Introduction
A living organism continually faces intrusion by
various pathogens such as bacteria, viruses and fungi. Inflammatory
reactions occur to protect the organism from extracellular threats
(1). The intensity and duration of
inflammatory responses are dependent on the balance between
pro-inflammatory and anti-inflammatory mediators (2). Therefore, an imbalance in these
mediators causes severe cellular damage that can lead to chronic
inflammatory disorders, including rheumatoid arthritis, autoimmune
diseases, cancers and atherosclerosis (3). Macrophages function as a first alarm of
the defense system against common pathogens and are activated in
response to cytokines and pathogen-associated molecular patterns
such as lipopolysaccharide (LPS), flagellin, and lipoteichoic acid
(4-6).
Toll-like receptor 4 (TLR4) at the surface of immune cells
recognizes LPS, which is a cell-wall component of gram-negative
bacteria (7). In the presence of
LPS, activated macrophages induce a wide range of pro-inflammatory
mediators, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β, IL-6, nitric oxide (NO), and prostaglandin E2
(PGE2), to stimulate other immune cells (8,9).
Pro-inflammatory cytokines exert biological activities related to
acute or chronic inflammatory diseases (10). TNF-α is a potent stimulator of
inducible nitric oxide synthase (iNOS) expression in vascular
smooth muscle cells (11). When
activated, iNOS produces a high concentration of NO, a regulatory
molecule of various physiological functions such as vasodilatation
(12). Another major event during
inflammation is the arachidonic acid cascade produced from membrane
phospholipids (13). Arachidonic
acid is degraded by cyclooxygenase (COX) to prostaglandins (PGs),
which mediates inflammatory responses (13). COX-2 is induced by cytokines and is
responsible for releasing a high concentration of PGE2
(14,15). Both NO and PGE2 are
involved in pain-induction and -perception (16). These inflammatory mediators function
to protect the host cells from infection. However, the unnatural
expression or activation of inflammatory mediators is associated
with inflammatory disease (17,18). RAW
264.7 is a monocyte/macrophage-like Abelson leukemia virus
transformed cell line derived from BALB/c mice. This cell line is a
commonly used model of mouse macrophages for the study of cellular
responses to anti-inflammatory extracts. Upon stimulation with LPS,
RAW 264.7 cells increase nitric oxide (NO) production and enhance
phagocytosis. Furthermore, these cells are able to kill target
cells by antibody-dependent cytotoxicity (19). The RAW 264.7 cell line is designed to
grow and differentiate more stably than other macrophage cells.
These cells are suitable for monitoring anti-inflammatory
mechanisms mediated by various cytokines or LPS (20).
In folk medicinal remedies, Synedrella
nodiflora (Linn.) Gaertn. (S. nodiflora), which belongs
to the Asteraceae family, has been used for the prevention and
treatment of diverse diseases in Asia. It is a traditional plant of
tropical America and is also found in Ghana, India, China,
Malaysia, Japan, and other Indopacific countries (21). In Ghana, traditional medical
practitioners have used aqueous extracts of S. nodiflora for
epilepsy and pain management after boiling the whole plant
(22). S. nodiflora has been
treated as an external medicine to cure earache, headache, and
inflammation in Malaysia (23). The
leaves of S. nodiflora have also been used to treat
stomachache and hiccup and in threatened abortion cases (24). Furthermore, a methanol extract of
S. nodiflora leaves (MSN) proved to have insecticidal,
sedative, anti-oxidative, anti-convulsant, and anti-inflammatory
effects (25,26). The components of MSN are already well
reported by many researchers (21,27,28). The
phytochemical profile indicates that extracts of S.
nodiflora contain alkaloids, flavonoids, triterpenes, saponins,
simple phenolics, glycosides, and polyose (29,30).
Glycosides suppress the expression of inflammatory mediators via
TNF-α inhibition (31). Triterpenes
inhibit nuclear factor (NF)-κB-regulated gene expression and
transforming growth factor-β-activated kinase 1 (TAK1)-mediated
NF-κB activation (32). In general,
flavonoids regulate the inflammatory responses associated with
activating protein-1 or NF-κB, thereby suppressing chronic
inflammatory diseases (33,34). Several researchers have reported that
total alkaloids show anti-inflammatory effects and regulate
proto-oncogene tyrosine-protein kinase (Src)/spleen tyrosine kinase
(Syk) of NF-κB signaling (35).
Saponins have been reported to suppress the inflammatory response
by inhibiting the PI3K/Akt signaling pathway in macrophages
(36).
Although S. nodiflora has been evaluated for
its pharmacological activities, there has been no systematic study
of the mechanisms underlying the anti-inflammatory effects of MSN.
Therefore, this study focused on the analysis of the potential
anti-inflammatory effects of MSN at the protein level in
macrophages activated by LPS.
Materials and methods
MSN preparation
S. nodiflora was collected from the Slamet
Mountains, Central Java, Indonesia. Plant samples were collected
and identified by staff at the Center for Pharmaceutical and
Medical Technology (PTFM), and verified at the Herbarium Bogoriense
(LIPI). Voucher specimens recorded as KRIB 0039477 and PMT 1171,
were deposited in the herbarium (KRIB) of the Korean Research
Institute of Bioscience and Biotechnology (Daejeon, Korea) as well
as in the Center for Pharmaceutical and Medical Technology (PTFM)
and the Herbarium Bogoriense. The extract was deliquesced in
dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) and added to
the culture media to the final concentration as indicated. It was
confirmed that S. nodiflora is not a protected or endangered
species (37,38).
Cell culture and reagents
RAW 264.7 macrophages were purchased from ATCC. RAW
264.7 cells were cultured under the following condition: 10% fetal
bovine serum (FBS, Invitrogen; Thermo Fisher Scientific, Inc.) and
1% penicillin/streptomycin (Thermo Fisher Scientific, Inc.) in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C in 5% CO2. LPS for activation
of RAW 264.7 cells was purchased from Sigma-Aldrich; Merck
KGaA.
Cell viability assay
RAW 264.7 cells were seeded in 96-well plates
(4.5x104 cells/well), pre-treated with MSN (100, 200,
300, 400 and 600 µg/ml) for 2 h, and then incubated with LPS (1
µg/ml) at 37˚C for 24 h. The cell viability was measured using the
EZ-Cytox cell viability assay kit (Daeil Tech Co., Ltd.) according
to the manufacturer's instructions. Cell viability was calculated
following the absorbance for viable cells at 450 nm and reference
absorbance at 650 nm (A450-A650) with the
Synergy H1 Microplate Reader (BioTek Instruments, Inc.).
Nitrite assay
Cells (4.5x104 cells/well; 96-well plate)
were incubated with MSN (100, 200, 300 and 400 µg/ml) for 2 h and
then with LPS (1 µg/ml) at 37˚C for 24 h. Nitrite assay was
performed as described in a previous study (39).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RAW 264.7 cells (2x105 cells/well;
12-well plate) were pre-treated with MSN (100, 200, 300 and 400
µg/ml) for 2 h and activated by LPS (1 µg/ml) for 3 h at 37℃. Total
RNA preparation, cDNA synthesis, and quantification of mRNA were
performed as previously described (39). Quantification of gene expression was
analyzed using the 2-∆∆Cq method (40). Calculated gene expression was
normalized to reference genes, glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) and β-actin. The sequences of the PCR primers
are listed in a previous study (41).
Enzyme-linked immunosorbent assay
(ELISA)
RAW 264.7 macrophages were seeded in 96-well plates
(4.5x104 cells/well) and incubated at 37˚C overnight.
The cells were pre-treated with MSN (100, 200, 300 and 400 µg/ml)
for 2 h and then incubated with LPS (1 µg/ml) at 37˚C for 24 h.
Culture supernatants were collected by centrifugation at 1,500 x g
for 1 min at room temperature (RT). ELISA kit for the detection of
IL-6 (cat. no. 88-7064) and TNF-α (cat. no. 88-7324) were from
eBioscience (Thermo Fisher Scientific, Inc.). TNF-α and IL-6 levels
in cell supernatants were measured using sandwich ELISA with
monoclonal antibodies specific to each mediator according to the
manufacturer's instructions. Briefly, a 96-well-ELISA plate was
pre-coated with the capture antibody at 4˚C for overnight. The
plate was washed 4 times with 1X phosphate-buffered saline (PBS)/5%
Tween 20 (PBST) and blocked with 1X assay diluent at RT for 1 h.
