Introduction
Chronic lymphocytic leukaemia (CLL), the most common
leukaemia in adults (1), is a
malignancy characterised by the accumulation of mature monoclonal B
cells in the peripheral blood, the lymphoid organs and the bone
marrow of patients with a circulatory
CD5+CD19+CD20dimIgdimCD23+CD43+CD27+
surface phenotype (2,3). Despite advances in CLL therapy over the
years, the survival rate of CLL patients remains low (4).
Although CLL pathophysiology is complicated, B-cell
receptor signalling is considered a driving factor of CLL tumour
cell survival (5). Bruton's tyrosine
kinase (BTK), which is downstream of B-cell receptors, serves an
important role in the activation of pathways associated with CLL
cell survival (6), including protein
kinase B (3), extracellular
signal-regulated kinase (7) and
nuclear factor-kB pathways (8). It
has previously been reported that inhibition of BTK may be used as
a treatment strategy in untreated and relapsed CLL patients
(9).
MicroRNAs (miRNAs) are small endogenous RNAs
involved in various biological processes and diseases, including
tumourigenesis, cell proliferation, apoptosis and autophagy
(10,11). It has been demonstrated that miRNAs
are differentially expressed between normal and tumour cells,
including in CLL (12). miRNAs are
involved in the proliferation and apoptosis of CLL cells (13); however, a deeper insight into the
function of miRNAs in CLL is lacking. miR-425 is an miRNA
associated with numerous diseases and bioprocesses, including
anti-angiogenesis (14),
tumourigenesis (15) and
inflammatory cytokine production (16). HE et al (17) reported that miR-425 contributes to
tumour development by inhibiting the tumour suppressor catenin α-3
in hepatocellular carcinoma. miR-425 is also reportedly upregulated
in gastric cancer (18). However, to
the best of our knowledge, no previous study has focused on the
role of miR-425 in CLL.
In the present study, the effect of miR-425 on the
proliferation of CLL cells and the possible mechanism whereby this
regulation occurs were assessed, identifying the BTK/phospholipase
Cγ2 (PLCγ2) signalling pathway. The current study provides a deeper
insight for understanding the development of CLL and identifies a
novel potential therapeutic target for CLL patients.
Materials and methods
Clinical samples and cell culture
Normal and CLL B lymphocytes were isolated from
peripheral blood collected from 15 healthy individuals and 15 CLL
patients, respectively, using the MagCellect Human B Cell Isolation
kit (cat. no. MAGH103; R&D Systems) as described previously
(19). In addition, the CLL cell
line MEC-1 was purchased from ATCC (Manassas, VA, USA), and cells
were maintained in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% foetal
bovine serum (FBS) under 37˚C and 5% CO2. The present
study was approved by the Ethics Committee of Hunan Provincial
People's Hospital (Changsha, China). Informed consent was obtained
from participants.
Cell transfection
The miR-425 negative control and miR-425 mimic were
purchased from RiboBio Co., Ltd. (Guangzhou, China). In addition, a
recombinant plasmid was used to overexpress BTK in MEC-1 cells.
Briefly, the BTK promoter was cloned and inserted into the pcDNA3.1
vector (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to form
pcDNA3.1-BTK. MEC-1 cells were cultured to 30-50% confluence in
96-well plates and transfected with miR-425 mimic, pcDNA3.1-BTK or
pcDNA3.1-negative control (NC; 50 nmol/l). Samples were also
co-transfected with miR-425 mimic and pcDNA3.1-BTK (50 nmol/l for
each) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) in serum-free Opti-Minimum Essential
Medium (Gibco; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
MTT assay
Cells were inoculated into 96-well plates at a
density of 1x103 cells/well and incubated for 24, 48 or
72 h. An MTT assay was then performed to evaluate cell viability at
24, 48 and 72 h after transfection, as described previously
(20). Briefly, 25 ml MTT solution
(5 mg/ml) was added to each well and incubated at 37˚C for 4 h.
Following centrifugation at a speed of 1,200 x g for 5 min at room
temperature, the supernatant was then replaced with 180 ml dimethyl
sulfoxide, and absorbance at 490 nm was evaluated using a
Synergy-HT Multi-Detection microplate reader (Bio-Tek Instruments,
Inc., Winooski, VT, USA).
