Introduction
Pseudomonas aeruginosa (P. aeruginosa)
is a gram-negative non-fermenting bacillus that is prevalent
in the community and hospital environment. Carbapenem-resistant
P. aeruginosa (CRPA) is a major cause of life-threatening
infections worldwide (1,2). CRPA is considered to be a multidrug
resistant (MDR) pathogen, as it is intrinsically resistant to
different types of antimicrobial drugs. CRPA also has the capacity
to develop resistance to various antimicrobial agents, thereby
reducing the number of available treatment options. In the previous
decade, the resistance rate of carbapenem has increased 3-fold in
various countries, including the United States of America (USA),
Singapore, Brazil, Iran and China, reaching 50-80% in certain areas
(3-6).
Since the 1950s, polymyxins have been popular for the treatment of
carbapenem-resistant enterobacteriaceae (CRE) infections
(7). However, their use has been
restricted, due to significant neurotoxicity and nephrotoxicity.
With an increasing number of CRE infections observed over recent
years, polymyxin B and colistin have become increasingly popular
treatment choices. Although the resistance rate of polymyxin B is
low in most countries, it appears to be increasing. Globally, the
polymyxin B resistance rate is <5%; however, it has been
reported to be 50% in Singapore. Therefore, clinicians should be
vigilant in regards to the rising rate of resistance (3-6,
8). Identifying an appropriate
method for antimicrobial susceptibility testing (AST) of polymyxin
B and colistin is important for the treatment of CRPA
infections.
A reliable method for testing polymyxin
susceptibility remains elusive. In 2017, the Clinical and
Laboratory Standards Institute® (CLSI®) no
longer considered the disc diffusion (DD) method to be appropriate
for colistin susceptibility testing. Furthermore, the European
Committee on Antimicrobial Susceptibility Testing (EUCAST) did not
previously deem DD to be an appropriate method for colistin
susceptibility testing (9). CLSI and
EUCAST guidelines have suggested broth microdilution (BMD) as the
reference method for polymyxin B and colistin susceptibility
testing (9). However, technical
issues have been reported by clinicians worldwide, as polymyxin B
and colistin adhere to microtiter plates, contributing to
inaccurate results. Therefore, many clinical laboratories have used
Etest® strips as an alternative method.
Studies describing the use of Etest® for
polymyxin B testing in CRPA are scarce and previous data have
disputed the reliability of this method (10,11). In
addition, EUCAST has revised the breakpoints for colistin in its
guidelines of 2017 and 2018 (9,12). As
novel data has been generated over the last two years, it is
necessary to compare the Etest® and BMD methods in
accordance with CLSI/EUCAST standards in larger CRPA populations.
The present study analyzed CRPA resistance to polymyxin B in the
Suzhou district of China. A comparison analysis of polymyxin B
resistance rates from different countries or regions was also
performed to determine resistance trends. Additionally, the present
study assessed the effectiveness and reliability of
Etest® in a clinical laboratory setting.
Materials and methods
Bacterial isolates
A total of 50 non-duplicated clinical CRPA isolates
that were non-susceptible (resistant or intermediate) to any
carbapenem (imipenem or meropenem) were identified and collected
from patients admitted to the First Affiliated Hospital of Soochow
University, the leading tertiary hospital of Suzhou district with
3,000 beds, between October 2017 and February 2019. All isolates
were stored at -80˚C in 10% glycerol and sub-cultured twice prior
to testing. Isolates were identified using an automated system
(Vitek2 compact; bioMérieux). P. aeruginosa [American Type
culture collection (ATCC)® 27853™; 0.5-4 µg/ml] and
Escherichia coli (ATCC® 25922™; 0.25-2 µg/ml)
served as quality control strains in the 2 susceptibility methods
assessed. The present study was approved by the Ethics Committee of
the First Affiliated Hospital of Soochow University and was
performed in accordance with the 1975 Declaration of Helsinki. All
patients provided written informed consent.
Susceptibility testing
All susceptibility testing was conducted in
accordance with the CSLI® recommendations (12). The range of susceptible, resistant
and intermediate polymyxin B concentrations were ≤2, ≥8 and 4
µg/ml, respectively. Additionally, the carbapenems that were
assessed (imipenem or meropenem) demonstrated the same ranges as
polymyxin B (susceptible, ≤2 µg/ml; resistant, ≥8 µg/ml;
intermediate, 4 µg/ml). BMD was performed using a cation-adjusted
Mueller Hinton II broth (Wenzhou Kangtai Biotechnology Co., Ltd.)
in accordance with CLSI® guidelines. Each test was
duplicated and a third test was performed for discrepant minimal
inhibitory concentrations (MICs) or for MICs exceeding 1 log2
dilution. The polymyxin B Etest® (Wenzhou Kangtai
Biotechnology Co., Ltd.) was performed in the clinical microbiology
laboratory in accordance with the manufacturer's protocol. The
results were compared with those obtained via BMD.
Search strategy
The PubMed and Embase databases updated on April
2019 were searched using the following terms: ‘Pseudomonas
aeruginosa’ or ‘Polymyxin B’, together with ‘antimicrobial
resistance’. Entire manuscripts associated with the resistance rate
of polymyxin B and P. aeruginosa infection were then
identified.
Selection of literature
The titles and abstracts of previous studies
obtained from PubMed and Embase were reviewed. If the titles
appeared to be associated with the research strategy abstracts were
reviewed. If abstracts correlated with the research strategy the
full texts were reviewed. The inclusion criteria were as follows:
i) An original article or research article; ii) short
communications; and iii) correspondence or letters. The exclusion
criteria were as follows: i) Reviews or case reports; and ii)
animal experiments.
Statistical analysis
The present study utilized CLSI®
susceptibility breakpoints in CRPA isolates to obtain all
descriptive statistics including susceptibility (%), resistance
(%), the MIC90 concentration (mg/l) and the range
(mg/l). Essential agreement (EA) was defined as samples with MICs
that were equivalent to the ± 1-log2 dilution between the polymyxin
B Etest® methodology and the reference method. A result
was deemed inconsistent if there was a difference of ± 2-log2 in
the dilution of results. Categorical agreement (CA) was determined
if the results from both methods belonged to the same category of
susceptibility. A serious major error rate was defined as the
percentage of CRPA isolates reported to be susceptible using the
Etest® method, but resistant when using the reference
method (false susceptibility). A major error rate was defined as
the percentage of CRPA isolates reported to be resistant using the
Etest® method, but susceptible using the reference
method (false resistance). Finally, a minor error rate was
determined if acceptable levels were defined as <1.5% for very
major errors, <3% for major errors and <10% for minor errors,
all of which were indicated in the CLSI® document
M23-A2(9). The odds ratio and 95%
confidence interval (CI) were determined to evaluate the
association power. The χ2 test-based Q-statistic and
I2 statistics were also utilized as previously described
(13,14). If there was no evident heterogeneity,
the fixed-effects model was applied (15). If there was heterogeneity, a
random-effects model was utilized (16). All statistics were performed using
Stata software (v.14.0; StataCorp LLC).
