1. Introduction
Venous thromboembolism (VTE) is a common and
potentially fatal disease that are comprised of deep vein
thrombosis (DVT) and pulmonary embolism (PE) (1). The estimated annual incidence rates of
VTE among people of European ancestry range from 104 to 183 per
100,000 person-years (2,3), but is more prevalent in elderly
subjects. Annually, there are ~72.4 cases per 100,000 adults aged
40-54 years, compared with 280 cases per 100,000 people aged 85-89
years (Table I) (4,5).
Although the exact incidence rate of PE in younger populations is
unknown, it is typically lower in patients aged <40 years
compared with that in the elderly (5), with peak incidence in the sixth decade
(6). Nevertheless, PE remains to be
a major cause of mortality among young patients (7).
 | Table IOutline and key points of young
patients with PE. |
Table I
Outline and key points of young
patients with PE.
| Outline | Key point | (Refs) |
|---|
| Incidence | In 238 patients
tested, (19.7%) of PE cases affect young patients <50 years old
(Medical University of Bialystok) | (7) |
| | In 387 patients
examined, PE incidence was 5% in patients <45 years old (North
Carolina) | (27) |
| | In 631 patients
tested, PE incidence was 9.4% in patients between 20 and 50 years
old (A USA retrospective study) | (28) |
| | In 232 patients
examined, 25% of patients with PE were <65 years old
(Italy) | (29) |
| | In 250 patients
examined, 25% of patients with PE were aged ≤50 years old
(France) | (30) |
| | In a study of 540
patients with PE, 62% of were of <65 years old (Boston) | (31) |
| Unprovoked
factors | Factor V Leiden
mutations and prothrombin gene mutations | (40,41) |
| | Deficiencies in
protein S, protein C and antithrombin | (39) |
| | Increased levels of
factor VIII or IX, heparin cofactor II deficiencies | (42-44) |
| | Abnormal functions
of plasminogen and Factor XII and fibrinogenemia | (42-44) |
| | Increased protein C
antigen, protein S antigen and protein S activity, antithrombin III
antigen and factor VIII | (45) |
| Provoked (acquired
or secondary) factors | Recent surgery or
trauma, inflammation, immobilization, malignancy, pregnancy,
contraceptive use, hormone replacement therapy | (30,45,51) |
| | Heart failure,
atrial fibrillation, chronic obstructive pulmonary disease,
hematologic diseases, obesity and smoking | (58) |
| Pathogenesis and
pathophysiology | There is no single
sign or symptom that can accurately diagnose or exclude PE in young
patients. The common symptoms are dyspnea and chest pain.
Additional signs and symptoms include hemoptysis, cough,
tachycardia, syncope, fatigue, hypoxemia, cyanosis and leg
pain. | (66-68) |
Younger individuals typically present with fewer
comorbidities than elderly patients and are generally in better
health. Since a substantial number of previous studies mainly
focused on PE in elderly instead of that in younger patients
(8,9), the risk factors, clinical features and
anticoagulation strategies in younger patients with PE remain
poorly defined. Guidelines for PE treatment were not designed with
age as a parameter, resulting in gaps in the study data for younger
patients.
In recent decades, the vitamin K antagonist (VKA)
warfarin has been used for PE treatment and prophylaxis (10,11).
However, the introduction of the non-vitamin K oral anticoagulant
(NOAC) rivaroxaban changed the choice of pharmacological treatment
in young patients with PE. Rivaroxaban has several advantages over
VKAs, including a rapid onset of action and a more predictable
pharmacokinetic profile, allowing for simplified drug
administration in a single, standardized dose, which negates the
need for frequent laboratory monitoring and dose adjustment
(12,13).
In the EINSTEIN-DVT dose-ranging phase II clinical
trials (12) and subsequent phase
III clinical trials (EINSTEIN- DVT, EINSTEIN-PE and EINSTEIN-EXT)
(14,15), rivaroxaban was found to be
non-inferior to VKA in terms of efficacy but conferred a lower risk
of serious adverse drug reactions, especially cerebral hemorrhages
in all participants with PE (15).
Although the aforementioned study did not specify the condition of
young patients with PE, the study included young patients (>18
and <50 years) with PE. Following the treatment recommendations
of the 2016 American College of Chest Physicians and the 2014 and
2017 European Society of Cardiology guidelines for both DVT and PE
(16-18),
numerous clinical studies and case reports of rivaroxaban use in
real-life have been published, including data from young patients
(19-23).
A review of the current literature would allow for
the identification of specific risk factors and clinical
characteristics in younger patients with PE, in addition to
clarifying the current status of rivaroxaban use in this age group.
The present article reviews the current literature published prior
to January 1, 2019. ‘Rivaroxaban’ ivaroxabans age group and oxabans
age group. The present article reviews the current literature
published prior to January 1, 2019. ‘acteristics in younger
patients with PE, in additvant original research, review articles,
guidelines and personal experience were used to summarize the
collective understanding regarding the clinical characteristics of
PE and rivaroxaban use in young patients to guide clinical decision
making in daily practice.
2. Epidemiology data of PE among younger
populations
Although the reported incidence of PE in young
subjects is inconsistent among studies, it is generally considered
to be lower compared with that in the elderly population. The
estimated incidence of the first acute VTE is between 0.7 and 1.4
per 1,000 person-years and is mostly observed in patients >55
years old (1). An epidemiological
study in Norway suggested that the incidence rates for all first
VTE events, DVT and PE to be 1.43, 0.93 and 0.50 per 1,000
person-years, respectively (24).
Another study in United States demonstrated that the average annual
incidence of DVT alone is 48 per 100,000, whilst the incidence of
PE with or without DVT is 23 per 100,000(25). A Seville prospective study of
forensic autopsies reported a similar PE incidence rate of 0.65 per
100,000(26).
Previously studies on the incidence of PE in young
patients have produced controversial data, as the cut-off
thresholds for defining the younger and older age groups were set
differently. A number of previous studies defined the younger
population as those aged ranging from 40 to 65 years. In a Medical
University of Bialystok study including 238 retrospectively
enrolled patients with confirmed PE, patients <50 years
accounted for 19.7% of the cohort (7), whilst another North Carolina study
consisting of 387 patients aged >45 years suggested a 5%
incidence of PE in a population aged <45 years (27). A USA retrospective study of 631
patients with PE demonstrated a 9.4% PE incidence in a cohort of
patients aged between 20 and 50 years (28). In another 232-subject study in Italy,
25% of the patients with PE were found to be aged <65 years
(29). A previous France study
consisting of 250 patients diagnosed with the first episode of VTE
found 25% of the patients were aged <50 years (30). In a Boston cohort study of 547
consecutive patients with PE from 2005 to 2011, 62% of the patients
were <65 years old (Table I)
(31).