Cell cycle measurements
Cell cycle distribution was evaluated using flow
cytometry analysis, as described previously (21). Briefly, cells in different groups
were harvested by trypsinization, washed twice with ice cold PBS
and then fixed with 70% ethanol overnight at 4˚C. Next, cells were
resuspended in 100 µl PBS containing a final concentration of 50
µg/ml RNase A for 30 min at room temperature. Propidium iodide from
the FITC Annexin V kit (BD Biosciences, San Jose, CA, USA) was used
to stain the cells at a concentration of 20 µg/ml for 20 min. A
flow cytometer (BD-LSR; BD Biosciences) was used to detect the DNA
content, while the cell cycle distribution was analysed using the
Cell Quest software, version 5.1 (BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the B lymphocytes or
MEC-1 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and RNA concentration was determined using a
NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA). A High Capacity cDNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was then used to convert RNA into cDNA. qPCR
reactions were performed using SYBR Green Master Mix (Solarbio
Science & Technology Co., Ltd., Beijing, China) in an
Exicycler™ 96 (Bioneer Corporation, Daejeon, Korea). The
thermocycling conditions were as follows: Initial activation at
95˚C for 15 min, followed by 40 cycles of denaturation at 94˚C for
15 sec, annealing at 55˚C for 30 sec and extension at 72˚C for 30
sec. The Primers utilized for the reverse transcription of mature
miR-425-5p and U6 were as follows: hsa-miR-425-5p stem-loop RT
primer, 5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAACGGG-3'; U6-RT,
5'-CAACTGGTGTCGTGGAGTCGGC-3'. The primers utilized in PCR were as
follows: miR-425-5p forward, 5'-ACACTCCAGCTGGGAATGACACGATCACTCC-3'
and reverse, 5'-TGGTGTCGTGGAGTCG-3'; BTK forward,
5'-GAGAAGCTGGTGCAGTTGTAT-3', and reverse,
5'-GGCCGAAATCAGATACTTTAAC-3'; PLCγ2 forward,
5'-GCTGCTTCACCATCCTATATG-3', and reverse
5'-GGAACTTGGCACTGCTCACT-3'; Ki-67 forward,
5'-GAGAATCTGTGAATCTGGGTAA-3', and reverse,
5'-CAGGCTTGCTGAGGGAAT-3'; proliferating cell nuclear antigen (PCNA)
forward, 5'-ACTCGTCTCATGTCTCCTTG-3', and reverse,
5'-TCATTCATCTCTATGGCAACAG-3'; GAPDH forward,
5'-CACCCACTCCTCCACCTTTG-3', and reverse,
5'-CCACCACCCTGTTGCTGTAG-3'. The relative RNA levels were calculated
by the 2-ΔΔCq method (22). U6 and GAPDH were utilized as an
internal control.
Plasmid construction and
dual-luciferase reporter assay
The predicted binding of BTK and miR-425 was
obtained via bioinformatic prediction using targetscan 5.1 software
(http://www.targetscan.org; Whitehead
Institute for Biomedical Research, Cambridge, MA, USA). Wild-type
(WT) constructs of the BTK 3'-untranslated region (3'-UTR)
contained the predicted miR-425 binding region. Mutant (MUT)
constructs of BTK 3'-UTR lacked the predicted miR-425 responsive
element. Each were inserted downstream of the firefly luciferase
gene in the pmiR-REPORT™ plasmid (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China). MEC-1 cells were co-transfected with the
luciferase reporter plasmids and miR-425 mimics/miR-425-NC.
Following transfection for 48 h, luciferase activity was measured
by a Dual-Luciferase Reporter System (Promega Corporation, Madison,
WI, USA) using an Centro LB 960 microplate luminometer (Berthold
Technologies, Bad Wildbad, Germany). All reactions were repeated in
triplicate for at least three independent experiments.
Western blotting
Western blotting was used to test the protein
expression levels of BTK, PLCγ2, Ki-67 and PCNA, with β-actin used
as a loading control. Briefly, protein was extracted from MEC-1
cells using radioimmunoprecipitation assay buffer (Vazyme,
Piscataway, NJ, USA). Protein was quantitated using a protein assay
reagent Colorimetric assay kit (cat. no. 5000002) from Bio-Rad
(Hercules, CA, USA). Samples were then subjected to 10% SDS-PAGE
and then transferred to polyvinylidene difluoride membranes.
Following blocking with 5% non-fat milk at room temperature for 1
h, membranes were incubated with the following primary antibodies
(all purchased from Abcam, Cambridge, UK) at 4˚C overnight:
Anti-BTK (cat. no. ab137503; 1:1,000), anti-PLCγ2 (cat. no.
ab227129; 1:500), anti-Ki67 (cat. no. ab16667; 1:500), anti-PCNA
antibodies (cat. no. ab29; 1:1,000) and β-actin (cat. no. ab8226;
1:1,000). Next, membranes were incubated with a horseradish
peroxidase-conjugated anti-rabbit (ab6721) or anti-mouse (ab6785)
immunoglobulin G secondary antibody (Abcam) at 37˚C for 45 min.
Films were scanned using Super Signal West Pico Chemiluminescent
Substrate kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. Relative protein
expression was quantified using Image-Pro Plus software (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons between two groups were performed using Student's
t-test. Comparisons among three or more groups were conducted using
one-way analysis of variance with Tukey's post-hoc test. The
results were considered to be statistically significant when the
P-value was <0.05. All calculations were performed using SPSS
software (version 18.0; SPSS, Inc., Chicago, IL, USA).
