Introduction
Purulent meningitis (PM), caused by pyogenic
bacteria, is common in children aged ≤5 years (1). The incidence of PM in children is
increasing, with the number of new cases of infantile PM globally
exceeding 300,000 as of 2015(2).
Additionally, it was previously reported that the incidence of PM
is exhibiting major geographical differences, with higher rates of
incidence observed in developing countries (3). Infants are particularly vulnerable to a
variety of PM-causing pyogenic bacterial infections, the most
common species being Neisseria meningitidis.,
Streptococcus pneumoniae and Haemophilus influenzae
(4). Since acute infant PM is severe
and harmful, missing the optimal treatment time directly endangers
the life of the patients (5); if not
treated on time, the mortality rate associated with this disease
can reach 50-70% (6).
Accumulating evidence has demonstrated that the
optimal treatment strategy for PM is antibiotic therapy (7). However, in recent years, novel
pharmacological agents for the effective treatment of PM such as
ceftriaxone have been developed with advancing technology (8). The antibacterial spectrum of
ceftriaxone sodium is comparable to that of cefotaxime sodium,
which it has potent effects against Escherichia coli,
Klebsiella pneumoniae, Proteus mirabilis,
Serratia, Meningococcus and Neisseria
gonorrhoeae (9). Although it has
been demonstrated in a number of previous studies to be highly
effective for the treatment of infant PM (10-12),
the efficacy of ceftriaxone sodium in infantile PM is deteriorating
due to a surge of bacterial resistance in the population (13). Dexamethasone is a synthetic
corticosteroid which exhibits anti-inflammatory properties and that
is considered safe in pregnant women and newborns (14,15).
Previous studies have shown that the additive use of dexamethasone
can greatly enhance the efficacy of antibiotics (16,17). At
present, limited information exist on the efficacy of ceftriaxone
sodium combined with dexamethasone on the treatment of infant PM.
The present study retrospectively analyzed the role of ceftriaxone
sodium combined with dexamethasone for the treatment of infant PM
at the Department of Pediatrics, Yongchuan Hospital of Chongqing
Medical University (Chongqing, China) and its associated effects on
brain-derived neurotrophic factor (BDNF) levels. The study provides
an effective reference and guidance for future clinical management
of PM.
Patients and methods
Patients
A retrospective analysis was performed on 177
children (sex, 114 males and 63 females; age range, between 5
months and 6 years; mean age, 3.27±1.42 years) who were admitted to
Yongchuan Hospital of Chongqing Medical University (Chongqing,
China) between January 2015 and February 2016. The present study
was approved by the Ethics Committee of Yongchuan Hospital of
Chongqing Medical University (Chongqing, China) and informed
consent was obtained from the parents of all subjects.
The inclusion criteria were: i) Age of patient <8
years; ii) Early symptoms of meningitis, including nausea and
vomiting; fever; headache and a stiff neck; muscle pain;
sensitivity to light; confusion; cold hands or feet and mottled
skin; in some cases, subjects had a rash that did not fade under
pressure. Later symptoms included seizures and coma. iii) diagnosed
with PM with severity evaluated following cerebrospinal fluid (CSF)
and CT examination at the hospital using the diagnostic criteria of
the 2015 PM Diagnostic Guidelines (14); iv) was receiving follow-up treatment
in our hospital after diagnosis; v) cooperation with hospital
staff; and vi) having complete set of medical records.
The exclusion criteria were: i) Patients with
cancer, diseases of the immune system, blood or severe organ
disorders, hepatocellular failure or renal failure, other
infectious diseases and drug allergies; ii) patients who received
medical treatments other than prescribed drugs from our hospital
following diagnosis; and iii) patients who were transferred from
other hospitals.
Methods
Following explanation of the mechanism of action and
effects of ceftriaxone sodium and dexamethasone, the families of
the respective patients selected the treatment regimens
independently. Of the 177 patients enrolled into the present study,
92 were treated with ceftriaxone sodium combined with
dexamethasone, which served as the combination group; the other 85
patients who received ceftriaxone sodium treatment only served as
the monotherapy group. The treatment regimens performed in the
present study were determined in accordance with the bacterial
species found. For any cases of unidentified pathogenic bacterial
suppurative meningitis, third-generation ceftriaxone or cefotaxime
was used as the first choice of treatment, whilst for pneumococcal
disease, high-dose penicillin was used. For those who were
resistant to penicillin, ceftriaxone was considered in addition to
vancomycin. For meningococcal infection, penicillin was preferred,
whereas those who were penicillin-resistant were treated with
cefotaxime or ceftriaxone. Ceftazidime was used for meningitis
caused by Pseudomonas aeruginosa, whilst ceftriaxone,
cefotaxime or ceftazidime was used for other forms of meningitis
caused by gram-negative bacilli.
The monotherapy group was administered ~70-90 mg/kg
ceftriaxone sodium once daily (Southwest Pharmaceutical Co., Ltd.),
whereas the combination group was administered additively with 0.3
mg/kg dexamethasone once daily (Guizhou Tiandi Pharmaceutical Co.,
Ltd.). Both groups of treatment regimens lasted for two weeks,
which would be stopped immediately if any patient developed an
adverse reaction. The recovery of a set of PM indicators to normal
healthy levels was considered as the completion of treatment. CSF
samples (3 ml) were obtained within three days prior to treatment
(T1), at one week after treatment (T2) and two weeks after
treatment (T3) for further analysis. After centrifugation at 1,500
x g for 5 min at 4˚C, the supernatant was taken for subsequent
testing.
