Introduction
Among the most common primary malignant tumors of
the brain, human glioblastoma accounts for ~40% of intracranial
tumors (1). The main clinical
features of glioblastoma include high malignancy, a high relapse
rate and poor prognosis (2). Local
abnormal proliferation and infiltration of human glioblastoma cells
in the brain is the main cause of death in patients, and at
present, there are no safe and effective treatments for the disease
(3). Despite significant advances in
treatment strategies, including surgical resection, radiotherapy
and chemotherapy, the overall 5-year survival rate for patients
with glioblastoma remains poor (~10%) (4). Currently, there are only two drugs
clinically used for the standardized treatment of human
glioblastoma, temozolomide and carmustine; however, both drugs
display serious side effects (5).
Therefore, identifying novel therapeutics for human glioblastoma is
essential.
As a member of the G-protein coupled receptor
superfamily, adrenergic receptors play an important role in the
sympathetic nervous system (6).
There are three subtypes of adrenergic receptors: α1A, α1B and α1D
(7). Commonly used adrenergic
receptor antagonists include quinazoline-based prazosin, doxazosin
and terazosin, as well as the sulfonamide derivative tamsulosin.
The aformentioned drugs are not only used to treat essential
hypertension (8), but are also used
for the treatment of prostate cancer as they inhibit progression,
induce apoptosis and reduce prostate specific antigen levels
(9,10). Kyprianou et al (8) reported for the first time that
α-adrenergic antagonists can induce apoptosis in the glandular
epithelium and smooth muscle, which are present in benign prostatic
hyperplasia (8). Further studies
have reported that α-adrenergic antagonists can also induce
apoptosis in malignant prostate cancer cells (11-13).
Based on the aforementioned studies, further investigation into the
effects of α1-adrenergic antagonists on other human malignancies,
including mesothelioma, as well as breast and bladder cancer, has
been conducted (14-16).
These further studies have reported that α-adrenergic antagonists
have a significant proapoptotic effect on malignant tumors.
Doxazosin is an α-adrenergic receptor blocker that inhibits tumor
growth and angiogenesis (17,18).
Prazosin is also an α-adrenergic antagonist used to treat essential
hypertension and although the effects of prazosin on human
glioblastoma have been reported, the underlying mechanism remains
unclear (19). In the present study,
the potential antitumor effects of prazosin in glioblastoma cells
were investigated. Referring to recently published studies
investigating the mechanism underlying glioblastoma progression,
U251 and U87 cell lines were used in the present study (20,21).
Furthermore, the PI3K/AKT signaling pathway was investigated as a
potential molecular mechanism underlying the effects of prazosin on
glioblastoma cells.
Materials and methods
Cell lines and cell culture
Human malignant glioblastoma cell lines U251MG
(astrocytoma) and U87 (glioblastoma of unknown origin) were
purchased from Nianjing KeyGen Biotech Co., Ltd. U87 cells were
authenticated by a short tandem repeat profiling method using the
PowerPlex 18D system kit (cat. no. DC1802; Promega Corporation) and
an ABI 3500 Genetic analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Cells were cultured in DMEM (HyClone; GE
Healthcare Life Sciences) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Beijing Solarbio Science & Technology,
Co., Ltd.) at 37˚C with 5% CO2.
Drug sensitivity assays
U251 and U87 cells were seeded (1x103
cells/well) into 96-well microtiter plates containing 100 µl
culture medium. Prazosin was dissolved in DMSO to 10 different
concentrations: 0, 2.5, 5, 7.5, 10, 15, 20, 30, 40 and 50 µM.
Different concentrations of prazosin were added to each well and
incubated for 48 h at 37˚C. Subsequently, 10 µl Cell Counting Kit-8
(CCK-8) reagent (Beijing Solarbio Science & Technology Co.,
Ltd.) was added to each well and the plates were incubated for 1.5
h at 37˚C. The optical density value of each well was detected at a
wavelength of 450 nm using a microplate reader and a dose-response
curve was plotted. The IC50 concentration of prazosin
was calculated using GraphPad Prism software (version 7; GraphPad
Software, Inc.). Each drug concentration was tested 3 times.
CCK-8 assay for cell
proliferation
U251 and U87 cells were seeded (1x103
cells/well) into 96-well plates and cultured for 24 h at 37˚C with
5% CO2. U251 and U87 cells were treated with 13.16 and
11.57 µM prazosin, respectively. Cells were incubated for 24, 48 or
72 h 37˚C. Subsequently, 10 µl CCK-8 solution was added to each
well and the plates were incubated for 1.5 h at 37˚C. The
absorbance was measured at a wavelength of 450 nm using a
microplate reader.
Cell invasion and migration
assays
The upper chamber of the Transwell plate was
precoated with Matrigel® for 30 min at 70˚C.
Subsequently, the Transwell membrane was hydrated with serum-free
medium containing 10 g/l bovine serum albumin (BSA; Sigma-Aldrich;
Merck KGaA) for 30 min at 37˚C. Prior to plating, 1x105
U251 or U87 cells were serum-starved for 12-24 h at 37˚C.
Subsequently, cells were detached by trypsinization and resuspended
in serum-free medium containing 10 g/l BSA. Cell invasion was
evaluated using Transwell invasion assays. Subsequently, cells
treated with prazosin for 24 h at 37˚C (1x105) were
seeded into the upper chambers. Medium containing 10% FBS (500 µl)
was plated in the lower chamber of the Transwell plates. Following
incubation at 37˚C for 24 h, the invading cells were fixed with 4%
paraformaldehyde for 10 min at room temperature and stained with
0.1% crystal violet for 20 min at room temperature. Stained cells
were counted in five randomly-selected fields using a light
microscope (magnification, x200).
The Transwell migration assay followed the same
protocol as the Transwell invasion assay, however, the Transwell
membranes were not precoated with Matrigel and 5x103
cells were plated in the upper chamber of the Transwell plates.