Results
Downregulation of miR-425 in B
lymphocytes of CLL patients
Initially, the expression of miR-425 was compared
between the B lymphocytes of CLL patients and normal B lymphocytes
using RT-qPCR. The B lymphocytes were isolated from peripheral
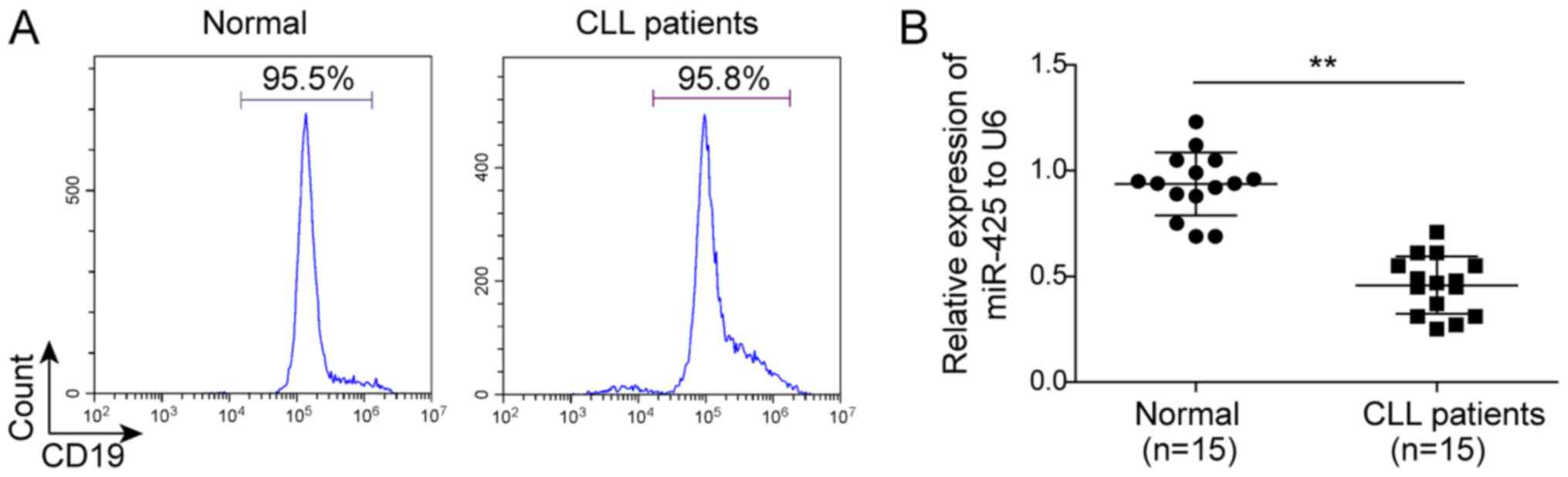
blood collected from healthy individuals and CLL patients, and the
purity was >95%, as indicated in Fig.
1A. As shown in Fig. 1B, the
expression of miR-425 was significantly downregulated in the B
lymphocytes of CLL patients compared with that in normal B
lymphocytes (P<0.05).
Upregulation of miR-425 inhibits the
proliferation of MEC-1 cells
To further investigate the effects of miR-425 on CLL
cells, an miR-425 mimic was used to transfect the CLL cell line
MEC-1, and its effect on cell proliferation was subsequently
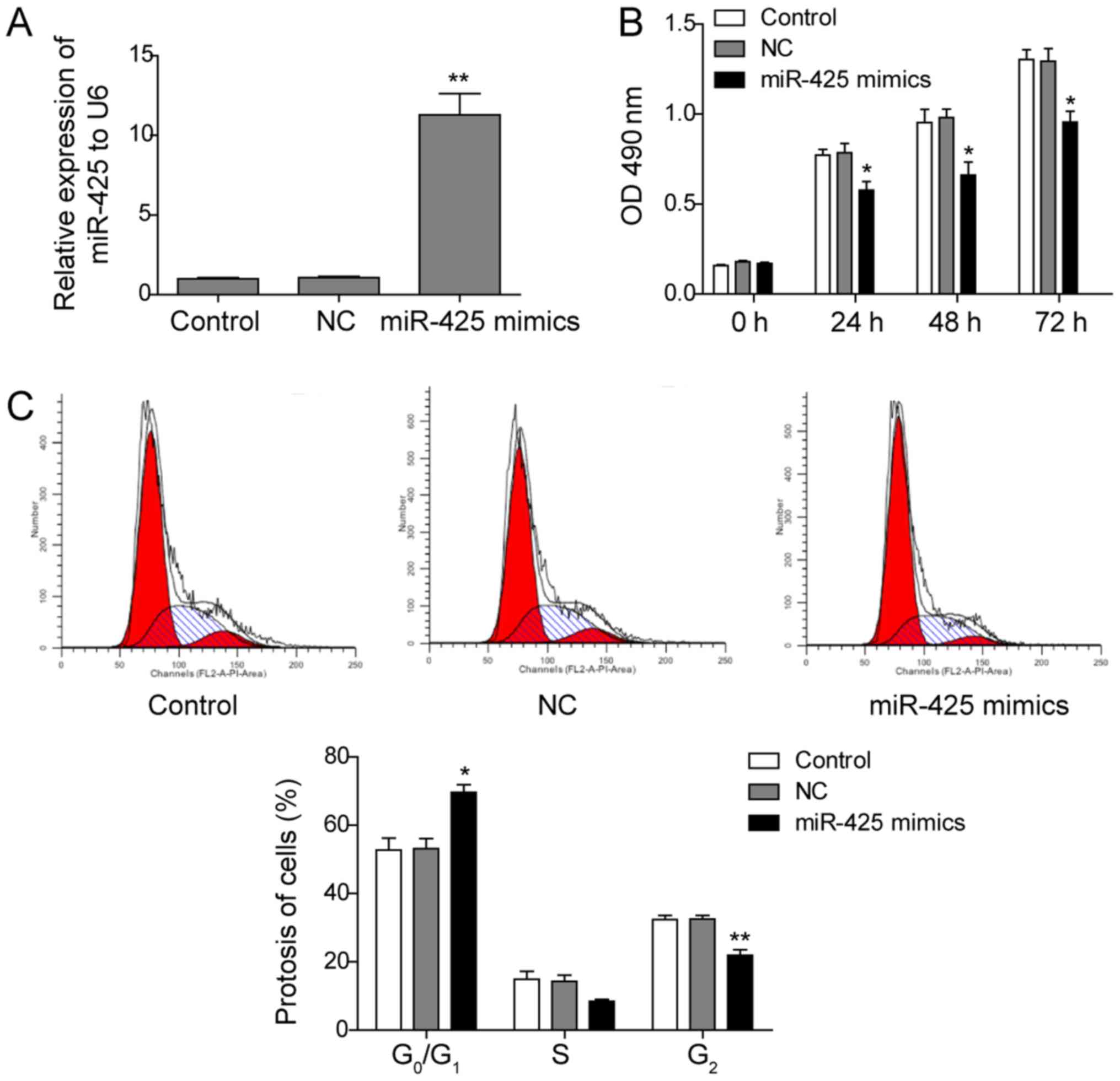
investigated. As shown in Fig. 2A,
transfection with the miR-425 mimic significantly enhanced the
expression of miR-425, as expected, compared with that in the
control groups (P<0.05). Furthermore, when miR-425 was
upregulated, the proliferation of MEC-1 cells was significantly
inhibited at 24, 48 and 72 h compared with the proliferation of
control cells (P<0.05; Fig. 2B).
Next, the effect of miR-425 on cell cycle distribution in MEC-1
cells was evaluated by flow cytometry analysis. The results