Then, 100 µl of the sample was added to each well and incubated at
RT for 2 h. Subsequently, a biotinylated detection antibody
solution was added at RT for 1 h. After this, the plate was treated
with HRP-streptavidin solution at RT for 30 min, and then 100 µl of
3,3',5,5'-tetramethylbenzidine was added for further incubation at
RT for 10 min in the dark. The further reaction was blocked by
adding 50 µl of 1N H3PO4. The absorbance was
measured (450 nm) with a Synergy H1 Microplate Reader. The
concentrations of the secreted cytokines were calculated based on a
standard curve.
Preparation of total cell lysates
RAW 264.7 cells were treated with MSN (100, 200, 300
and 400 µg/ml) at 37˚C for 2 h and then stimulated with LPS (1
µg/ml) at 37˚C for 3 min (for IκBα, Src, Syk, Akt, TAK1), 15 min
(for MAPKs), or 24 h (for iNOS and COX-2), and subsequently washed
twice with cold PBS (pH 7.4). Cells were collected and lysed in
lysis buffer containing 150 mM NaCl, 20 mM trisaminomethane
hydrochloride (Tris-HCl) (pH 8.0), 0.5% IGEPAL CA-630 (NP-40), 1 mM
ethylenediaminetetraacetic acid, 0.5% Triton X-100, 1% glycerol, 10
mM sodium fluoride, 2 mM phenylmethylsulfonyl fluoride, and 1 mM
sodium orthovanadate. The lysates were centrifuged at 15,814 x g at
4˚C for 30 min. Supernatants were transferred to a new tube.
Immunoblot analysis
Briefly, the protein concentration was measured
using Bradford protein assay (Bio-Rad) according to the
manufacturer's instructions. Aliquots of cell lysates were mixed
with 5X sodium dodecyl sulfate (SDS)-polyacrylamide sample buffer
including 12 mM Tris-HCl (pH 6.8), 5% glycerol, 1%
β-mercaptoethanol, 0.4% SDS, and 0.02% bromophenol blue and boiled
at 95˚C for 5 min. Samples were separated on 10% SDS-polyacrylamide
gels and transferred to nitrocellulose membranes (GE Healthcare).
The membranes were blocked with 5% nonfat-dried skim milk in 1X
TBST solution, followed by incubation at 4˚C overnight with the
following primary antibodies: Mouse polyclonal anti-p38 (cat no.
sc-7972), mouse monoclonal anti-c-Jun N-terminal kinase (JNK; cat
no. sc-7345), rabbit polyclonal phosphorylated (p)-anti-IκBα
(Ser32/36; cat no. sc-101713), mouse polyclonal anti-spleen
tyrosine kinase (Syk; cat no. sc-1240), mouse monoclonal
anti-c-proto-oncogene tyrosine-protein kinase Src (c-Src; cat no.
sc-19), rabbit polyclonal anti-p-c-Src (Tyr424; cat no. sc-81521),
rabbit polyclonal anti-Akt1/2/3 (cat no. sc-8312), rabbit
polyclonal anti-p-Akt1/2/3 (Ser473; cat no. sc-7985), goat
polyclonal anti-cyclooxygenase 2 (COX-2; cat no. sc-1745), rabbit
polyclonal anti-inducible NO synthase (iNOS; cat no. sc-651) and
mouse monoclonal anti-α-tubulin (cat no. sc-5286) antibodies were
purchased from Santa Cruz Biotechnology, Inc. Rabbit polyclonal
transforming growth factor-β-activated kinase 1 (TAK1; cat no.
4505), rabbit monoclonal anti-p-TAK1 (Thr184/187; cat no. 4508),
rabbit polyclonal anti-p-p38 (Thr180/Tyr182; cat no. 9211), rabbit
polyclonal anti-extracellular signal-regulated kinase (ERK; cat no.
9102), rabbit monoclonal anti-p-ERK (Thr202/Tyr204; cat no. 9106),
rabbit polyclonal anti-p-JNK (Thr183/Tyr185; cat no. 9252) and
rabbit polyclonal anti-p-Syk (Tyr525/526; cat no. 2711) antibodies
were purchased from Cell Signaling Technology, Inc. All primary
antibodies were diluted to 1:1,000 in 5% non-fat dried milk. Each
membrane was washed 4 times with 1X TBST and incubated with the
following secondary antibodies: Polyclonal anti-rabbit IgG-HRP
(1:5,000; cat no. LF-SA8002) and polyclonal anti-mouse IgG Fc-HRP
(1:5,000; cat no. LF-SA8001) were from AbFrontier; Young In
Frontier Co., Ltd., at RT for 2 h. Each protein level was
visualized using an enhanced chemiluminescence (ECL) immunoblotting
detection reagent (Pierce; Thermo Fisher Scientific, Inc.). Protein
levels were scanned and analyzed with LabWorks v.4.0 software (UVP
Inc.).
Statistical analysis
All experiments were performed 3 times. The results
are represented as means ± standard error of the mean (SEM).
Differences between experimental conditions were assessed using
one-way ANOVA followed by Tukey's test in Prism v.3.0 (GraphPad
Software, Inc.). P<0.05 was concidered to indicate a
statistically significant difference.
Results
Effect of MSN on cell viability
Since ethnopharmacological records indicate that
S. nodiflora has been shown to exhibit potent
anti-inflammatory effects (42), MSN
was evaluated to identify its molecular mechanisms of action in the
present study. To determine the maximal non-cytotoxic concentration
of MSN, a cell viability assay was performed. RAW 264.7 cells were
treated with MSN in the presence or absence of LPS. The cell
viability was not altered until treatment with 400 µg/ml of MSN
(Fig. 1A). When cells were treated
with 600 µμg/ml of MSN, cell viability was significantly reduced.
Therefore, concentrations <400 µg/ml of MSN were used in the
subsequent experiments.