Results
Bacterial isolates
In total, 50 CRPA clinical isolates were collected
from the First Affiliated Hospital of Soochow University. The
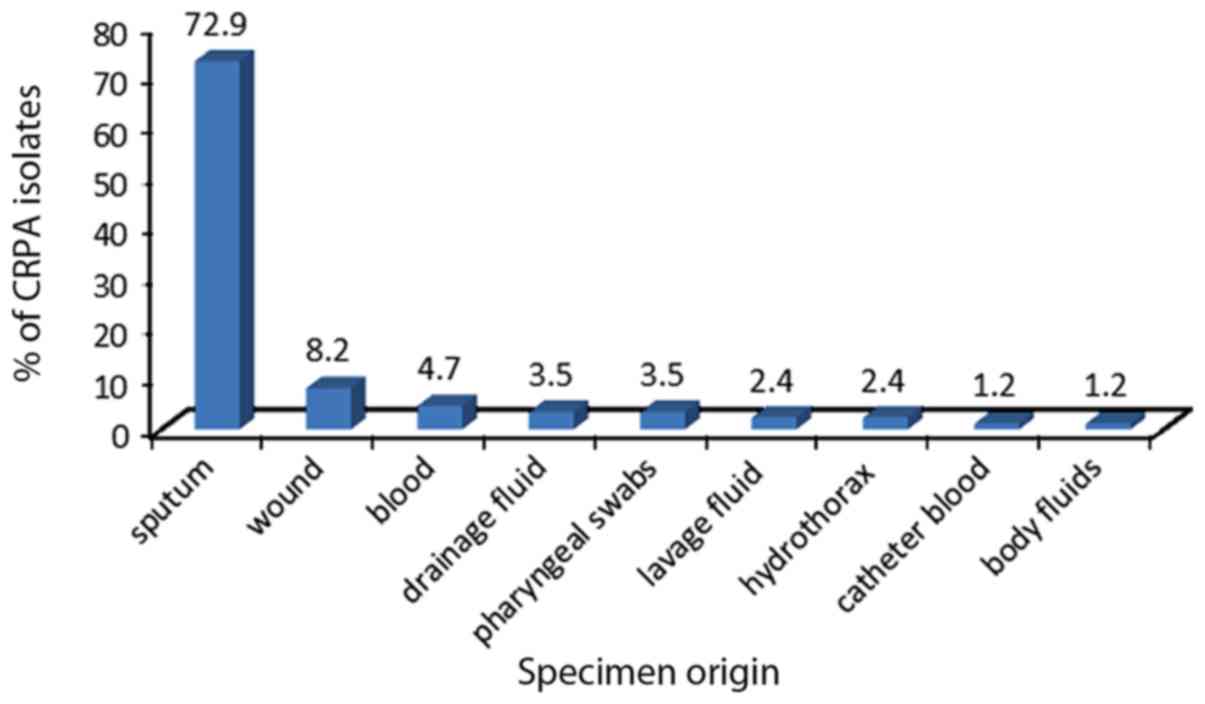
isolates were obtained from different clinical departments and
specimen types (Figs. 1 and 2), and were determined to be
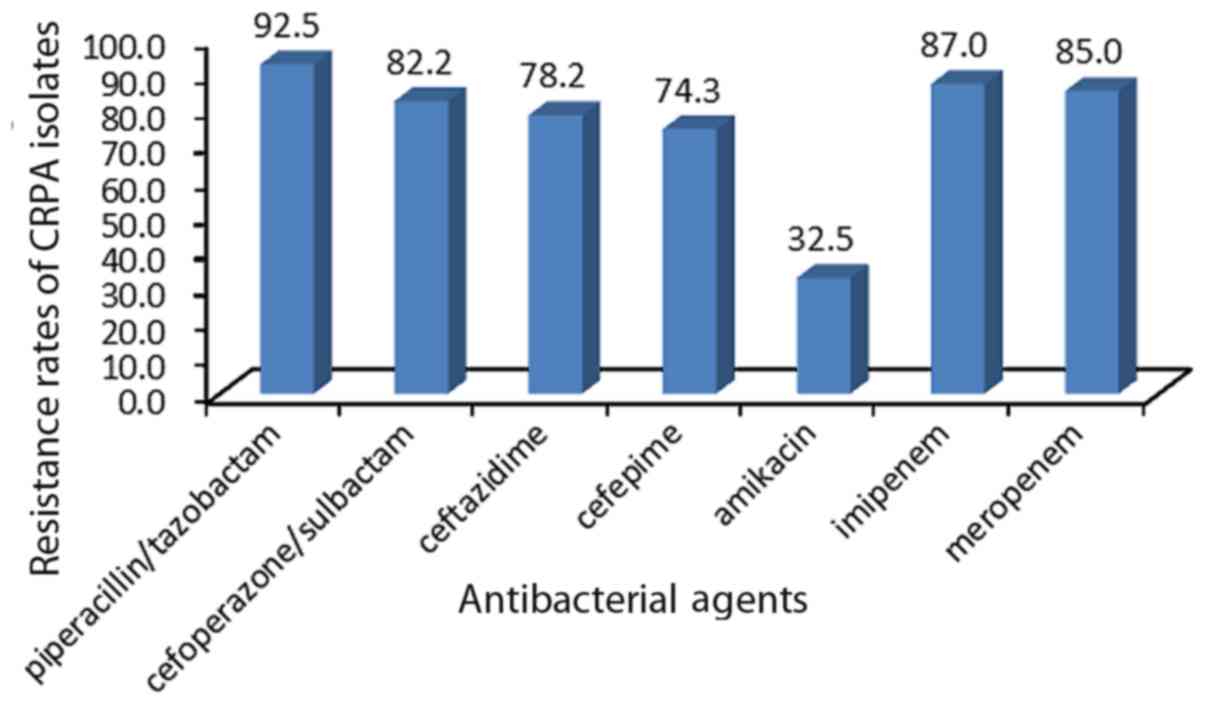
non-susceptible to imipenem or meropenem. Fig. 3 presents the resistance rate of CRPA
isolates to several antibacterial agents.
