Introduction
Doxorubicin (DOX) is a highly effective and widely
used chemotherapeutic agent for the treatment of human tumors,
including solid tumors and hematological malignancies (1,2).
However, the severe cardiotoxic side effects associated with DOX
have limited its application in the clinic (1). DOX-induced cardiomyopathy may be caused
by a variety of factors, including oxidative stress (3); however, the exact mechanisms underlying
DOX-induced cardiomyopathy are not completely understood. Cancer
chemotherapy-associated heart disease is a main cause of the
increased mortality of cancer survivors (4,5);
therefore, investigating the mechanisms of action of
anthracyclines, including DOX, is important for the treatment of
tumors and cardiovascular diseases.
As the main active ingredient in Rehmannia,
catalpol belongs to the class of iridoid monosaccharides (6). Previous studies have demonstrated that
catalpol exhibits a variety of biological activities, including
anti-inflammatory (7-9),
antioxidant (10,11), antiapoptotic (12,13) and
hypoglycemic (14,15) effects. Previous studies investigating
catalpol have primarily focused on its protective effects in the
nervous system, and research into the effects of the compound on
the cardiovascular system is in the early stages (16-18).
Previous studies have reported that catalpol displays protective
effects on cardiomyocytes at the cellular level, protecting against
myocardial injury through antioxidation and ameliorating cardiac
dysfunction in rat models (19,20).
However, further studies are required to verify the cardiovascular
protective effects of catalpol.
Peroxisome proliferator-activated receptor (PPAR) is
a ligand-inducible transcription factor that is a member of the
type II nuclear receptor superfamily. PPAR is primarily expressed
in vascular smooth muscle cells, where it activates receptors,
prevents proliferation and migration of vascular smooth muscle
cells, weakens vascular remodeling, exerts anti-inflammatory and
antiproliferative effects, and protects against pulmonary
hypertension (21,22). It has also been reported that PPAR-γ
receptors are also involved in the development of a variety of
cardiovascular diseases. Moreover, PPAR-γ displays a range of
physiological effects, including anti-inflammatory and
antiatherosclerotic activities, and improving left ventricular
remodeling (23,24). PPAR-γ also displays a protective
effect against kidney and brain tissue ischemia-reperfusion injury
(25,26); however, whether PPAR-γ displays a
protective effect against myocardial ischemia-reperfusion injury
has not been previously reported.
The present study aimed to investigate the
protective effects of catalpol against DOX-induced inflammation and
oxidative stress in H9C2 cardiomyoblasts, and to explore the
possible mechanisms associated with PPAR-γ. The results of the
present study may provide a theoretical basis for the treatment of
DOX-induced inflammation and oxidative stress in H9C2
cardiomyoblasts.
Materials and methods
Cell lines and reagents
H9C2 cells were obtained from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. Cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% inactivated fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 2 ml glutamine, 100 U/ml penicillin and
streptomycin at 37˚C with 5% CO2. Catalpol (purity
>98.0%) and DOX were purchased from the National Institute for
the Control of Pharmaceutical and Biological Products. H9C2 cells
were cultured with various concentrations of DOX (0, 0.1, 1 and 10
µM) for 12 and 24 h at 37˚C. The same cells were also treated with
various concentrations of catalpol (0, 10, 20, 40 and 80 µM) for 24
h at 37˚C.
Cell Counting Kit-8 (CCK-8) assay
H9C2 cells were seeded (0.5x103 cells/ml)
into 96-well plates and incubated with various concentrations of
DOX (0, 0.1, 1 and 10 µM) for 12 and 24 h at 37˚C (0 µM as control
group). Subsequently, 10 µl CCK-8 reagent (Roche Diagnostics) was
added to each well according to the manufacturer's protocol and
incubated for 2 h at 37˚C. The optical density of each well was
measured at a wavelength of 490 nm using a microplate reader.
To confirm the effect of catalpol on H9C2 cells,
H9C2 cells were cultured with various concentrations of catalpol
(0, 10, 20, 40 and 80 µmol/l) for 24 h at 37˚C (0 µM as control
group). Then, to confirm the effect of DOX-induced H9C2
cardiomyocytes, catalpol administration in each dose group (10, 20,
40 and 80 µmol/l) after H9C2 cells treatment with DOX (1 µM) for 24
h at 37˚C, respectively. According to the aforementioned method, 10
µl CCK-8 regent was added to each well in accordance with the
manufacturer's protocol and incubated for 2 h at 37˚C. The optical
density of each well was measured at a wavelength of 490 nm using a
microplate reader.
Transfection
The lentiviral vectors encoding PPAR-γ shRNA or
control shRNA lentiviral particles were generated via 293T cell
co-transfection with the PPAR-γ-shRNA plasmid vector (sc-156077-V)
or the control shRNA Lentiviral Particles (sc-108080; each, Santa
Cruz Biotechnology, Inc.). Short hairpin (sh)RNAs targeting PPAR-γ
(shRNA-PPAR-γ-1 and shRNA-PPAR-γ-2) and an appropriate negative
control (NC; non-targeting shRNA) were transfected into H9C2 cells
(all, 4 µm). Then, the cells H9C2 transfected with shRNA-PPAR-γ
subsequently treated either Dox/catalpol. shRNAs were designed and
synthesized by Guangzhou RiboBio Co., Ltd. H9C2 cells were seeded
into 6-well plates and at 70-80% confluence, transfection was
performed using Opti-MEM Medium, serum-free DMEM and
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Subsequently, cells were cultured for 24-72 h at 37˚C. Transfection
efficiency was determined by western blotting and RT-qPCR.
Western blotting
H9C2 cells were harvested and total protein was
extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using the
bicinchoninic acid method. Proteins (30 µg/lane) were separated by
10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore).
After blocking with 5% milk for 2 h at room temperature, the
membranes were incubated overnight at 4˚C with primary antibodies
against: PPAR-γ (cat. no. sc-7273; 1:1,000; Santa Cruz
Biotechnology, Inc.) and GAPDH (cat. no. ab181602; 1:2,000; Abcam).
Following primary incubation, the membranes were incubated with a
goat anti-mouse IgG horseradish peroxidase-conjugated secondary
antibody (cat. no. sc-2005; 1:10,000; Santa Cruz Biotechnology,
Inc.) and a horseradish peroxidase-conjugated goat anti-rabbit IgG
H&L antibody (cat. no. ab205718; 1:10,000; Abcam) at room
temperature for 2 h. Protein bands were visualized using an ECL
detection reagent (EMD Millipore). Blots were performed in
triplicate and protein expression was quantified using ImageJ
software (version 1.43; National Institutes of Health) with GAPDH
as the loading control and for normalization.