Cell apoptosis assay
Following treatment with prazosin for 24 h, U251 and
U87 cells were resuspended in binding buffer (5x106
cells/ml; CoWin Biosciences). Cells were stained with 5 µl annexin
V-FITC and 10 µl propidium iodide (CoWin Biosciences) in the dark
at room temperature for 5 min. Subsequently, the cell suspensions
were centrifuged for 5 min at 4˚C and a speed of 447 x g, the
supernatant was discarded and the pellets were resuspended in 400
µl PBS. Apoptotic cells were analyzed using a flow cytometer (BD
Biosciences) and FlowJo software 7.6.1 (FlowJo, LLC).
Colony formation assay
U251 and U87 cells in the logarithmic growth phase
were trypsinized, seeded (2x102 cells/dish) into 35 mm
cell culture dishes and gently rotated to uniformly disperse the
cells. Cells were suspended in DMEM containing 10% FBS.
Subsequently, complete medium containing prazosin (IC50
concentration: U251, 13.16 µM; U87, 11.57 µM) was added to the
experimental group and the same concentration of DMSO was added to
the control group. The cells were cultured for 2-3 weeks at 37˚C.
When macroscopic clones appeared in the culture dish, the medium
was discarded and the cells were carefully washed three times with
PBS. The cells were fixed with 4% paraformaldehyde for 20 min at
room temperature and stained with crystal violet for 30 min at room
temperature. Colony number was counted in 5 random fields of view
using a light microscope (magnification, x40).
Western blot analysis
U251 and U87 cells were treated with prazosin for 48
h at 37˚C. Total protein was extracted from the cells using RIPA
buffer (CoWin Biosciences). Total protein was quantified using a
bicinchoninic acid assay. Protein (20 mg) was separated by 10%
SDS-PAGE and transferred to PVDF membranes. Subsequently, the
membranes were blocked with 5% fat-free milk for 1 h at room
temperature. The membranes were incubated overnight at 4˚C with
primary antibodies targeted against the following: AKT (cat. no.
ab18785; 1:2,000; Abcam), phosphorylated (p)-AKT (cat. no. ab38449;
1:1,000; Abcam), mTOR (cat. no. ab2732; 1:1,000; Abcam), p-mTOR
(cat. no. ab109268; 1:1,000; Abcam), Bcl-2 (cat. no. 12789-1-AP;
1:1,000; ProteinTech Group, Ltd.), Bax (cat. no. 50599-2-Ig;
1:1,000; ProteinTech Group, Ltd.), Caspase-3 (cat. no. ab32351;
1:1,000; Abcam), cyclin D1 (cat. no. ab134175; 1:1,000; Abcam), P70
(cat. no. ab184551, 1:1,000; Abcam) CDK4 (cat. no. ab108357;
1:1,000; Abcam), CDK6 (cat. no. ab124821; 1:1,000; Abcam), NUSAP1
(cat. no. ab169083; 1:500; Abcam) and GAPDH (cat. no. 10494-1-AP;
1:5,000; ProteinTech Group, Ltd.). Subsequently, the membranes were
incubated with an anti-Rabbit secondary antibody (cat. no. ab6721;
1:5,000; Abcam) for 1 h at room temperature. Protein bands were
visualized by ECL (CoWin Biosciences). Protein expression was
quantified using Quantity One 4.6.6 (Bio-Rad Laboratories, Inc.)
and Image J 1.41 (National Institutes of Health) software with
GAPDH as the loading control.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 18.0; SPSS, Inc.). Data are presented as the mean
± SD. Differences were assessed using an unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were performed in triplicate.
Results
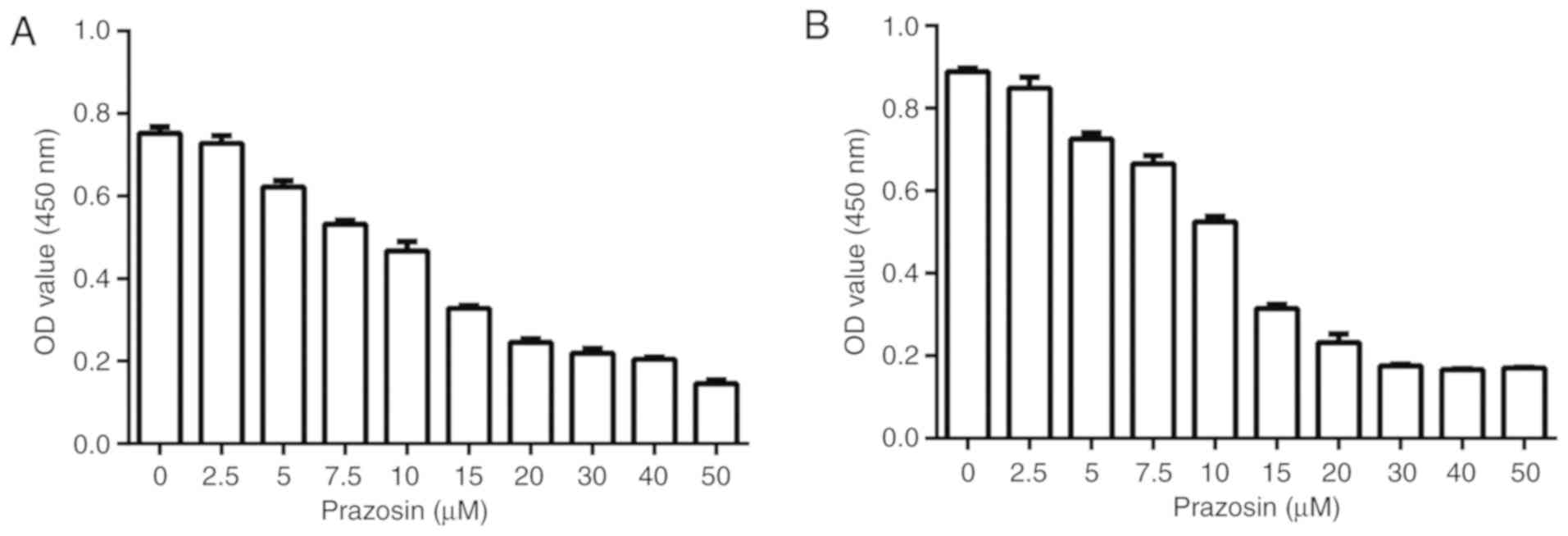
Dose-response experiment to determine
the IC50 of prazosin
A dose-response experiment was conducted to
investigate whether prazosin inhibited the proliferation of U87 and
U251 cells. The IC50 of prazosin for glioblastoma cells
was also determined using a CCK-8 assay. The IC50 was
13.16±0.95 and 11.57±0.79 µM prazosin for U251 and U87 cells,
respectively (Fig. 1).