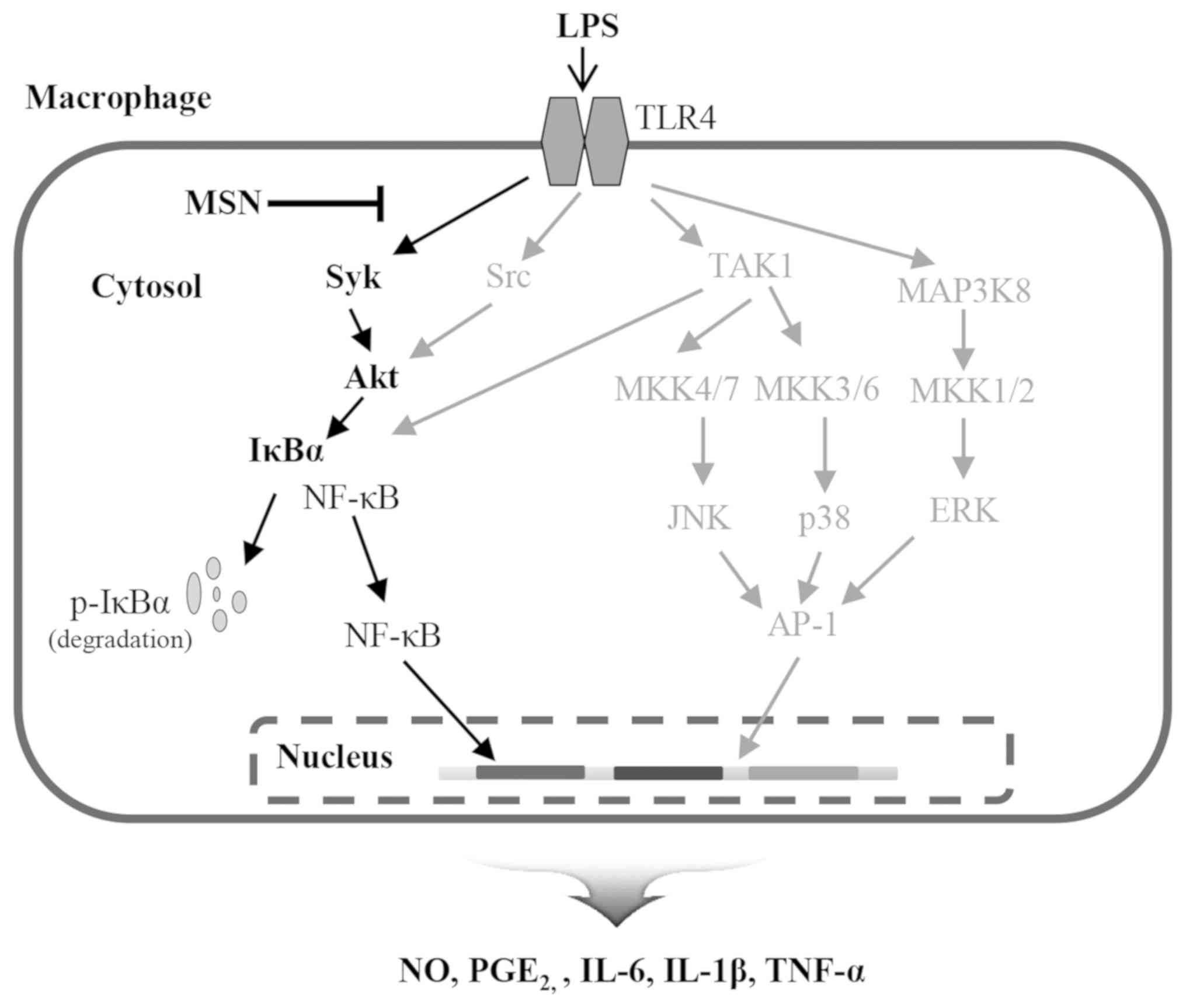 | Figure 1Effect of MSN on cell viability. (A)
RAW 264.7 macrophages were treated with MSN (100, 200, 300, 400 and
600 µg/ml) at 37˚C for 2 h and incubated in the absence or presence
of LPS (1 µg/ml) at 37˚C for an additional 24 h. The cell viability
was examined using EZ-Cytox assay kit. Cell viability of each group
was calculated based on the LPS-treated or LPS-untreated control
group. (B) RAW 264.7 macrophages were pre-treated with MSN (100,
200, 300, and 400 µg/ml) at 37˚C for 2 h and then stimulated with
LPS (1 µg/ml). After 24 h stimulation, the levels of NO secretion
in the culture media were measured using Griess reagent. The
amounts of NO secretion were calculated according to a standard
curve according to a nitrite standard solution. (C) Total cell
lysates were collected after 24 h LPS stimulation, and RT-qPCR
analysis was performed. Relative expression levels of iNOS are
represented as a bar graph. (D) After 3 h LPS stimulation, iNOS was
analyzed by immunoblotting. The expression of iNOS was
detected using ECL reagent and quantified by analysis with LabWorks
software. All the protein levels were normalized to corresponding
tubulin levels. Data are represented as the mean ± SEM.
*P<0.01 vs. LPS-untreated control group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. LPS-treated and MSN-untreated control
group. MSN, methanol extract of Synedrella nodiflora (Linn.)
Gaertn.; LPS, lipopolysaccharide; iNOS, inducible nitric oxide
synthase. |
Inhibitory effect of MSN on the
production of nitric oxide (NO) in LPS-stimulated macrophages
NO is an essential cellular signaling molecule
involved in diverse physiological and pathological processes in
mammals (43). In the inflammatory
response, NO levels are increased due to induced iNOS in cells, and
the produced NO plays a dual role in immune and inflammatory
responses (44). To investigate the
anti-inflammatory effects of MSN, the MSN-regulated NO expression
was examined in LPS-stimulated macrophages that showed inflammatory
responses. MSN effectively inhibited NO production in a
dose-dependent manner (Fig. 1B).
Since iNOS is the essential enzyme of NO production, iNOS
expression at the mRNA and protein levels following MSN treatment
was evaluated. RT-qPCR analysis showed that upregulated iNOS
expression by LPS stimulation was suppressed following MSN
treatment (Fig. 1C). In addition,
immunoblot analysis showed that increased protein expression of
iNOS by LPS treatment was dose-dependently reduced by MSN (Fig. 1D). These results suggest that MSN
negatively regulates NO production by suppression of iNOS at the
transcriptional level.
Inhibitory effect of MSN on the
production of pro-inflammatory mediators
Since COX-2 catalyzes the production of
PGE2, which causes fever, diarrhea, and excessive
uterine contraction (45), the
anti-inflammatory effects of MSN were assessed by evaluating mRNA
and protein expression of COX-2. LPS-induced expression of
COX-2 gene and COX-2 protein was suppressed by MSN in a
dose-dependent manner (Fig. 2A and
B). Therefore, these results suggest
that MSN regulates COX-2 in LPS-activated macrophages through the
inhibition of the COX-2 gene and COX-2 protein
expression.
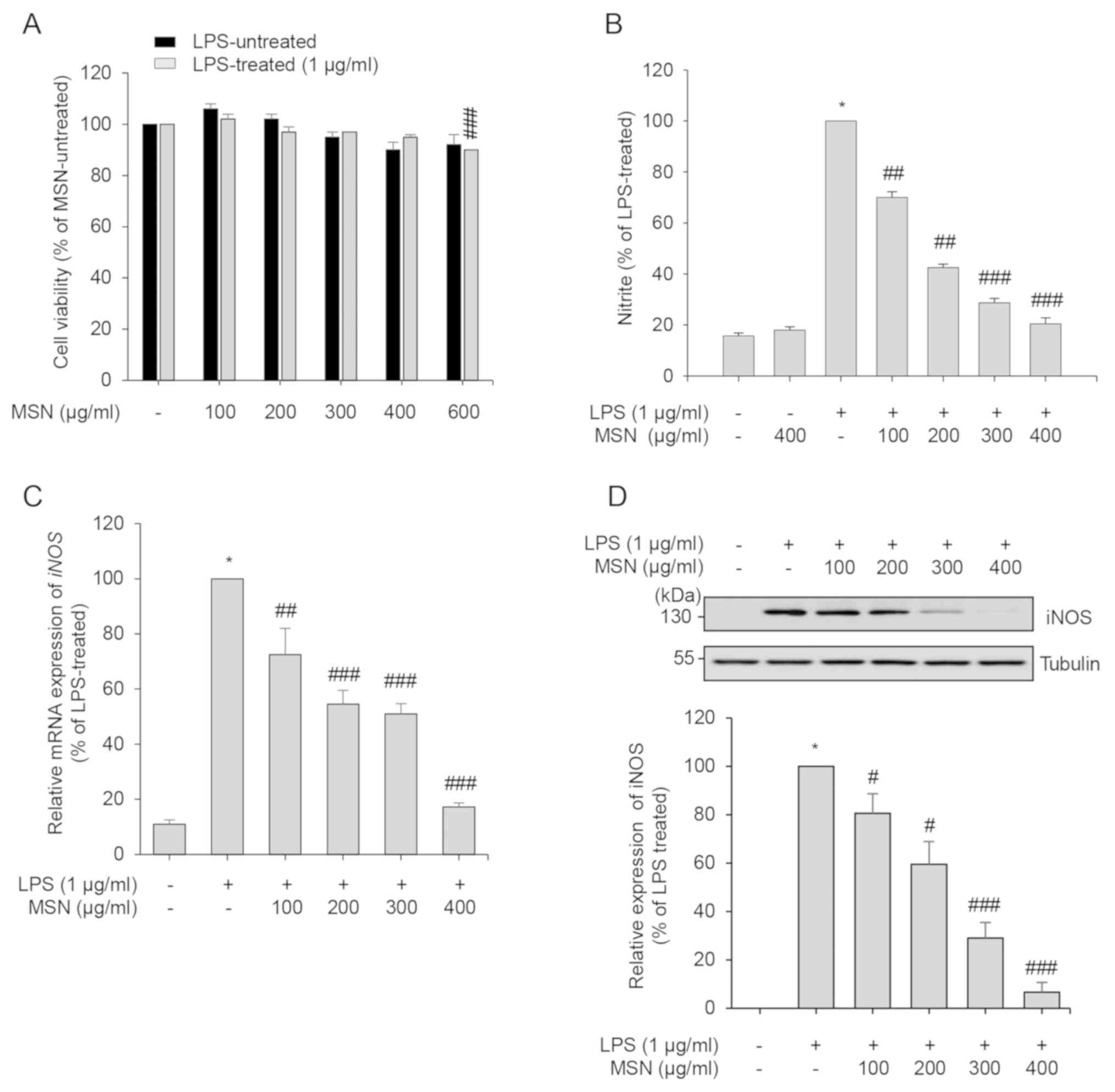 | Figure 2Inhibitory effects of MSN on
pro-inflammatory mediators. RAW 264.7 macrophages were pre-treated
with MSN (100, 200, 300, and 400 µg/ml) at 37˚C for 2 h and then
stimulated with LPS (1 µg/ml) for an indicated time. (A) After 3 h
LPS stimulation, COX-2 was amplified by RT-qPCR, and the expression
of each group was compared to that in the LPS-treated group. (B)
Total cell lysates were collected after 24 h stimulation, and
immunoblot analyses were performed. The protein expression levels
of COX-2 were detected using ECL reagent and quantified by analysis
with LabWorks software. All the protein levels were normalized to
corresponding tubulin levels. Relative expression levels of COX-2
are represented as a bar graph. After stimulation for 24 h, the
levels of (C) IL-6 and (D) TNF-α in the cultured media were
quantified using ELISA. The secretion of each cytokine was
calculated using a standard curve. (E) After 24 h stimulation,
IL-1β, IL-6 and TNF-α were amplified by RT-qPCR, and the expression
of each group was compared to that in the LPS-treated and
MSN-untreated control group. Data are represented as the mean ±
SEM. *P<0.01 vs. LPS-untreated control group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. LPS-treated and MSN-untreated control
group. MSN, methanol extract of Synedrella nodiflora (Linn.)