Outcome indicators
Clinical data from the two groups of children,
including age, course of disease, weight, red blood cell (RBC)
count, white blood cell (WBC) count, platelet count, sex, place of
residence and first onset of symptoms were compared. The
rehabilitation indicators measured were time taken for the recovery
of body temperature and WBC counts in both the peripheral blood
(PB) and CSF returning to normal, healthy levels. The CSF
biochemical indicators included WBC count, concentration of protein
and sugar in the CSF and BDNF levels. BDNF levels in the CSF were
analyzed using an ELISA kit (Shanghai Yubo Biological Technology
Co., Ltd.; cat. no. KT11531), whilst the biochemical parameters in
CSF were determined using an automatic biochemical analyzer
(AU5800; Beckman Coulter, Inc.).
Effective rate
The effective treatment rates were determined using
the evaluation criteria referred to as the 2015 PM Rehabilitation
Guidelines (14). Clinical symptoms
and normal CSF examination findings were defined as ‘effective’;
normalized clinical symptoms and CSF examination findings or
results indicating significant improvement were defined ‘improved’;
and clinical symptoms and CSF examination findings revealing
uniformity or even deterioration were defined as ‘ineffective.’ The
effective treatment rate was calculated using the following
formula: [(‘Effective’ + ‘Improved’)/total number of cases] x100%.
The incidence of adverse reactions was using the following formula:
(Number of patients with adverse reactions during treatment/total
number of cases) x100%.
Statistical analysis
The data were analyzed using SPSS version 24.0 (IBM
Corp.). Categorical variables and effective treatment rates between
the two groups were compared using the Chi-square test. Continuous
variables, including the recovery time of body temperature and WBC
counts, were presented as the mean ± standard deviation and
compared between the two groups using Student's t-test. Multiple
time points were compared using repeated measures ANOVA followed by
Bonferroni test. P<0.05 was considered to indicate a
statistically significant difference. The experiments were repeated
3 times.
Results
General data and rehabilitation
outcomes
There were no significant differences in age,
