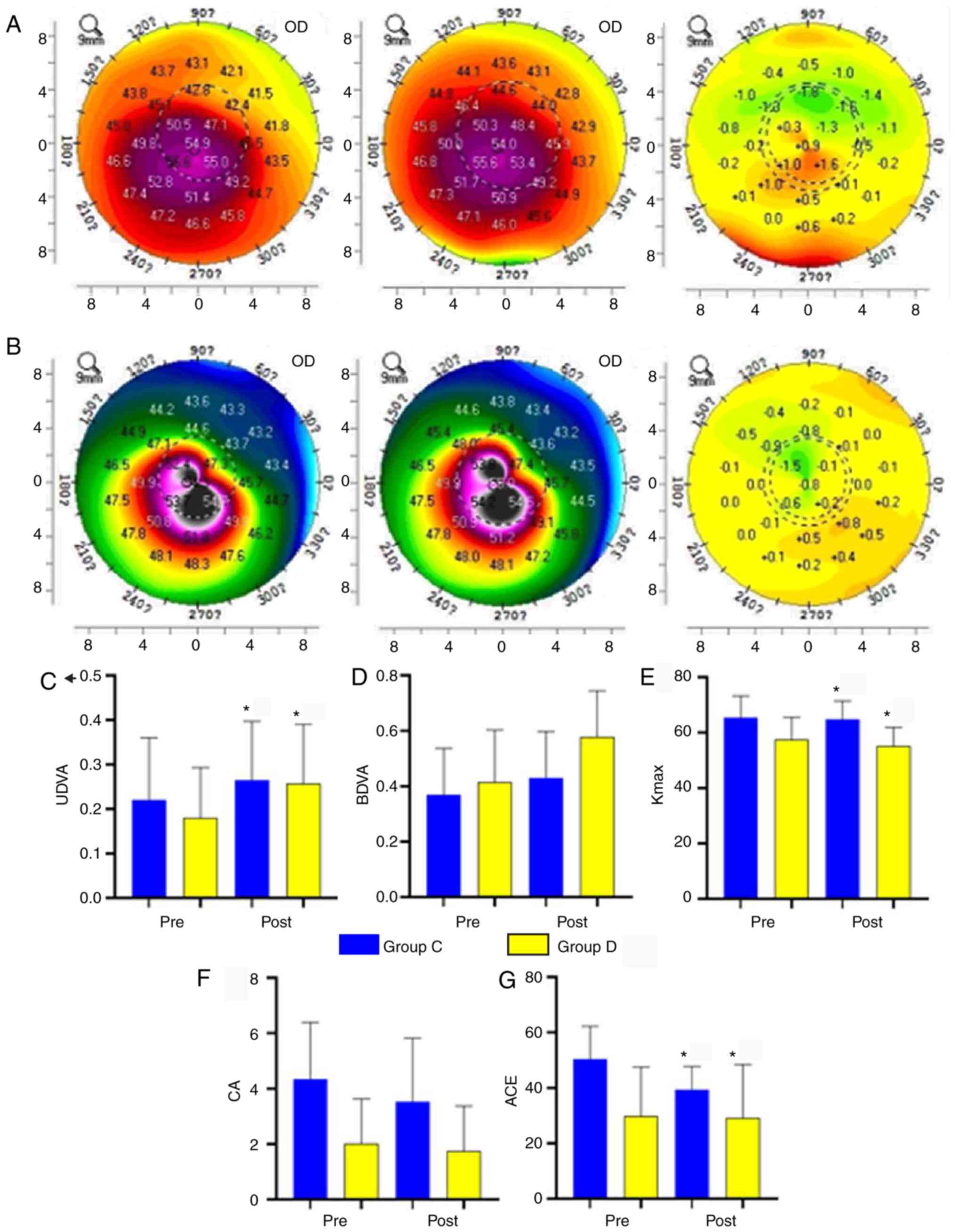 | Figure 3Pre- and post-operative results of
groups C and D. (A) Left: Pre-operative topographic map of group C
comprising a steep region on the temporal side with high curvature
and low curvature in the peripheral region. Centre: After 3 years,
the cornea had an eccentric irregular shape with a steep region in
the center with high curvature. Right: Differences between the pre-
and post-operative topographic maps, which indicates that the
curvature of the topographic region with high pre-operative
curvature continued to increase (i.e., became steeper), whereas the
post-operative curvature was lower in areas with lower
pre-operative curvature. (B) Left: Pre-operative topographic map of
group D, revealing a steep region on the temporal side with high
curvature and reduced curvature in the peripheral region. Centre:
After 3 years, the cornea had an eccentric elliptical shape with a
steep region in the center and high curvature. Right: Differences
in the pre- and post-operative topographic maps, which indicates
that the curvature of the region with high pre-operative curvature
was reduced (i.e., became flattened), whereas that of the region
with low pre-operative curvature was increased (i.e., became
steeper). (C-G) Comparison of parameters post-surgery with the
baseline. (C) UDVA was increased. (D) The increase in BDVA was
insignificant. (E) Kmax was decreased. (F) CA was not
significantly changed. (G) ACE was significantly decreased.