Gaertn.; LPS, lipopolysaccharide; COX-2, cyclooxygenase-2; IL-6,
interleukin-6; IL-1β, interleukin-1β; TNF-α, tumor necrosis
factor-α. |
To further investigate the anti-inflammatory effects
of MSN, the production of pro-inflammatory cytokines, which are
induced by LPS in macrophages, was measured in the presence or
absence of MSN. As shown in Fig. 2C
and D, pro-inflammatory cytokines
including IL-6 and TNF-α were significantly increased upon
stimulation with LPS and decreased by MSN treatment. The effect of
MSN on the gene expression of pro-inflammatory cytokines, including
IL-6, IL-1β, and TNF-α, was also analyzed using RT-qPCR. The mRNA
expression of the pro-inflammatory cytokine genes was inhibited by
MSN in LPS-activated RAW 264.7 macrophages (Fig. 2E). Taken together, these results
suggest that MSN suppresses the LPS-induced production of
pro-inflammatory cytokines by inhibition of IL-6,
IL-1β, and TNF-α transcription.
Differential inhibitory role of MSN on
MAPK and NF-κB in RAW 264.7 macrophages
NF-κB and mitogen-activated protein kinase (MAPK)
regulate the genes involved in inflammatory responses of RAW 264.7
cells (46). Phosphorylation at
Ser32/36 of IκBα was found to lead to the degradation of IκBα bound
to NF-κB, and freed and activated NF-κB translocated to the
nucleus, inducing transcription of target inflammatory genes
(47). We, therefore, measured the
phosphorylation levels at Ser32/36 of IκBα and the protein level of
IκBα to examine whether MSN caused a change in IκBα phosphorylation
and protein levels. As presented in Fig.
3A, LPS induced the phosphorylation and degradation of IκBα,
while the phosphorylation of IκBα decreased and IκBα levels
increased in the MSN-treated groups. These results suggest that MSN
inhibits NF-κB activation through the change in IκBα
phosphorylation.
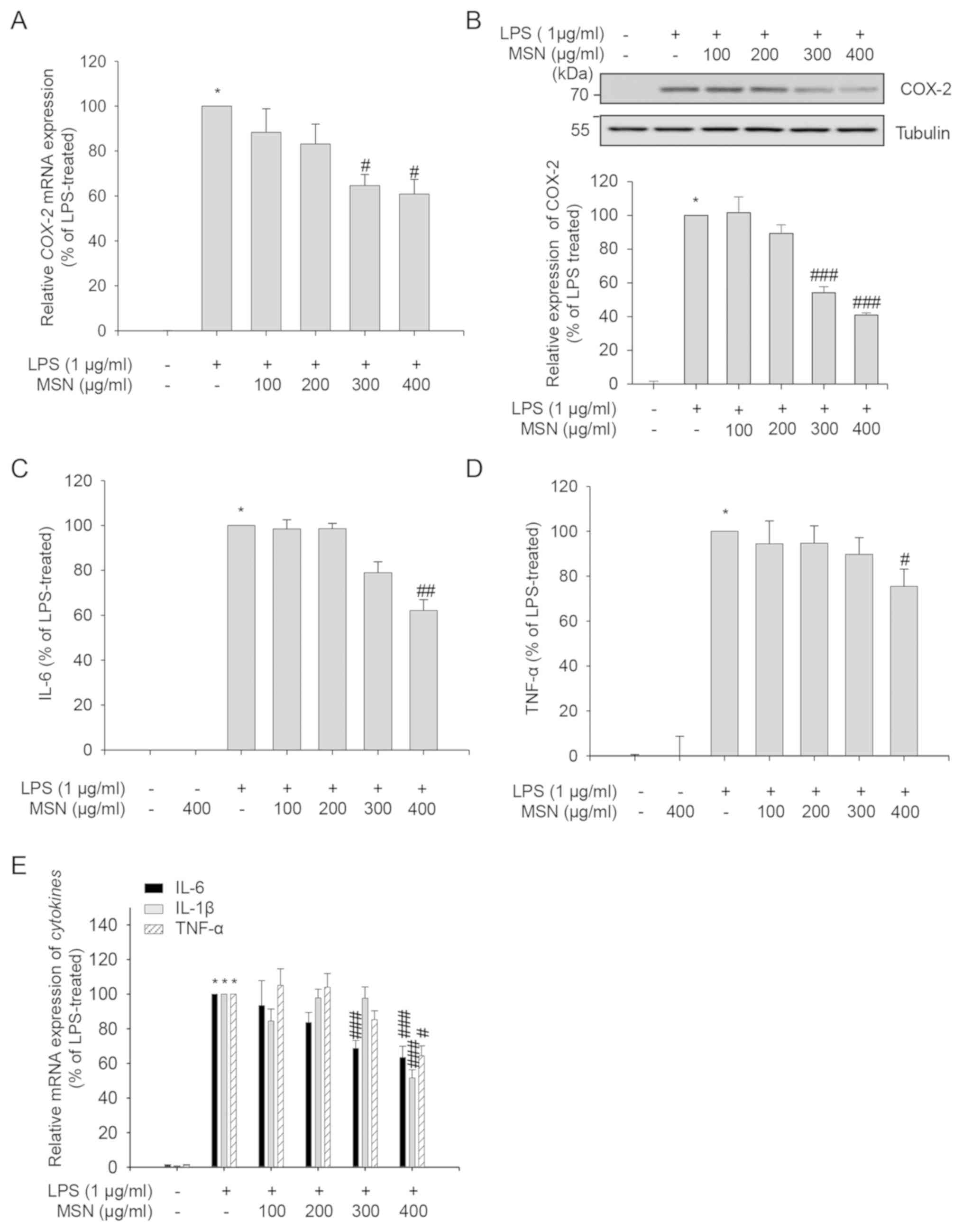 | Figure 3Inflammatory inhibition of MSN on
MAPK phosphorylation and NF-κB activation in RAW 264.7 macrophages.