gender, body weight, location of residence, total bilirubin levels,
BUN, number of siblings, family medical history, course of disease,
species of pathogens, severity of disease, red blood cell (RBC),
WBC and platelet (PLT) counts in routine blood examinations between
the two groups, suggesting that the two groups were comparable
prior to treatment (Table I).
 | Table IGeneral characteristics of
patients. |
Table I
General characteristics of
patients.
| Characteristic | Combination
(n=92) | Monotherapy
(n=85) | X2 or
t | P-value |
|---|
| Age | 3.42±1.67 | 3.18±1.84 | 0.91 | 0.36 |
| Course of disease
(days) | 5.23±1.04 | 5.51±1.22 | 1.647 | 0.1 |
| Body weight (kg) | 16.63±5.27 | 17.52±6.04 | 1.047 | 0.3 |
| RBC
(x1012/l) | 4.12±0.84 | 4.09±1.15 | 0.199 | 0.84 |
| WBC
(x109/l) | 47.24±7.68 | 45.81±8.54 | 1.173 | 0.24 |
| PLT
(x109/l) | 247.52±24.16 | 241.34±26.54 | 1.622 | 0.11 |
| Total bilirubin at T1
(µmol/l) | 16.72±2.51 | 17.21±2.66 | 1.261 | 0.21 |
| Total bilirubin at T3
(µmol/l) | 15.62±2.16 | 15.16±2.38 | 1.348 | 0.18 |
| BUN at T1 | 5.65±2.06 | 5.87±2.05 | 0.712 | 0.48 |
| BUN at T3 | 5.12±1.04 | 5.25±1.27 | 0.747 | 0.46 |
| Sex | | | | |
|
Male | 62 (67.39) | 52 (61.18) | | |
|
Female | 30 (32.61) | 33 (38.82) | | |
| Place of
residence | | | 0.689 | 0.41 |
|
Town | 69 (75.00) | 59 (69.41) | | |
|
Rural | 23 (25.00) | 26 (30.59) | | |
| Only Child | | | 0.431 | 0.51 |
|
Yes | 50 (54.35) | 42 (49.41) | | |
|
No | 42 (45.65) | 43 (50.59) | | |
| First onset | | | 1.221 | 0.27 |
|
Yes | 81 (88.04) | 79 (92.94) | | |
|
No | 11 (11.96) | 6 (7.06) | | |
| Pathogen
species | | | 0.701 | 0.7 |
|
Meningococcus | 38 (41.30) | 32 (37.65) | | |
|
Gram-negative
bacilli | 30 (32.61) | 26 (30.59) | | |
|
Pneumococcus | 24 (26.09) | 27 (31.76) | | |
| Family medical
history | | | 0.255 | 0.61 |
|
Yes | 12 (13.04) | 9 (10.59) | | |
|
No | 80 (86.96) | 76 (89.41) | | |
| Severity of
disease | | | 0.099 | 0.95 |
|
Ordinary
type | 47 (51.09) | 44 (51.76) | | |
|
Sudden | 19 (20.65) | 16 (18.82) | | |
|
Light | 26 (28.26) | 25 (29.41) | | |
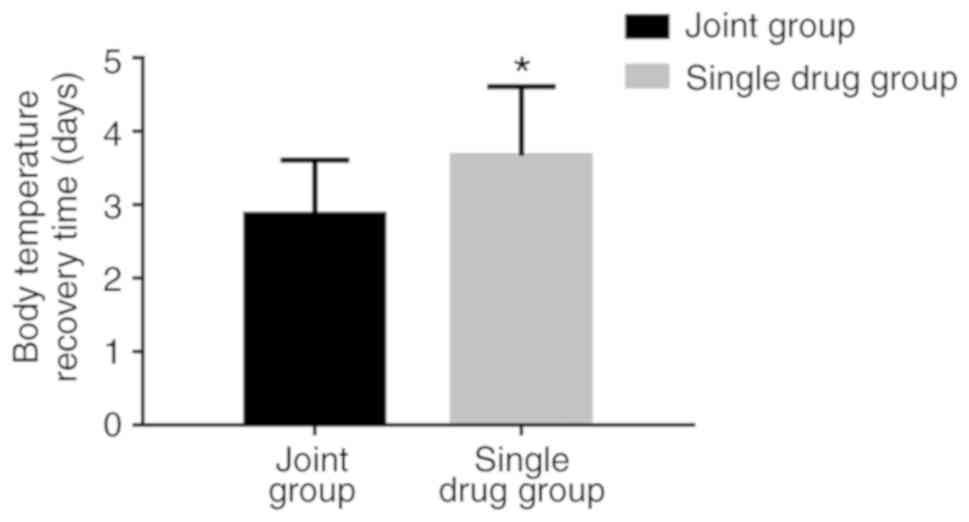
The recovery time of body temperature in the
combination group was 2.87±0.74 days, which was significantly
shorter compared with that in the monotherapy group (3.67±0.94
days; P<0.05; Fig. 1). The