Reverse transcription-quantitative PCR
(RT-qPCR)
H9C2 cell total RNA was extracted using the TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and stored at
-40˚C. Total RNA samples were thawed on ice and was reverse
transcribed into cDNA using the PrimeScript RT reagent (Takara Bio,
Inc.), according to the manufacturer's protocol. Subsequently, qPCR
was performed using SYBR Green Master Mix I (Takara Bio, Inc.) and
the ABI 7900 Fast Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The following primers were used for qPCR:
PPAR-γ, forward 5'-CAAGACAACCTGCTACAAGC-3', reverse
5'-TCCTTGTAGATCTCCTGCAG-3'; GAPDH, forward
5'-CCAGGGGTGCCTTCTCTT-3', reverse 5'-CCGTGGGTAGAGTCATACTGG-3'. The
following thermocycling conditions were used for qPCR: Initial
denaturation at 95˚C for 5 min; followed by 45 cycles of
amplification, including denaturation at 95˚C for 30 sec, annealing
at 60˚C for 30 sec and a final extension at 72˚C for 10 min. mRNA
expression levels were quantified using the 2-∆∆Cq
method and normalized to the internal reference gene GAPDH
(27).
ELISA
Detection of reactive oxygen species (ROS),
malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione
peroxidase (GSH-Px) levels in the H9C2 cell culture medium were
measured by ELISA using commercial ELISA kits for Reactive Oxygen
Species (ROS) Assay Kit 520 nm (cat. no. 88-5930; Thermo Fisher
Scientific, Inc.), MDA ELISA kit (cat. no. AMS.E-EL-0060; Whuan
Boster Biological Technology, Ltd.), SOD Human ELISA kit (cat. no.
BMS222; Thermo Fisher Scientific, Inc.) and High Throughput
Glutathione Peroxidase Assay kit (cat. no. 7512-100-K; R&D
Systems, Inc.). The expression levels of tumor necrosis factor
(TNF)-α (cat. no. ADI-901-099; NeoBioscience Technology Co., Ltd.),
interleukin (IL)-1β (cat. no. EHC002b.48; NeoBioscience Technology
Co., Ltd.) and IL-6 (cat. no. ADI-901-033; NeoBioscience Technology
Co., Ltd.) were detected using ELISA kits according to the
manufacturer's protocol. The levels were quantified using Multiskan
Mk3 microplate reader (Thermo Fisher Scientific, Inc.) to detect
the concentration.
DCF-DA staining
H9C2 cells were seeded into 6-well plates, incubated
for 24 h, washed twice with Earle's Balanced Salt Solution and
subsequently incubated with 25 µM DCFH-DA for 30 min at 37˚C.
Following the incubation, cells were washed twice with sugar
Earle's solution. Fluorescence intensity was analyzed using a
fluorescence reader (Fluoroscan Ascent FL; Thermo Labsystems) at an
excitation wavelength of 488 nm and an emission wavelength of 525
nm. The images were obtained via confocal laser scanning microscopy
(Olympus FV500; Olympus Corporation). Images were analyzed using
ImageJ software (version 1.45; National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± SD. All experiments
were performed in triplicate. Statistical analyses were performed
using SPSS (version 11.5; SPSS, Inc.) and GraphPad Prism (version
5; GraphPad Software, Inc.) software. Comparisons among groups were
determined using one-way ANOVA followed by Tukey's or Dunnett's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
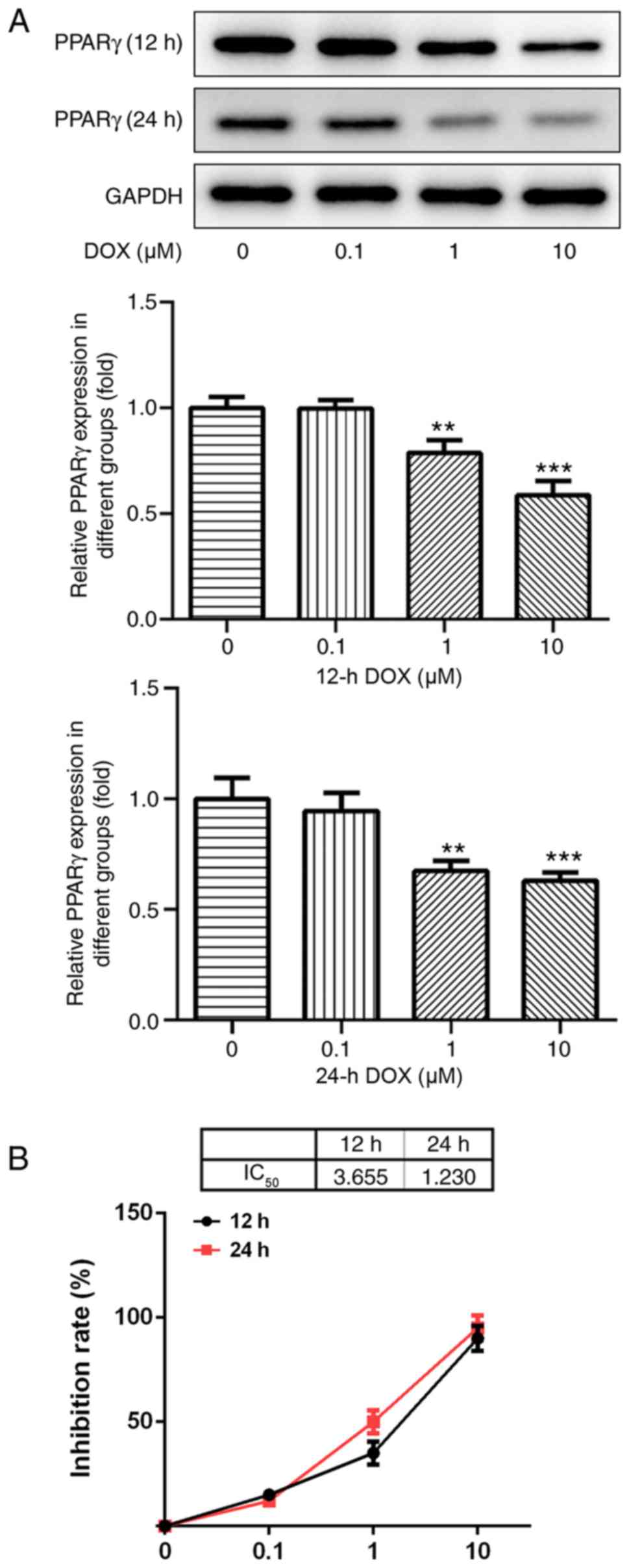
DOX inhibits the expression of
PPAR-γ
Following treatment with various concentrations of
DOX (0, 0.1, 1 and 10 µM) for 12 and 24 h, the protein expression
levels of PPAR-γ in H9C2 cells were detected by western blotting.
PPAR-γ expression levels decreased with increasing DOX
concentrations (Fig. 1A). The
results indicated that DOX inhibited the expression of PPAR-γ in a
dose-dependent manner.