demonstrated that the ratio of G0/G1 cells was significantly
increased in miR-425-overexpressing cells, while the ratio of G2/M
cells was significantly decreased, compared with that in the
controls (P<0.05; Fig. 2C). These
results indicate that overexpression of miR-425 significantly
inhibits the proliferation of MEC-1 cells.
BTK expression is regulated by
miR-425
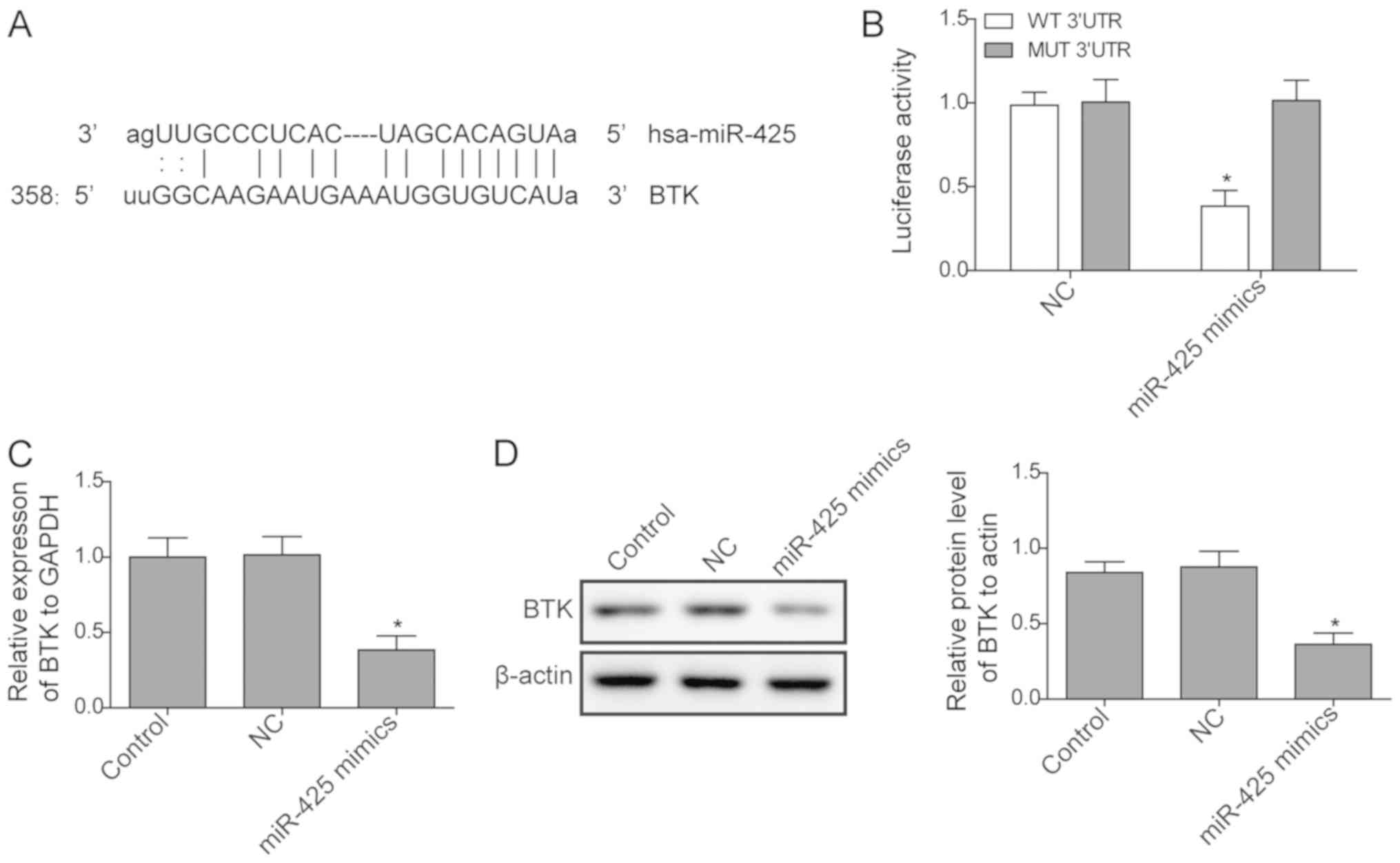
As reported in a previous study (23), miR-425 may target the BTK gene. In
the present study, a dual-luciferase reporter assay was used to
confirm whether BTK was a target of miR-425. As presented in
Fig. 3A and B, luciferase activity significantly
decreased when cells were transfected with miR-425 mimics of WT BTK
3'-UTR vectors, while no significant difference was identified in
MUT BTK 3'-UTR. Next, the expression of BTK in cells transfected
with miR-425 mimic and in control cells was measured. The results
revealed that the expression of BTK was significantly inhibited at
the mRNA and protein levels when miR-425 was upregulated by mimic
transfection (Fig. 3C and D), indicating that the expression of BTK is
negatively regulated by miR-425.
miR-425 inhibits the proliferation of
MEC-1 cells by regulating the BTK/PLCγ2 signalling pathway
To investigate the mechanisms underlying the