Although PE is less prevalent in younger subjects,
it remains to be a life-threatening disorder (32,33). A
previous study estimated the number of clear cases of fatal PE
among the Danish population and reported an annual mortality rate
to be 0.36 cases per 100,000 person-years in the age group of 0-35
years (34). In a University of
California retrospective study of 3,456 patients who had idiopathic
PE ageing between 18 and 56 years from 1994 to 2001, 10 (0.29%)
died due to first-time recurrent thromboembolism between 1 month
and 5 years following diagnosis (35).
3. Risk factors for PE in young
patients
Unprovoked factors of PE (neither an important
transient nor persistent provoking risk factor for thrombosis, with
no apparent clinical risk factors and environmental risk factor)
are either hereditary or idiopathic, in a manner that is not
associated with environmental risk factors. PE do not normally
occur spontaneously, but the proportion of unprovoked PE is
unclear. These unprovoked causes tend to be more common in young
patients with PE (Table II)
(36-38).
Hereditary thrombophilia typically confers the greatest risk for PE
(39). Most cases of inherited
thrombophilia are due to Factor V Leiden and prothrombin gene
mutations (40,41), whilst the remaining causes are due to
deficiencies in protein S, protein C and antithrombin (39). Unprovoked causes also include
increased levels of factor VIII or IX, heparin co-factor II
deficiency and dysfunctions in plasminogen, factor XII and
fibrinogenemia (42-44).
A recent study involving 237 patients demonstrated that the levels
of protein C antigen, protein S antigen, protein S activity,
antithrombin III antigen and factor VIII were significantly
increased in patients with unprovoked PE, compared with those with
provoked PE (45). A prospective
observational study of 331 patients reported that unprovoked PE was
more likely to occur at a young age (36). These factors lead to hypercoagulative
states, which can result in blood clot formation and frequently
affect multiple first-degree family members. Consequently, affected
individuals have a higher lifetime probability of developing
thrombosis compared with those in the normal population (46-48).
Recurrent VTE events often occur when patients discontinue
anticoagulation treatments (49,50).
 | Table IICommon risk factors of PE in young
and elderly patients. |
Table II
Common risk factors of PE in young
and elderly patients.
| Patient
population | Risk factor | (Refs) |
|---|
| Young | Hereditary
thrombophilia; deficiencies in protein S, protein C; deficiencies
in antithrombin factor V Leiden mutations | (39-41,45) |
| | Trauma; obesity;
smoking; oral contraceptive use; pregnancy; postpartum hormone
replacement therapy | (45,59,60) |
| Elderly | Recent
hospitalization; cancer, infection, immobility | (63,64) |
| | Chronic
cardiopulmonary disease; renal dysfunction; diabetes mellitus;
heart failure; venous insufficiency; increasing levels of blood
plasma fibrinogen and plasminogen activator inhibitor-1 | (65) |
Provoked risk factors (acquired or secondary) for PE
include the presence of underlying conditions and/or precipitating
factors, including recent surgery or trauma, inflammation,
immobilization, malignancy, pregnancy, contraceptive use, hormone
replacement therapy, heart failure, atrial fibrillation, obesity,
chronic obstructive pulmonary disease and hematological diseases
(45,51). Provoked factors can be transient, as
it is the case in recent surgery, where patients have a reduced
risk of recurrence of PE after the cessation of therapy (52). In addition, provoked risk factors can
also be persistent and progressive, such as metastatic cancer,
which is associated with a high risk of recurrence of PE after
ceasing therapy (53,54). A retrospective study of younger
patients aged <50 years with confirmed PE suggested that obesity
and smoking were significantly more prevalent in young patients
with PE, whilst malignancies, hypertension, diabetes, ischemic
heart disease, atrial fibrillation and other comorbidities were
less frequently observed compared with older patients (7). By contrast, another previous study
reported that tobacco use, immobilization or estrogen use were more
common in patients with PE aged <50 years old (30). Smoking was reported as an independent
risk factor for VTE in all populations, especially in younger
female patients (55,56). A population-based study concluded
that the relative risk of VTE in the presence of cancer was highest
in patients <50 years of age (57), whilst a study of female patients
between the ages of 10 and 29 with PE suggested that oral
contraceptive use, pregnancy and postpartum status were predominant
factors (58). Other previous
studies also demonstrated pregnancy, puerperium, estrogen and oral
contraceptive use, family history of VTE and a history of trauma to
be more prevalent in younger patients with PE (ages in three
articles were between 21-50 years, 18-40 years and <40 years,
respectively) (32,59,60).
Inflammatory bowel disease and antiphospholipid syndrome have also
been documented to increase the risk of early PE (61,62). The
rates of respiratory failure and infection were found to be
significantly higher in patients with provoked, compared with those
with unprovoked PE (45). Although
some risk factors for PE are common among both the young and the
elderly, some differences are apparent between the two populations
(Table II) (24,63,64).
4. PE pathogenesis and pathophysiology in
young patients
PE affects the circulatory and respiratory systems
and first develops in the right ventricle (RV), leading to
significant right ventricular dysfunction due to increased pressure
overload. Severe cases of PE can lead to hemodynamic instability
and death at any age (65). Due to
RV dysfunction and exacerbated ventricle desynchronization, blood
volume in the left ventricle (LV) is reduced, lowering the LV
ejection fraction, resulting in systemic hypotension, hemodynamic
instability and can lead to death (66). Additionally, low cardiac output
results in the mixed venous blood desaturation and a
ventilation-perfusion mismatch, contributing to hypoxemia (17). Hypoxemia leads to neurohumoral
activation, systemic vasoconstriction, increased pulmonary artery
pressure and right ventricular failure (17).
Pulmonary artery pressure increases when blocked by
thrombi that are >30-50% of the total cross-sectional area of
the artery lumen. When LV filling and ejection fraction are
reduced, secondary angina develops, which can result in cardiogenic
shock, increasing the risk of mortality (67). PE can induce vasoconstriction and
subsequent release of inflammatory cytokines and epinephrine,
further contributing to the increased pressure in the pulmonary
artery, increases in arterial wall tension, myocyte stretching,
elevated biomarkers of myocardial injury, neurohumoral activation
and the further activation of coagulation factor (68-70).
Although most of the observed direct effects of PE are manifested
on the circulatory system, respiratory failure is predominantly a
consequence of hemodynamic disturbances as a result of PE (71).
5. Clinical presentation of PE in young
patients
PE may be completely asymptomatic, where it is
diagnosed incidentally during check-up for other unrelated
condition or even at autopsy. In younger patients, presenting signs
and symptoms are often nonspecific and insufficient for accurate
diagnosis because the incidence is generally lower in young
patients compared with that in the elderly and the clinical
features of PE in younger patients have not been clearly
characterized.
Dyspnea and chest pain are among the most common
symptoms reported in younger patients with PE. Additional signs and
symptoms of PE include hemoptysis, coughing, tachypnea,
tachycardia, syncope, fatigue and hypoxemia (60). Previous studies compared the clinical
manifestations of PE between younger and older patients (72,73).