DOX affects H9C2 cell viability
Results from the CCK-8 assay indicated that the
concentration of DOX (0.1 µM) had no significant effect on the
viability of H9C2 cells, and that various concentrations of DOX (1
and 10 µM) significantly decreased H9C2 cell viability compared
with the control group (P<0.05; Fig.
1B). As the IC50 of DOX at 24 h was ~1 µM, this
concentration and duration were used for subsequent
experiments.
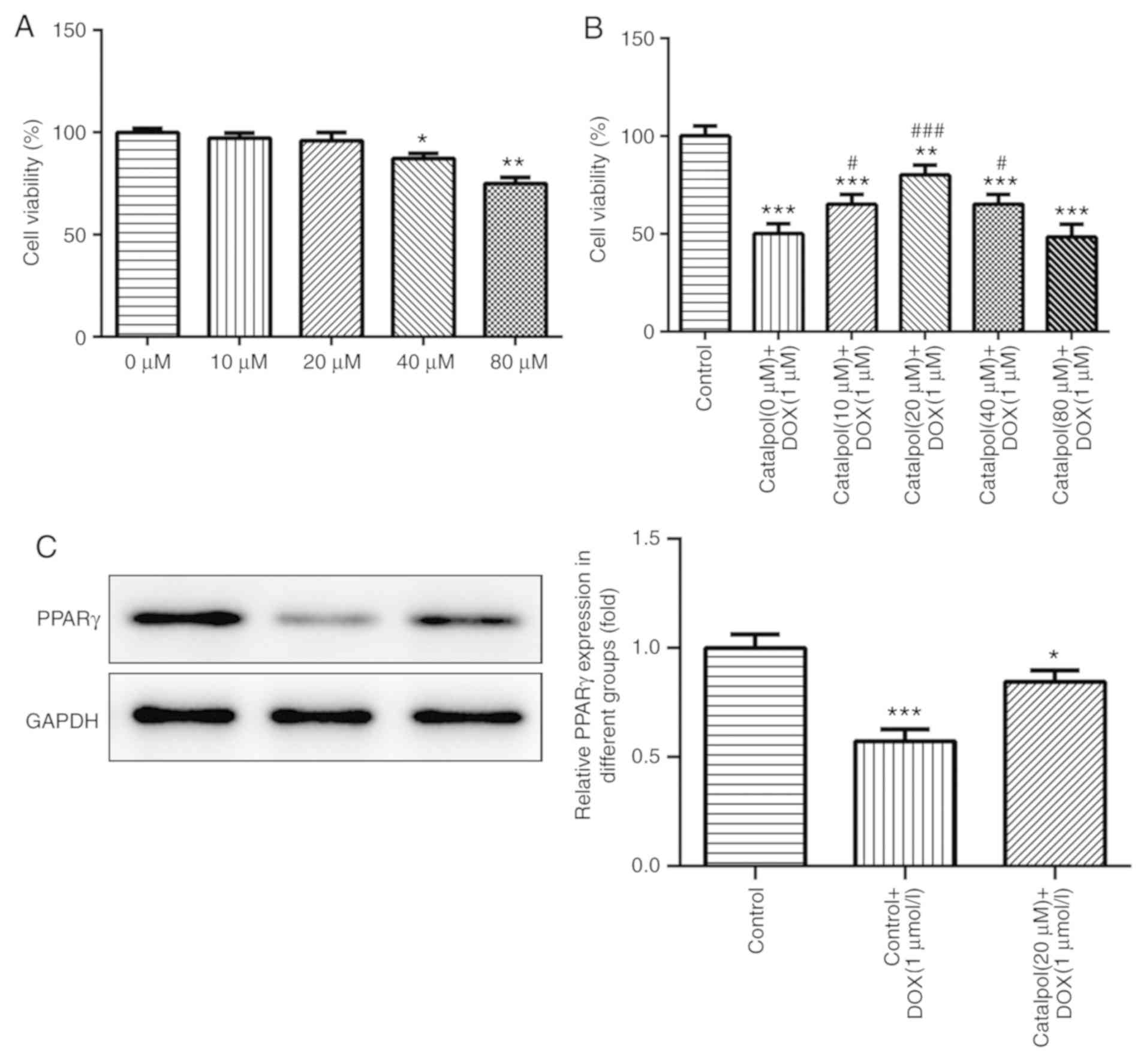
Effect of catalpol on H9C2 cell
viability
The results of the CCK-8 assay also indicated that
H9C2 cell viability was not significantly altered following
treatment with various concentrations of catalpol (0, 10, and 20
µM) for 24 h, but 40 and 80 µM catalpol significantly decreased
H9C2 cell viability compared with the untreated control group
(Fig. 2A).
Effect of catalpol on DOX-induced
reductions to H9C2 cell viability
DOX treatment reduced viability, but co-treatment
with catalpol reduced these effects. Additionally, H9C2 cell
viability was significantly increased in the 10 µM catalpol+1 µM
DOX group, the 20 µM catalpol+1 µM DOX group and the 40 µM
catalpol+1 µM DOX group when compared with the 0 µM catalpol+1 µM
DOX group (P<0.05; Fig. 2B). The
results indicated that catalpol (10, 20 and 40 µM) attenuated
DOX-induced effects on H9C2 cell viability and 80 µM catalpol
significantly decreased H9C2 cell viability. Furthermore, 20 µM
catalpol increased H9C2 cell viability to the highest level
compared with other catalpol concentrations. Therefore, 20 µM
catalpol was used for subsequent experimentation.
Catalpol serves a role in reducing
DOX-induced cell damage via PPAR-γ
The expression of PPAR-γ was detected by western
blotting. PPAR-γ expression levels in the DOX group were
significantly lower compared with the control group; however,
catalpol co-treatment increased PPAR-γ expression levels in the DOX
group (Fig. 2C). The results
indicated that catalpol serves a role in reducing DOX-induced cell
damage via PPAR-γ.