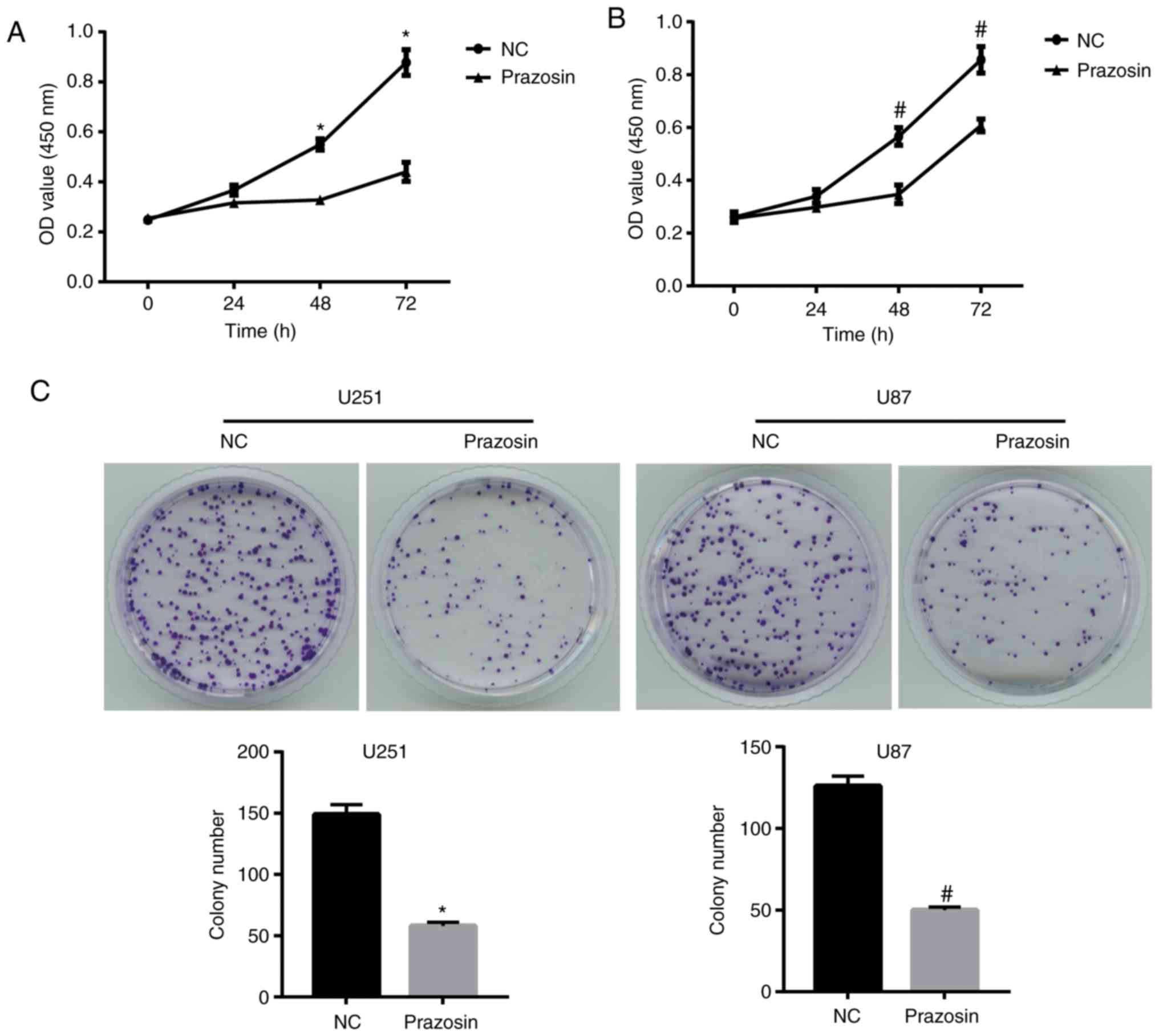
Prazosin inhibits U251 and U87 cell
proliferation
To investigate the potential antitumor effects of
prazosin, the proliferation of U251 and U87 cells treated with
13.16 and 11.57 µM prazosin, respectively, for 24, 48 or 72 h was
assessed. The results suggested that prazosin significantly
decreased the proliferation of U251 and U87 cells after treatment
for 48 and 72 h (Fig. 2A and
B).
Furthermore, the colony formation assay suggested
that prazosin-treated U251 and U87 cells displayed significantly
decreased colony formation compared with the NC group (58±3 vs.
149±8 and 50±2 vs. 126±6, respectively; Fig. 2C). The colony number of
prazosin-treated U251 and U87 cells displayed a similar trend to
colony formation (11.6 vs. 29.8 and 10 vs. 25.2%, respectively;
P<0.05; Fig. 2C). Therefore, the
results suggested that prazosin effectively inhibited the
proliferation of U251 and U87 cells.