EA and CA
Following BMD, only 2 isolates were determined to be
non-susceptible to polymyxin B according to CLSI®
criteria (both 4.0 mg/l). The susceptibility rate reached 96.0 and
98.0%, as determined via BMD and Etest® methods,
respectively. The EA and CA reached 98.0%. No very major or major
errors were detected in the 50 CRPA strains. Furthermore, only 2.0%
minor errors were detected (Tables I
and II). The detailed results of
antimicrobial susceptibility testing are provided in Table III.
 | Table IMIC comparison analysis between
Etest® and BMD. |
Table I
MIC comparison analysis between
Etest® and BMD.
| Variable | MIC50
(mg/l) | MIC90 (mg/l) | Range (mg/l) | Susceptible
(%) | Non-susceptible
(%) |
|---|
| BMD | 1.0 | 1.0 | 0.5-4.0 | 96.0 | 4.0 |
|
Etest® | 1.0 | 1.0 | 0.5-2.0 | 98.0 | 2.0 |
 | Table IIEA and CA comparison analysis between
Etest® and BMD. |
Table II
EA and CA comparison analysis between
Etest® and BMD.
| Comparison | EA | CA | Very major error
(%) | Major error
(%) | Minor error
(%) |
|---|
| BMD vs.
Etest® | 98.0 | 98.0 | 0 | 0 | 2.0 |
 | Table IIIAntimicrobial susceptibility testing
of Etest® and BMD from carbapenem-resistant
Pseudomonas aeruginosaisolates. |
Table III
Antimicrobial susceptibility testing
of Etest® and BMD from carbapenem-resistant
Pseudomonas aeruginosaisolates.
| | Antimicrobial
susceptibility testing |
|---|
| Number of
isolates | IPM | MEM | PB
(Etest®) | PB (BMD) |
|---|
| Pae-503 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-504 | Resistant | Resistant | 2.0 | 1.0 |
| Pae-505 | Resistant | Intermediate | 1.0 | 1.0 |
| Pae-506 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-507 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-510 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-511 | Resistant | Intermediate | 1.0 | 1.0 |
| Pae-512 | Resistant | Resistant | 1.0 | 0.5 |
| Pae-514 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-515 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-516 | Resistant | Resistant | 2.0 | 1.0 |
| Pae-517 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-518 | Resistant | Resistant | 0.5 | 1.0 |
| Pae-520 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-521 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-522 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-523 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-524 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-525 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-526 | Resistant | Intermediate | 1.0 | 1.0 |
| Pae-529 | Resistant | Resistant | 1.0 | 0.5 |
| Pae-531 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-532 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-533 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-534 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-535 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-536 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-537 | Resistant | Resistant | 1.0 | 4.0 |
| Pae-538 | Resistant | Resistant | 2.0 | 1.0 |
| Pae-539 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-540 | Resistant | Resistant | 2.0 | 2.0 |
| Pae-541 | Resistant | Resistant | 0.5 | 1.0 |
| Pae-542 | Resistant | Resistant | 2.0 | 2.0 |
| Pae-555 | Resistant | Resistant | 2.0 | 2.0 |
| Pae-561 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-562 | Resistant | Resistant | 1.0 | 2.0 |
| Pae-565 | Resistant | Intermediate | 2.0 | 2.0 |
| Pae-566 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-567 | Resistant | Resistant | 2.0 | 1.0 |
| Pae-568 | Resistant | Resistant | 2.0 | 2.0 |
| Pae-569 | Resistant | Resistant | 2.0 | 2.0 |
| Pae-571 | Resistant | Resistant | 2.0 | 2.0 |
| Pae-572 | Resistant | Resistant | 2.0 | 1.0 |
| Pae-573 | Resistant | Resistant | 2.0 | 2.0 |
| Pae-574 | Resistant | Resistant | 1.0 | 1.0 |
| Pae-575 | Resistant | Resistant | 2.0 | 1.0 |
| Pae-576 | Resistant | Resistant | 4.0 | 4.0 |
| Pae-578 | Resistant | Resistant | 2.0 | 1.0 |
| Pae-579 | Resistant | Resistant | 2.0 | 1.0 |
| Pae-580 | Resistant | Resistant | 2.0 | 1.0 |
A total of 34 previous studies assessing the
resistance rate of polymyxin B in P. aeruginosa were
reviewed and analyzed (Table IV)
(4-6,8,17-46).