inhibitory effect of miR-425 on the proliferation of MEC-1 cells,
the expression levels of proteins associated with the BTK/PLCγ2
signalling pathway were measured by western blotting in different
experimental groups. The results indicated that upregulation of
miR-425 significantly inhibited the expression levels of BTK and
its downstream protein PLCγ2, as compared with the control
treatment (P<0.05; Fig. 4A and
B). The levels of the
proliferation-associated proteins Ki-67 and PCNA were also
downregulated in miR-425-overexpressing cells. Subsequently, BTK
overexpression was conducted by plasmid transfection, and western
blotting indicated that BTK protein level was successfully
increased (Fig. 4C). An MTT assay
was then used to assess whether BTK overexpression reduces the
inhibition of cell proliferation in response to miR-425. As
indicated in Fig. 4D, following 24 h
culture, the optical density value was significantly higher in the
miR-425 mimic + BTK-treated group at all time points (24, 48 and 72
h), as compared with that in the group treated with miR-425 mimic
alone, indicating that BTK overexpression partially rescued the
inhibitory effect of miR-425 on cell proliferation. These results
suggest that miR-425 regulates the BTK/PLCγ2 signalling pathway to
further inhibit expression of Ki-67 and PCNA, inhibiting the
proliferation of MEC-1 cells.