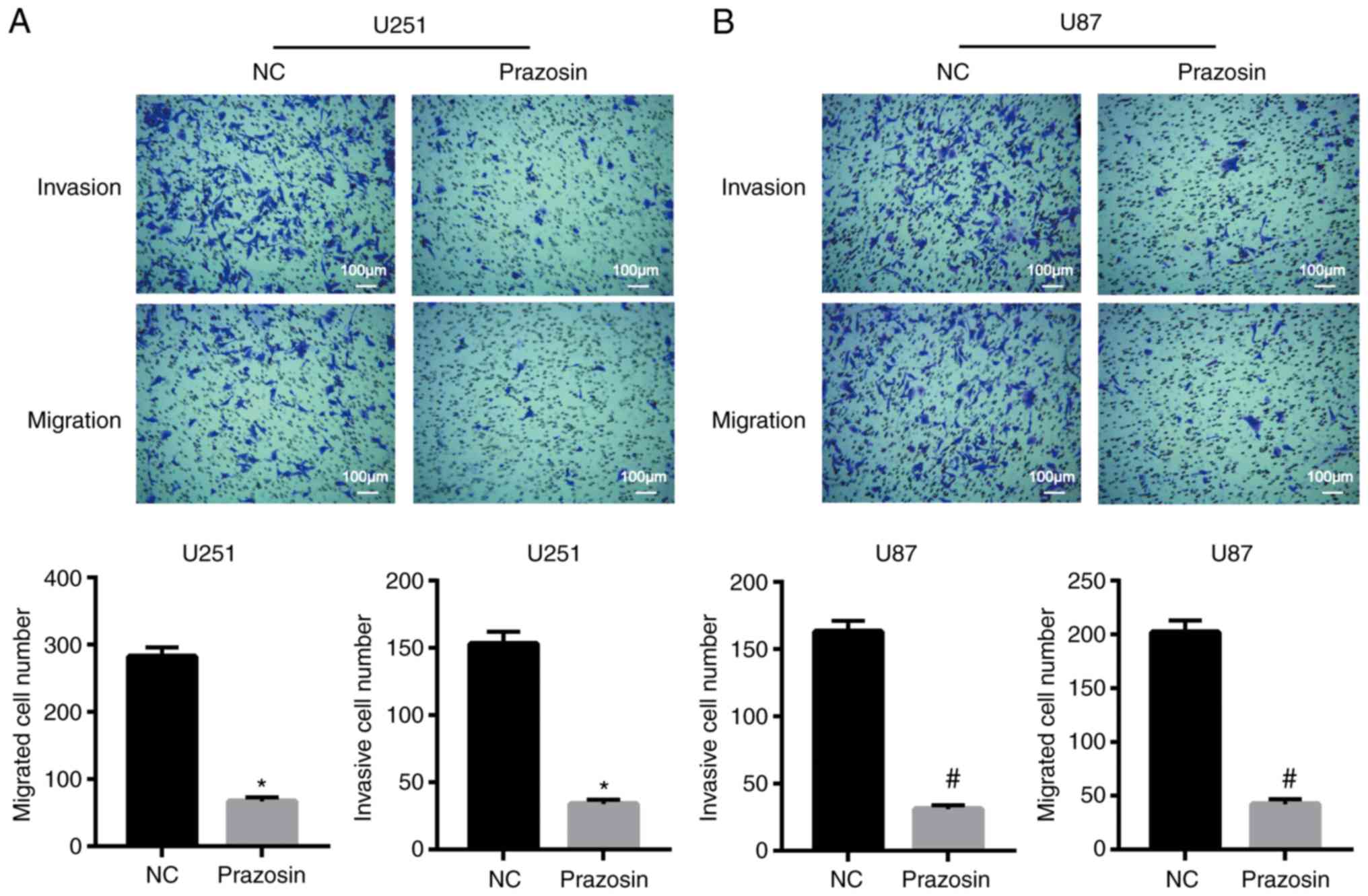
Prazosin inhibits U251 and U87 cell
invasion and migration
The effect of prazosin on the migration and invasion
of U251 and U87 cells was detected using Transwell assays. The
migratory ability of U251 and U87 cells was significantly decreased
following prazosin treatment compared with the negative control
cells (282±14 vs. 67±6 and 202±11 vs. 42±5, respectively;
P<0.05; Fig. 3). The invasive
ability of U251 and U87 cells decreased significantly following
prazosin treatment compared with the negative control cells (153±9
vs. 34±3 and 163±8 vs. 31±3; P<0.05; Fig. 3A). The results indicated that
prazosin inhibited the migration and invasion of U251 and U87
cells.