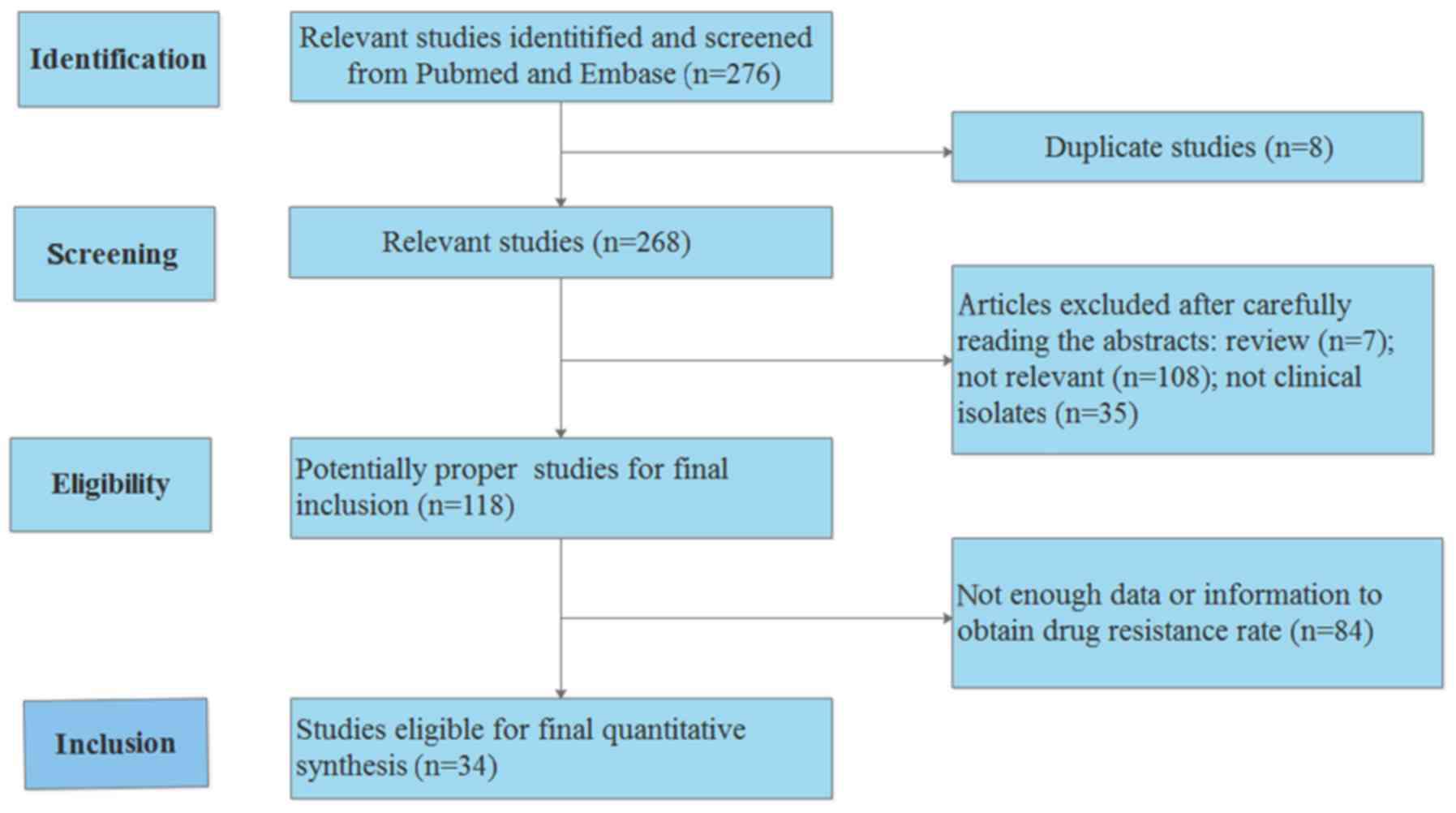
The process used to search the literature is presented in Fig. 4. The results revealed that the
resistance rate of polymyxin B was relatively low in the majority
of countries and regions, with the exception of Singapore. The
resistance rate of polymyxin B in Singapore reached 53% (95% CI,
12-93%). A summary of the susceptibility analyses in different
countries or regions is presented in Table V.
 | Table IVDetailed literature review of data
obtained from various countries or regions. |
Table IV
Detailed literature review of data
obtained from various countries or regions.
| First author | Year (ref) |
Country/district | Number of
isolates | Method |
Carbapenem-resistant Pseudomonas
aeruginosa (%) |
|---|
| Landman | 2005(10) | USA | 527 | AD | 36.0 |
| Yang | 2005(18) | China | 320 | AD | 20.6 |
| Kirby | 2006(19) | USA | 351 | BMD | 8.0 |
| Gales (a) | 2006(4) | North America | 3,036 | BMD | 12.5 |
| Gales (b) | 2006(4) | Latin America | 1,626 | BMD | 12.5 |
| Gales (c) | 2006(4) | Europe | 3,145 | BMD | 12.5 |
| Gales (d) | 2006(4) | Asia-Pacific | 898 | BMD | 12.5 |
| van der
Heijden | 2007(20) | Brazil | 109 |
Etest® | 0.0 |
| Raja | 2007(21) | Malaysia | 505 | DD | 9.9 |
| Tan | 2008(22) | Singapore | 188 | BMD | 17.6 |
| Scheffer | 2010(23) | Brazil | 29 | AD | 100.0 |
| Tam | 2010(24) | USA | 18 |
Etest® | 100.0 |
| Cereda | 2011(25) | Brazil | 94 | BMD | 44.1 |
| Lim | 2011(8) | Singapore | 22 | BMD | 100.0 |
| Memish | 2012(27) | Saudi Arabia | 1,734 | DD | 15.9 |
| Haeili | 2013(28) | Iran | 112 | DD | 50.0 |
| YN Liu | 2012(26) | China | 82 | AD | 74.4 |
| Qi Wang | 2013(21) | China | 178 | AD | 28.7 |
| Xiao | 2013(30) | China | 16 | BMD | 25.0 |
| Ameen | 2015(32) | Pakistan | 230 | DD | 49.5 |
| Ali | 2015(31) | Pakistan | 204 | DD | 22.0 |
| Kim | 2015(39) | South Korea | 100 | BMD | NR |
| Vaez | 2015(5) | Iran | 45 | DD | 100.0 |
| Habibi | 2015(33) | Iran | 8 | DD | 12.5 |
| Bangera | 2015(36) | India | 224 | DD | 7.14 |
| Qi Wang | 2015(34) | China | 201 | AD | 28.9 |
| Zhang | 2015(35) | China | 42 | BMD | 79.0 |
| Yang | 2015(18) | China | 256 | DD | 34.4 |
| Zowalaty | 2016(37) | Qatar | 86 | AD | 1.1 |
| Gong | 2016(38) | China | 43 | DD | 61.5 |
| Grewal | 2017(40) | India | 190 | DD | 16.3 |
| Wilhelm | 2018(6) | Brazil | 6 | BMD | 100.0 |
| Sader (a) | 2018(45) | USA | 417 | BMD | 13.9 |
| Sader (b) | 2018(45) | Europe | 491 | BMD | 19.3 |
| Sader (c) | 2018(45) | China | 311 | BMD | 22.2 |
| Ismail | 2018(43) | Iraq | 22 | DD | 22.7 |
| Azimi | 2018(41) | Iran | 160 | DD | 98.8 |
| Dogonchi | 2018(42) | Iran | 71 | DD | 28.2 |
| Kuti | 2018(44) | China | 112 | AD | 40.2 |
 | Table VOverall analysis of susceptibility
trends in different countries or districts. |
Table V
Overall analysis of susceptibility
trends in different countries or districts.