*P<0.05. Groups: C, accelerated transepithelial CXL
peripheral; D, accelerated epithelial-off CXL peripheral. CXL,
corneal collagen crosslinking; Pre, prior to surgery; Post,
following surgery; OD, optical density; UDVA, uncorrected distant
visual acuity; ACE, anterior corneal elevation; BDVA,
best-corrected distant visual acuity; CA, corneal astigmatism; ECD,
corneal endothelial cell density; Kmax, maximum corneal
curvature. |
The differences between pre- and post-surgery UDVA,
Kmax and ACE in group D were significant (P<0.05);
however, there was no significant difference in BDVA (P>0.05;
Fig. 3 and Table II). Post-operative Kmax,
TCT and ECD values differed significantly from those prior to
surgery (P<0.05; Fig. 3; Table II).
The change in UDVA (post- vs. pre-operative) was
similar between groups C and D (P>0.05); however, changes in
Kmax differed significantly, with values of -2.5±1.8 and
-3.2±2.8 D, respectively (P=0.01). The changes in CA values were
also significant at -0.2±0.6 and -0.2±0.8 D, respectively
(P<0.05). There were no differences in the changes in TCT, ACE
and ECD between groups C and D (P=0.20; Table II).
Table III indicates
that there was no statistical difference between group C and group
D before treatment. After treatment, there was remission in both
groups, but group D had a statistically significant improvement in
BDVA (P=0.047), CA (P=0.045) and ACE (P=0.012) compared with group
C.
 | Table IIIThe differences (post-operative vs.
pre-operative) between groups C and D. |
Table III
The differences (post-operative vs.
pre-operative) between groups C and D.
| Group C (n=18) |
|---|
| Examination
Items | Pre-therapy | Post-therapy | t | P-value |
|---|
| UDVA | 0.22±0.14 | 0.25±0.14 | -2.825 | 0.023 |
| BDVA | 0.28±0.19 | 0.41±0.18 | -6.97 | 0.244 |
| Kmax | 51.2±5.4 | 45.8±3.5 | 0.743 | 0.032 |
| CA | 2.3±1.8 | 2.1±1.5 | 6.05 | 0.570 |
| TCT | 418.22±45.77 | 402.33±43.42 | 1.056 | 0.837 |
| ACE | 36.6±14.7 | 30.8±12.3 | 2.749 | 0.035 |
| ECD |
2,508.26±252.63 |
2,343.16±110.05 | 6.088 | 0.099 |
| IOP | 16.07±0.42 | 16.49±0.54 | 0.772 | 0.445 |
| Group D (n=18) | | | | |
|
UDVA | 0.16±0.12 | 0.24±0.15 | -4.484 | 0.031 |
|
BDVA | 0.41±0.20 | 0.57±0.15 | -7.198 | 0.064 |
|
Kmax | 56.8±9.6 | 51.6±8.2 | 3.947 | 0.048 |
|
CA | 2.4±2.0 | 2.0±1.9 | 2.281 | 0.032 |
|
TCT | 463.55±48.22 | 442.36±38.44 | 11.039 | 0.110 |
|
ACE | 34.6±13.8 | 32.5±17.2 | 0.288 | 0.042 |
|
ECD |
2,496.45±162.38 |
2,324.43±165.67 | 2.154 | 0.131 |
|
IOP | 14.34±1.36 | 15.71±0.54 | 2.493 | 0.177 |
| P-value | | | | |
|
UDVA | 0.109 | 0.884 | - | - |
|
BDVA | 0.066 | 0.047 | - | - |
|
Kmax | 0.068 | 0.066 | - | - |
|
CA | 0.074 | 0.045 | - | - |
|
TCT | 0.069 | 0.053 | - | - |
|
ACE | 0.158 | 0.012 | - | - |
|
ECD | 0.875 | 0.995 | - | - |
|
IOP | 0.793 | 0.837 | - | - |
The intergroup comparison shows that ATE-CXL
achieved better control of central keratoconus UDVA, Kmax and CA as
compared with A-CXL (P<0.05).