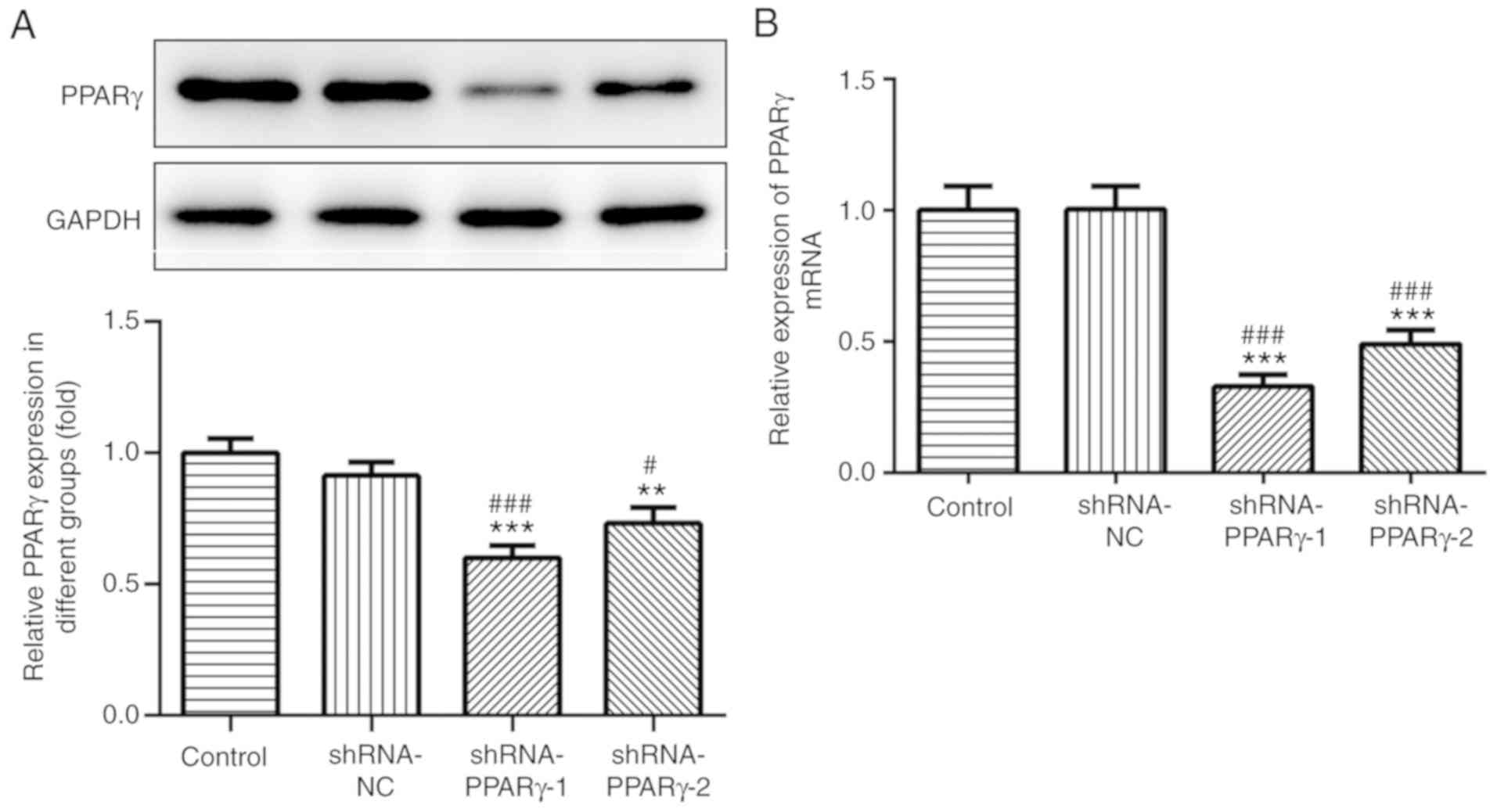
Catalpol reduces DOX-induced
inflammation and oxidative stress in H9C2 cells
The transfection efficiency of shRNA-PPAR-γ was
detected by western blotting and RT-qPCR (Fig. 3A and B, respectively). The results suggested that
shRNA-PPAR-γ-1 and shRNA-PPAR-γ-2 significantly decreased PPAR-γ
expression levels compared with the control group; however, the
inhibitory effects of shRNA-PPAR-γ-1 were superior compared with
shRNA-PPAR-γ-2 in H9C2 cells and was used in subsequent
experiments.
The results of ELISA revealed that concentrations of
the inflammatory factors TNF-α, IL-1β and IL-6 in the DOX group was
significantly higher compared with the control group, and catalpol
treatment significantly downregulated DOX-induced inflammatory
factor expression (Fig. 4A).
Furthermore, the expression of TNF-α, IL-1β and IL-6 in the 20 µM
catalpol+1 µM DOX+ shRNA-PPAR-γ group was significantly upregulated
compared with the 20 µM catalpol+1 µM DOX+ shRNA-NC group (Fig. 4A). Subsequently, the levels of
oxidative stress-associated factors were examined. The levels of
ROS and MDA were significantly increased in the DOX group compared
with the control group, and catalpol co-treatment significantly
downregulated DOX-induced ROS and MDA expression. The levels of ROS
and MDA in the 20 µM catalpol+1 µM DOX+ shRNA-PPAR-γ group were
significantly upregulated compared with the 20 µM catalpol+1 µM
DOX+shRNA-NC group. SOD and GSH-Px displayed the opposite trend to
ROS and MDA (Fig. 4B).
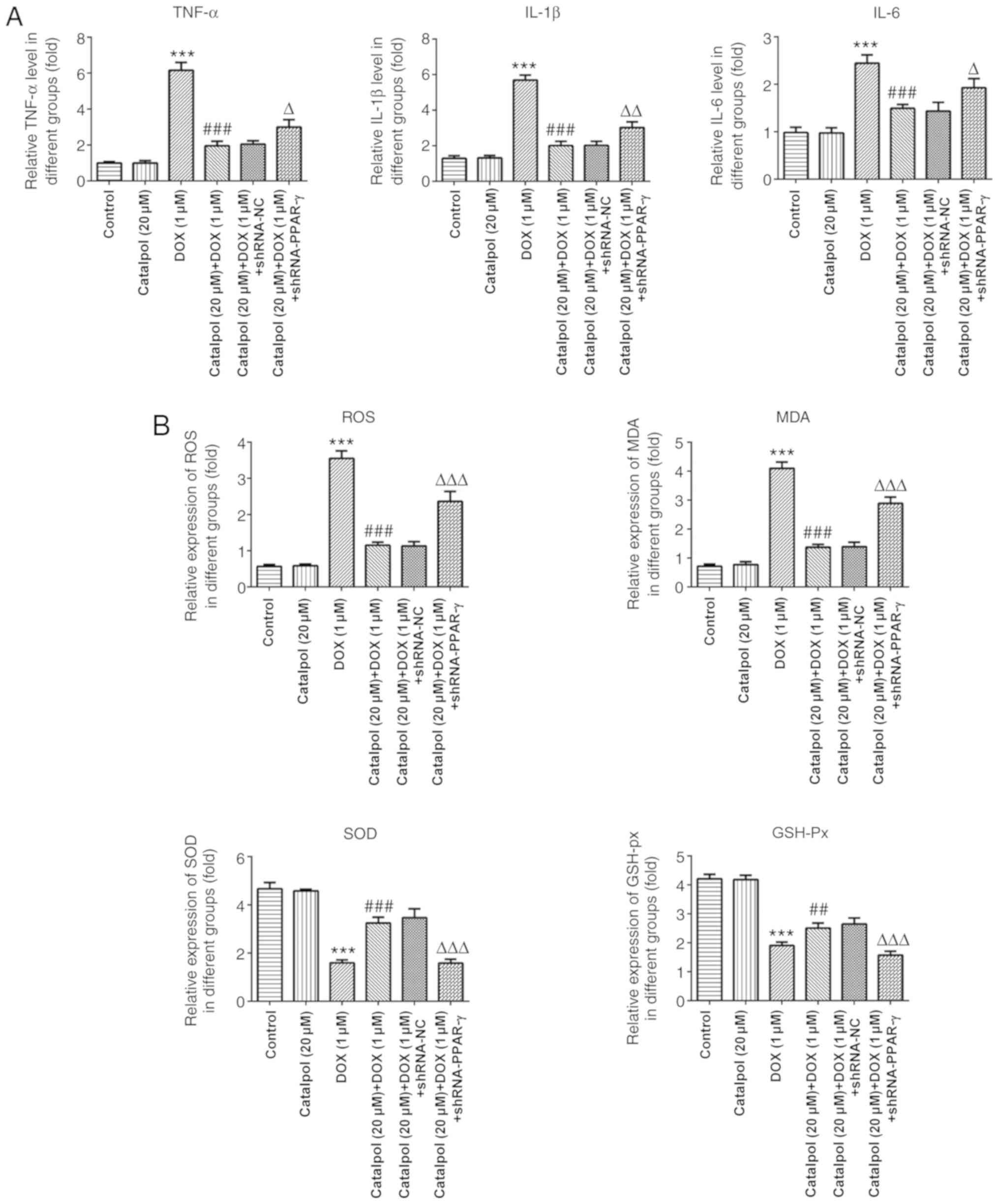 | Figure 4Catalpol relieves DOX-induced
inflammation and oxidative stress in H9C2 cells. (A) Catalpol