| Country or
region | Non-susceptibility
(95% CI) | Weight (%) |
|---|
| United States | 0.03
(0.02-0.04) | 17.17 |
| China | 0.01
(0.01-0.02) | 26.10 |
| Latin America | 0.01
(0.01-0.02) | 12.36 |
| Europe | 0.01
(0.01-0.02) | 8.70 |
| Malaysia | 0.01
(0.00-0.02) | 4.16 |
| Singapore | 0.53
(0.12-0.93) | 0.68 |
| Saudi Arabia | 0.02
(0.02-0.03) | 4.33 |
| Iran | 0.05
(0.01-0.09) | 6.83 |
| Pakistan | 0.07
(0.01-0.18) | 4.49 |
| South Korea | 0.06
(0.01-0.11) | 1.08 |
| India | 0.01
(0.00-0.02) | 7.25 |
| Qatar | 0.01
(0.00-0.03) | 2.77 |
| Iraq | 0.14
(0.01-0.28) | 0.14 |
Discussion
The issue of limited treatment options for CRPA has
attracted increasing attention in the previous decade. Despite the
neurotoxicity and nephrotoxicity generated by polymyxin B, it
remains a viable treatment option to which the majority of CRPA
strains remain susceptible (47).
However, the resistance rates of antibacterial agents may differ
between geographical locations; for example, the resistance rate of
polymyxin B is relatively low in the majority of countries and
regions, with the exception of Singapore, whose resistance rate has
been reported to be as high as 53% (95% CI, 12-93%). Polymyxin B
resistance depends on a complicated multi-factorial process that
includes polymyxin B exposure, the inappropriate usage of other
antibacterial agents, such as carbapenems, and resistance to
transmission via plasmids (48). The
high resistance rates observed in Singapore may be due to the early
usage of polymyxin B in the late 1990 s, which was earlier than the
majority of countries and regions globally (22). In addition, polymyxin B is used as
the primary polymyxin for the treatment of multidrug-resistant
gram-negative infections (22).
Locally, the combination of polymyxin B and other antibiotics for
the treatment of infections is also common (22). Polymyxin B is rarely administered in
the USA, Europe, Africa and Asia for several reasons: i) Physicians
in the USA and Europe frequently use polymyxin E to treat patients
with CRPA; ii) polymyxin B is only used in Brooklyn and New York
city; iii) The majority of Asian countries, including China, have
not approved the prescription and sale of polymyxin B (49,50).
The meta-analysis in the present study offers
important data regarding the trends in CRPA resistance to polymyxin
B in different global regions. Generally, the susceptibility rate
of polymyxin B is high in most countries. However, an efficient and
reliable method of antibiotic susceptibility testing for polymyxin
B has yet to be established. There are several concerns regarding
polymyxin B and colistin in vitro susceptibility testing.
Firstly, and primarily, conflicting data regarding the AST
procedure exists in the literature (12). Secondly, it is not clear which
reference method is the most appropriate for making comparisons
(51). Thirdly, the testing
population that represents the MIC spectrum of polymyxin (highly
resistant or highly sensitive) is restricted and inaccessible
(51). Fourthly, it is difficult to
obtain reproducible susceptibility information due to the
heteroresistance exhibited within bacterial isolates (52). Finally, despite the MICs obtained in
the present study, a single value may not accurately represent the
populations that exhibit heteroresistance.
It has been demonstrated that DD is not a reliable
method for susceptibility testing, and the CLSI® and
EUCAST do not recommended it for polymyxin B testing (53,54).
Although BMD has been recommended as a reference method by the
CLSI® and EUCAST, it is time-consuming and laborious
procedure, which represents a burden in routine clinical practices.
In recent years, the majority of studies have focused their
attention on the effectiveness and reliability of Etest®
(10,20). However, certain issues remain
unresolved. Studies comparing the MIC of Etest® with BMD
in CRPA are rare. In addition, there are uncertainties and
contrasting opinions surrounding the reliability of the
Etest® method. Simar et al (10) demonstrated that the Etest®
was not a reliable method for the detection of the polymyxin B MIC
in CRPA strains. A high inconsistency rate between polymyxin B
Etest® and BMD MICs was also revealed. Additionally, van
der Heijden et al (20)
revealed that only 1.2% of very major errors were detected and no
major errors were determined. However, 48.7% of minor errors were
detected, with the EA reaching 61%. It is well known that the
acceptable rate of EA and minor errors should be ≥90 and ≤10%,
respectively. In the present study, almost no difference was
detected between the Etest® and BMD, as the EA reached
98.0%. No very major errors or major errors were identified, and
only 2.0% minor errors were detected. All of the measurable
indicators including EA, CA, very major errors, major errors and
minor errors were within the acceptable level. Despite similarities
in the aims and techniques utilized in a previous study by van der
Heijden et al (20), the
present study was valuable, as few studies have performed
methodological comparisons between BMD and Etest®
testing methods for polymyxin B. Furthermore, the breakpoint of
polymyxin B MIC antibiotic susceptibility tests was updated in the
2017 edition of the CLSI® (12). As new data have been generated in the
past decade, it is necessary to compare the Etest® with
BMD methods using the new CLSI/EUCAST standards for CRPA
strains.
The results of the present study differ to those
published previously. There are several reasons that may account
for this. Firstly, Western countries began administering polymyxins
earlier than Asian countries. In 2003, it was reported that at
least nine P. aeruginosa isolates were non-susceptible to
colistin in Greece (55).
Furthermore, in 2005, a marked decrease in polymyxin susceptibility
was detected in Brooklyn and New York city, in the USA (17). However, polymyxins have not been
employed by clinical physicians in China. The resurgence of
polymyxin use in Malaysia occurred in 2009 due to the lack of
effective treatment options for MDR gram-negative superbugs
(56). Therefore, the sensitivity
rate of polymyxins for CRPA in Asian countries has been identified
to be increased compared with Western countries. The results of the
present study may differ from previous studies due to the
resistance mechanisms utilized. For example, Tan et al
(53) identified the activity of
mobilized colistin resistance (mcr-1), which was a resistance gene
in the majority of polymyxin-resistant enterobacteriaceae
isolates, but this was not observed in the study by Rojas et
al (57). The present study did
not investigate mcr-1 and it was challenging to elucidate the
resistant mechanisms utilized by polymyxins. At present, the PhoPQ
regulatory system is the only mechanism considered to serve an
important role in polymyxin resistance (58). Heteroresistance may also have had a
significant effect on the results of the present study.