Most studies defined those aged <40-50 years old as young
patients, whilst others used 65 years as a cut-off point. A
previous group study showed that patients <65 years old
presented with less dyspnea and syncope compared with those aged
>65 years (7), whilst in another
study, using a cut-off of 65 years, pleuritic chest pain was found
to be more prevalent in patients <65 years, with cyanosis and
hypoxia being less frequent (73).
An observational retrospective study of younger patients (age ≤45
years) reported that the most frequent symptoms were dyspnea, chest
pain and cough (74). Among 61 cases
of fatal PE (age, 0-35 years), the predominant symptoms were
revealed to be dyspnea, syncope, leg pain and chest pain (34). Other previous studies found no
difference in the clinical signs and symptoms between young and
elderly patients (7,60,72). An
insufficient number of studies have comprehensively and
prospectively compared PE symptoms in different age groups, where
no single indicator or symptom can accurately diagnose or exclude
PE in young patients. Therefore, clinicians should use more precise
clinical tools and maintain a high index of suspicion when
considering PE in younger patients.
6. Anticoagulation therapy in young patients
with PE
For decades, antithrombotic regimens for PE
consisted of initial treatment with heparin followed by warfarin
(75-77).
This treatment poses a number of problems associated with the
adverse effects of warfarin and the high risk of bleeding events,
including frequent laboratory monitoring and dosage changes, a
narrow therapeutic range, variable pharmacokinetic profiles,
unpredictable anticoagulation outcomes, interactions between food
and drug and genetic polymorphism (78). In a previous study, it was found that
>70% patients with VTE with high risks of recurrence did not
comply with warfarin therapy, where >50% discontinued warfarin
therapy within 1 year (79). The
primary reasons for changing the treatment regimen included
difficulties in managing the international normalized ratio (INR)
instability and patient choice. In the EINSTEIN-DVT and EINSTEIN-PE
trials, the INRs of patients receiving warfarin were only 62.7 and
57.7% within the therapeutic range, respectively (14,15).
These values underscore the difficulties associated with managing
the warfarin treatment regimen. Due to unstable vitamin K
absorption and metabolism, frequent hospital visits are required
for the routine monitoring of coagulation whilst under warfarin
treatment (80,81), which is a source of great
inconvenience for patients.
NOACs are small molecules that directly inhibit the
activated coagulation factor Xa (12). NOACs, including apixaban, edoxaban
and rivaroxaban, have similar pharmacological characteristics. The
EINSTEIN-DVT and EINSTEIN-PE trials involving patients aged >18
years (14,15) indicated that rivaroxaban was
effective in patients with PE of all ages, including young patients
(>18 and <50 years). Apixaban and edoxaban also demonstrated
promising results for the treatment of VTE in all age groups
(82,83). The application of apixaban for the
Initial Management of Pulmonary Embolism and Deep Vein Thrombosis
as First-line Therapy (AMPLIFY) trial compared single oral apixaban
treatments with conventional therapy in 5,395 patients with acute
VTE (84). The primary efficacy
outcome of the AMPLIFY study was recurrent symptomatic VTE or death
related to VTE and the results demonstrated that apixaban was
non-inferior compared with conventional therapy for the primary
efficacy outcome, where major bleeding occurred less frequently
than with conventional therapy. The composite outcome of major
bleeding occurred in 4.3% of the patients in the apixaban group,
compared with 9.7% of those in the conventional therapy group
(82). In another study, Hokusai-VTE
Investigators et al (83)
compared edoxaban treatment with conventional therapy in 8,240
patients with acute VTE. Edoxaban was also found to be non-inferior
compared with warfarin with respect to the primary efficacy outcome
of recurrent symptomatic VTE or fatal PE, where the principal
safety outcome, major bleeding, occurred less frequently in the
edoxaban group. In a study of 938 patients who presented with acute
PE and elevated N-terminal-pro hormone brain natriuretic peptide
(NT-proBNP) concentrations (≥500 pg/ml), the rate of recurrent VTE
was found to be 3.3% in the edoxaban group and 6.2% in the warfarin
group (83). In a meta-analysis of
NOAC-treated or VKA-treated patients, major bleeding in the
critical sites was found to occur less frequently in NOAC-treated
patients (85). In particular, there
was a significant reduction in intracranial bleeding and in fatal
bleeding with patient groups treated with NOACs compared with those
treated with VKAs (85).
Rivaroxaban targets specific sites which is a
selective direct inhibitor of activated coagulation factor X within
the coagulation cascade (86).
Advantages of using rivaroxaban include fewer drug interactions and
a more predictable pharmacological profile compared with warfarin,
thereby minimizing the need for routine laboratory monitoring and
frequent dose adjustments. Rivaroxaban has garnered attention for
the treatment of PE, especially in young patients (87). It is readily available and was
approved in many countries for treating and preventing recurrent
VTE whilst offering the same efficacy as warfarin with a lower risk
of bleeding. At present, rivaroxaban is approved for the prevention
of stroke in nonvalvular atrial fibrillation (88), prevention and treatment of DVT and PE
(14,15) and prophylaxis against DVT after knee
and hip replacement surgery (89). A
recent study indicated that patients with stable atherosclerotic
vascular disease achieved superior cardiovascular outcomes
following treatment with rivaroxaban and aspirin (90). Results from the EINSTEIN-DVT (65.5%
of participants aged <65 years), EINSTEIN-PE (60.9% of
participants aged ≤65 years) and EINSTEIN-EXT trials (61.4% of
participants aged ≤65 years) demonstrated that rivaroxaban was
non-inferior compared with warfarin with respect to efficacy
(Table III) and may confer a
superior safety profile (14,15).
These findings also applied to younger patients.
 | Table IIIPhase III rivaroxaban safety and
efficacy clinical trials in selected subgroups (14,15). |
Table III
Phase III rivaroxaban safety and
efficacy clinical trials in selected subgroups (14,15).