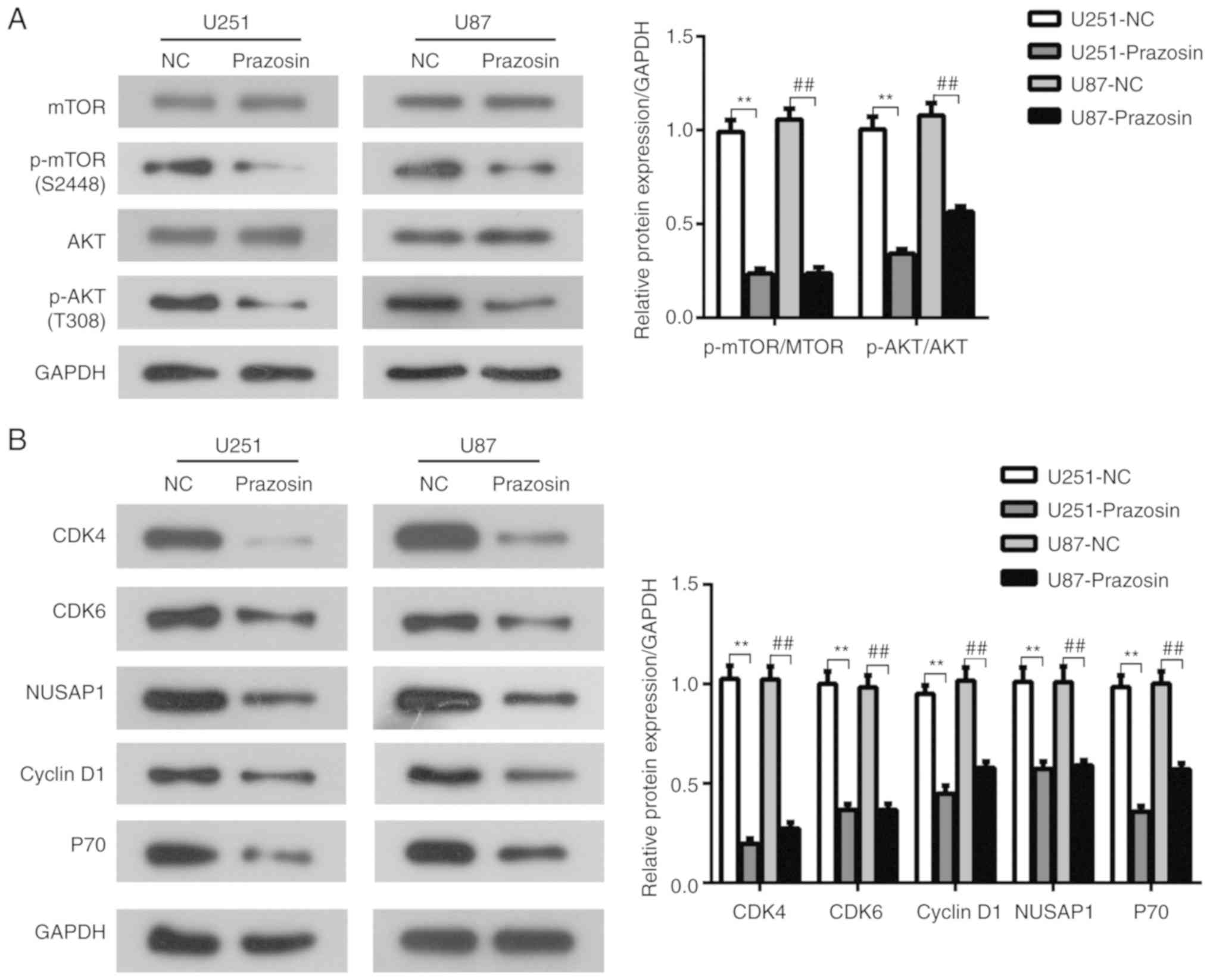
Prazosin suppresses the PI3K/AKT/mTOR
signaling pathway in U251 and U87 cells
The PI3K/AKT/mTOR signaling pathway plays a key role
in the regulation of cell proliferation (22). To investigate the effects of prazosin
on the PI3K/AKT/mTOR signaling pathway in glioblastoma, U251 and
U87 cells were treated with prazosin and protein expression levels
were determined by western blotting. Following prazosin treatment,
the expression of p-AKT and p-mTOR was significantly reduced in
U251 and U87 cells compared with the negative control cells
(P<0.05; Fig. 4A). Furthermore,
the expression levels of P70 and cyclin D1, which are downstream
target genes of the PI3K/AKT/mTOR signaling pathway (23), were decreased in prazosin-treated
cells compared with negative control cells (P<0.05; Fig. 4B). The results suggested that
prazosin treatment decreased the protein expression of components
of the PI3K/AKT/mTOR signaling pathway in U251 and U87 cells.
Based on the result that cyclin D1 expression was
reduced by prazosin treatment, the expression levels of cell cycle
related genes, cyclin dependent kinase (CDK)4/6 and nucleolar and
spindle associated protein 1 (NUSAP1), were measured to investigate
whether prazosin affected the cell cycle. The expression levels of
CDK4/6 and NUSAP1 were significantly decreased in the prazosin
treatment groups compared with the NC groups (P<0.05; Fig. 4B). The results suggested that
prazosin may inhibit the proliferation of U251 and U87 cells by
blocking the cell cycle.
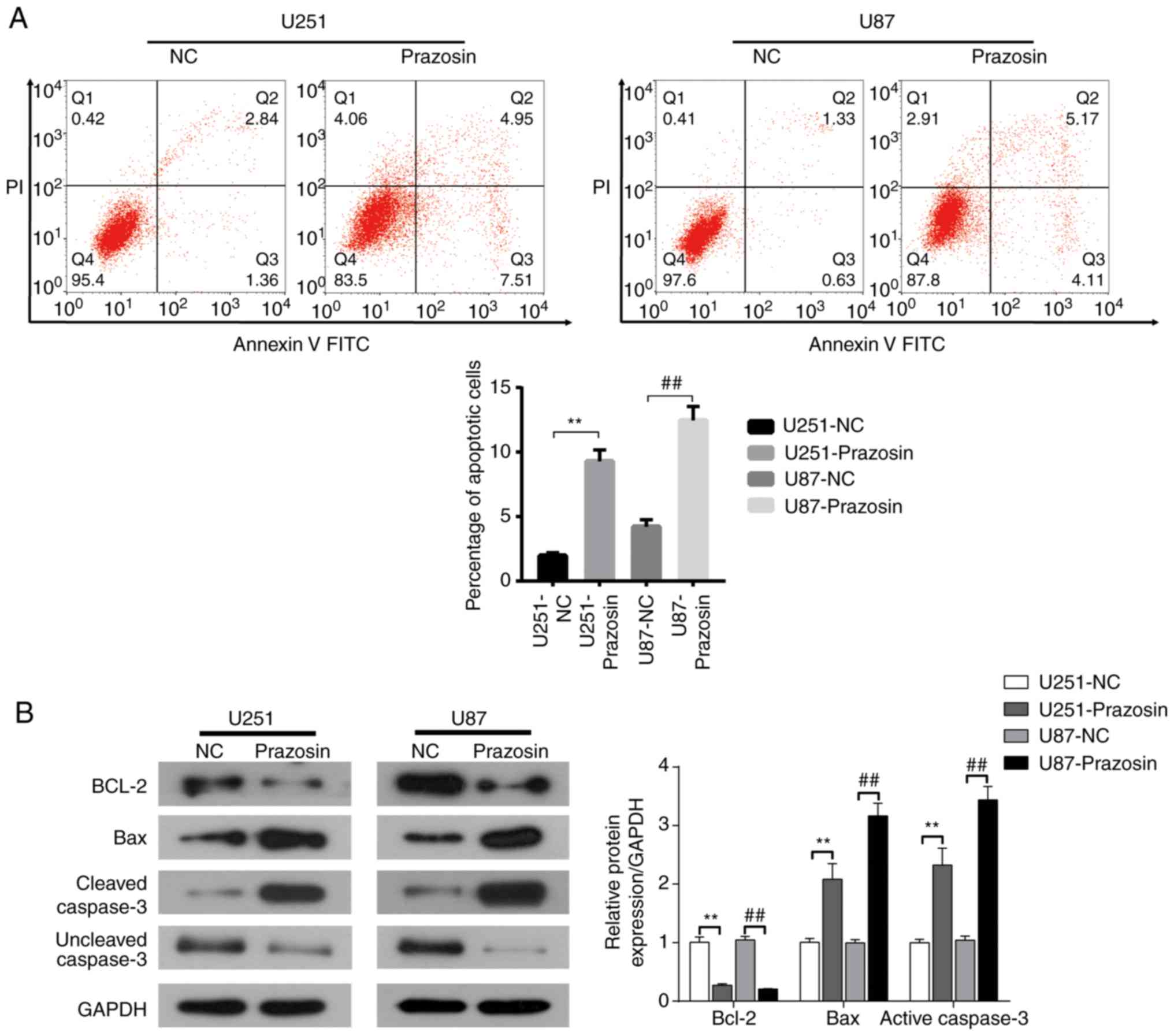
Prazosin induces apoptosis in U251 and
U87 cells
The effect of prazosin on U251 and U87 cell
apoptosis was detected by flow cytometry. The percentage of
apoptotic U251 and U87 cells was significantly increased in the
prazosin treatment groups compared with the NC groups (9.28±0.89
vs. 1.96±0.23 and 12.46±1.07 vs. 4.20±0.56%, respectively; Fig. 5A). Western blot analysis indicated
that the expression of the antiapoptotic protein Bcl2 was
decreased, and the expression of the proapoptotic proteins Bax and
active Caspase-3 was increased in the prazosin treatment groups
compared with the NC groups (Fig.
5B). The results suggested that prazosin induced apoptosis in
U251 and U87 cells, indicating that the antitumor activity of
prazosin may be related to apoptosis induction.
Discussion
To the best of our knowledge, the present study
suggested for the first time that prazosin inhibits the
proliferation, migration and invasion of U251 and U81 cells via the
PI3K/AKT/mTOR signaling pathway.
Glioblastoma is one of the most common adult
malignant brain tumors worldwide, with a median survival time of
13-15 months (24). At present,