downregulates DOX-induced expression of the inflammatory factors
TNF-α, IL-6 and IL-1β. (B) Catalpol downregulates DOX-induced
expression of the oxidative stress factors ROS, MDA, SOD and GSH-Px
in H9C2 cells. **P<0.001 vs. control;
##P<0.05 and ###P<0.001 vs. 1 µM DOX;
ΔP<0.01, ΔΔP<0.05 and
ΔΔΔP<0.001 vs. shRNA-NC. DOX, doxorubicin; GSH-Px,
glutathione peroxidase; IL, interleukin; MDA, malondialdehyde; NC,
negative control; PPAR-γ, peroxisome proliferator-activated
receptor-γ; ROS, reactive oxygen species; shRNA, short hairpin RNA;
SOD, superoxide dismutase; TNF-α, tumor necrosis factor-α. |
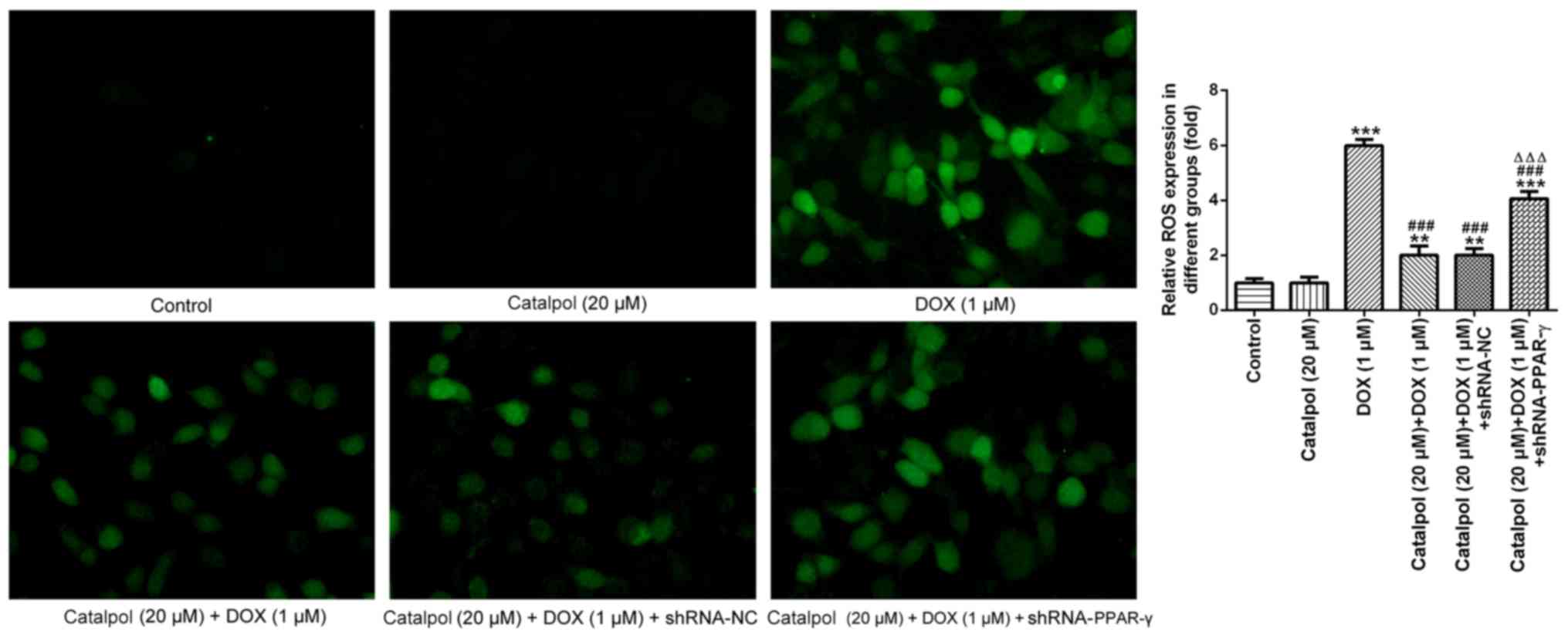
To further investigate ROS expression levels, DCF-DA
staining was performed to detect the expression of ROS in H9C2
cells (Fig. 5). The fluorescence
intensity in normal cardiomyocytes was generally weak with low ROS
content. Following treatment with DOX for 24 h, the fluorescence
intensity of the myocardial cells was enhanced and the fluorescence
value was notably increased compared with the control group,
indicating that the intracellular ROS content had increased.
Compared with the DOX group, catalpol treatment significantly
reduced the intracellular fluorescence intensity, the fluorescence
value and the ROS content of H9C2 cells. However, the addition of
shRNA-PPAR-γ significantly reversed catalpol-mediated
downregulation of intracellular ROS content. These results
indicated that catalpol treatment significantly reduced DOX-induced
ROS production. Collectively, the results suggested that catalpol
reversed DOX-induced inflammation and oxidative stress in H9C2
cells through PPAR-γ activation.