| A,
EINSTEIN-DVT |
|---|
| | Recurrent VTE | Clinically relevant
bleeding |
|---|
| Age (years) | Rivaroxaban, n/N
(%) | Enoxaparin + VKA,
n/N (%) | Rivaroxaban, n/N
(%) | Enoxaparin + VKA,
n/N (%) |
|---|
|
2<65 | 26/1145 (2.3) | 30/1111 (2.7) | 86/1134 (7.6) | 70/1107 (7.1) |
|
65-75 | 6/371 (1.6) | 11/382 (2.9) | 34/369 (9.2) | 39/381 (10.2) |
|
>75 | 4/215 (1.9) | 10/225 (4.4) | 19/215 (8.8) | 20/223 (9.0) |
| B, EINSTEIN-PE |
|
<65 | 29/1461 (2.0) | 23/1479 (1.6) | 132/1458 (9.1) | 136/1472 (9.2) |
|
65-75 | 10/517 (1.9) | 8/532 (1.5) | 59/514 (11.5) | 71/532 (13.3) |
|
>75 | 11/441 (2.5) | 13/401 (3.2) | 58/440 (13.2) | 67/401 (16.7) |
| C,
EINSTEIN-EXT |
|
<65 | 4/360
(1.1)a | 23/374
(6.2)a | 22/358
(6.2)a | 3/373
(0.8)a |
|
65-75 | 3/153 (2.0) | 8/121 (6.6) | 7/152 (4.6) | 1/119 (0.8) |
|
>75 | 1/89
(1.1)a | 11/99
(11.1)a | 7/88 (8.0) | 3/98 (3.1) |
A real-world study (a non-randomized, practical
clinical study based on the patient's actual condition and
willingness) involving 103 patients with PE (including 27 patients
aged <50 years old) suggested that rivaroxaban provided
advantages over warfarin (exhibiting, fast-start action
pharmacokinetic and pharmacodynamic characteristics, and has an
enhanced predictable anticoagulant effect with fewer drug-drug
interactions) (22), whilst another
study found that patients with VTE who continued rivaroxaban
therapy after the initial 3- or 6 month treatment period had a
significantly lower risk of VTE recurrence without a statistically
significant increased risk for major bleeding (91). Among 13,609 rivaroxaban and 32,244
warfarin users with VTE, rivaroxaban was found to be associated
with less recurrent VTE and reduced risk of major bleeding compared
with warfarin (92). In a clinical
setting, rivaroxaban use in patients with unprovoked VTE was
demonstrated to be associated with reduced risk of recurrent VTE
compared with standard treatment (93). In a study comparing 2,619 patients on
rivaroxaban and 2,149 on standard anticoagulant therapy,
rivaroxaban-treated patients had a lower risk profile at baseline
compared with those treated with standard anticoagulation, where
the rates of major bleeding and recurrent VTE were low in
rivaroxaban-treated patients (94).
Despite the lack of younger PE patient analysis, the previous
studies aforementioned included younger patients with PE and found
that they achieved good outcomes with rivaroxaban.
In a retrospective observational study comparing the
length of stay in hospitals (LOS) and hospitalization costs for
patients with VTE treated with rivaroxaban compared with those
treated with warfarin, the mean LOS was found to be significantly
shortened by 1.57 days in the rivaroxaban treatment group, where
the hospitalization costs were also significantly lower (95,96). In
the EINSTEIN trials, rivaroxaban was demonstrated to be associated
with a shorter LOS, which was consistent across all included
hospitals and countries as patients did not need to remain
hospitalized during the transition from heparin/warfarin to
warfarin (14,15). Subsequent economic assessments of the
EINSTEIN trials demonstrated that rivaroxaban was associated with
increased cost effectiveness and increased quality-adjusted years
of life (82,97).
7. Alternative treatment methods in young
patients with PE
Although anticoagulation is crucial to PE treatment,
including that in younger patients, other treatment methods can
provide better outcomes in certain cases. Thrombolytic therapy
using recombinant tissue-type plasminogen activator, streptokinase
or urokinase have been shown to result in faster improvements in
pulmonary obstruction (98) with the
associated significant reductions in the risk of hemodynamic
decompensation or collapse, despite an increased risk of severe
extracranial and intracranial bleeding (99). Percutaneous catheter-directed
treatment and endovascular thrombolysis by means of catheter are
also important alternatives for PE treatment (100). Vena cava filters can mechanically
prevent venous clots from reaching the pulmonary circulation. Most
filters in current use are inserted percutaneously and can be
retrieved after several weeks or months, or left in place long-term
in patients with contraindications to anticoagulant treatment or
recurrent PE despite adequate anticoagulation (101).
8. Adverse effects or toxicity associated
with rivaroxaban
Despite fewer interactions which may cause
unpredictable anticoagulation outcomes with rivaroxaban and other
NOACs, every patient should be considered on a personalized basis,
especially when a combination of interfering underlying factors is
present. NOACs differ in their rates of absorption, distribution,
metabolism and excretion. An important interaction for all NOACs
involves significant gastrointestinal re-secretion through the
P-glycoprotein (P-gp) transporter following absorption (88). Many drugs used in patients with PE
are either inhibitors of P-gp and cytochrome P450 family 3
subfamily A member 4 (CYP3A4) or activators affecting plasma NOAC
concentrations (102). Rivaroxaban
is generally not recommended in combination with drugs that are
strong inhibitors of CYP3A4 and/or P-gp. Conversely, strong
activators of P-gp and/or CYP3A4 markedly reduce NOAC plasma levels
(88). In phase III VTE trials, the
dosages of rivaroxaban and apixaban were not reduced in patients
with creatinine clearance (CrCl) at 30-60 ml/min (mild-moderate
renal dysfunction), whilst patients with CrCl <30 ml/min were
required to avoid rivaroxaban and edoxaban and were not enrolled in
the study (12,82,83).
Advanced age, frailty and low weight are associated with higher
risks of bleeding, where a reduction in the dose of rivaroxaban is
required (103).
9. A case report
A 28 year-old male with a 10 year smoking history
was admitted to the hospital following a cough and gradually
worsening dyspnea over 10 days. Upon arrival, the heart rate was 95
beats/min, blood pressure at 140/84 mmHg, respiratory rate at 23
breaths/min and oxygen saturation of 93% on room air. His physical
examination results were normal. Complete blood cell count, liver
function and renal function tests did not reveal abnormalities.
Cardiac troponin-T and NT-proBNP levels were also normal. However,
urinalysis showed 3+ proteinuria, where blood tests indicated low
plasma total protein and albumin, high low-density lipoprotein
(LDL) and elevated D-dimer levels. Arterial blood gas analysis was
indicative of hypoxemia (Table IV).