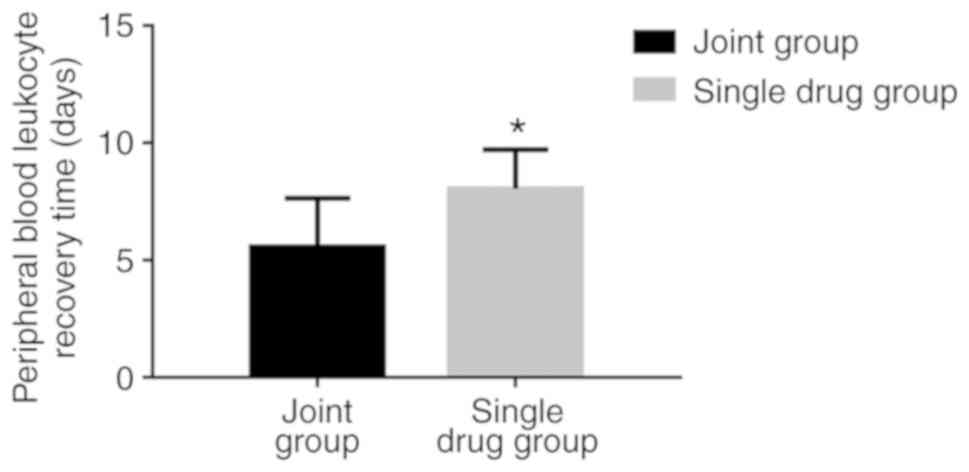
recovery time of the PB WBC count in the combination group was
5.57±2.07, which was significantly shorter compared with that in
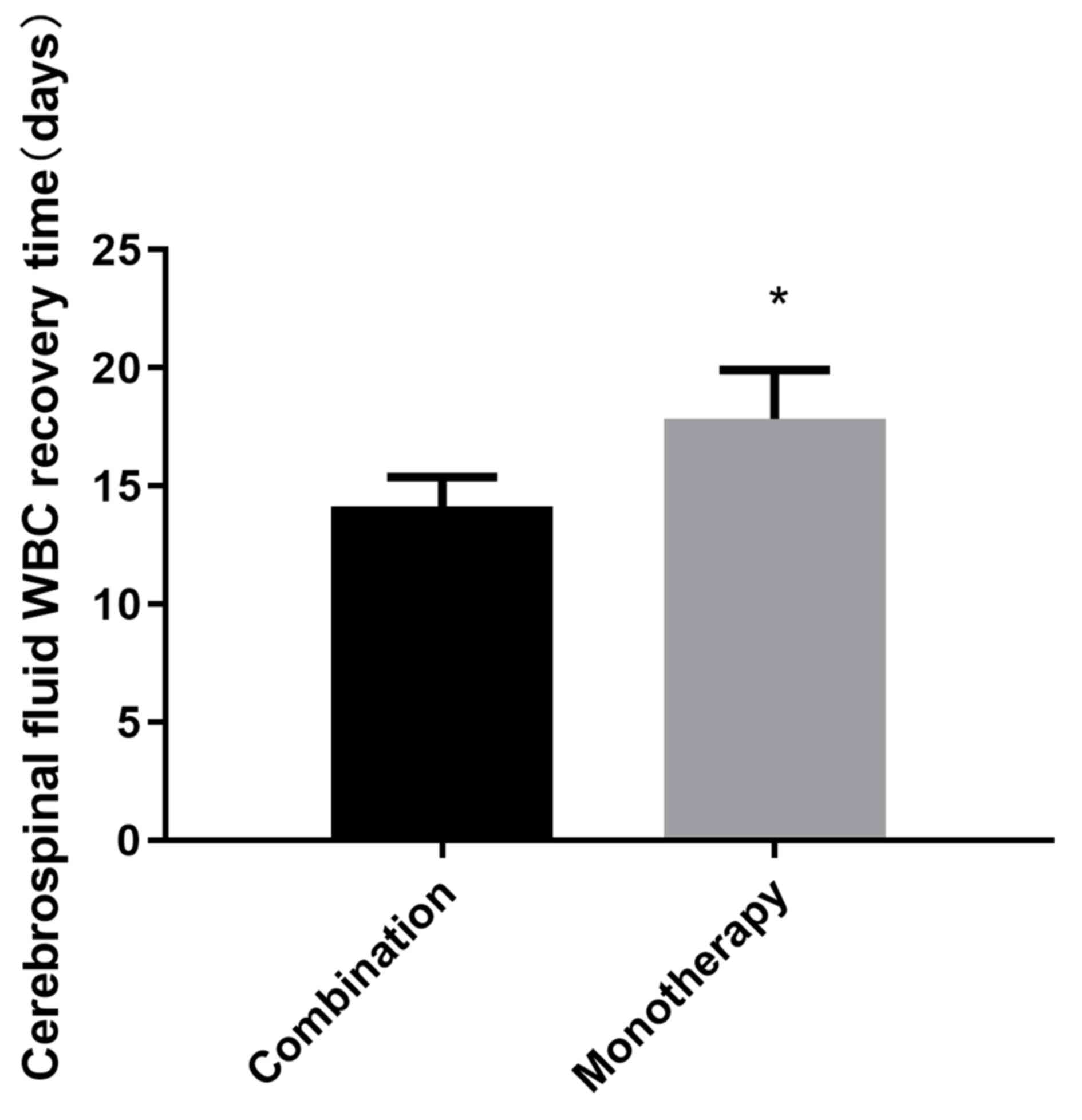
the monotherapy group (8.04±1.68 days; P<0.05; Fig. 2). The recovery time for CSF WBC count
to normal level in the combination group was 14.14±1.24 days, which
was also significantly shorter compared with that in the
monotherapy group (17.84±2.07 days; P<0.05; Fig. 3).
Comparison of the CSF biochemical
parameters
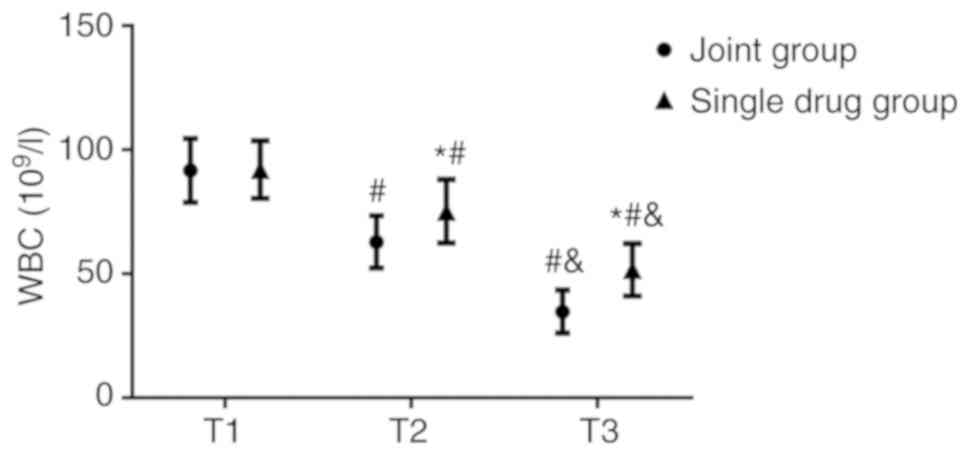
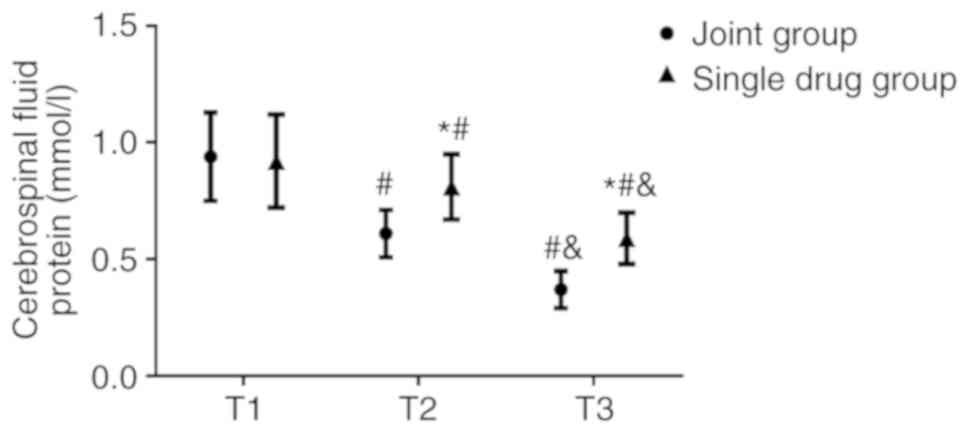
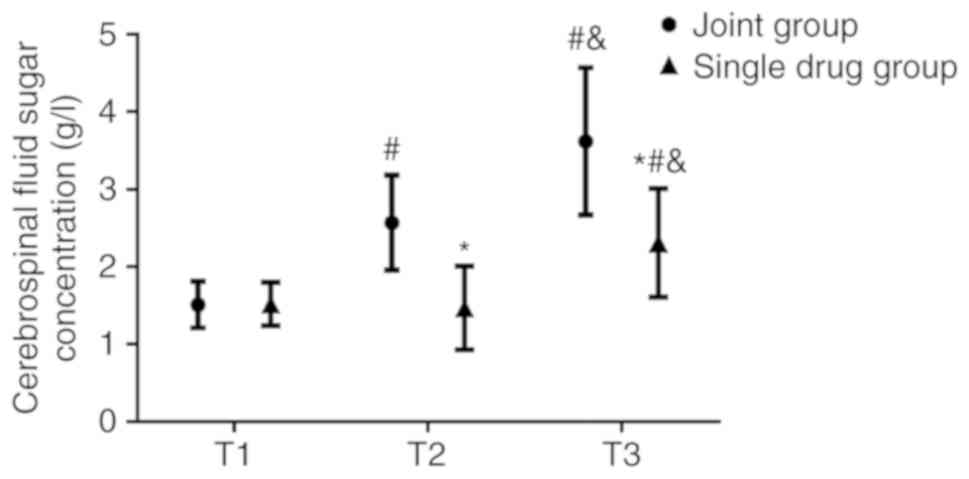
No significant differences were observed in the WBC
count (Fig. 4), CSF protein
(Fig. 5) and sugar concentrations
(Fig. 6) between the combination and
the monotherapy groups at T1. At T2, the WBC count
(62.87±10.54x106/l) and CSF protein concentration
(0.61±0.10 g/l) in the combination group were significantly lower
(both P<0.05) compared with those in the monotherapy group (WBC
count, 75.24±12.84x106/l; CSF protein concentration,
0.81±0.14 g/l). The CSF sugar concentration in the combination
group was 2.57±0.61 mmol/l, which was significantly higher compared
with that in the monotherapy group (1.47±0.54 mmol/l; P<0.05) at
T2. At T3, the WBC count and CSF protein concentration in the
combination group were 34.71±8.68x106/l and 0.37±0.08
g/l, respectively, both of which were also significantly lower
(both P<0.05) compared with those in the monotherapy group (WBC
count, 51.63±10.54x106/l; CSF protein concentration,
0.59±0.11 g/l). The CSF sugar concentration in the combination
group was 3.62±0.95 mmol/l, which was also significantly higher
compared with that in the monotherapy group (2.31±0.70 mmol/l;
P<0.05).
The WBC count and CSF protein concentrations in both
groups were lower at T2 compared with those at T1 (P<0.05),
which decreased further at T3 (P<0.05). By contrast, the CSF
sugar concentration of the combination group increased at T2
compared with T1 (P<0.050), which increased further at T3
(P<0.05). No significant differences in the CSF sugar
concentration could be identified between T1 and T2 in the
monotherapy group, although it was higher at T3 compared with T1
(P<0.05; Figs.
4-6).