RAW 264.7 macrophages were pre-treated with MSN (100, 200, 300, and
400 µg/ml) for 2 h and stimulated with LPS (1 µg/ml) at 37˚C for
indicated times. (A) After stimulation for 3 min, total cell
lysates were prepared and immunoblot analyses were performed.
Tubulin was used as a loading control. Protein levels were
quantified with LabWorks software. Relative expression levels of
IκBα and p-IκBα were normalized to tubulin levels and then the
ratio of phosphorylated (p)-IκBα vs. total IκBα protein level is
shown as a bar graph. (B) Total cell lysates were collected after
15 min of LPS treatment and subjected to immunoblot analysis using
appropriate antibodies. Tubulin was used as a loading control.
Protein levels were measured using LabWorks software. Levels of
phosphorylated MAPKs were normalized to corresponding total MAPK
levels. The ratio of phosphorylated vs. total protein level for
each MAPK is shown as a bar graph after normalization. (C) Cells
were lysed after 3 min stimulation. Samples were subjected to
immunoblot analysis. p-TAK1, p-Syk, p-Src, p-Akt, TAK1, Syk, Src,
and Akt protein expression levels were determined. The
phosphorylation levels of TAK1, Syk, Src, and Akt were normalized
to the corresponding total protein levels. The ratios of
phosphorylated vs. total protein levels are shown as a bar graph.
*P<0.01 vs. LPS-untreated control group.
#P<0.05 and ###P<0.001 vs. LPS-treated
and MSN-untreated control group. MSN, methanol extract of
Synedrella nodiflora (Linn.) Gaertn.; LPS,
lipopolysaccharide; IκBα, inhibitor of κBα; JNK, c-Jun N-terminal
kinase; ERK, extracellular signal-regulated kinase; TAK1,
transforming growth factor-β-activated kinase 1; Syk, spleen
tyrosine kinase; Src, proto-oncogene tyrosine-protein kinase. |
Since MAPK signaling, another LPS-induced
inflammatory signaling pathway, regulates the production of
inflammatory cytokines and mediators (48), the regulation of MAPK signaling by
MSN was investigated in the present study. Phosphorylation levels
of p38, JNK, and ERK in their activation loop were increased in RAW
264.7 cells upon exposure to LPS (Fig.
3B). When the cells were treated with MSN prior to LPS
stimulation, the levels of p-ERK, p-JNK, and p-p38 were not
altered, suggesting that MSN has little effect on MAPK
signaling.
TAK1 is an upstream signaling kinase of NF-κB and
MAPK signaling transduction (49).
In addition, the TAK1-independent Syk/Src/Akt signaling pathway
plays a vital role in NF-κB activation (50). The effect of MSN on the
phosphorylation of TAK1/Syk/Src/Akt at their activation sites was
investigated by immunoblot analysis to identify the targets of MSN
in the regulation of NF-κB activation. The LPS-induced
phosphorylation of Syk and Akt was reduced by MSN without changing
the protein levels, whereas the phosphorylation of TAK1 and Src was
not affected by MSN (Fig. 3C). These
results suggest that MSN suppresses the phosphorylation of Syk and
its downstream factors of the NF-κB pathway independently of TAK1
and Src.
Discussion
Despite the swift and proven efficacy of
non-steroidal anti-inflammatory drugs, these drugs have the
drawbacks of adverse side effects (51). Therefore, there have been efforts to
develop anti-inflammatory drugs with fewer side effects and
excellent healing effects (52).
Among them, traditional and natural medicines have great benefits
and potential (53,54). Several traditional medicines such as
Euphorbia cooperi and Thunbergia alata have been
reported to exert anti-inflammatory effects in folk remedies
(24,29,55,56).
In the present study, a methanol extract of S.
nodiflora (MSN) strongly suppressed the expression of inducible
nitric oxide synthase (iNOS) in lipopolysaccharide (LPS)-stimulated
RAW 264.7 cells in a dose-dependent manner. However, the 100 µg/ml
MSN-treated group showed a prominent reduction on mRNA level
compared that on protein level. This difference may be due to a
variety of reasons, including transcriptional,
post-transcriptional, translational and post-translational
regulation, mRNA stability and protein stability (57-60).
Moderate inhibitory activity on the production of tumor necrosis
factor (TNF)-α, interleukin (IL)-6, IL-1β, and cyclooxygenase-2
(COX-2) was observed in the resent study. These results indicate
that MSN may contain small amounts of anti-inflammatory components,
inhibitors, or selective anti-inflammatory drugs. Analysis of the
inflammation-regulated signaling pathways following treatment with
MSN showed that only the NF-κB pathway was affected by MSN. The
MAPK signaling pathway, the other major signaling pathway
regulating inflammation, was not inhibited by MSN. Since both the
NF-κB and MAPK pathways function in the regulation of inflammation
(46,48), these data indicate why MSN showed a
mild effect on the expression of TNF-α, IL-6, IL-1β, and COX-2.
Furthermore, analysis of spleen tyrosine kinase (Syk),
proto-oncogene tyrosine-protein kinase (Src), and transforming
growth factor-β-activated kinase 1 (TAK1), which are upstream
regulators of the NF-κB signaling pathway, showed that Syk was
inhibited by MSN while Src and TAK1 were not regulated by MSN. The
specific inhibition of Syk by MSN suggests that the
anti-inflammatory role of MSN may be dependent on highly selective
anti-inflammatory components constituting MSN, such as alkaloids,
flavonoids, triterpenes, saponins, simple phenolics, glycosides,
and polyose. Even though the constituents of MSN have been
previously reported (29,30), High-performance liquid chromatography
(HPLC) analysis of MSN is still needed for further study of MSN and
its constituents together with the anti-inflammatory mechanisms,
which is the limitation of the present study. In the present study,
these anti-inflammatory components were interpreted as responsible
for the selective inhibition of Syk, Akt, and NF-κB pathways. Thus,
further studies are needed to identify the specific functions of
each anti-inflammatory component comprising MSN on selective
inhibition.
One of the most prominent steps of inflammation is
NO production by iNOS (61). Several
studies have attempted to identify candidates for anti-inflammatory
drugs and elucidate their mechanisms of action (62). In LPS-activated macrophages, induced
NO serves as a pro-inflammatory mediator due to overproduction by
iNOS. MSN inhibited the production of NO via decreased expression
of both iNOS protein and mRNA in the present study. Furthermore, it
is well known that the excessive production of prostaglandin
E2 (PGE2) is related to inflammatory states
(63), and COX-2 mediates the
synthesis of PGE2 (64).
MSN negatively regulated COX-2 expression at both the mRNA and
protein levels in the LPS-stimulated RAW 264.7 macrophages. Several
studies have attempted to confirm novel anti-inflammatory agents
that inhibit iNOS and COX-2 expression and have demonstrated them
as novel drugs for inflammatory treatment (64,65).
Therefore, data in the present study suggest that MSN may be used
as a drug candidate to treat inflammatory disease.
In response to LPS stimulation, RAW 264.7
macrophages produce several cytokines, including IL-6, IL-1β, and
TNF-α (66). Previous studies have
shown that IL-1β and IL-6 genes have both NF-κB and
STAT binding domain whereas the TNF-α promoter has NF-κB
binding region but not STAT binding region (67,68). In
the present study, MSN inhibited LPS-induced IL-6 and TNF-α
production in macrophages. In addition, mRNA expression of
IL-6, IL-1β, and TNF-α was also reduced. This
suggests that MSN is possibly involved in the regulation of the
STAT3 signaling pathway and that both NF-κB and STAT3 function in
collaboration to regulate IL-6 and IL-1β genes,
resulting in potent inhibition of these genes.
Binding of LPS to Toll-like receptors induces the
activation of both MAPKs and NF-κB (64). However, the activities of MAPKs and
NF-κB are regulated by different upstream molecules, such as
specific MAP3Ks and MAP2Ks for MAPKs and IκBα kinases for NF-κB
(69). Following the LPS
stimulation, IκBα is phosphorylated and degraded (69,70).