Echocardiography and lower extremity venous compression ultrasound
results were also normal. Since PE was highly suspected based on
the patient's clinical presentation, hypoalbuminemia, hypoxemia and
high D-dimer levels, computed tomography pulmonary angiography
(CTPA) was performed to confirm the diagnosis, which showed
intraluminal filling defects representing thromboses in the
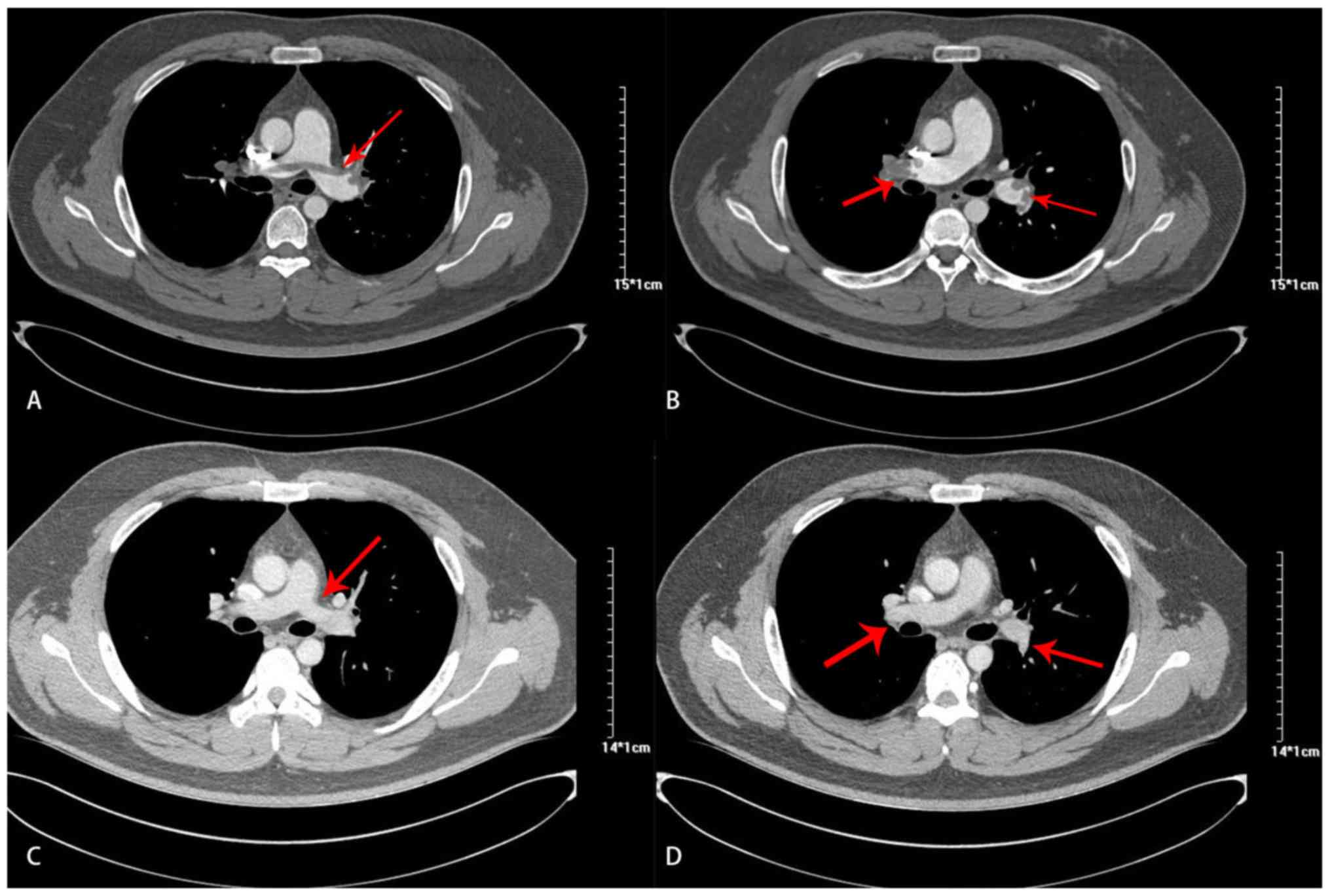
bilateral pulmonary artery trunk and branches (Fig. 1A and B). Nephrotic syndrome (NS) due to minimal
change disease and PE were diagnosed by renal biopsy and CTPA,
respectively. The patient therefore received oral prednisone
treatment. PE risk stratification was performed to determine the
simplified PE severity index (sPESI) and guide treatment strategy.
His initial stratification was determined as ‘not high-risk’ with a
sPESI of 0. Therefore, anticoagulation therapy with 15 mg
rivaroxaban twice daily was initiated. The patient was discharged
from the hospital after 10 days. After 3 weeks, the rivaroxaban
dose was reduced to 20 mg once daily and prednisone was continued.
At follow-up 90 days after discharge, the symptoms had disappeared
and the laboratory results, including those of plasma total protein
and albumin, 24 h urine protein quantification, total cholesterol
and LDL, D-dimer and arterial blood gas levels had nearly
normalized (Table IV). Repeat CTPA
yielded normal results after 90 days on rivaroxaban (Fig. 1C and D), following which the drug was
discontinued.
 | Table IVLaboratory test results on admission
and at 90-day follow-up. |
Table IV
Laboratory test results on admission
and at 90-day follow-up.
| Result | On admission | Follow-up at 90
days | Reference
range |
|---|
| Total protein
(g/l) | 46.40 | 64.2 | 65.0-85.0 |
| Albumin (g/l) | 16.80 | 38.4 | 40.0-55.0 |
| Urine protein/24 h
(mg) | 2,668.00 | 208.12 | 0-00.00 |
| Proteinuria | 3+ | Negative | Negative |
| TC (mmol/l) | 8.97 | 6.75 | 2.60-6.00 |
| LDL-C (mmol/l) | 6.10 | 3.3 | 2.07-3.10 |
| D-dimer
(pg/ml) | >20 | 1.04 | <0.50 |
| PaO2
(mmHg) | 59.00 | 93.2 | 80.0-100.0 |
| PaCO2
(mmHg) | 35.00 | 43.5 | 35.0-45.0 |
| SaO2
(%) | 93.00 | 97.5 | 95.0-98.0 |
In summary, NS was identified as a risk factor in
the young patient with PE. The pathological process of NS involves
increased glomerular permeability resulting in leakage of albumin
through the glomerulus into the urine. As hypoalbuminemia occurs,
the plasma colloid osmotic pressure decreases, inducing water
movement from the blood to the tissues. This, in turn, decreases
the circulating blood volume and leads to increased levels of blood
coagulation factors. Concurrently, the liver increases production
of many substances, including albumin, coagulation factors,
cholesterol and LDL, whereas the kidney reduces the excretion of
these substances (except albumin), leading to an imbalance between
procoagulant and anticoagulant factors, thereby triggering
thrombosis (104). The patient, in
this case, presented with normotension without RV injury and
elevated biomarkers and was classified as ‘low-risk’ based on the
2014 European Society of Cardiology guidelines for PE (17). Therefore, rivaroxiban was used for 3
months as recommended by the guidelines (17), producing satisfactory results.
10. Conclusions
The incidence of PE in young populations remain
unclear due to differing definitions of youth across previous
studies, ranging from 40 to 65 years. Since there is also limited
understanding on the clinical features of PE, anticoagulant
treatment selection difficult for younger patients. Although
existing guidelines, randomized controlled trials and large
clinical studies lacked subgroups of young patients, they did
include younger patients. Real-world studies also provided valuable
insights into PE in this particular population. The present
literature review suggests that the incidence of PE in the younger
population should not be ignored, especially for individuals
presenting with the various risk factors mentioned in the present
article. Unprovoked risk factors pose a potential threat to young
subjects and can lead to long-term hypercoagulation. Screening for
hereditary causes should also be performed, followed by monitoring.