Comparison of effective treatment
rates and adverse reactions
The effective treatment rate for the combination
group was calculated to be 94.57%, which was significantly higher
compared with that of the monotherapy group (76.47%; P=0.006;
Table II). In the combination
group, the patients were primarily ‘effective’ to the treatment,
accounting for 61.96%, whilst in the monotherapy group, a slight
majority of the patients were categorized as ‘improved’ (38.82%).
The incidence of adverse reactions in the combination group was
calculated to be 5.43%, which was not significantly different
compared with the monotherapy group (Table III).
 | Table IIComparison of treatment
effectiveness. |
Table II
Comparison of treatment
effectiveness.
| Outcome | Combination group
(n=92) | Monotherapy group
(n=85) | X2 | P-value |
|---|
| Effective [n
(%)] | 57 (61.96) | 32 (37.65) | | |
| Improved [n
(%)] | 30 (32.61) | 33 (38.82) | | |
| Ineffective [n
(%)] | 5 (5.43) | 20 (23.53) | | |
| Effective treatment
rate (%) | 94.57% | 76.47% | 11.930 | 0.006 |
 | Table IIIComparison of incidence of adverse
reactions. |
Table III
Comparison of incidence of adverse
reactions.
| Adverse
reaction | Combination group
(n=92) | Monotherapy group
(n=85) | X2 | P-value |
|---|
| Rash [n (%)] | 0 (0.00) | 1 (1.18) | 1.089 | 0.297 |
| Jaundice [n
(%)] | 2 (2.17) | 2 (2.35) | 0.006 | 0.936 |
| Flatulence [n
(%)] | 1 (1.09) | 0 (0.00) | 0.929 | 0.335 |
| Diarrhea [n
(%)] | 3 (3.26) | 4 (4.71) | 0.243 | 0.622 |
| Adverse reaction
rate (%) | 5.43% | 8.24% | 0.548 | 0.459 |
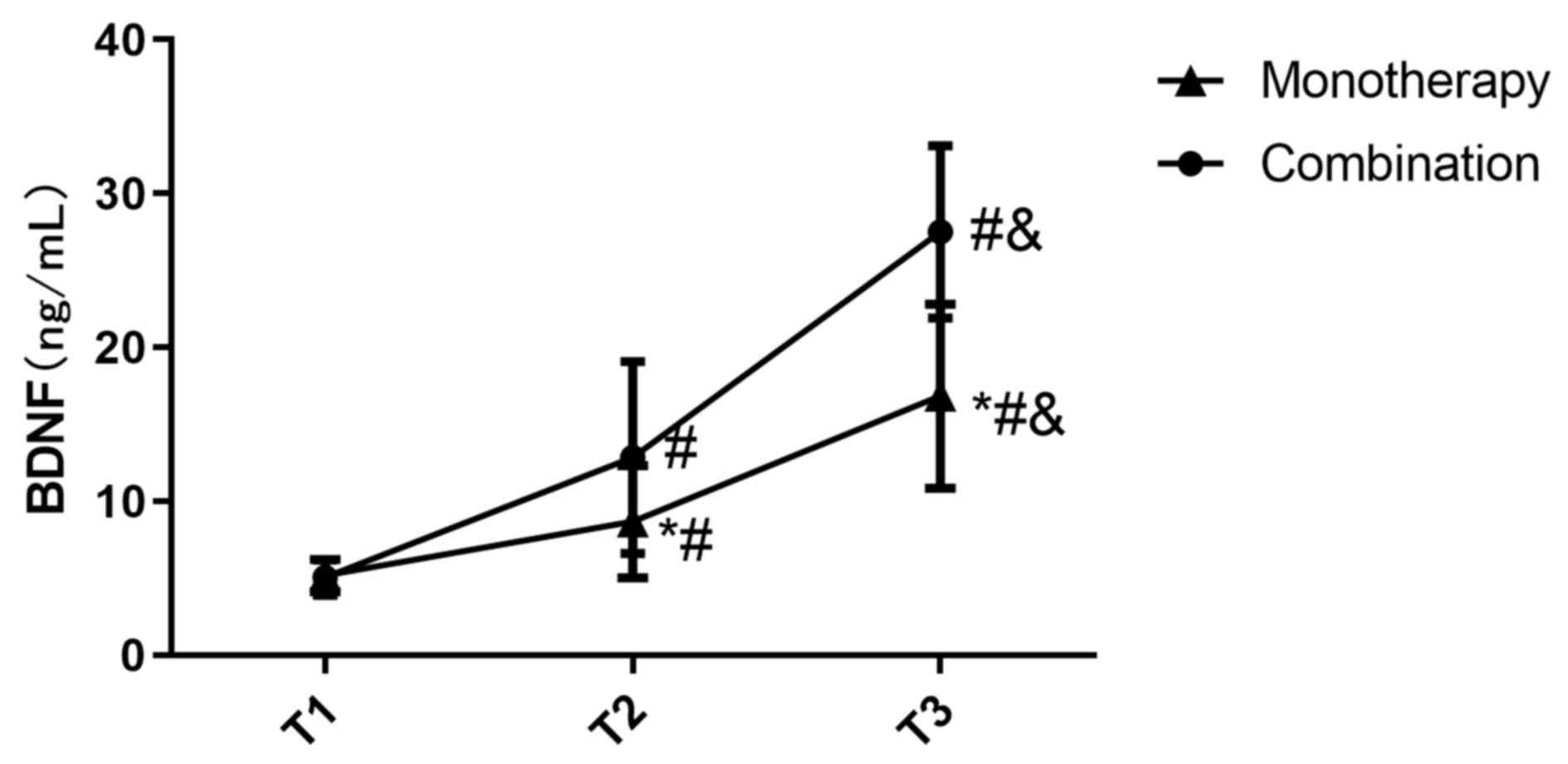
Comparison of BDNF levels
There was no significant difference in the BDNF
levels between the two groups at T1 (Fig. 7). The BDNF levels in the combination
group at T2 was 12.84±6.24, which was significantly higher compared
with that in the monotherapy group (8.67±3.65; P<0.05; Fig. 7). The BDNF levels in the combination
group at T3 was 27.52±5.61, which was also significantly higher
compared with that in the monotherapy group (16.84±5.99; P<0.05;
Fig. 7).
The BDNF levels in both groups were significantly
increased at T2 (P<0.05) which increased further at T3
(P<0.05; Fig. 7).
Discussion
PM is an infectious disease caused by purulent
bacterial infection in the central nervous system (CNS).