Discussion
The histopathologic changes observed in keratoconus
are linked to the structural changes in corneal collagen fibers and
biomechanical weakening caused by corneal cell apoptosis. The
UV-riboflavin CXL procedure was developed by a research team at the
University of Dresden, Germany for the treatment of keratoconus and
has been indicated to increase the mechanical strength of collagen
fiber and their capacity for resisting corneal expansion (4,5). In
addition to increasing corneal hardness, cross-linking surgery may
reduce resource utilization and thus alleviate the economic burden
on patients (6). Since its first
application to the treatment of progressive keratoconus in 2003,
corneal collagen cross-linking has been expanded to include a
variety of corneal diseases (7). The
increased tensile strength of the corneal matrix after
cross-linking increases corneal resistance to degradation by
proteolytic enzymes, including matrix metalloproteinase and thermal
damage (8-10).
One study suggested that corneal topography and wavefront
aberration values were stable at 7 years after cross-linking
(11).
There are multiple methods of CXL. EDTA has been
used in patients with keratoconus to enhance riboflavin penetration
and TE-CXL; at the 12-month follow-up, safety indices, including
average uncorrected VA, mean equivalent spherical reduction, mean
simulated corneal curvature K-value and mean surface variance index
were higher in the TE-CXL group compared with those in the control
group (A-CXL) (12), indicating that
TE-CXL effectively inhibits keratoconus progression.
The traditional CXL procedure includes removal of
epithelial tissue with a central corneal diameter of 5-9 mm under
topical anesthesia (13) to
facilitate the entry of riboflavin into the corneal stroma. During
the addition of riboflavin, the corneal surface temperature is
constant and does not result in thermal burns to the corneal tissue
(14). After irradiation, antibiotic
eye ointment is applied and a contact lens soaked with 3 g/l
ofloxacin is worn by the patient until the corneal epithelium has
healed by 8% (15). The
cross-linking therapy for keratoconus is relatively safe; the major
post-operative complication is aseptic infiltration of the corneal
stromal and scarring of the central cornea, which occur at
estimated rates of 7.6 and 2.8%, respectively (16). For patients with a corneal thickness
<400 µm, a hypotonic riboflavin preparation has been used to
induce edema of the corneal stroma and thus increase the thickness
to >400 µm, after which cross-linking therapy achieved good
results (17). An occasional
complication is haze, which reduces the efficacy of steroid eye
drops without affecting best-corrected VA (18). One patient developed diffuse lamellar
keratitis after surgery and another developed herpes simplex
keratitis that healed after treatment (19). Histological sections revealed an
obvious boundary at a depth of 300 µm in the stromal layer,
suggesting that the extent of cross-linking was limited to only the
superficial tissue (17). Thus, the
efficacy of cross-linking therapy for the posterior corneal cone
remains to be determined; this is particularly important in the
light of the fact that changes in corneal hardness and biomechanics
after cross-linking therapy may increase the IOP (20).