Smoking and obesity are among the concerning provoked causes of PE
in younger patients. Based on existing guideline, the results of
large phase III clinical studies and real-life studies, rivaroxaban
is demonstrated to be safe and effective, providing a new
anticoagulant treatment option for young patients with PE.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HW and HC made substantial contributions to the
conception and design of the study and wrote the original draft of
the manuscript. ZS and XX conducted data analysis and
interpretation. MT, SY and YL were responsible for data
acquisition. LQ designed the current article and revised it
critically for important intellectual content. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethics approval was waived by The First Hospital of
Jilin University Ethical Board (Changchun, China), based on their
policy of reviewing all intervention and observational studies,
except for case reports.
Patient consent for publication
All patients provided informed consent for the
publication of his clinical data. The presented data are anonymized
and the risk of identification is minimal.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tritschler T, Kraaijpoel N, Le Gal G and
Wells PS: Venous thromboembolism: Advances in diagnosis and
treatment. JAMA. 320:1583–1594. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Heit JA: Epidemiology of venous
thromboembolism. Nat Rev Cardiol. 12:464–474. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bartholomew JR: Update on the management
of venous thromboembolism. Cleve Clin J Med. 84:39–46.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kniffin WD Jr, Baron JA, Barrett J,
Birkmeyer JD and Anderson FA Jr: The epidemiology of diagnosed
pulmonary embolism and deep venous thrombosis in the elderly. Arch
Intern Med. 154:861–866. 1994.PubMed/NCBI
|
|
5
|
Silverstein MD, Heit JA, Mohr DN,
Petterson TM, O'Fallon WM and Melton LJ III: Trends in the
incidence of deep vein thrombosis and pulmonary embolism: A 25 year
population-based study. Arch Intern Med. 158:585–593.
1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kumasaka N, Sakuma M and Shirato K:
Clinical features and predictors of in-hospital mortality in
patients with acute and chronic pulmonary thromboembolism. Intern
Med. 39:1038–1043. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kiluk IE, Krajewska A, Kosacka U, Tycińska
A, Milewski R, Musiał W and Sobkowicz B: Different manifestations
of pulmonary embolism in younger compared to older patients:
Clinical presentation, prediction rules and long-term outcomes. Adv
Med Sci. 62:254–258. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jaquet E, Tritschler T, Stalder O,
Limacher A, Méan M, Rodondi N and Aujesky D: Prediction of
short-term prognosis in elderly patients with acute pulmonary
embolism: Validation of the RIETE score. J Thromb Haemost.
16:1313–1320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Engbers MJ, van Hylckama Vlieg A and
Rosendaal FR: Venous thrombosis in the elderly: Incidence, risk
factors and risk groups. J Thromb Haemost. 8:2105–2112.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gallus A, Jackaman J, Tillett J, Mills W
and Wycherley A: Safety and efficacy of warfarin started early
after submassive venous thrombosis or pulmonary embolism. Lancet.
2:1293–1296. 1986.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lotke PA and Ecker ML:
Low-Molecular-weight heparin vs. Warfarin for prophylaxis against
deep-vein thrombosis. N Engl J Med. 330(863)1994.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Buller HR, Lensing AW, Prins MH, Agnelli
G, Cohen A, Gallus AS, Misselwitz F, Raskob G, Schellong S and
Segers A: Einstein-DVT Dose-Ranging Study investigators. A
dose-ranging study evaluating once-daily oral administration of the
factor xa inhibitor rivaroxaban in the treatment of patients with
acute symptomatic deep vein thrombosis: The Einstein-DVT
dose-ranging study. Blood. 112:2242–2247. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scaglione F: New oral anticoagulants:
Comparative pharmacology with vitamin K antagonists. Clin
Pharmacokinet. 52:69–82. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
EINSTEIN Investigators. Bauersachs R,
Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing
AW, Misselwitz F, Prins MH, et al: Oral rivaroxaban for symptomatic
venous thromboembolism. N Engl J Med. 363:2499–2510.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
EINSTEIN–PE Investigators. Buller HR,
Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J,
Verhamme P, Wells P, et al: Oral rivaroxaban for the treatment of
symptomatic pulmonary embolism. N Engl J Med. 366:1287–1297.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kearon C, Akl EA, Ornelas J, Blaivas A,
Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et
al: Antithrombotic therapy for VTE disease: CHEST guideline and
expert panel report. Chest. 149:315–352. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Konstantinides SV, Torbicki A, Agnelli G,
Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M,
Kucher N, et al: 2014 ESC guidelines on the diagnosis and
management of acute pulmonary embolism. Eur Heart J. 35:3033–3069.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mazzolai L, Aboyans V, Ageno W, Agnelli G,
Alatri A, Bauersachs R, Brekelmans MPA, Büller HR, Elias A, Farge
D, et al: Diagnosis and management of acute deep vein thrombosis: A
joint consensus document from the European society of cardiology
working groups of aorta and peripheral vascular diseases and
pulmonary circulation and right ventricular function. Eur Heart J.
39:4208–4218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang Y, Wang C, Chen Z, Zhang J, Liu Z,
Jin B, Ying K, Liu C, Shao Y, Jing Z, et al: Rivaroxaban for the
treatment of symptomatic deep-vein thrombosis and pulmonary
embolism in Chinese patients: A subgroup analysis of the EINSTEIN
DVT and PE studies. Thromb J. 11(25)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou Q, Wu Y, Jiang X, Liu X, Lei H, Jing
Z and Huang W: [Efficacy comparison of 3 rivaroxaban regimen in
patients with venous thromboembolism]. Zhonghua Xin Xue Guan Bing
Za Zhi. 43:782–784. 2015.PubMed/NCBI
|
|
21
|
Song Z, Wu H, Cao H, Tang M, Yang S and
Qin L: Nephrotic syndrome with acute pulmonary embolism in young
adults: Two case reports. Medicine (Baltimore).
97(e11495)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jara-Palomares L, Sanchez-Oro-Gomez R,
Elias-Hernandez T, Morillo-Guerrero R, Ferrer-Galvan M,
Asensio-Cruz MI, Barrot-Cortes E and Otero-Candelera R: Rivaroxaban
for the treatment of venous thromboembolism. A ‘real-life’
perspective in 103 patients. Thromb Res. 134:617–621.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pesavento R and Iori I: Gruppo Italiano
Survey TEV. [Use of rivaroxaban in real-life treatment of venous
thromboembolism: Results of the TEV Survey, an Italian
epidemiological study]. G Ital Cardiol (Rome). 18:239–246.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Naess IA, Christiansen SC, Romundstad P,
Cannegieter SC, Rosendaal FR and Hammerstrom J: Incidence and
mortality of venous thrombosis: A population-based study. J Thromb
Haemost. 5:692–699. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Anderson FA Jr, Wheeler HB, Goldberg RJ,
Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A and Dalen JE: A
population-based perspective of the hospital incidence and
case-fatality rates of deep vein thrombosis and pulmonary embolism.