Specifically, toxins produced by the bacteria can induce aberrant
inflammatory responses in the arachnoid and pia mater. If not
treated immediately, the bacterial toxins can spread to the brain
parenchyma and spinal cord, at which point more intensive
treatments would be required with a guarded prognosis (18). The main pathogens responsible for
infant PM are gram-negative bacteria and Staphylococcus
aureus (19). PM is frequently
accompanied with fibrin exudation and neutrophil infiltration,
resulting in susceptibility to inflammatory small vessel embolism,
focal cerebral infarction and encephalorrhagia (20). On clinical suspicion of PM, an
empirical antibacterial therapeutic strategy is first adopted,
where targeted antibiotic treatment is initiated as soon as the
presence of the pathogen is confirmed (21).
Among the antibacterial pharmacological agents
currently applied for PM, ceftriaxone sodium is the most frequently
used. Mechanistically, ceftriaxone sodium operates by increasing
the expression of intracellular glutamate transporters to reduce
the levels of excitatory glutamate and enhance neuroprotection,
reducing the risk of brain tissue damage (22). However, with the rise in
drug-resistant bacterial strains, the use of ceftriaxone alone has
not achieved desirable outcomes for the treatment of PM. In such
cases, administration of dexamethasone, a commonly used
glucocorticoid, is applied. In addition to the suppression of
inflammation by mainly inhibiting macrophage activity,
dexamethasone has also been previously demonstrated to reduce
intracranial pressure and cerebral edema (23).
BDNF is a vital neurotrophic factor in CNS that
serves a role in promoting neuronal survival and differentiation in
the human body (24). For the
treatment of PM, BDNF application can significantly reduce neuronal
damage in the hippocampus, which may accelerate rehabilitation and
improve prognosis. However, insufficient studies regarding the
association between PM and BDNF exist. By comparing the efficacy of
ceftriaxone sodium combined with dexamethasone to ceftriaxone
sodium alone in patients with PM and monitoring changes in BDNF
levels, the present study demonstrated a potential clinical role of
ceftriaxone sodium combined with dexamethasone for infant PM
treatment.