there are no safe and effective treatments for the disease, and the
standard treatment involves a combination of surgery, radiotherapy
and chemotherapy (25). However, the
standard therapeutic strategies display a number of problems and
limitations: i) Glioblastoma invades the adjacent brain parenchyma,
which makes it difficult to completely remove the tumor (26); ii) orthotropic tumors grow
malignantly, which causes surrounding tumors to grow and leads to
brain tissue edema, which is one of the major causes of the high
mortality rate observed in patients with glioblastoma (27); iii) the maximum dose of radiation
does not completely eradicate tumor cells (27); iv) movement, language and cognitive
deficits can occur following surgery, resulting in poor patient
quality of life and increased mortality (28,29); and
v) the anatomy of the human brain, for example, the blood-brain
barrier, limits the efficacy of cancer therapeutics (30). Therefore, there is an urgent
requirement for the identification of novel therapeutic targets for
glioblastoma.
Previous studies have reported that the activation
of α1-adrenergic receptors increases the proliferation of nerve
cells, vascular smooth muscle cells and vascular endothelial cells
during development (31-35).
Furthermore, it has been reported that α1-adrenergic receptors play
a crucial role in embryonic brain development (36). In the present study, U251 and U87
cells were treated with prazosin to investigate the effect of the
drug on human glioblastoma in vitro. Prazosin inhibited the
proliferation, migration and invasion of U251 and U87 cells, as
indicated by CCK-8, colony formation and Transwell assays. However,
a limitation of the present study is that the Transwell assay
detects the 3D migration of cells, whereas the wound healing assay,
which detects the horizontal migration of cells, was not performed
in the present study.
The molecular mechanism underlying the anticancer
activity of prazosin on human glioblastoma cells was also
investigated. The PI3K/AKT/mTOR signaling pathway plays a crucial
role in tumor cell proliferation, migration, invasion and apoptosis
(22). Numerous studies have
reported that the PI3K/AKT/mTOR signaling pathway is abnormally
activated during human glioblastoma (23,37).
mTOR is a downstream molecule in the PI3K/AKT signaling pathway
that plays a key role in the activation of P70 and cyclin
D1(38), which are associated with
apoptosis (39,40). The results of the present study
indicated that the expression levels of p-AKT, p-mTOR, P70 and
cyclin D1 were significantly reduced in the prazosin-treated group
compared with the control group, suggesting that prazosin inhibited
the PI3K/AKT/mTOR signaling pathway in U251 and U87 cells.