With the degradation of IκBα, NF-κB is unconstrained to enter the
nucleus, where it chains to and triggers the transcription of
target genes (71,72). In the present study, MSN treatment
suppressed IκBα phosphorylation dose-dependently, which in turn
reduced IκBα degradation. When the degradation of IκBα is
suppressed, NF-κB nuclear translocation is inhibited, which fails
to transcribe its downstream target genes (72). Since the activity of NF-κB is mostly
regulated by IκBα binding, NF-κB target genes are potential targets
of MSN treatment. In addition, upstream signaling of IκBα includes
the TAK1/Syk/Src/Akt signaling pathway (73). MSN showed inhibitory effects by
reducing the phosphorylation of Syk and Akt. Therefore, these
findings support the hypothesis that MSN shows an anti-inflammatory
effect by inhibiting NF-κB activation.
Anti-oxidant, anti-bacterial, and even
anti-psychotic effects as well as anti-inflammatory effects of MSN
have been previously reported (74,75).
However, previous studies have focused on the chemical analysis of
the constituents of MSN or the physiological effects of MSN in
mouse models (76). In the present
study, the anti-inflammatory effects and the underlying
anti-inflammatory mechanisms of MSN were evaluated. The
Syk/Akt/IκBα pathway that is significantly suppressed by MSN
provides insight for the further application of MSN in the
development of anti-inflammatory agents. In addition, the mode of
action needs to be investigated to reveal the target pathway of the
extract or to avoid unexpected side effects.
In conclusion, the present study demonstrates that
MSN exhibits anti-inflammatory properties via inhibition of the
production of various inflammatory mediators such as NO,
PGE2, TNF-α, IL-6, and IL-1β. The inhibitory effects of
MSN are dependent on the suppression of the Syk/Akt/IκBα signaling
pathway and NF-κB activation (Fig.
4). Therefore, these results support the traditional use of MSN
in the treatment of several inflammation-associated diseases and
suggest that the novelty of the present study lies in the revealed
pathway. Thus, MSN is a candidate anti-inflammatory agent for the
treatment of inflammation.
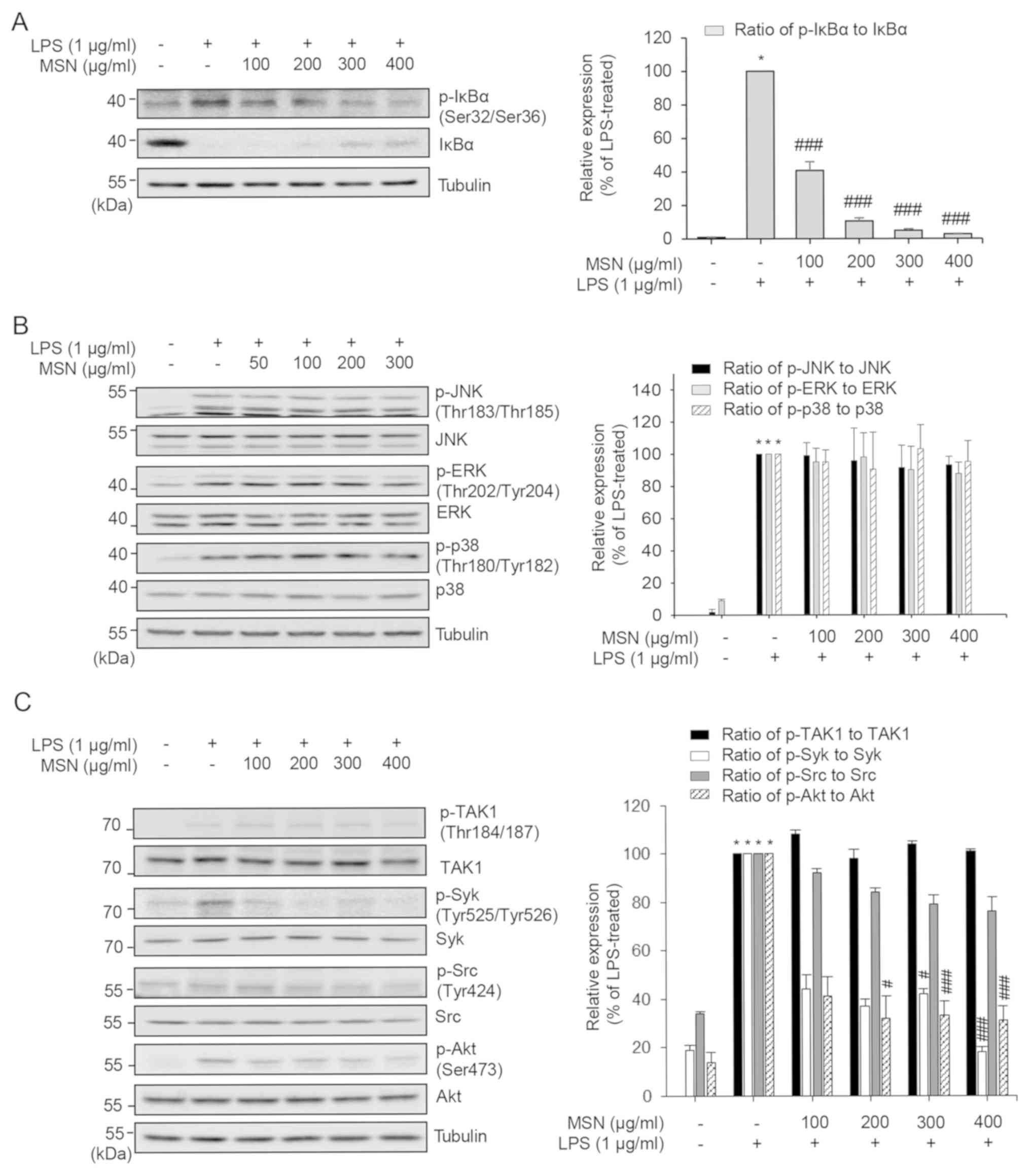 | Figure 4The molecular pathway of the
inflammatory effects of MSN in LPS-stimulated RAW 264.7 cells. MSN,
methanol extract of Synedrella nodiflora (Linn.) Gaertn.;
LPS, lipopolysaccharide; IL-6, interleukin-6; IL-1β,
interleukin-1β; TNF-α, tumor necrosis factor-α; NO, nitric oxide;
PGE2, prostaglandin E2; COX-2,
cyclooxygenase-2; IκBα, inhibitor of κBα; NF-κB, nuclear factor κB;
Akt, protein kinase B; Src, proto-oncogene tyrosine-protein kinase;
Syk, spleen tyrosine kinase; TLR4, toll-like receptor 4; TAK1,
transforming growth factor-β-activated kinase 1; MAP3K8,
mitogen-activated protein kinase kinase kinase 8; MKK,
mitogen-activated protein kinase kinase; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; AP-1,
activator protein 1. |
Acknowledgements
The authors would like to thank the KRIBB Initiative
Program of the Republic of Korea for information on plant extract
list and MSN.
Funding
The present study was financially supported by the
Chung-Ang University Research Scholarship Grants in 2018 and by the
National Research Foundation of Korea (NRF) grant funded by the
Ministry of Science and ICT (grant no. NRF-2018R1A2B6005084).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HTTL and SC designed the study. HTTL and JP
performed the data acquisition. JP performed the cell viability and
immunoblot assays. HTTL, JP, JH, SK, JHP, and SC analyzed and
interpreted the data. SK and JHP provided extraction methods and
MSN. HTTL wrote the draft. JP, JH, SK, JHP, and SC revised the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Belkaid Y and Hand TW: Role of the
microbiota in immunity and inflammation. Cell. 157:121–141.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tamayo E, Fernández A, Almansa R, Carrasco
E, Heredia M, Lajo C, Goncalves L, Gómez-Herreras JI, de Lejarazu
RO and Bermejo-Martin JF: Pro- and anti-inflammatory responses are
regulated simultaneously from the first moments of septic shock.
Eur Cytokine Netw. 22:82–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Galkina E and Ley K: Immune and
inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol.