The results from the present study indicated that
the effective treatment rate of the combination group was superior
to that of the monotherapy group. The recovery time of body
temperature, PB and CF WBC counts in the combination group was
shorter compared with that of the monotherapy group, suggesting
that ceftriaxone sodium combined with dexamethasone in PM was more
effective compared with ceftriaxone alone. It could be hypothesized
that these observations may be due to the anti-inflammatory
properties of dexamethasone or the expansion of the ceftriaxone
antibacterial spectrum. The use of ceftriaxone sodium alone has
poor antibacterial effect and is likely to cause resistance in
children. In children with PM, the metabolic function of central
nervous system is abnormal due to the influence of bacterial
toxins, which has an impact on glucose transporters and blood
circulation fluidity (25).
There was no significant difference in the incidence
of adverse reactions between the two treatment groups, suggesting
that both therapeutic strategies are safe and worthy of clinical
promotion. Some bacteria are able to convert glucose in the CSF to
lactic acid (26), where the
resultant inflammatory response can increase the level of
antibodies in the CSF of patients with PM (27). Compared with the monotherapy group,
CSF sugar concentrations were higher in the combination group,
whilst the CSF protein concentrations were lower, suggesting that
ceftriaxone sodium combined with dexamethasone was more effective
in improving CSF function and CNS metabolism.
Dexamethasone inhibits the release of chemokines by
reducing the stimulation of the inflammatory cells such as
monocytes (28). As a result, damage
to the neurovascular system is greatly reduced. In addition,
dexamethasone contributed to the stabilization of the vascular
endothelial structure and was of great significance to the
protection of neurons and blood vessels (29). This was speculated to be a reason for
the higher BDNF levels observed in the combination group compared
with the monotherapy group in the present study. Previous studies
have demonstrated that BDNF can regulate the expression of cortical
neurons and hippocampal neurons through the MARK/ERK pathway
(30,31). Although it could be speculated that
dexamethasone inhibited the MAPK/ERK pathway, further research is
required to explore the intracellular mechanism of BDNF action.
Small sample size was a limitation of this study.
Further research and discussion are needed to clarify the mechanism
of the effects of the combination of ceftriaxone sodium and
dexamethasone on BDNF levels in infant PM. A longer follow-up of
patients should be performed in a future study.
In conclusion, the effects of ceftriaxone combined
with dexamethasone for the treatment of infant PM was found to be
superior compared with ceftriaxone alone. In addition, ceftriaxone
combined with dexamethasone improved the effective treatment rate
and rehabilitation of patients with PM and also improved the BDNF
levels in patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WYZ and WZ conceived the study and designed the
experiments, contributed to the data collection, performed the data
analyses, and interpreted the results. WYZ drafted the manuscript.
ZW contributed to the critical revision of the article. All authors
read and approved the final draft of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yongchuan Hospital of Chongqing Medical University
(Chongqing, China), and informed consent was obtained from all the
parents of the subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Qian Y, Wong CC, Lai SC, Lin ZH, Zheng WL,
Zhao H, Pan KH, Chen SJ and Si JM: Klebsiella pneumoniae
invasive liver abscess syndrome with purulent meningitis and septic
shock: A case from mainland China. World J Gastroenterol.
22(2861)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
He Z, Li X and Jiang L: Clinical analysis
on 430 cases of infantile purulent meningitis. Springerplus.
5(1994)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Agossou J, Adédémy JD, Noudamadjo A,
Houessou MRM, Tsawlassou P, Assogba R, Sagbo GG, Lalya HF, Alao MJ,
Bankolé H, et al: Serotypes of bacteria encountered in childhood
purulent meningitis in children in Parakou (Benin) in 2011. Open J
Pediat. 6(109)2016.
|
|
4
|
Du H, Liu E, Xu C, Zhao S, Xiang H and Li
Z: Prognostic value of funisitis and/or chorionic vasculitis
compared to histologic chorioamnionitis in full-term infants. The J
Matern Fetal Neonatal Med. 30:169–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu C and Zhao D: Correlation between CD64
and PCT levels in cerebrospinal fluid and degree of hearing
impairment sequelae in neonates with purulent meningitis. Exp Ther
Med. 14:5997–6001. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lan SY, Lin JJ, Hsia SH, Wang HS, Chiu CH
and Lin KL: CHEESE Study Group: Analysis of fulminant cerebral
edema in acute pediatric encephalitis. Pediatr Neonatol.
57:402–407. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ai J, Xie Z, Liu G, Chen Z, Yang Y, Li Y,
Chen J, Zheng G and Shen K: Etiology and prognosis of acute viral
encephalitis and meningitis in Chinese children: A multicentre
prospective study. BMC Infect Dis. 17(494)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yimer EM, Hishe HZ and Tuem KB:
Repurposing of the β-lactam antibiotic, ceftriaxone for
neurological disorders: A review. Front Neurosci.
13(236)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xu W, Yin M, Huo MC, Yan JL, Yang Y and
Liu CF: Changes in blood CD4+ CD25+
regulatory T cells in children with severe purulent meningitis.
Zhongguo Dang Dai Er Ke Za Zhi. 18:821–825. 2016.(In Chinese).