Heteroresistance occurs when sensitized bacteria are mixed with a
small drug-resistant subpopulation, leading to the unexplained
failure of clinical treatment. Heteroresistance is affected by
diverse factors including bacteria species, antibacterial agents,
resistance phenotypes or mechanisms and local epidemiology
(59-61).
The present study hypothesized that the discrepancy between BMD and
Etest® results may be explained by the fact that BMD is
more sensitive to heteroresistant subpopulations than
Etest®. However, a small number of heteroresistant
colonies growing in the inhibition zone appeared to contribute to
the results of the Etest® strip. If equal quantities of
heteroresistant and sensitive colonies grew in the specific
microtiter wells and turned the wells turbid, then an elevated MIC
of polymyxin B would be recorded. Furthermore, the degree of
heteroresistance may determine the very major errors, major errors
and minor errors between the present study and previous studies.
However, studies that assess heteroresistance are scarce and rarely
investigate polymyxin B in P. aeruginosa (62).
The identification of feasible and reliable
susceptibility testing methods to determine the MIC of polymyxin B
are urgently required. The results of the present study identified
a good concordance between BMD and Etest®. However,
there are certain limitations: The present study is single-center
investigation and does not contain genetic data regarding the
resistant mechanisms utilized by polymyxins. Furthermore, the CRPA
populations in the present study lacked isolates with an MIC of
polymyxin B>2 mg/l (n=1). Additionally, detailed information
regarding clinical outcome data was not obtained.
Despite the existence of several studies from
various geographical regions assessing trends of polymyxin B in the
antimicrobial resistance of CRPA, to the best of our knowledge, the
present study is the first that provides global data and compares
the MIC of Etest® with BMD for CRPA isolates in China.
In conclusion, polymyxin B resistance rates are relatively low in
the majority of countries and regions, with the exception of
Singapore. The Etest® may serve as a potentially
reliable clinical method of polymyxin B MIC determination in
CRPA.
Acknowledgements
The authors would like to thank Ms Li Yan
(Zhangjiagang Hospital of Traditional Chinese Medicine) and Mr
Haitao Hu (People's Hospital of Taizhou) who assisted in the
collection and organization of the experiment data.
Funding
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant no. BK20170364), the
National Natural Science Foundation of China (grant no. 81702065)
and Jiangsu Province Medical Innovation Team (grant no.
CXTDB2017009).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ and XC conceived and designed the experiments of
the current study. XC, QZ, YR and JX performed the experiments,
analyzed the data, contributed reagents, materials and analysis
tools, and wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and informed consent
Patient data were collected retrospectively from
electronic health records. The present study was approved by the
Ethics Committee of First Affiliated Hospital of Soochow University
and was in accordance with the 1975 Declaration of Helsinki. The
patients provided written informed consent.
Patient consent for publication
The patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khosravi AD, Shafie F, Abbasi Montazeri E
and Rostami S: The frequency of genes encoding exotoxin A and
exoenzyme S in Pseudomonas aeruginosa strains isolated from
burn patients. Burns. 42:1116–1120. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Leseva M, Arguirova M, Nashev D, Zamfirova
E and Hadzhyiski O: Nosocomial infections in burn patients:
Etiology, antimicrobial resistance, means to control. Ann Burns
Fire Disasters. 26:5–11. 2013.PubMed/NCBI
|
|
3
|
Liu PY, Sun YX, Gu DQ and Cheng JL: Drug
resistance of imipenem-resistant Gram-negative bacilli in coal
worker's pneumoconiosis chronic obstructive pulmonary disease
patients with lower respiratory tract infection. Zhonghua Lao Dong
Wei Sheng Zhi Ye Bing Za Zhi. 31:700–702. 2013.PubMed/NCBI(In Chinese).
|
|
4
|
Gales AC, Jones RN and Sader HS: Global
assessment of the antimicrobial activity of polymyxin B against 54
731 clinical isolates of Gram-negative bacilli: Report from the
SENTRY antimicrobial surveillance programme (2001-2004). Clin
Microbiol Infect. 12:315–321. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vaez H, Moghim S, Nasr Esfahani B and
Ghasemian Safaei H: Clonal relatedness among imipenem-resistant
Pseudomonas aeruginosa isolated from ICU-hospitalized
patients. Crit Care Res Pract. 2015(983207)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wilhelm CM, Nunes LS, Martins AF and Barth
AL: In vitro antimicrobial activity of imipenem plus amikacin or
polymyxin B against carbapenem-resistant Pseudomonas
aeruginosa isolates. Diagn Microbiol Infect Dis. 92:152–154.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nation RL, Velkov T and Li J: Colistin and
polymyxin B: Peas in a pod, or chalk and cheese? Clin Infect Dis.
59:88–94. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lim TP, Lee W, Tan TY, Sasikala S, Teo J,
Hsu LY, Tan TT, Syahidah N and Kwa AL: Effective antibiotics in
combination against extreme drug-resistant Pseudomonas
aeruginosa with decreased susceptibility to polymyxin B. PLoS
One. 6(e28177)2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bakthavatchalam YD and Veeraraghavan B:
Challenges, issues and warnings from CLSI and EUCAST working group
on polymyxin susceptibility testing. J Clin Diagn Res.
11:DL03–DL04. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Simar S, Sibley D, Ashcraft D and Pankey
G: Colistin and polymyxin B minimal inhibitory concentrations
determined by Etest found unreliable for Gram-negative bacilli.
Ochsner J. 17:239–242. 2017.PubMed/NCBI
|
|
11
|
Nhung PH, Miyoshi-Akiyama T, Phuong DM,
Shimada K, Anh NQ, Binh NG, Thanh do V, Ohmagari N and Kirikae T:
Evaluation of the Etest method for detecting colistin
susceptibility of multidrug-resistant Gram-negative isolates in
Vietnam. J Infect Chemother. 21:617–619. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kulengowski B, Ribes JA and Burgess DS:
Polymyxin B Etest® compared with gold-standard broth
microdilution in carbapenem-resistant Enterobacteriaceae exhibiting
a wide range of polymyxin B MICs. Clin Microbiol Infect. 25:92–95.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
16
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Landman D, Bratu S, Alam M and Quale J:
Citywide emergence of Pseudomonas aeruginosa strains with
reduced susceptibility to polymyxin B. J Antimicrob Chemother.