The worcester DVT study. Arch Intern Med. 151:933–938.
1991.PubMed/NCBI
|
|
26
|
Lucena J, Rico A, Vazquez R, Marín R,
Martínez C, Salguero M and Miguel L: Pulmonary embolism and
sudden-unexpected death: Prospective study on 2477 forensic
autopsies performed at the institute of legal medicine in seville.
J Forensic Leg Med. 16:196–201. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Heredia V, Ramalho M, Zapparoli M and
Semelka RC: Incidence of pulmonary embolism and other chest
findings in younger patients using multidetector computed
tomography. Acta Radiol. 51:402–406. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kuroki M, Nishino M, Takahashi M, Mori Y,
Raptopoulos VD, Boiselle PM, Tamura S and Hatabu H: Incidence of
pulmonary embolism in younger versus older patients using CT. J
Thorac Imaging. 21:167–171. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Castelli R, Bergamaschini L, Sailis P,
Pantaleo G and Porro F: The impact of an aging population on the
diagnosis of pulmonary embolism: Comparison of young and elderly
patients. Clin Appl Thromb Hemost. 15:65–72. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Roupie AL, Dossier A, Goulenok T,
Perozziello A, Papo T and Sacre K: First venous thromboembolism in
admitted patients younger than 50 years old. Eur J Intern Med.
34:e18–e20. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cefalo P, Weinberg I, Hawkins BM,
Hariharan P, Okechukwu I, Parry BA, Chang Y, Rosovsky R, Liu SW,
Jaff MR and Kabrhel C: A comparison of patients diagnosed with
pulmonary embolism who are ≥65 years with patients ≥65 years. Am J
Cardiol. 115:681–686. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kreidy R, Salameh P and Waked M: Lower
extremity venous thrombosis in patients younger than 50 years of
age. Vasc Health Risk Manag. 8:161–167. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tsai AW, Cushman M, Rosamond WD, Heckbert
SR, Polak JF and Folsom AR: Cardiovascular risk factors and venous
thromboembolism incidence: The longitudinal investigation of
thromboembolism etiology. Arch Intern Med. 162:1182–1189.
2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Theilade J, Winkel BG, Holst AG,
Tfelt-Hansen J, Svendsen JH and Haunso S: A nationwide,
retrospective analysis of symptoms, comorbidities, medical care and
autopsy findings in cases of fatal pulmonary embolism in younger
patients. J Thromb Haemost. 8:1723–1729. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
White RH, Zhou H and Murin S: Death due to
recurrent thromboembolism among younger healthier individuals
hospitalized for idiopathic pulmonary embolism. Thromb Haemost.
99:683–690. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stoeva N, Kirova G, Staneva M, Lekova D,
Penev A and Bakalova R: Recognition of unprovoked (idiopathic)
pulmonary embolism-Prospective observational study. Respir Med.
135:57–61. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bruwer G, Limperger V, Kenet G,
Klostermeier UC, Shneyder M, Degenhardt F, Finckh U, Heller C,
Holzhauer S, Trappe R, et al: Impact of high risk thrombophilia
status on recurrence among children and adults with VTE: An
observational multicenter cohort study. Blood Cells Mol Dis.
62:24–31. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Alhassan S, Pelinescu A, Gandhi V, Naddour
M, Singh AC and Bihler E: Clinical presentation and risk factors of
venous thromboembolic disease. Crit Care Nurs Q. 40:201–209.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
De Stefano V, Simioni P, Rossi E, Tormene
D, Za T, Pagnan A and Leone G: The risk of recurrent venous
thromboembolism in patients with inherited deficiency of natural
anticoagulants antithrombin, protein C and protein S.
Haematologica. 91:695–698. 2006.PubMed/NCBI
|
|
40
|
Segal JB, Brotman DJ, Necochea AJ, Emadi
A, Samal L, Wilson LM, Crim MT and Bass EB: Predictive value of
factor V Leiden and prothrombin G20210A in adults with venous
thromboembolism and in family members of those with a mutation: A
systematic review. JAMA. 301:2472–2485. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lijfering WM, Middeldorp S, Veeger NJ,
Hamulyák K, Prins MH, Büller HR and van der Meer J: Risk of
recurrent venous thrombosis in homozygous carriers and double
heterozygous carriers of factor V leiden and prothrombin G20210A.
Circulation. 121:1706–1712. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kyrle PA, Minar E, Hirschl M, Bialonczyk
C, Stain M, Schneider B, Weltermann A, Speiser W, Lechner K and
Eichinger S: High plasma levels of factor VIII and the risk of
recurrent venous thromboembolism. N Engl J Med. 343:457–462.
2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Eischer L, Gartner V, Schulman S, Kyrle PA
and Eichinger S: AUREC-FVIII Investigators. 6 versus 30 months
anticoagulation for recurrent venous thrombosis in patients with
high factor VIII. Ann Hematol. 88:485–490. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Weltermann A, Eichinger S, Bialonczyk C,
Minar E, Hirschl M, Quehenberger P, Schönauer V and Kyrle PA: The
risk of recurrent venous thromboembolism among patients with high
factor IX levels. J Thromb Haemost. 1:28–32. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gjonbrataj E, Kim JN, Gjonbrataj J, Jung
HI, Kim HJ and Choi WI: Risk factors associated with provoked
pulmonary embolism. Korean J Intern Med. 32:95–101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Baglin T, Luddington R, Brown K and Baglin
C: Incidence of recurrent venous thromboembolism in relation to
clinical and thrombophilic risk factors: Prospective cohort study.
Lancet. 362:523–526. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Palareti G, Legnani C, Cosmi B, Valdré L,
Lunghi B, Bernardi F and Coccheri S: Predictive value of D-dimer
test for recurrent venous thromboembolism after anticoagulation
withdrawal in subjects with a previous idiopathic event and in
carriers of congenital thrombophilia. Circulation. 108:313–318.
2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Christiansen SC, Cannegieter SC, Koster T,
Vandenbroucke JP and Rosendaal FR: Thrombophilia, clinical factors,
and recurrent venous thrombotic events. JAMA. 293:2352–2361.
2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kyrle PA, Rosendaal FR and Eichinger S:
Risk assessment for recurrent venous thrombosis. Lancet.
376:2032–2039. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cannegieter SC and van Hylckama Vlieg A:
Venous thrombosis: Understanding the paradoxes of recurrence. J
Thromb Haemost. 11:161–169. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kearon C, Ageno W, Cannegieter SC, Cosmi
B, Geersing GJ and Kyrle PA: Subcommittees on Control of
Anticoagulation and Predictive and Diagnostic Variables in
Thrombotic Disease. Categorization of patients as having provoked
or unprovoked venous thromboembolism: Guidance from the SSC of
ISTH. J Thromb Haemost. 14:1480–1483. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Iorio A, Kearon C, Filippucci E, Marcucci
M, Macura A, Pengo V, Siragusa S and Palareti G: Risk of recurrence
after a first episode of symptomatic venous thromboembolism
provoked by a transient risk factor: A systematic review. Arch
Intern Med. 170:1710–1716. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Prandoni P, Lensing AW, Piccioli A,
Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins
MH, Noventa F and Girolami A: Recurrent venous thromboembolism and
bleeding complications during anticoagulant treatment in patients
with cancer and venous thrombosis. Blood. 100:3484–3488.