Riboflavin/UV (370 nm) CXL is the first treatment
that was indicated to effectively suppress keratoconus progression
(3). The efficacy has been
demonstrated experimentally and the technique lacks numerous
disadvantages of other cross-linking methods (21). The adhesion of collagen fibers
between adjacent layers of the keratoconus that are weakened by CXL
was demonstrated by X-ray (22). In
patients with a corneal thickness of >400 µm, CXL did not cause
any damage to corneal endothelial cells and is therefore considered
to be safe (7,23). It was also reported that CXL is able
to increase corneal stress and strain, reduce swelling and increase
the shrinkage temperature, as well as the tolerance of corneal
stromal tissue to digestive enzymes (24-26).
After CXL, the diameter of collagen fibers within 300 µm of the
pre-corneal stroma was increased and no corneal endothelial cell
damage occurred (27).
The present study indicated that the reduction in
ACE persisted 3 years after ATE-CXL or A-CXL surgery, suggesting
that the hardness of the corneal matrix fibers and tensile strength
were increased, whereas the curvature was decreased by the
cross-linking. At 3 years after CXL, most patients in the ATE-CXL
group had improved UDVA and BDVA and reduced Kmax, CA,
and ACE; however, the differences in Kmax, CA and ACE
were attenuated compared with those in the A-CXL group, indicating
that the transepithelial treatment was less effective than the
epithelial approach.
After the surgery, UDVA and BDVA were significantly
increased in groups A and B. A superior curative effect was
observed in group A, which was reflected in the Kmax
value. On the other hand, group B exhibited greater improvements in
CA and ACE, indicating that while ATE-CXL and A-CXL may be used to
treat central keratoconus, the latter achieves superior
results.
In groups C and D, UDVA was increased, whereas
Kmax and CA were decreased after CXL. VA of the naked
eye was higher in group C than in group D but Kmax and
CA were lower. This provides further evidence that the
transepithelial method is the less effective of the two
cross-linking methods, particularly in patients with peripheral
keratoconus.
Intra-group correlation analysis revealed that the
difference between pre- and post-operative IOP in group C decreased
with increasing difference in BDVA, whereas that of BC increased
with increasing difference in TCT. TCT, IOP and diopter are
presumed to be associated, although this is debated. The corneal
thickness is reduced in myopia (28)
and it has been determined that for each 70-µm decrease, the IOP as
measured with a tonometer is 3.5 mmHg lower than the baseline value
(29); however, other studies have
not indicated any correlation between diopter and IOP (30). By contrast, a larger diopter was
suggested to be associated with higher IOP (31). In the present study, a correlation
was observed between diopter and IOP (P<0.05) that may be linked
to the decrease in corneal thickness caused by reduced IOP, which
increases TCT and results in a superior curative effect. In group
C, a larger change in IOP was correlated with a greater change in
Kmax. The changes in intraocular pressure and BDVA in
group B before and after operation were larger than those in group
A, which indicated that treatment received by Group B patients was
more effective. In group D, the differences between pre- and
post-operative UDVA and ACE values were correlated, which may be
attributed to alterations in corneal shape caused by post-operative
changes in ACE; however, further experimental data are required to
confirm this possibility.
As conical cornea is a progressive complication of
corneal degeneration, it is unable to self-heal, and the incidence
and progression rate of the two eyes in the same patient are
different. The progression rate in different patients also varied.
Therefore, a pre- and post-control study design was adopted in the
present study. By acute observation, changes in patients with
ATE-CXL (UVA parameters: 45 mW/cm2 x320 sec, 7.2
J/cm2) and A-CXL (UVA parameters: 30 mW/cm2
x480 sec, 7.2 J/cm2) were assessed, and observation and
analysis of long-term effects and associated factors of ATE-CXL in
advanced keratoconus treatment were performed to verify the
long-term safety and effectiveness of the surgical treatment of
progressive cone cornea. The predictiveness and stability of
outcomes determined in the present study are expected to contribute
to the guidance and provide a reference for the clinical treatment
of pyramidal cornea.
Of note, the present study had certain limitations.
The left and the right eyes of the patients were not distinguished
at the time of sample collection, which may have affected the
accuracy of the results. In future experiments, the left or right
eye should be used consistently in order to improve the accuracy of
the experimental results.