Discussion
Drug-induced cardiomyopathy is a severe disease that
occurs independent of other cardiovascular risk factors, but is a
widespread side effect of a number of therapeutic agents, such as
Daunorubicin, DOX and Paclitaxel (28-30).
As it is difficult to identify symptoms and signs during the early
stages of the disease, drug-induced cardiomyopathy often leads to
refractory heart disease in the late stages, with increasing
detrimental effects (31,32). DOX-induced cardiomyopathy is the most
common type of drug-induced heart disease (33). DOX-induced damage to the heart has
significantly limited its therapeutic use in the clinic (34). The main mechanism of action of DOX is
inhibition of DNA double-strand break, DNA replication and DNA
transcription by topoisomerase 2 inhibition. DOX also directly
embeds into the DNA, induces reactive oxygen species production and
regulates the binding of histone DNA (35,36).
Furthermore, DOX sequesters iron ions to produce free radicals,
which in turn trigger the initiation of apoptotic factor expression
and promotes cell death (37).
Therefore, DOX induces cardiomyocyte damage when it destroys tumor
cells (4). Early myocardial damage
manifests as myocarditis and arrhythmia; however, when DOX reaches
a specific dose, irreversible myocardial expansion occurs, which
may eventually develop into heart failure (38). Although numerous efforts have been
made to reduce the toxicity of DOX, no significant progress in the
prevention and treatment of DOX-induced toxicity has been reported.
Therefore, the development of novel therapeutic agents and
strategies for the prevention of DOX-induced myocardial damage is
important.
Rehmannia glutinosa is one of the most
commonly used Traditional Chinese Medicine, and it has been
reported to lower blood sugar, regulate immunity, enhance
hematopoietic functions and inhibit tumors. R. glutinosa
also displays antiaging properties by exerting protective effects
on the cardiovascular and vascular systems. Catalpol, an iridoid
glycoside isolated from the roots of R. glutinosa, has been
reported to display neuroprotective effects (38,39).
Previous studies have also demonstrated the cardioprotective and
anti-inflammatory properties of catalpol, including apoptosis
inhibition, reduced neuronal death and promotion of differentiation
(40,41). In a mouse model of
lipopolysaccharide-induced acute lung injury, catalpol prevents
injury by inhibiting TNF-α, IL-1β and IL-6 expression (42). However, the mechanisms underlying the
effects of catalpol on inflammation are not completely
understood.
DOX has a potent toxic effect on cardiomyocytes and
can alter cell morphology, induce cell death and promote apoptosis
through a series of molecular mechanisms (2,43,44).
Therefore, identifying whether catalpol can attenuate the effects
of DOX on myocardial cell survival is important. In the present
study, H9C2 cell viability was significantly reduced in the DOX
group compared with the control group, which indicated that DOX
displayed an inhibitory effect on cardiomyocyte survival.
Furthermore, compared with the DOX group, H9C2 cells treated with
catalpol displayed significantly increased cell viability,
suggesting that catalpol attenuated the inhibitory effects of DOX
on myocardial cell survival. The results indicated that the 20 µM
catalpol group displayed the optimal protective effect, which
suggested that catalpol reduced DOX-induced cardiomyocyte
damage.
A previous study has reported that catalpol displays
potent antioxidant effects, and DOX-induced cell damage is
primarily induced via cellular oxidative stress (45). The initiation of oxidative stress in
cardiomyocytes increases intracellular oxygen free radical
production, and damages cells by attacking cell membranes and the
mitochondria (46). Catalpol can
reduce the generation of oxygen free radicals to decrease cell
damage (47). The present study
demonstrated that DOX increased the toxicity of cardiomyocytes, and
reduced the ability of cells to resist oxidation. Our results
indicating that catalpol reduced cardiomyocyte toxicity compared
with the DOX group.
The inflammatory response is a defensive response of
the body to damaging factors, involving several types of cells and
cytokines, such as white blood cells, neutrophils, TNF-α, IL-1β and
IL-6 (48,49). An increase in inflammatory cytokine
levels is a sign of an inflammatory reaction in the body, which can
induce the adhesion and migration of neutrophils and vascular
endothelial cells, as well as the accumulation of neutrophils in
myocardial tissue, the release of lysosomal enzymes and myocardial
cell damage (50). TNF-α induces
inflammation by activating inflammatory cells, including
neutrophils, which mediate damage. TNF-α also displays direct
cytotoxic effects, leading to alterations in the myocardial calcium
balance and excitation-contraction coupling, as well as inducing
apoptosis (51). A previous study
has demonstrated that the mechanism underlying DOX-induced
myocardial injury is complex (52).
DOX damages myocardial tissue by increasing the expression of
inflammatory factors, including TNF-α, IL-1β and IL-6(53). Similarly, TNF-α, IL-1β, IL-6 and
other inflammatory factors are involved in the process of
isoproterenol-induced myocardial injury (54). In the present study, the expression
of TNF-α, IL-1β and IL-6 in the DOX group was significantly
increased compared with the control group, which was consistent
with the results of previous studies. The expression of
inflammatory factors in the catalpol co-treatment group was
significantly decreased, indicating that catalpol effectively
prevented the DOX-induced inflammatory reaction in cardiomyocytes
by inhibiting the release of inflammatory factors, thereby exerting
a protective effect against myocardial injury.
To identify the possible mechanism underlying the
anti-inflammatory activity of catalpol in cardiomyocytes, the
present study focused on the role of PPAR-γ, as it has been
reported that PPAR-γ receptors are also involved in the development
of a variety of cardiovascular diseases, including inflammation,
atherosclerosis and left ventricular remodeling (55-57).
The present study demonstrated that catalpol acted to significantly
increase PPAR-γ expression. Furthermore, to verify the effect of
catalpol on PPAR-γ expression, H9C2 cells were treated with
shRNA-PPAR-γ and 20 µM catalpol. The results suggested that PPAR-γ
protein expression was inhibited at the transcriptional level after
the addition of shRNA-PPAR-γ, and catalpol downregulated
DOX-induced proinflammatory cytokine production. Collectively, the
results indicated that catalpol played a role in DOX-induced cell
damage by regulating PPAR-γ expression.
In conclusion, results from the present study
indicated that DOX inhibited the expression of PPAR-γ and decreased
H9C2 cardiomyocyte cell viability. Catalpol alleviated DOX-induced
damage to H9C2 cardiomyocytes, potentially by reducing oxidative
stress in cardiomyocytes. Therefore, the present study suggested
that catalpol reversed DOX-induced H9C2 cardiomyocyte inflammation
and oxidative stress by increasing PPAR-γ expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ designed the study, collected and analyzed the
data, and drafted the manuscript. QZ conceived the study,
contributed to the study design and critically revised the
manuscript for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rochette L, Guenancia C, Gudjoncik A,
Hachet O, Zeller M, Cottin Y and Vergely C:
Anthracyclines/trastuzumab: New aspects of cardiotoxicity and
molecular mechanisms. Trends Pharmacol Sci. 36:326–348.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Smith LA, Cornelius VR, Plummer CJ, Levitt
G, Verrill M, Canney P and Jones A: Cardiotoxicity of anthracycline
agents for the treatment of cancer: Systematic review and
meta-analysis of randomised controlled trials. BMC Cancer.