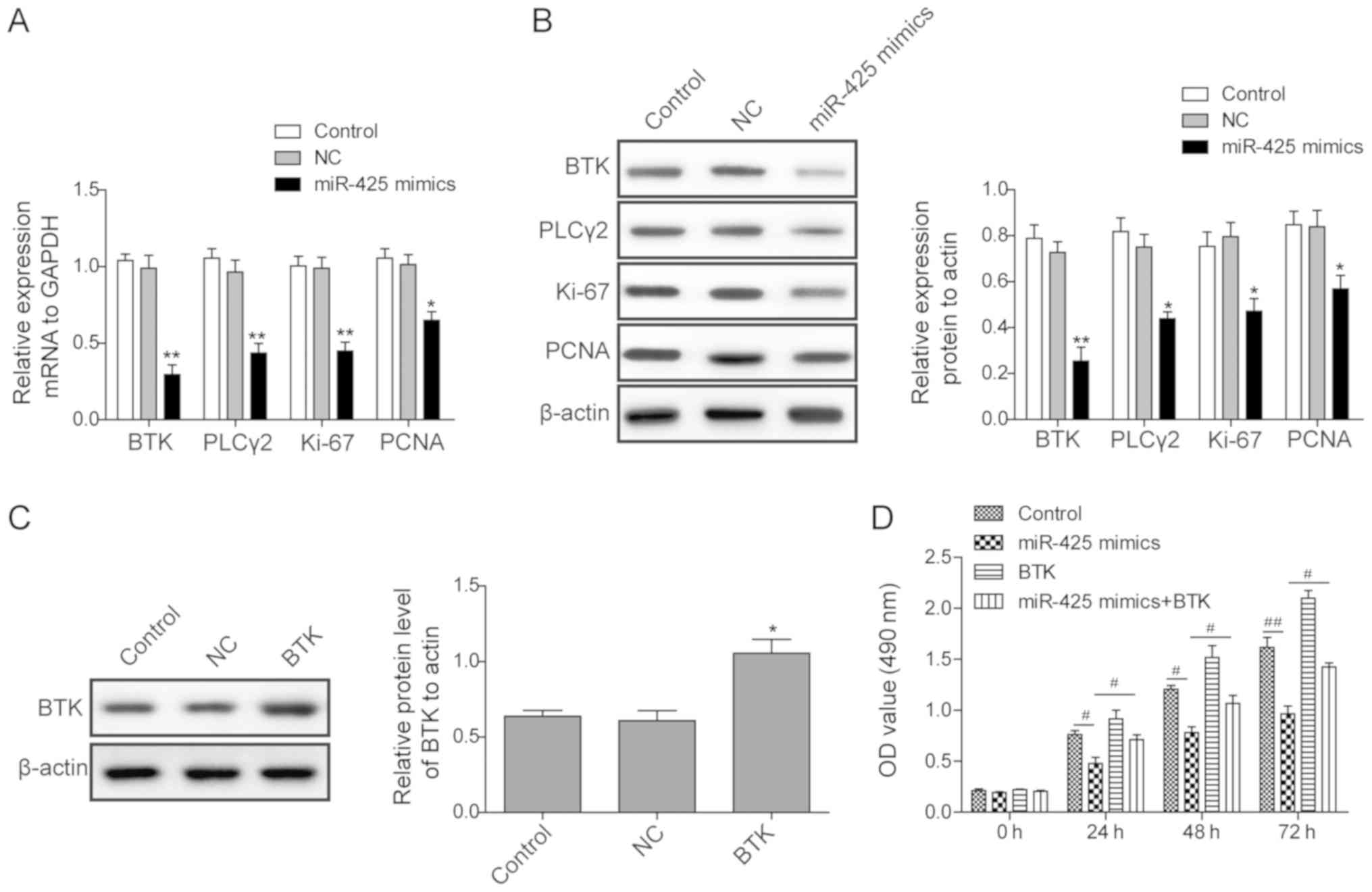 | Figure 4miR-425 inhibits the proliferation of
MEC-1 cells through regulation of the BTK/PLCγ2 signalling pathway.
(A) mRNA and (B) protein expression levels of BTK, PLCγ2, Ki-67 and
PCNA in cells transfected with miR-425 or NC at 48 h after
transfection, as detected by reverse transcription-quantitative
polymerase chain reaction and western blotting, respectively. (C)
BTK protein expression levels in cells transfected with
pcDNA3.1-BTK or pcDNA3.1-NC, and control cells, detected by western
blotting at 48 h after transfection. (D) Proliferation of cells in
different experimental groups, determined by MTT assay at 24, 48
and 72 h after transfection. All experiments were conducted in
triplicate. Statistical analysis was conducted using Student t-test
for comparisons between two groups and analysis of variance for
comparisons among three groups. *P<0.05 and
**P<0.01 vs. control/NC group; #P<0.05
and ##P<0.01. miR, microRNA; BTK, Bruton's tyrosine
kinase; PLCγ2, phospholipase Cγ2; PCNA, proliferating cell nuclear
antigen; NC, negative control. |
Discussion
As the most common leukaemia in adults, CLL remains
a worldwide health problem and accounts for ~30% of all leukaemia
cases in Western countries (24).
Recently, Bottoni et al (23)
demonstrated that BTK protein expression was targeted and reduced
most strongly by miR-210 and miR-425 in CLL, and that miR-425 was
expressed at lower levels in primary CLL samples in comparison with
that in normal B cells. However, whether miR-425 affects CLL
development and the mechanisms of these effects remained unclear.
In the present study, the data demonstrated that miR-425 inhibits
the proliferation of CLL cells through regulation of the BTK/PLCγ2
signalling pathway. It was observed that miR-425 was significantly
downregulated in the B lymphocytes of CLL patients as compared with
its expression in normal B lymphocytes. Furthermore, overexpression
of miR-425 significantly inhibited the proliferation of MEC-1 cells
and altered the cell cycle distribution.
miR-425 is involved in the regulation of cell
proliferation in various diseases. For instance, Ma et al
(25) demonstrated that upregulation
of miR-425 promotes the cell growth of gastric cancer cells by
targeting phosphatase and tensin homolog (PTEN). Feng et al
(26) reported that cell invasion
and metastasis of hepatocellular carcinoma cells was promoted by
miR-425-5p through suppressor of cancer cell invasion
(SCAI)-mediated dysregulation of multiple signalling pathways. Di
Leva et al (27) also
demonstrated that in vitro and in vivo miR-425
expression reduced proliferation, and impaired tumourigenesis and
metastasis in aggressive breast cancer cells. A recent study also
revealed that miR-425-5p inhibits differentiation and proliferation
in porcine intramuscular preadipocytes (28). In the present study, it was
demonstrated that miR-425 inhibits the proliferation of MEC-1
cells, indicating that miR-425 serves an important role in the
development of CLL, which is consistent with the results published
by Bottoni et al (23).