27:165–197. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mogensen TH: Pathogen recognition and
inflammatory signaling in innate immune defenses. Clin Microbiol
Rev. 22:240–273. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Balamayooran G, Batra S, Fessler MB,
Happel KI and Jeyaseelan S: Mechanisms of neutrophil accumulation
in the lungs against bacteria. Am J Respir Cell Mol Biol. 43:5–16.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thapa B and Lee K: Metabolic influence on
macrophage polarization and pathogenesis. BMB Rep. 52:360–372.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Janssens S and Beyaert R: Role of
Toll-like receptors in pathogen recognition. Clin Microbiol Rev.
16:637–646. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang JM and An J: Cytokines,
inflammation, and pain. Int Anesthesiol Clin. 45:27–37.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Penna G, Mondaini N, Amuchastegui S, Degli
Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M and
Adorini L: Seminal plasma cytokines and chemokines in prostate
inflammation: Interleukin 8 as a predictive biomarker in chronic
prostatitis/chronic pelvic pain syndrome and benign prostatic
hyperplasia. Eur Urol. 51:524–533; discussion 533. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arango Duque G and Descoteaux A:
Macrophage cytokines: Involvement in immunity and infectious
diseases. Front Immunol. 5(491)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu D, Liu J, Pang X, Wang S, Zhao J, Zhang
X and Feng L: Palmitic acid exerts pro-inflammatory effects on
vascular smooth muscle cells by inducing the expression of
C-reactive protein, inducible nitric oxide synthase and tumor
necrosis factor-α. Int J Mol Med. 34:1706–1712. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Forstermann U and Sessa WC: Nitric oxide
synthases: regulation and function. Eur Heart J 33: 829-837,
837a-837d, 2012. https://doi.org/10.1093/eurheartj/ehr304.
|
|
13
|
Sun GY, Shelat PB, Jensen MB, He Y, Sun AY
and Simonyi A: Phospholipases A2 and inflammatory responses in the
central nervous system. Neuromolecular Med. 12:133–148.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang H and Chen C: Cyclooxygenase-2 in
synaptic signaling. Curr Pharm Des. 14:1443–1451. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mbonye UR and Song I: Posttranscriptional
and posttranslational determinants of cyclooxygenase expression.
BMB Rep. 42:552–560. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ricciotti E and FitzGerald GA:
Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol.
31:986–1000. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cekici A, Kantarci A, Hasturk H and Van
Dyke TE: Inflammatory and immune pathways in the pathogenesis of
periodontal disease. Periodontol 2000. 64:57–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lucas SM, Rothwell NJ and Gibson RM: The
role of inflammation in CNS injury and disease. Br J Pharmacol. 147
(Suppl 1):S232–S240. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fuentes AL, Millis L, Vapenik J and Sigola
L: Lipopolysaccharide-mediated enhancement of zymosan phagocytosis
by RAW 264.7 macrophages is independent of opsonins, laminarin,
mannan, and complement receptor 3. J Surg Res. 189:304–312.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Taciak B, Białasek M, Braniewska A, Sas Z,
Sawicka P, Kiraga Ł, Rygiel T and Król M: Evaluation of phenotypic
and functional stability of RAW 264.7 cell line through serial
passages. PLoS One. 13(e0198943)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Amoateng P, Adjei S, Osei-Safo D, Kukuia
KKE, Bekoe EO, Karikari TK and Kombian SB: Extract of Synedrella
nodiflora (L) Gaertn exhibits antipsychotic properties in murine
models of psychosis. BMC Complement Altern Med.
17(389)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gruca M, van Andel TR and Balslev H:
Ritual uses of palms in traditional medicine in sub-Saharan Africa:
A review. J Ethnobiol Ethnomed. 10(60)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Amoateng P, Woode E and Kombian SB:
Anticonvulsant and related neuropharmacological effects of the
whole plant extract of Synedrella nodiflora (L.) Gaertn
(Asteraceae). J Pharm Bioallied Sci. 4:140–148. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Amoateng P, Adjei S, Osei-Safo D, Ahedor
B, Mahmood SA, N'guessan BB, Asiedu-Gyekye IJ and Nyarko AK:
Long-term continuous administration of a hydro-ethanolic extract of
Synedrella nodiflora (L) Gaertn in male Sprague-Dawley rats:
Biochemical, haematological and histopathological changes. Ghana
Med J. 50:163–171. 2016.PubMed/NCBI
|
|
25
|
Mulla W, Kuchekar S, Thorat V, Chopade A
and Kuchekar B: Antioxidant, antinociceptive and anti-inflammatory
activities of ethanolic extract of leaves of Alocasia indica
(Schott.). J Young Pharm. 2:137–143. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ghaisas MM, Dandawate PR, Zawar SA, Ahire
YS and Gandhi SP: Antioxidant, antinociceptive and
anti-inflammatory activities of atorvastatin and rosuvastatin in
various experimental models. Inflammopharmacology. 18:169–177.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ghayal N, Dhumal K, Deshpande N, Ruikar A
and Phalgune U: Phytotoxic effects of leaf leachates of an invasive
weed Synedrella nodiflora and characterization of its
allelochemical. Int J Pharm Sci Rev Res. 19:79–86. 2013.
|
|
28
|
Aalbersberg WG and Singh Y: Essential oils
from two medicinal plants of Fiji: Dysoxylum richii (A Gray) CDC
fruit and Synedrella nodiflora (L.) Gaertn. leaves. Flavour
Fragrance J. 6:125–128. 1991.
|
|
29
|
Amoateng P, Adjei S, Osei-Safo D, Ameyaw
EO, Ahedor B, N'guessan BB and Nyarko AK: A hydro-ethanolic extract
of Synedrella nodiflora (L.) Gaertn ameliorates hyperalgesia and
allodynia in vincristine-induced neuropathic pain in rats. J Basic
Clin Physiol Pharmacol. 26:383–394. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Adjibode A, Tougan U, Youssao A, Mensah G,
Hanzen C and Koutinhouin G: Synedrella nodiflora (L.) Gaertn: A
review on its phytochemical screening and uses in animal husbandry
and medicine. Int J Adv Sci Tech Res. 3:436–443. 2015.
|
|
31
|
Cho JY, Yoo ES, Cha BC, Park H-J, Rhee MH
and Han YN: The inhibitory effect of triterpenoid glycosides
originating from Sanguisorba officinalis on tissue factor activity
and the production of TNF-α. Planta Med. 72:1279–1284.
2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sethi G, Ahn KS, Pandey MK and Aggarwal
BB: Celastrol, a novel triterpene, potentiates TNF-induced
apoptosis and suppresses invasion of tumor cells by inhibiting
NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB
activation. Blood. 109:2727–2735. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Serafini M, Peluso I and Raguzzini A:
Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 69:273–278.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee S, Kim YJ, Kwon S, Lee Y, Choi SY,
Park J and Kwon HJ: Inhibitory effects of flavonoids on
TNF-alpha-induced IL-8 gene expression in HEK 293 cells. BMB Rep.
42:265–270. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wen H, Jiang L, Zhang D, Yuan X, Dang J,
Mei L, Shao Y and Tao Y: Anti-inflammatory activity of total
alkaloids from Hypecoum leptocarpumhook. f. et Thoms. Pharmacogn
Mag. 14:397–403. 2018.
|
|
36
|
Obasi TC, Braicu C, Iacob BC, Bodoki E,
Jurj A, Raduly L, Oniga I, Berindan-Neagoe I and Oprean R:
Securidaca-saponins are natural inhibitors of AKT, MCL-1, and
BCL2L1 in cervical cancer cells. Cancer Manag Res. 10:5709–5724.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Glenn CR: Earth's Endangered Creatures,
2006. http://earthsendangered.com.
Accession date: January 31, 2020.
|
|
38
|
International Union for Conservation of
Nature and Natural Resources (IUCN): The IUCN Red List of
Threatened Species. 2019. https://www.iucn.org.
Accession date: January 31, 2020.
|
|
39
|
Le HTT, Cho YC and Cho S: Methanol extract
of Guettarda speciosa Linn. inhibits the production of inflammatory
mediators through the inactivation of Syk and JNK in macrophages.