10(337)2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang YW, Shi J, Li YJ and Wei L:
Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch
Immunol Ther Exp (Warsz). 57:435–445. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cardinale D, Colombo A, Bacchiani G,
Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N,
Curigliano G, et al: Early detection of anthracycline
cardiotoxicity and improvement with heart failure therapy.
Circulation. 131:1981–1988. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gallucci G and Simeon V: Letter by
Gallucci and Simeon regarding article, ‘early detection of
anthracycline cardiotoxicity and improvement with heart failure
therapy’. Circulation. 133(e362)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen Y, Liu Q, Shan Z, Zhao Y, Li M, Wang
B, Zheng X and Feng W: The protective effect and mechanism of
catalpol on high glucose-induced podocyte injury. BMC Complement
Altern Med. 19(244)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang X, Jin C, Li Y, Guan S, Han F and
Zhang S: Catalpol improves cholinergic function and reduces
inflammatory cytokines in the senescent mice induced by
D-galactose. Food Chem Toxicol. 58:50–55. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fu Q, Zhou Z, Li X, Guo H, Fan X, Chen J,
Zhuang J, Zheng S and Zhu P: Protective effect of adenosine
preconditioning against spinal cord ischemia-reperfusion injury in
rats. Nan Fang Yi Ke Da Xue Xue Bao. 34:92–95. 2014.PubMed/NCBI(In Chinese).
|
|
9
|
Bi J, Jiang B, Zorn A, Zhao RG, Liu P and
An LJ: Catalpol inhibits LPS plus IFN-γ-induced inflammatory
response in astrocytes primary cultures. Toxicol In Vitro.
27:543–550. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Z, Liu Y, Xue B and Wei L:
Protective effects of catalpol against
H2O2-induced oxidative damage in astrocytes.
Zhongguo Zhong Yao Za Zhi. 34:1955–1958. 2009.PubMed/NCBI(In Chinese).
|
|
11
|
Mao YR, Jiang L, Duan YL, An LJ and Jiang
B: Efficacy of catalpol as protectant against oxidative stress and
mitochondrial dysfunction on rotenone-induced toxicity in mice
brain. Environ Toxicol Pharmacol. 23:314–318. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu L, Sun Y and Hu J: Catalpol inhibits
apoptosis in hydrogen peroxide-induced endothelium by activating
the PI3K/Akt signaling pathway and modulating expression of Bcl-2
and Bax. Eur J Pharmacol. 628:155–163. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li DQ, Bao YM, Li Y, Wang CF, Liu Y and An
LJ: Catalpol modulates the expressions of Bcl-2 and Bax and
attenuates apoptosis in gerbils after ischemic injury. Brain Res.
1115:179–185. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang WJ, Niu HS, Lin MH, Cheng JT and Hsu
FL: Antihyperglycemic effect of catalpol in streptozotocin-induced
diabetic rats. J Nat Prod. 73:1170–1172. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang CF, Li DQ, Xue HY and Hu B: Oral
supplementation of catalpol ameliorates diabetic encephalopathy in
rats. Brain Res. 1307:158–165. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yuan H, Ni X, Zheng M, Han X, Song Y and
Yu M: Effect of catalpol on behavior and neurodevelopment in an
ADHD rat model. Biomed Pharmacother. 118(109033)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Q, Yang T, Guo AC and Fan YP: Role of
catalpol in ameliorating the pathogenesis of experimental
autoimmune encephalomyelitis by increasing the level of
noradrenaline in the locus coeruleus. Mol Med Rep. 17:4163–4172.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zou G, Zhong W, Wu F, Wang X and Liu L:
Inhibition of lncRNA Neat1 by catalpol via suppressing
transcriptional activity of NF-κB attenuates cardiomyocyte
apoptosis. Cell Cycle. 18:3432–3441. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu YR, Li PW, Suo JJ, Sun Y, Zhang BA, Lu
H, Zhu HC and Zhang GB: Catalpol provides protective effects
against cerebral ischaemia/reperfusion injury in gerbils. J Pharm
Pharmacol. 66:1265–1270. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cai Q, Yao Z and Li H: Catalpol promotes
oligodendrocyte survival and oligodendrocyte progenitor
differentiation via the Akt signaling pathway in rats with chronic
cerebral hypoperfusion. Brain Res. 1560:27–35. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Youssef J and Badr M: Role of peroxisome
proliferator-activated receptors in inflammation control. J Biomed
Biotechnol. 2004:156–166. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bishop-Bailey D: Peroxisome
proliferator-activated receptors in the cardiovascular system. Br J
Pharmacol. 129:823–834. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Geng DF, Wu W, Jin DM, Wang JF and Wu YM:
Effect of peroxisome proliferator-activated receptor gamma ligand.
Rosiglitazone on left ventricular remodeling in rats with
myocardial infarction. Int J Cardiol. 113:86–91. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Singh AP, Singh N, Pathak D and Bedi PMS:
Estradiol attenuates ischemia reperfusion-induced acute kidney
injury through PPAR-γ stimulated eNOS activation in rats. Mol Cell
Biochem. 453:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Otero-Losada M, LC Udovin L, Kobiec T,
Toro-Urrego N, A KR and Capani F: Long-term effects of
hypoxia-reoxygenation on thioredoxins in rat central nervous
system. Curr Pharm Des. 25:4791–4798. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Messinis DE, Melas IN, Hur J, Varshney N,
Alexopoulos LG and Bai JPF: Translational systems
pharmacology-based predictive assessment of drug-induced
cardiomyopathy. CPT Pharmacometrics Syst Pharmacol. 7:166–174.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yanagimoto K, Okamoto Y, Kodama Y,
Nishikawa T, Tanabe T and Kawano Y: Decrease of cardiac base
rotation in 2D speckle tracking indicates drug-induced
cardiomyopathy after chemotherapy in children with cancer. J
Pediatr Hematol Oncol. 39:10–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Helbock HJ, Beckman KB and Ames BN:
8-Hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of
oxidative DNA damage. Methods Enzymol. 300:156–166. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rasheed S, Hashim R and Yan JS: Possible
biomarkers for the early detection of HIV-associated heart
diseases: A proteomics and bioinformatics prediction. Comput Struct
Biotechnol J. 13:145–152. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bizino MB, Jazet IM, Westenberg JJM, van
Eyk HJ, Paiman EHM, Smit JWA and Lamb HJ: Effect of liraglutide on
cardiac function in patients with type 2 diabetes mellitus:
Randomized placebo-controlled trial. Cardiovasc Diabetol.