To further investigate the mechanisms underlying the
inhibitory effect of miR-425 on MEC-1 cells, a luciferase reporter
assay was used to confirm that miR-425 targeted BTK mRNA. In
addition, the expression levels of BTK, PLCγ2, and the
proliferation-associated proteins Ki-67 and PCNA were assessed by
RT-qPCR and western blotting. The results displayed that miR-425
upregulation significantly inhibited the expression of all the
aforementioned proteins. BTK is considered crucial for CLL cell
survival and functions as an activator of PLCγ2; therefore, it is
proposed that miR-425 transfection inhibits the expression of PLCγ2
as a secondary effect of BTK loss. A study by Byrd et al
(1) demonstrated that the BTK
inhibitor ibrutinib was effective in relapsed CLL. Singh et
al (3) also reported that
inhibition of BTK or phosphoinositide 3-kinase resulted in reduced
viability, proliferation and fibronectin-dependent cell adhesion in
CLL in vitro and in vivo. In the present study,
miR-425 was found to inhibit the proliferation of MEC-1 cells, and
it is propose that this effect was mediated through the inhibition
of BTK/PLCγ2 signalling, and of Ki-67 and PCNA expression levels.
Furthermore, the proliferation of MEC-1 cells was only partially
rescued when BTK was overexpressed in cells transfected with
miR-425 mimics, which indicated that other factors may be involved.
Taken together, more targets of miR-425 may exist, including PTEN
and SCAI, which may potentially affect CLL cell proliferation,
warranting further investigation in future studies.
In conclusion, the present study investigated the
effect of miR-425 on the proliferation of CLL cells and the
possible mechanisms responsible for this regulation. The results
demonstrated that miR-425 upregulation inhibited the proliferation
of MEC-1 cells, and this effect appeared to be mediated through the
inhibition of BTK/PLCγ2 signalling, and of Ki-67 and PCNA
expression levels. These results provide a deeper insight for
understanding the development of CLL and reveal a potential novel
target for the treatment of CLL patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from Hunan
Provincial Engineering Research Center of Traditional Chinese
Medicine Technology for Anti-tumor [grant no. Xiangfagaigaoji
(2015) 1085].
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
CY designed the current study, performed clinical
experiments and edited and reviewed the manuscript. LH performed
the experiments and acquired the data. XL analyzed the data and
reviewed the manuscript. All authors approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hunan Provincial People's Hospital (Changsha,
China).
Patient consent for publication
Informed consent was obtained from participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Byrd JC, Furman RR, Coutre SE, Flinn IW,
Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, et
al: Targeting BTK with ibrutinib in relapsed chronic lymphocytic
leukemia. N Engl J Med. 369:32–42. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Singh SP, Pillai SY, de Bruijn MJW,
Stadhouders R, Corneth OBJ, van den Ham HJ, Muggen A, van IJcken W,
Slinger E, Kuil A, et al: Cell lines generated from a chronic
lymphocytic leukemia mouse model exhibit constitutive Btk and Akt
signaling. Oncotarget. 8:71981–71995. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gribben JG and O'Brien S: Update on
therapy of chronic lymphocytic leukemia. J Clin Oncol. 29:544–550.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seda V and Mraz M: B-cell receptor
signalling and its crosstalk with other pathways in normal and
malignant cells. Eur J Haematol. 94:193–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Y, Dong Y, Jiang QL, Zhang B and Hu
AM: Bruton's tyrosine kinase: Potential target in human multiple
myeloma. Leuk Lymphoma. 55:177–181. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yaktapour N, Meiss F, Mastroianni J, Zenz
T, Andrlova H, Mathew NR, Claus R, Hutter B, Fröhling S, Brors B,
et al: BRAF inhibitor-associated ERK activation drives development
of chronic lymphocytic leukemia. J Clin Invest. 124:5074–5084.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Rushworth SA, Bowles KM, Barrera LN,
Murray MY, Zaitseva L and MacEwan DJ: BTK inhibitor ibrutinib is
cytotoxic to myeloma and potently enhances bortezomib and
lenalidomide activities through NF-κB. Cell Signal. 25:106–112.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barrientos J and Rai K: Ibrutinib: A novel
Bruton's tyrosine kinase inhibitor with outstanding responses in
patients with chronic lymphocytic leukemia. Leuk Lymphoma.