In conclusion, ATE-CXL and A-CXL are safe and
effective treatment methods for inhibiting keratoconus progression
after 3 years. Central (and to a lesser extent, peripheral)
keratoconus may be effectively controlled by either approach, with
disease stabilization 3 years later. ATE-CXL is associated with
fewer adverse reactions and better patient compliance, which may
reduce post-operative pain and complications caused by
epithelialization. ATE-CXL is the most suitable treatment for
keratoconus of <400 µm with a corneal thickness of >400 µm;
however, A-CXL yields superior long-term outcomes. The relatively
small sample size precluded observation of the outcomes of ATE-CXL
in patients with secondary keratoconus after refractive surgery.
Larger studies with longer follow-up are required to verify the
effectiveness of ATE-CXL for controlling keratoconus.
Acknowledgements
The authors thank Dr Kang Yu (Department of
Ophthalmology, The First Affiliated Hospital of Nanchang
University, Nanchang, China) for assistance with the
preparation/writing of this manuscript.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81660158) and the Natural
Science Key Project of Jiangxi Province (grant no.
20161ACB21017).
Availability of data and materials
The datasets generated and/or analyzed in this study
are available from the corresponding author on reasonable
request.
Authors' contributions
JRH and YS designed the study. CHW, LMG, LFH, HFL,
BL and HJJ collected and analyzed the data. JRH wrote the
manuscript. All authors read and approved the final manuscript.
Ethical approval and informed consent
The study protocol was approved by The Medical
Research Ethics Committee of the Affiliated Eye Hospital of
Nanchang University (Nanchang, China). The experiments were
performed according to relevant guidelines and regulations. All
study subjects were informed of the study purpose and design and
provided written informed consent prior to their participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shalchi Z, Wang X and Nanavaty MA: Safety
and efficacy of epithelium removal and transepit helial corneal
collagen crosslinking for keratoconus. Eye (Lond). 29:15–29.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baum J: On the location of the cone and
the etiology of keratoconus. Cornea. 14:142–143. 1995.PubMed/NCBI
|
|
3
|
Wollensak G: Crosslinking treatment of
progressive keratoconus: New hope. Curr Opin Ophthalmol.
17:356–360. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alió JL and Shabayek MH: Corneal higher
order aberrations: A method to grade keratoconus. J Refract Surg.
22:539–545. 2006.PubMed/NCBI
|
|
5
|
Prausnitz MR and Noonan JS: Permeability
of cornea, sclera, and conjunctiva: A literature analysis for drug
delivery to the eye. J Pharm Sci. 87:1479–1488. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hoyer A, Raiskup-Wolf F, Spörl E and
Pillunat LE: Collagen cross-linking with riboflavin and UVA light
in keratoconus.results from Dresden. Ophthalmologe. 106:133–140.
2009.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
7
|
Wollensak G, Spoerl E and Serler T:
Riboflavin/ultraviolet-a-induced collagen crosslinking for the
treatment of keratoconus. Am J Ophthalmol. 135:620–627.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Santhiago MR, Giacomin NT, Medeiros CS,
Smadja D and Bechara SJ: Intense early flattening after corneal
collagen cross-linking. J Refract Surg. 31:419–422. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sel S, Nass N, Pötzsch S, Trau S, Simm A,
Kalinski T, Duncker GI, Kruse FE, Auffarth GU and Brömme HJ: UVA
irradiation of riboflavin genetates oxygen-dependent hydroxyl
radicals. Redox Rep. 19:72–79. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kopsachilis N, Tsaousis KT, Tsinopoulos
IT, Kruse FE and Welge-Luessen U: A novel mechanism of UVA and
ribollavin-mediated corneal cross-linking through induction of
tissue transglutaminases. Cornea. 32:1034–1039. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
O'Brart DP: Corneal collagen
cross-1inking: A review. J Optom. 7:113–124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Leccisotti A and Islam T: Transepithelial
corneal collagen cross-linking in keratoconus. J Refract Surg.