PubMed/NCBI
|
|
10
|
Rahman M, Khan MA, Abdul Rub M, Hoque MA
and Asiri AM: Investigation of the effect of various additives on
the clouding behavior and thermodynamics of polyoxyethylene (20)
sorbitan monooleate in absence and presence of ceftriaxone sodium
trihydrate drug. J Chem Eng Data. 62:1464–1474. 2017.
|
|
11
|
Rahman M, Khan MA, Rub MA and Hoque MA:
Effect of temperature and salts on the interaction of
cetyltrimethylammonium bromide with ceftriaxone sodium trihydrate
drug. J Mol Liquids. 223:716–724. 2016.
|
|
12
|
Patel N, Lalwani D, Gollmer S, Injeti E,
Sari Y and Nesamony J: Development and evaluation of a calcium
alginate based oral ceftriaxone sodium formulation. Prog Biomater.
5:117–133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guo X, Wan J, Yu X and Lin Y: Study on
preparation of SnO2-TiO2/Nano-graphite
composite anode and electro-catalytic degradation of ceftriaxone
sodium. Chemosphere. 164:421–429. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fu Y, Jing J, Ren T and Zhao H:
Intratympanic dexamethasone for managing pregnant women with sudden
hearing loss. J Int Med Res. 47:377–382. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dileep A, Khan NB and Sheikh SS: Comparing
neonatal respiratory morbidity in neonates delivered at term by
elective Caesarean section with and without dexamethasone:
Retrospective cohort study. J Pak Med Assoc. 65:607–611.
2015.PubMed/NCBI
|
|
16
|
Tange M, Yoshida M, Nakai Y and Uchida T:
The role of an impurity in ceftriaxone sodium preparation for
injection in determining compatibility with calcium-containing
solutions. Chem Pharm Bull (Tokyo). 64:207–214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Di Cicco M, Bellino EM, Marabotti A, Luti
L, Peroni DG and Baroncelli GI: Acute dacryocystitis with giant
lacrimal abscess: A case report. Ital J Pediatr.
46(15)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo LY, Zhang ZX, Wang X, Zhang PP, Shi W,
Yao KH, Liu LL, Liu G and Yang YH: Clinical and pathogenic analysis
of 507 children with bacterial meningitis in Beijing, 2010-2014.
Int J Infect Dis. 50:38–43. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rodriguez WJ, Ross S, Khan WN and
Goldenberg R: Clinical and laboratory evaluation of cefamandole in
infants and children. J Infect Dis. 137:S150–S154. 1978.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ekhtiyari E, Barzegar M, Mehdizadeh A,
Shaaker M, Ghodoosifar S, Abhari A and Darabi M: Differential fatty
acid analysis of cerebrospinal fluid in infants and young children
with suspected meningitis. Child's Nerv Sys. 33:111–117.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kępa L, Oczko-Grzesik B, Stolarz W and
Boroń-Kaczmarska A: Cerebrospinal fluid ferritin concentration in
patients with purulent, bacterial meningitis-own observations.
Przegl Epidemiol. 70:593–603. 2016.(In English, Polish). PubMed/NCBI
|
|
22
|
Sharma VD, Singla A, Chaudhary M and
Taneja M: Population pharmacokinetics of fixed dose combination of
ceftriaxone and sulbactam in healthy and infected subjects. AAPS
PharmSciTech. 17:1192–1203. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dimopoulos MA, Moreau P, Palumbo A, Joshua
D, Pour L, Hájek R, Facon T, Ludwig H, Oriol A, Goldschmidt H, et
al: Carfilzomib and dexamethasone versus bortezomib and
dexamethasone for patients with relapsed or refractory multiple
myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre
study. Lancet Oncol. 17:27–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jha A, Dwivedi NC, Verma SK and Chaurasia
AK: Role of cerebrospinal fluid, creatine kinase and lactate
dehydrogenase enzyme levels in diagnostic and prognostic evaluation
of tubercular and pyogenic meningitis. Int J Adv Med. 4:824–829.
2017.
|
|
25
|
Scoppetta TLPD, da Rocha AJ and Nunes RH:
Meningitis, empyema, and brain abscess in adults. In: Editors.
Critical Findings in Neuroradiology. Springer. 141–154. 2016.
|
|
26
|
Ahmed R: Gestational dexamethasone alters
fetal neuroendocrine axis. Toxicol Lett. 258:46–54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Panackal AA, Chittboina P, Marr KA,
Bielekova B and Williamson PR: Dexamethasone in cryptococcal
meningitis. N Engl J Med. 375(188)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Miao YL, He P, Zhang WX, Zhang WZ, Feng M
and Ni Y: Anti-inflammatory mechanism of Crepis crocea based on
NF-κB signaling pathway and ~1H-NMR metabonomics. Zhongguo Zhong
Yao Za Zhi. 45:946–954. 2020.(In Chinese). PubMed/NCBI View Article : Google Scholar
|
|
29
|
Barna L, Walter FR, Harazin A, Bocsik A,
Kincses A, Tubak V, Jósvay K, Zvara Á, Campos-Bedolla P and Deli
MA: Simvastatin, edaravone and dexamethasone protect against
kainate-induced brain endothelial cell damage. Fluids Barriers.
17(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang JC, Yao W and Hashimoto K:
Brain-derived neurotrophic factor (BDNF)-TrkB signaling in
inflammation-related depression and potential therapeutic targets.
Curr Neuropharmacol. 14:721–731. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
El Morsy EM and Mae A: Protective effects
of lycopene on hippocampal neurotoxicity and memory impairment
induced by bisphenol A in rats. Hum Exp Toxicol.
960327120909882:2020.PubMed/NCBI View Article : Google Scholar
|