54:1817–1820. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chang HM, Martinez NJ, Thornton JE, Hagan
JP, Nguyen KD and Gregory RI: Trim71 cooperates with microRNAs to
repress Cdkn1a expression and promote embryonic stem cell
proliferation. Nat Commun. 3(923)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kovaleva V, Mora R, Park YJ, Plass C,
Chiramel AI, Bartenschlager R, Döhner H, Stilgenbauer S, Pscherer
A, Lichter P and Seiffert M: miRNA-130a targets ATG2B and DICER1 to
inhibit autophagy and trigger killing of chronic lymphocytic
leukemia cells. Cancer Res. 72:1763–1772. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Audrito V, Vaisitti T, Rossi D, Gottardi
D, D'Arena G, Laurenti L, Gaidano G, Malavasi F and Deaglio S:
Nicotinamide blocks proliferation and induces apoptosis of chronic
lymphocytic leukemia cells through activation of the
p53/miR-34a/SIRT1 tumor suppressor network. Cancer Res.
71:4473–4483. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gao Y, Yin Y, Xing X, Zhao Z, Lu Y, Sun Y,
Zhuang Z, Wang M, Ji W and He Y: Arsenic-induced anti-angiogenesis
via miR-425-5p-regulated CCM3. Toxicol Lett. 254:22–31.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu L, Zhao Z, Zhou W, Fan X, Zhan Q and
Song Y: Enhanced expression of miR-425 promotes esophageal squamous
cell carcinoma tumorigenesis by targeting SMAD2. J Genet Genomics.
42:601–611. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nakagawa R, Muroyama R, Koike K, Saeki C,
Ito S, Morimoto S, Goto K, Matsubara Y, Kato N and Zeniya M:
Decreased mir-425 induced inflammatory cytokine production via
N-RAS upregulation in CD4+ T cells of primary biliar cholangitis. J
Hepatol. 64 (Suppl):S643–S644. 2016.
|
|
17
|
He B, Li T, Guan L, Liu FE, Chen XM, Zhao
J, Lin S, Liu ZZ and Zhang HQ: CTNNA3 is a tumor suppressor in
hepatocellular carcinomas and is inhibited by miR-425. Oncotarget.
7:8078–8089. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Peng WZ, Ma R, Wang F, Yu J and Liu ZB:
Role of miR-191/425 cluster in tumorigenesis and diagnosis of
gastric cancer. Int J Mol Sci. 15:4031–4048. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fu Y, Xu X, Xue J, Duan W and Yi Z:
Deregulated lncRNAs in B cells from patients with active
tuberculosis. PLoS One. 12(e0170712)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cai BL, Xu XF, Fu SM, Shen LL, Zhang J,
Guan SM and Wu JZ: Nuclear translocation of MRP1 contributes to
multidrug resistance of mucoepidermoid carcinoma. Oral Oncol.
47:1134–1140. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cai BL, Li Y, Shen LL, Zhao JL, Liu Y, Wu
JZ, Liu YP and Yu B: Nuclear multidrug resistance-related protein 1
is highly associated with better prognosis of human mucoepidermoid
carcinoma through the suppression of cell proliferation, migration
and invasion. PLoS One. 11(e0148223)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bottoni A, Rizzotto L, Lai TH, Liu C,
Smith LL, Mantel R, Reiff S, El-Gamal D, Larkin K, Johnson AJ, et
al: Targeting BTK through microRNA in chronic lymphocytic leukemia.
Blood. 128:3101–3112. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kaderi MA, Kanduri M, Buhl AM, Sevov M,
Cahill N, Gunnarsson R, Jansson M, Smedby KE, Hjalgrim H, Jurlander
J, et al: LPL is the strongest prognostic factor in a comparative
analysis of RNA-based markers in early chronic lymphocytic
leukemia. Haematologica. 96:1153–1160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ma J, Liu J, Wang Z, Gu X, Fan Y, Zhang W,
Xu L, Zhang J and Cai D: NF-kappaB-dependent microRNA-425
upregulation promotes gastric cancer cell growth by targeting PTEN
upon IL-1β induction. Mol Cancer. 13(40)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Feng F, Song T, Zhang T, Cui Y, Zhang G
and Xiong Q: MiR-425-5p promotes invasion and metastasis of
hepatocellular carcinoma cells through SCAI-mediated dysregulation
of multiple signaling pathways. Oncotarget. 8:31745–31757.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Di Leva G, Piovan C, Gasparini P, Ngankeu
A, Taccioli C, Briskin D, Cheung DG, Bolon B, Anderlucci L, Alder
H, et al: Estrogen mediated-activation of miR-191/425 cluster
modulates tumorigenicity of breast cancer cells depending on
estrogen receptor status. PLoS Genet. 9(e1003311)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen FF, Xiong Y, Peng Y, Gao Y, Qin J,
Chu GY, Pang WJ and Yang GS: miR-425-5p inhibits differentiation
and proliferation in porcine intramuscular preadipocytes. Int J Mol
Sci. 18(pii: E2101)2017.PubMed/NCBI View Article : Google Scholar
|