18(55)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xin YF, Wan LL, Peng JL and Guo C:
Alleviation of the acute doxorubicin-induced cardiotoxicity by
Lycium barbarum polysaccharides through the suppression of
oxidative stress. Food Chem Toxicol. 49:259–264. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hu J, Wu Q, Wang Z, Hong J, Chen R, Li B,
Hu Z, Hu X and Zhang M: Inhibition of CACNA1H attenuates
doxorubicin-induced acute cardiotoxicity by affecting endoplasmic
reticulum stress. Biomed Pharmacother. 120(109475)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Riba A, Deres L, Eros K, Szabo A, Magyar
K, Sumegi B, Toth K, Halmosi R and Szabados E: Doxycycline protects
against ROS-induced mitochondrial fragmentation and ISO-induced
heart failure. PLoS One. 12(e0175195)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chaudhari U, Nemade H, Gaspar JA,
Hescheler J, Hengstler JG and Sachinidis A: MicroRNAs as early
toxicity signatures of doxorubicin in human-induced pluripotent
stem cell-derived cardiomyocytes. Arch Toxicol. 90:3087–3098.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mjos KD, Cawthray JF, Jamieson G, Fox JA
and Orvig C: Iron (III)-binding of the anticancer agents
doxorubicin and vosaroxin. Dalton Trans. 44:2348–2358.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Medeiros-Lima DJM, Carvalho JJ, Tibirica
E, Borges JP and Matsuura C: Time course of cardiomyopathy induced
by doxorubicin in rats. Pharmacol Rep. 71:583–590. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu L, Zhang W, Zeng L and Jin JO:
Rehmannia glutinosa polysaccharide induced an anti-cancer effect by
activating natural killer cells. Int J Biol Macromol. 105:680–685.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Choi EM, Suh KS, Jung WW, Yun S, Park SY,
Chin SO, Rhee SY and Chon S: Catalpol protects against
2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cytotoxicity in
osteoblastic MC3T3-E1 cells. J Appl Toxicol. 39:1710–1719.
2019.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Zou G, Zhong W, Wu F, Wang X and Liu L:
Catalpol attenuates cardiomyocyte apoptosis in diabetic
cardiomyopathy via Neat1/miR-140-5p/HDAC4 axis. Biochimie.
165:90–99. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fu K, Piao T, Wang M, Zhang J, Jiang J,
Wang X and Liu H: Protective effect of catalpol on
lipopolysaccharide-induced acute lung injury in mice. Int
Immunopharmacol. 23:400–406. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Stěrba M, Popelová O, Vávrová A, Jirkovský
E, Kovaříková P, Geršl V and Simůnek T: Oxidative stress, redox
signaling, and metal chelation in anthracycline cardiotoxicity and
pharmacological cardioprotection. Antioxid Redox Signal.
18:899–929. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yu X, Cui L, Zhang Z, Zhao Q and Li S:
α-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in
rats through suppression of oxidative stress and apoptosis. Acta
Biochim Biophys Sin (Shanghai). 45:817–826. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao X, Jin Y, Li L, Xu L, Tang Z, Qi Y,
Yin L and Peng J: MicroRNA-128-3p aggravates doxorubicin-induced
liver injury by promoting oxidative stress via targeting Sirtuin-1.
Pharmacol Res. 146(104276)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P,
Mao X, Huang K, Xie Z and Zou MH: Hyperglycemia-driven inhibition
of AMP-activated protein kinase α2 induces diabetic cardiomyopathy
by promoting mitochondria-associated endoplasmic reticulum
membranes in vivo. Circulation. 139:1913–1936. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li Y, Bao Y, Jiang B, Wang Z, Liu Y, Zhang
C and An L: Catalpol protects primary cultured astrocytes from in
vitro ischemia-induced damage. Int J Dev Neurosci. 26:309–317.
2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chakraborty RK and Burns B: Systemic
inflammatory response syndrome. In: StatPearls, Treasure Island
(FL), 2020.
|
|
49
|
Ramadori P, Ahmad G and Ramadori G:
Cellular and molecular mechanisms regulating the hepatic
erythropoietin expression during acute-phase response: A role for
IL-6. Lab Invest. 90:1306–1324. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hu L, Mauro TM, Dang E, Man G, Zhang J,
Lee D, Wang G, Feingold KR, Elias PM and Man MQ: Epidermal
dysfunction leads to an age-associated increase in levels of serum
inflammatory cytokines. J Invest Dermatol. 137:1277–1285.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yamashita M and Passegue E: TNF-α
coordinates hematopoietic stem cell survival and myeloid
regeneration. Cell Stem Cell. 25:357–372, e357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Faridvand Y, Haddadi P, Vahedian V, Nozari
S, Nejabati HR, Pezeshkian M, Afrasiabi A, Safaie N, Jodati A and
Nouri M: Human amnion membrane proteins prevent doxorubicin-induced
oxidative stress injury and apoptosis in rat H9c2 cardiomyocytes.
Cardiovasc Toxicol: Feb 21, 2020 doi: 10.1007/s12012-020-09564-8
(Epub ahead of print).
|
|
53
|
Han MS, White A, Perry RJ, Camporez JP,
Hidalgo J, Shulman GI and Davis RJ: Regulation of adipose tissue
inflammation by interleukin 6. Proc Natl Acad Sci USA.
117:2751–2760. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cen W, Chen Z, Gu N and Hoppe R:
Prevention of AMI induced ventricular remodeling: Inhibitory
effects of heart-protecting musk pill on IL-6 and TNF-alpha. Evid
Based Complement Alternat Med. 2017(3217395)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Terrasi M, Bazan V, Caruso S, Insalaco L,
Amodeo V, Fanale D, Corsini LR, Contaldo C, Mercanti A, Fiorio E,
et al: Effects of PPARγ agonists on the expression of leptin and
vascular endothelial growth factor in breast cancer cells. J Cell
Physiol. 228:1368–1374. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tan NS, Michalik L, Desvergne B and Wahli
W: Multiple expression control mechanisms of peroxisome
proliferator-activated receptors and their target genes. J Steroid
Biochem Mol Biol. 93:99–105. 2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Choo J, Lee Y, Yan XJ, Noh TH, Kim SJ, Son
S, Pothoulakis C, Moon HR, Jung JH and Im E: A novel peroxisome
proliferator-activated receptor (PPAR)γ agonist 2-hydroxyethyl
5-chloro-4,5-didehydrojasmonate exerts anti-inflammatory effects in
colitis. J Biol Chem. 290:25609–25619. 2015.PubMed/NCBI View Article : Google Scholar
|