55:954–957. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang QW, Xu YC, Chen MJ, Hu YJ, Ni YX, Sun
JY, Yu YS, Kong HS, He L, Wu WY, et al: Surveillance of
antimicrobial resistance among nosocomial gram-negative pathogens
from 15 teaching hospitals in China in 2005. Zhonghua Yi Xue Za
Zhi. 87:2753–2758. 2007.PubMed/NCBI(In Chinese).
|
|
19
|
Kirby JT, Fritsche TR and Jones RN:
Influence of patient age on the frequency of occurrence and
antimicrobial resistance patterns of isolates from
hematology/oncology patients: Report from the chemotherapy alliance
for neutropenics and the control of emerging resistance program
(North America). Diagn Microbiol Infect Dis. 56:75–82.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
van der Heijden IM, Levin AS, De Pedri EH,
Fung L, Rossi F, Duboc G, Barone AA and Costa SF: Comparison of
disc diffusion, Etest and broth microdilution for testing
susceptibility of carbapenem-resistant P. aeruginosa to
polymyxins. Ann Clin Microbiol Antimicrob. 6(8)2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Raja NS and Singh NN: Antimicrobial
susceptibility pattern of clinical isolates of Pseudomonas
aeruginosa in a tertiary care hospital. J Microbiol Immunol
Infect. 40:45–49. 2007.PubMed/NCBI
|
|
22
|
Tan TY, Hsu LY, Koh TH, Ng LS, Tee NW,
Krishnan P, Lin RT and Jureen R: Antibiotic resistance in
gram-negative bacilli: A Singapore perspective. Ann Acad Med
Singapore. 37:819–825. 2008.PubMed/NCBI
|
|
23
|
Scheffer MC, Bazzo ML, Steindel M, Darini
AL, Climaco E and Dalla-Costa LM: Intrahospital spread of
carbapenem-resistant Pseudomonas aeruginosa in a university
hospital in Florianópolis, Santa Catarina, Brazil. Rev Soc Bras Med
Trop. 43:367–371. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tam VH, Chang KT, Abdelraouf K, Brioso CG,
Ameka M, McCaskey LA, Weston JS, Caeiro JP and Garey KW:
Prevalence, resistance, mechanisms and susceptibility of
multidrug-resistant bloodstream isolates of Pseudomonas
aeruginosa. Antimicrob Agents Chemother. 54:1160–1164.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cereda RF, Azevedo HD, Girardello R,
Xavier DE and Gales AC: INVITA-A-CEFTO Brazilian Study Group.
Antimicrobial activity of ceftobiprole against gram-negative and
gram-positive pathogens: Results from INVITA-A-CEFTO Brazilian
study. Braz J Infect Dis. 15:339–348. 2011.PubMed/NCBI
|
|
26
|
Liu YN, Cao B, Wang H, Chen LA, She DY,
Zhao TM, Liang ZX, Sun TY, Li YM, Tong ZH, et al: Adult hospital
acquired pneumonia: a multicenter study on microbiology and
clinical characteristics of patients from 9 Chinese cities.
Zhonghua Jie He He Hu Xi Za Zhi. 35:739–746. 2012.PubMed/NCBI(In Chinese).
|
|
27
|
Memish ZA, Shibl AM, Kambal AM, Ohaly YA,
Ishaq A and Livermore DM: Antimicrobial resistance among
non-fermenting Gram-negative bacteria in Saudi Arabia. J Antimicrob
Chemother. 67:1701–1705. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Haeili M, Ghodousi A, Nomanpour B, Omrani
M and Feizabadi MM: Drug resistance patterns of bacteria isolated
from patients with nosocomial pneumonia at Tehran hospitals during
2009-2011. J Infect Dev Ctries. 7:312–317. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Q, Zhao CJ, Wang H, Yu YS, Zhu ZH,
Chu YZ, Sun ZY, Hu ZD, Xu XL, Liao K, et al: Antimicrobial
resistance of Gram-negative bacilli isolated from 13 teaching
hospitals across China. Zhonghua Yi Xue Za Zhi. 93:1388–1396.
2013.PubMed/NCBI(In Chinese).
|
|
30
|
Xiao H, Ye X, Liu Q and Li L: Antibiotic
susceptibility and genotype patterns of Pseudomonas
aeruginosa from mechanical ventilation-associated pneumonia in
intensive care units. Biomed Rep. 1:589–593. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ali Z, Mumtaz N, Naz SA, Jabeen N and
Shafique M: Multi-drug resistant pseudomonas aeruginosa: a threat
of nosocomial infections in tertiary care hospitals. J Pak Med
Assoc. 65:12–16. 2015.PubMed/NCBI
|
|
32
|
Ameen N, Memon Z, Shaheen S, Fatima G and
Ahmed F: Imipenem Resistant Pseudomonas aeruginosa: The fall
of the final quarterback. Pak J Med Sci. 31:561–565.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Habibi A and Honarmand R: Profile of
Virulence Factors in the Multi-Drug Resistant Pseudomonas
aeruginosa Strains of Human Urinary Tract Infections (UTI).
Iran Red Crescent Med J. 17(e26095)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Q, Wang H, Yu Y, Xu X, Sun Z, Lu J,
Yang B, Zhang L, Hu Z, Feng X, et al: Antimicrobial resistance
monitoring of gram-negative bacilli isolated from 15 teaching
hospitals in 2014 in China. Zhonghua Nei Ke Za Zhi. 54:837–845.
2015.PubMed/NCBI(In Chinese).