Both doxazosin and terazosin are clinically
effective α1-adrenergic receptor antagonists that trigger tumor
apoptosis (41). A number of
previous studies have reported that quinazoline-derived
α1-adrenergic receptor antagonists display anti-prostate cancer
effects. In addition, doxazosin induces apoptosis and inhibits
angiogenesis (11,42-45).
The results of the present study suggested that prazosin increased
the expression of proapoptotic proteins Bax and Caspase-3, and
decreased the expression of the antiapoptotic protein Bcl-2. The
results were consistent with the effect of prazosin on apoptosis,
as determined by flow cytometry, which indicated that the
percentage of apoptotic cells was increased in the prazosin-treated
group compared with the control group.
In conclusion, the present study suggested that
prazosin inhibited the proliferation, migration and invasion, and
promoted the apoptosis of U251 and U87 cells by inhibiting the
PI3K/AKT/mTOR signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and JF designed the experiments. JZ performed the
experiments; JZ and JF analyzed the data and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rousseau A, Mokhtari K and Duyckaerts C:
The 2007 WHO classification of tumors of the central nervous
system-what has changed? Curr Opin Neurol. 21(720)2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chargari C, Feuvret Lc, Bauduceau O,
Ricard D, Cuenca X, Delattre JY and Mazeron JJ: Treatment of
elderly patients with glioblastoma: From clinical evidence to
molecular highlights. Cancer Treat Rev. 38:988–995. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Franceschi E, Ermani M, Bartolini S,
Bartolotti M, Poggi R, Tallini G, Marucci G, Fioravanti A, Tosoni
A, Agati R, et al: Post progression survival in glioblastoma: Where
are we? J Neurooncol. 121:399–404. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Radin DP, Purcell R and Lippa AS:
Oncolytic properties of ampakines in vitro. Anticancer Res.
38:265–269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Piascik MT and Perez DM: Alpha1-adrenergic
receptors: New insights and directions. J Pharmacol Exp Ther.
298:403–410. 2001.PubMed/NCBI
|
|
7
|
Salomonsson M, Oker M, Kim S, Zhang H,
Faber JE and Arendshorst WJ: Alpha1-adrenoceptor subtypes on rat
afferent arterioles assessed by radioligand binding and RT-PCR. Am
J Physiol Renal Physiol. 281:F172–F178. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kyprianou N, Litvak JP, Borkowski A,
Alexander R and Jacobs SC: Induction of prostate apoptosis by
doxazosin in benign prostatic hyperplasia. J Urol. 159:1810–1815.
1998.PubMed/NCBI
|
|
9
|
Cal C, Uslu R, Gunaydin G, Ozyurt C and
Omay SB: Doxazos in: A new cytotoxic agent for prostate cancer? BJU
Int. 85:672–675. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kyprianou N and Benning CM: Suppression of
human prostate cancer cell growth by alpha1-adrenoceptor
antagonists doxazosin and terazosin via induction of apoptosis.
Cancer Res. 60(4550)2000.PubMed/NCBI
|
|
11
|
Benning CM and Kyprianou N:
Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate
cancer cell apoptosis via an alpha1-adrenoceptor-independent
action. Cancer Res. 62:597–602. 2002.PubMed/NCBI
|
|
12
|
Partin JV, Anglin IE and Kyprianou N:
Quinazoline-based α1-adrenoceptor antagonists induce prostate
cancer cell apoptosis via TGF-β signalling and IκBα induction. Br J
Cancer. 88:1615–1621. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cuellar DC, Rhee J and Kyprianou N:
Alpha1-adrenoceptor antagonists radiosensitize prostate cancer
cells via apoptosis induction. Anticancer Res. 22:1673–1679.
2002.PubMed/NCBI
|
|
14
|
Hui H, Fernando MA and Heaney AP: The
alpha1-adrenergic receptor antagonist doxazosin inhibits EGFR and
NF-kappaB signalling to induce breast cancer cell apoptosis. Eur J
Cancer. 44:160–166. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Siddiqui EJ, Shabbir M, Thompson CS,
Mumtaz FH and Mikhailidis DP: Growth inhibitory effect of doxazosin
on prostate and bladder cancer cells. Is the serotonin receptor
pathway involved? Anticancer Res. 25(4281)2005.PubMed/NCBI
|
|
16
|
Masachika E, Kanno T, Nakano T, Gotoh A
and Nishizaki T: Naftopidil induces apoptosis in malignant
mesothelioma cell lines independently of alpha1-adrenoceptor
blocking. Anticancer Res. 33:887–894. 2013.PubMed/NCBI
|
|
17
|
Park MS, Kim BR, Dong SM, Lee SH, Kim DY
and Rho SB: The antihypertension drug doxazosin inhibits tumor
growth and angiogenesis by decreasing VEGFR-2/Akt/mTOR signaling
and VEGF and HIF-1α expression. Oncotarget. 5:4935–4944.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hu ZW, Shi XY, Lin RZ, Chen J and Hoffman
BB: alpha1-Adrenergic receptor stimulation of mitogenesis in human
vascular smooth muscle cells: Role of tyrosine protein kinases and
calcium in activation of mitogen-activated protein kinase. J
Pharmacol Exp Ther. 290:28–37. 1999.PubMed/NCBI
|
|
19
|
Assad Kahn S, Costa SL, Gholamin S, Nitta
RT, Dubois LG, Fève M, Zeniou M, Coelho PL, El-Habr E, Cadusseau J,
et al: The anti-hypertensive drug prazosin inhibits glioblastoma
growth via the PKCδ-dependent inhibition of the AKT pathway. EMBO
Mol Med. 8:511–526. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oh SJ, Yang JI, Kim O, Ahn EJ, Kang WD,
Lee JH, Moon KS, Lee KH and Cho D: Human U87 glioblastoma cells
with stemness features display enhanced sensitivity to natural
killer cell cytotoxicity through altered expression of NKG2D
ligand. Cancer Cell Int. 17(22)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang J, Liu K, Wang XF and Sun DJ: Juglone
reduces growth and migration of U251 glioblastoma cells and
disrupts angiogenesis. Oncol Rep. 38:1959–1966. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Z, Wang F, Zhou ZW, Xia HC, Wang XY,
Yang YX, He ZX, Sun T and Zhou SF: Alisertib induces
G2/M arrest, apoptosis, and autophagy via PI3K/Akt/mTOR-
and p38 MAPK-mediated pathways in human glioblastoma cells. Am J
Transl Res. 9:845–873. 2017.PubMed/NCBI
|
|
23
|
Iżycka-Świeszewska E, Drożyńska E, Rzepko
R, Kobierska-Gulida G, Grajkowska W, Perek D and Balcerska A:
Analysis of PI3K/AKT/MTOR signalling pathway in high risk
neuroblastic tumours. Pol J Pathol. 61:192–198. 2010.PubMed/NCBI
|
|
24
|
Horvath S, Zhang B, Carlson M, Lu KV, Zhu
S, Felciano RM, Laurance MF, Zhao W, Qi S, Chen Z, et al: Analysis
of oncogenic signaling networks in glioblastoma identifies ASPM as
a molecular target. Proc Natl Acad Sci USA. 103:17402–17407.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Davis ME: Glioblastoma: Overview of
disease and treatment. Clin J Oncol Nurs. 20 (Suppl):S2–S8.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
D'Alessandro G, Catalano M, Sciaccaluga M,
Chece G, Cipriani R, Rosito M, Grimaldi A, Lauro C, Cantore G,
Santoro A, et al: KCa3.1 channels are involved in the infiltrative
behavior of glioblastoma in vivo. Cell Death Dis.