Int J Mol Med. 41:1783–1791. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kim BR, Cho YC, Le HTT, Vuong HL, Lee S
and Cho S: Suppression of inflammation by the rhizome of
Anemarrhena asphodeloides via regulation of nuclear factor-κB and
p38 signal transduction pathways in macrophages. Biomed Rep.
6:691–697. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Haque A, Zahan R, Nahar L, Mosaddik A and
Haque E: Anti-inflammatory and insecticidal activities of
Synedrella nodiflora. Mol Clin Pharmacol. 2012:60–67. 2012.
|
|
43
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Di Meo S, Reed TT, Venditti P and Victor
VM: Role of ROS and RNS sources in physiological and pathological
conditions. Oxid Med Cell Longev. 2016(1245049)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Giuliano F and Warner TD: Origins of
prostaglandin E2: Involvements of cyclooxygenase (COX)-1 and COX-2
in human and rat systems. J Pharmacol Exp Ther. 303:1001–1006.
2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Choi YH, Kim GY and Lee HH:
Anti-inflammatory effects of cordycepin in
lipopolysaccharide-stimulated RAW 264.7 macrophages through
Toll-like receptor 4-mediated suppression of mitogen-activated
protein kinases and NF-κB signaling pathways. Drug Des Devel Ther.
8:1941–1953. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Majumdar S and Aggarwal BB: Methotrexate
suppresses NF-kappaB activation through inhibition of IkappaBalpha
phosphorylation and degradation. J Immunol. 167:2911–2920.
2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Newton K and Dixit VM: Signaling in innate
immunity and inflammation. Cold Spring Harb Perspect Biol.
4(4)2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen F, Demers LM and Shi X: Upstream
signal transduction of NF-kappaB activation. Curr Drug Targets
Inflamm Allergy. 1:137–149. 2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lin YC, Huang DY, Chu CL, Lin YL and Lin
WW: The tyrosine kinase Syk differentially regulates Toll-like
receptor signaling downstream of the adaptor molecules TRAF6 and
TRAF3. Sci Signal. 6(ra71)2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ong CK, Lirk P, Tan CH and Seymour RA: An
evidence-based update on nonsteroidal anti-inflammatory drugs. Clin
Med Res. 5:19–34. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Weaver CS and Terrell KM: Evidence-based
emergency medicine. Update: Do ophthalmic nonsteroidal
anti-inflammatory drugs reduce the pain associated with simple
corneal abrasion without delaying healing? Ann Emerg Med.
41:134–140. 2003.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Luesch H and Abreu P: A natural products
approach to drug discovery: Probing modes of action of antitumor
agents by genome-scale cDNA library screening. Methods Mol Biol.
572:261–277. 2009.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Prisinzano TE: Natural products as tools
for neuroscience: Discovery and development of novel agents to
treat drug abuse. J Nat Prod. 72:581–587. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cho YC, Lee IS, Seo H, Ju A, Youn D, Kim
Y, Choun J and Cho S: Methanol extracts of Euphorbia cooperi
inhibit the production of inflammatory mediators by inhibiting the
activation of c-Jun N-terminal kinase and p38 in murine
macrophages. Mol Med Rep. 10:2663–2668. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cho YC, Kim YR, Kim BR, Bach TT and Cho S:
Thunbergia alata inhibits inflammatory responses through the
inactivation of ERK and STAT3 in macrophages. Int J Mol Med.
38:1596–1604. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
de Sousa Abreu R, Penalva LO, Marcotte EM
and Vogel C: Global signatures of protein and mRNA expression
levels. Mol Biosyst. 5:1512–1526. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Vogel C and Marcotte EM: Insights into the
regulation of protein abundance from proteomic and transcriptomic
analyses. Nat Rev Genet. 13:227–232. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Maier T, Güell M and Serrano L:
Correlation of mRNA and protein in complex biological samples. FEBS
Lett. 583:3966–3973. 2009.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Fekete T, Rásó E, Pete I, Tegze B, Liko I,
Munkácsy G, Sipos N, Rigó J Jr and Györffy B: Meta-analysis of gene
expression profiles associated with histological classification and
survival in 829 ovarian cancer samples. Int J Cancer. 131:95–105.
2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wojdasiewicz P, Poniatowski LA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gilroy DW: New insights into the
anti-inflammatory actions of aspirin-induction of nitric oxide
through the generation of epi-lipoxins. Mem Inst Oswaldo Cruz. 100
(Suppl 1):49–54. 2005.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Dinarello CA: Anti-inflammatory agents:
Present and future. Cell. 140:935–950. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Aggarwal BB, Prasad S, Reuter S, Kannappan
R, Yadev VR, Park B, Kim JH, Gupta SC, Phromnoi K, Sundaram C, et
al: Identification of novel anti-inflammatory agents from Ayurvedic
medicine for prevention of chronic diseases: ‘reverse pharmacology’
and ‘bedside to bench’ approach. Curr Drug Targets. 12:1595–1653.
2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Pan MH, Chiou YS, Tsai ML and Ho CT:
Anti-inflammatory activity of traditional Chinese medicinal herbs.
J Tradit Complement Med. 1:8–24. 2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Türe-Özdemir F, Tulunay A, Elbasi MO,
Tatli I, Maurer AM, Mumcu G, Direskeneli H and Eksioglu-Demiralp E:
Pro-inflammatory cytokine and caspase-1 responses to pattern
recognition receptor activation of neutrophils and dendritic cells
in Behcet's disease. Rheumatology (Oxford). 52:800–805.
2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hubackova S, Krejcikova K, Bartek J and
Hodny Z: Interleukin 6 signaling regulates promyelocytic leukemia
protein gene expression in human normal and cancer cells. J Biol
Chem. 287:26702–26714. 2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Friedrichsen S, Harper CV, Semprini S,
Wilding M, Adamson AD, Spiller DG, Nelson G, Mullins JJ, White MR
and Davis JR: Tumor necrosis factor-alpha activates the human
prolactin gene promoter via nuclear factor-kappaB signaling.
Endocrinology. 147:773–781. 2006.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Torres J, Enríquez-de-Salamanca A,
Fernández I, Rodríguez-Ares MT, Quadrado MJ, Murta J, Benítez del
Castillo JM, Stern ME and Calonge M: Activation of MAPK signaling
pathway and NF-kappaB activation in pterygium and ipsilateral
pterygium-free conjunctival specimens. Invest Ophthalmol Vis Sci.
52:5842–5852. 2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Bendoraite A, Knouf EC, Garg KS, Parkin
RK, Kroh EM, O'Briant KC, Ventura AP, Godwin AK, Karlan BY,
Drescher CW, et al: Regulation of miR-200 family microRNAs and ZEB
transcription factors in ovarian cancer: Evidence supporting a
mesothelial-to-epithelial transition. Gynecol Oncol. 116:117–125.
2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Limb JK, Yoon S, Lee KE, Kim BH, Lee S,
Bae YS, Jhon GJ and Kim J: Regulation of megakaryocytic
differentiation of K562 cells by FosB, a member of the Fos family
of AP-1 transcription factors. Cell Mol Life Sci. 66:1962–1973.
2009.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1(a000034)2009.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lowell CA: Src-family and Syk kinases in
activating and inhibitory pathways in innate immune cells:
Signaling cross talk. Cold Spring Harb Perspect Biol.
3(3)2011.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Nahar L, Zahan R, Morshed MTI, Haque A,
Alam Z and Mosaddik A: Antioxidant, analgesic and CNS depressant
effects of Synedrella nodiflora. Pharmacogn J. 4:29–36. 2012.
|
|
75
|
Wijaya S, Nee TK, Jin KT, Hon LK, San LH
and Wiart C: Antibacterial and antioxidant activities of Synedrella
nodiflora (L.) Gaertn. (Asteraceae). J Complement Integr Med.
8(8)2011.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gnanaraj C, Shah MD, Haque AT, Makki JS
and Iqbal M: Hepatoprotective and immunosuppressive effect of
Synedrella nodiflora L. on carbon tetrachloride (CCl4)-intoxicated
rats. J Environ Pathol Toxicol Oncol. 35:29–42. 2016.PubMed/NCBI View Article : Google Scholar
|


