26:942–948. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Caporossi A, Baiocchi S, Mazzotta C,
Traversi C and Caporossi T: Parasurgical therapy for keratoconus by
riboflavin-ultraviolet type A rays induced cross-linking of corneal
collagen: Preliminary refractive results in an Italian study. J
Cataract Refract Surg. 32:837–845. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mencucci R, Mazzotta C, Rossi F,
Ponchietti C, Pini R, Baiocchi S, Caporossi A and Menchini U:
Riboflavin and ultraviolet A Collagen crosslinking: In vivo
thermographic analysis of the corneal surface. J Cataract Refract
Surg. 33:1005–1008. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Spoerl E, Mrochen M, Sliney D, Trokel S
and Seiler T: Safty of UVA-riboflavin cross-linking of the cornea.
Cornea. 26:385–389. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Koller T, Mrochen M and Seiler T:
Complication and failure rates after corneal crosslinking. J
Cataract Refract Surg. 35:1358–1362. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hafezi F, Mrochen M, Iseli HP and Seiler
T: Collagen crosslinking with ultraviolet-A and hypoosmolar
riboflavin solution in thin corneas. J Cataract Refract Surg.
35:621–624. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mazzotta C, Balestrazzi A, Baiocchi S,
Traversi C and Caporossi A: Stromal haze after combined
riboflavin-UVA corneal collagen cross-linking in keratoconus: In
vivo confocal microscopic evaluation. Clin Exp Ophthalmol.
35:580–582. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kymionis GD, Portaliou DM, Bouzoukis DI,
Suh LH, Pallikaris AI, Markomanolakis M and Yoo SH: Herpetic
keratitis with iritis after corneal crosslinking with riboflavin
and ultraviolet A for keratoconus. J Cataract Refract Surg.
33:1982–1984. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nagy ZZ: Laser in situ keratomileusis
combined with topography-supported customized ablation after
repeated penetrating keratoplasty. J Cataract Refract Surg.
29:792–794. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Spöerl E, Huhle M, Kasper M and Seiler T:
Increased rigidity of the cornea caused by intrastromal
cross-linking. Ophthalmologe. 94:902–906. 1997.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
22
|
Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes
S, Newton RH and Bron AJ: Changes in collagen orientantion and
distribution in keratoconus corneas. Invest Ophthalmol Vis Sci.
46:1948–1956. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wollensak G, Spoerl E, Wilsch M and Seiler
T: Endothelial cell damage after riboflavin-ultraviolet-A treatment
in the rabbit. J Cataract Refract Surg. 29:1786–1790.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Spoerl E, Wollensak G and Seiler T:
Increased resistance of crosslinked cornea against enzymatic
digestion. Curr Eye Res. 29:35–40. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Spoerl E, Wollensak G, Dittert DD and
Seiler T: Thermomechanical behaviour of collagen-cross-linked
porcine cornea. Ophthalmologica. 218:136–140. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wollensak G, Spoerl E and Seiler T:
Stress-strain measurements of human and porcine corneas after
riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract
Surg. 29:1780–1785. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wen D, Song B, Li Q, Tu R, Huang Y, Wang
Q, McAlinden C, O'Brart D and Huang J: Comparison of epithelium-Off
versus transepithelial corneal collagen cross-linking for
keratoconus: A systematic review and meta-analysis. Cornea.
37:1018–1024. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim EJ, Sajjad A, Montes de Oca I, Koch
DD, Wang L, Weikert MP and Al-Mohtaseb ZN: Refractive outcomes
after multifocal intraocular lens exchange. J Cataract Refract
Surg. 43:761–766. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Whitacre MM, Stein RA and Hassanein K: The
effect of corneal thickness on applanationn tonometry. Am J
Ophthalmol. 115:592–596. 1993.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bonomi L, Mecca E and Massa F: Intracular
pressure in myopic anisometropia. Int Ophthalmol. 5:145–148.
1982.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wong TY, Klein BE, Klein R, Knudtson M and
Lee KE: Refractive errors, intraocular pressure, and glaucoma in a
white population. Ophthalmology. 110:211–217. 2003.PubMed/NCBI View Article : Google Scholar
|

