|
|
35
|
Zhang JF, Zhu HY, Sun YW, Liu W, Huo YM,
Liu DJ, Li J and Hua R: Pseudomonas aeruginosa infection
after pancreatoduodenectomy: risk factors and clinic impacts. Surg
Infect (Larchmt). 16:769–774. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bangera D, Shenoy SM and Saldanha DR:
Clinico-microbiological study of Pseudomonas aeruginosa in
wound infections and the detection of metallo-β-lactamase
production. Int Wound J. 13:1299–1302. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
El Zowalaty ME and Gyetvai B:
Effectiveness of Antipseudomonal Antibiotics and Mechanisms of
Multidrug Resistance in Pseudomonas aeruginosa. Pol J
Microbiol. 65:23–32. 2016.PubMed/NCBI
|
|
38
|
Gong YL, Yang ZC, Yin SP, Liu MX, Zhang C,
Luo XQ and Peng YZ: Analysis of the pathogenic characteristics of
162 severely burned patients with bloodstream infection. Zhonghua
Shao Shang Za Zhi. 32:529–535. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
39
|
Kim YH: Conditional probability analysis
of multidrug resistance in Gram-negative bacilli isolated from
tertiary medical institutions in South Korea during 1999-2009. J
Microbiol. 54:50–56. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Grewal US, Bakshi R, Walia G and Shah PR:
Antibiotic susceptibility profiles of non-fermenting gram-negative
Bacilli at a Tertiary Care Hospital in Patiala, India. Niger
Postgrad Med J. 24:121–125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Azimi A, Peymani A and Pour PK: Phenotypic
and molecular detection of metallo-β-lactamase-producing
Pseudomonas aeruginosa isolates from patients with burns in
Tehran, Iran. Rev Soc Bras Med Trop. 51:610–615. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dogonchi AA, Ghaemi EA, Ardebili A,
Yazdansetad S and Pournajaf A: Metallo-β-lactamase-mediated
resistance among clinical carbapenem-resistant Pseudomonas
aeruginosa isolates in northern Iran: A potential threat to
clinical therapeutics. Ci Ji Yi Xue Za Zhi. 30:90–96.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ismail SJ and Mahmoud SS: First detection
of New Delhi metallo-β-lactamases variants (NDM-1, NDM-2) among
Pseudomonas aeruginosa isolated from Iraqi hospitals. Iran J
Microbiol. 10:98–103. 2018.PubMed/NCBI
|
|
44
|
Kuti JL, Wang Q, Chen H, Li H, Wang H and
Nicolau DP: Defining the potency of amikacin against Escherichia
coli, Klebsiella pneumoniae, Pseudomonas
aeruginosa, and Acinetobacter baumannii derived from
Chinese hospitals using CLSI and inhalation-based breakpoints.
Infect Drug Resist. 11:783–790. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sader HS, Dale GE, Rhomberg PR and Flamm
RK: Antimicrobial Activity of Murepavadin Tested against Clinical
Isolates of Pseudomonas aeruginosa from the United States,
Europe, and China. Antimicrob Agents Chemother. 62: pii:e00311–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yang X, Xing B, Liang C, Ye Z and Zhang Y:
Prevalence and fluoroquinolone resistance of Pseudomonas
aeruginosa in a hospital of South China. Int J Clin Exp Med.
8:1386–1390. 2015.PubMed/NCBI
|
|
47
|
Meletis G and Skoura L: Polymyxin
Resistance Mechanisms: From Intrinsic Resistance to Mcr Genes.
Recent Pat Antiinfect Drug Discov. 13:198–206. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Teo JQ, Chang CW, Leck H, Tang CY, Lee SJ,
Cai Y, Ong RT, Koh TH, Tan TT and Kwa AL: Risk factors and outcomes
associated with the isolation of polymyxin B and
carbapenem-resistant Enterobacteriaceae spp.: A case-control
study. Antimicrob Agents. 53:657–662. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhan Y, Ma N, Liu R, Wang N, Zhang T and
He L: Polymyxin B and polymyxin E induce anaphylactoid response
through mediation of Mas-related G protein-coupled receptor X2.
Chem Biol Interact. 308:304–311. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li S, Jia X, Li C, Zou H, Liu H, Guo Y and
Zhang L: Carbapenem-resistant and cephalosporin-susceptible
Pseudomonas aeruginosa: a notable phenotype in patients with
bacteremia. Infect Drug Resist. 11:1225–1235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Humphries RM and Hindler JA: Emerging
Resistance, New Antimicrobial Agents ... but No Tests! The
Challenge of Antimicrobial Susceptibility Testing in the Current US
Regulatory Landscape. Clin Infect Dis. 63:83–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Meletis G, Tzampaz E, Sianou E, Tzavaras I
and Sofianou D: Colistin heteroresistance in
carbapenemase-producing Klebsiella pneumoniae. J Antimicrob
Chemother. 66:946–947. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tan TY and Ng LS: Comparison of three
standardized disc susceptibility testing methods for colistin. J
Antimicrob Chemother. 58:864–867. 2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gales AC, Reis AO and Jones RN:
Contemporary assessment of antimicrobial susceptibility testing
methods for polymyxin B and colist in: review of available
interpretative criteria and quality control guidelines. J Clin
Microbiol. 39:183–190. 2001.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Giamarellos-Bourboulis EJ, Sambatakou H,
Galani I and Giamarellou H: In vitro interaction of colistin and
rifampin on multidrug-resistant Pseudomonas aeruginosa. J
Chemother. 15:235–238. 2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zakuan ZD and Suresh K: Rational use of
intravenous polymyxin B and colistin: A review. Med J Malaysia.
73:351–359. 2018.PubMed/NCBI
|
|
57
|
Rojas LJ, Salim M, Cober E, Richter SS,
Perez F, Salata RA, Kalayjian RC, Watkins RR, Marshall S, Rudin SD,
et al: Colistin Resistance in Carbapenem-Resistant Klebsiella
pneumoniae: Laboratory Detection and Impact on Mortality. Clin
Infect Dis. 64:711–718. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lee JY and Ko KS: Mutations and expression
of PmrAB and PhoPQ related with colistin resistance in
Pseudomonas aeruginosa clinical isolates. Diagn Microbiol
Infect Dis. 78:271–276. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yamazumi T, Pfaller MA, Messer SA, Houston
AK, Boyken L, Hollis RJ, Furuta I and Jones RN: Characterization of
heteroresistance to fluconazole among clinical isolates of
Cryptococcus neoformans. J Clin Microbiol. 41:267–272.
2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Plipat N, Livni G, Bertram H and Thomson
RB Jr: Unstable vancomycin heteroresistance is common among
clinical isolates of methiciliin-resistant Staphylococcus
aureus. J Clin Microbiol. 43:2494–2496. 2005.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Tomasz A, Nachman S and Leaf H: Stable
classes of phenotypic expression in methicillin-resistant clinical
isolates of staphylococci. Antimicrob Agents Chemother. 35:124–129.
1991.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hermes DM, Pormann Pitt C, Lutz L,
Teixeira AB, Ribeiro VB, Netto B, Martins AF, Zavascki AP and Barth
AL: Evaluation of heteroresistance to polymyxin B among
carbapenem-susceptible and -resistant Pseudomonas
aeruginosa. J Med Microbiol. 62:1184–1189. 2013.PubMed/NCBI View Article : Google Scholar
|