4(e773)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wirth T, Samaranayake H, Pikkarainen J,
Määttä AM and Yläherttuala S: Clinical trials for glioblastoma
multiforme using adenoviral vectors. Curr Opin Mol Ther.
11:485–492. 2009.PubMed/NCBI
|
|
28
|
Jakola AS, Gulati S, Weber C, Unsgård G
and Solheim O: Postoperative deterioration in health related
quality of life as predictor for survival in patients with
glioblastoma: A prospective study. PLoS One.
6(e28592)2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mcgirt MJ, Mukherjee D, Chaichana KL, Than
KD, Weingart JD and Quinones-Hinojosa A: Association of surgically
acquired motor and language deficits on overall survival after
resection of glioblastoma multiforme. Neurosurgery. 65:463–470.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Juillerat-Jeanneret L: The targeted
delivery of cancer drugs across the blood-brain barrier: Chemical
modifications of drugs or drug-nanoparticles? Drug Discov Today.
13:1099–1106. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Popovik E and Haynes L: Survival and
mitogenesis of neuroepithelial cells are influenced by
noradrenergic but not cholinergic innervation in cultured embryonic
rat neopallium. Brain Res. 853:227–235. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen L, Xin X, Eckhart AD, Yang N and
Faber JE: Regulation of vascular smooth muscle growth by alpha
1-adrenoreceptor subtypes in vitro and in situ. J Biol Chem.
270:30980–30988. 1995.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Faber JE, Yang N and Xin X: Expression of
alpha-adrenoceptor subtypes by smooth muscle cells and adventitial
fibroblasts in rat aorta and in cell culture. J Pharmacol Exp Ther.
298:441–452. 2001.PubMed/NCBI
|
|
34
|
Xin X, Yang N, Eckhart AD and Faber JE:
Alpha1D-adrenergic receptors and mitogen-activated protein kinase
mediate increased protein synthesis by arterial smooth muscle. Mol
Pharmacol. 51:764–775. 1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bleeke T, Zhang H, Madamanchi N, Patterson
C and Faber JE: Catecholamine-induced vascular wall growth is
dependent on generation of reactive oxygen species. Circ Res.
94:37–45. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hiramoto T, Satoh Y, Takishima K and
Watanabe Y: Induction of cell migration of neural progenitor cells
in vitro by alpha-1 adrenergic receptor and dopamine D1 receptor
stimulation. Neuroreport. 19:793–797. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Westhoff MA, Karpel-Massler G, Brühl O,
Enzenmüller S, La Ferla-Brühl K, Siegelin MD, Nonnenmacher L and
Debatin KM: A critical evaluation of PI3K inhibition in
Glioblastoma and Neuroblastoma therapy. Mol Cell Ther.
2(32)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Halacli SO and Dogan AL: FOXP1 regulation
via the PI3K/Akt/p70S6K signaling pathway in breast cancer cells.
Oncol Lett. 9:1482–1488. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu W, Ren H, Ren J, Yin T, Hu B, Xie S,
Dai Y, Wu W, Xiao Z, Yang X and Xie D: The role of
EGFR/PI3K/Akt/cyclinD1 signaling pathway in acquired middle ear
cholesteatoma. Mediators Inflamm. 2013(651207)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Desiniotis A and Kyprianou N: Advances in
the design and synthesis of prazosin derivatives over the last ten
years. Expert Opin Ther Targets. 15:1405–1418. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ferrara N: Role of vascular endothelial
growth factor in regulation of physiological angiogenesis. Am J
Physiol Cell Physiol. 280:C1358–C1366. 2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Meyer RD and Rahimi N: Comparative
structure-function analysis of VEGFR-1 and VEGFR-2: What have we
learned from chimeric systems? Ann N Y Acad Sci. 995:200–207.
2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Meyer RD, Singh A, Majnoun F, Latz C,
Lashkari K and Rahimi N: Substitution of C-terminus of VEGFR-2 with
VEGFR-1 promotes VEGFR-1 activation and endothelial cell
proliferation. Oncogene. 23:5523–5531. 2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Clarke DE: Alpha adrenoceptor blockade in
the treatment of benign prostatic hyperplasia: Past, present and
future. Br J Urol. 82(167)1998.PubMed/NCBI
|