2002.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Heit JA, Mohr DN, Silverstein MD,
Petterson TM, O'Fallon WM and Melton LJ III: Predictors of
recurrence after deep vein thrombosis and pulmonary embolism: A
population-based cohort study. Arch Intern Med. 160:761–768.
2000.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pomp ER, Rosendaal FR and Doggen CJ:
Smoking increases the risk of venous thrombosis and acts
synergistically with oral contraceptive use. Am J Hematol.
83:97–102. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Blondon M, Wiggins KL, McKnight B, Psaty
BM, Rice KM, Heckbert SR and Smith NL: The association of smoking
with venous thrombosis in women. A population-based, case-control
study. Thromb Haemost. 109:891–896. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Blix K, Brækkan SK, le Cessie S,
Skjeldestad FE, Cannegieter SC and Hansen JB: The increased risk of
venous thromboembolism by advancing age cannot be attributed to the
higher incidence of cancer in the elderly: The tromso study. Eur J
Epidemiol. 29:277–284. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kröger K, Moerchel C, Moysidis T and
Santosa F: Incidence rate of pulmonary embolism in Germany: Data
from the federal statistical office. J Thromb Thrombolysis.
29:349–353. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Green RM, Meyer TJ, Dunn M and Glassroth
J: Pulmonary embolism in younger adults. Chest. 101:1507–1511.
1992.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Arima M, Kanoh T, Takagi A, Tanimoto K,
Oigawa T and Matsuda S: Clinical features of acute pulmonary
thromboembolism in younger patients. Circ J. 67:330–333.
2003.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kappelman MD, Horvath-Puho E, Sandler RS,
Rubin DT, Ullman TA, Pedersen L, Baron JA and Sørensen HT:
Thromboembolic risk among danish children and adults with
inflammatory bowel diseases: A population-based nationwide study.
Gut. 60:937–943. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lim W: Antiphospholipid syndrome.
Hematology. Hematology Am Soc Hematol Educ Program. 2013:675–680.
2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Spencer FA, Gore JM, Lessard D, Emery C,
Pacifico L, Reed G, Gurwitz JH and Goldberg RJ: Venous
thromboembolism in the elderly. A community-based perspective.
Thromb Haemost. 100:780–788. 2008.PubMed/NCBI
|
|
64
|
Ageno W, Agnelli G, Imberti D, Moia M,
Palareti G, Pistelli R, Rossi R and Verso M: MASTER Investigators.
Risk factors for venous thromboembolism in the elderly: Results of
the master registry. Blood Coagul Fibrinolysis. 19:663–667.
2008.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wood KE: Major pulmonary embolism: Review
of a pathophysiologic approach to the golden hour of
hemodynamically significant pulmonary embolism. Chest. 121:877–905.
2002.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mauritz GJ, Marcus JT, Westerhof N,
Postmus PE and Vonk-Noordegraaf A: Prolonged right ventricular
post-systolic isovolumic period in pulmonary arterial hypertension
is not a reflection of diastolic dysfunction. Heart. 97:473–478.
2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
McIntyre KM and Sasahara AA: The
hemodynamic response to pulmonary embolism in patients without
prior cardiopulmonary disease. Am J Cardiol. 28:288–294.
1971.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Lankhaar JW, Westerhof N, Faes TJ, Marques
KM, Marcus JT, Postmus PE and Vonk-Noordegraaf A: Quantification of
right ventricular afterload in patients with and without pulmonary
hypertension. Am J Physiol Heart Circ Physiol. 291:H1731–H1737.
2006.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lankeit M, Jimenez D, Kostrubiec M, Dellas
C, Hasenfuss G, Pruszczyk P and Konstantinides S: Predictive value
of the high-sensitivity troponin T assay and the simplified
pulmonary embolism severity index in hemodynamically stable
patients with acute pulmonary embolism: A prospective validation
study. Circulation. 124:2716–2724. 2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Mehta NJ, Jani K and Khan IA: Clinical
usefulness and prognostic value of elevated cardiac troponin I
levels in acute pulmonary embolism. Am Heart J. 145:821–825.
2003.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Burrowes KS, Clark AR and Tawhai MH: Blood
flow redistribution and ventilation-perfusion mismatch during
embolic pulmonary arterial occlusion. Pulm Circ. 1:365–376.
2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Gisselbrecht M, Diehl JL, Meyer G,
Collignon MA and Sors H: Clinical presentation and results of
thrombolytic therapy in older patients with massive pulmonary
embolism: A comparison with non-elderly patients. J Am Geriatr Soc.
44:189–193. 1996.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Timmons S, Kingston M, Hussain M, Kelly H
and Liston R: Pulmonary embolism: Differences in presentation
between older and younger patients. Age Ageing. 32:601–605.
2003.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Parenti NBA, Bonarelli S and Fanciulli A:
Pulmonary embolism in younger adults: Clinical presentation and
comparison of two scoring systems used to estimate pretest
probability of disease in the emergency department. Ann Emerg Med.
44(36)2004.
|
|
75
|
Witt DM, Clark NP, Kaatz S, Schnurr T and
Ansell JE: Guidance for the practical management of warfarin
therapy in the treatment of venous thromboembolism. J Thromb
Thrombolysis. 41:187–205. 2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Prandoni P, Lensing AW, Büller HR, Carta
M, Cogo A, Vigo M, Casara D, Ruol A and ten Cate JW: Comparison of
subcutaneous low-molecular-weight heparin with intravenous standard
heparin in proximal deep-vein thrombosis. Lancet. 339:441–445.
1992.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Columbus Investigtors. Büller HR, Gent M,
Gallus AS, Ginsberg J, Prins MH and Baildon R: Low-molecular-weight
heparin in the treatment of patients with venous thromboembolism. N
Engl J Med. 337:657–662. 1997.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Prins MH, Lensing AW, Bauersachs R, van
Bellen B, Bounameaux H, Brighton TA, Cohen AT, Davidson BL,
Decousus H, Raskob GE, et al: Oral rivaroxaban versus standard
therapy for the treatment of symptomatic venous thromboembolism: A
pooled analysis of the EINSTEIN-DVT and PE randomized studies.
Thromb J. 11(21)2013.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Lopez-Jimenez L, Montero M,
Gonzalez-Fajardo JA, Arcelus JI, Suárez C, Lobo JL, Monreal M and
RIETE Investigators: Venous thromboembolism in very elderly
patients: Findings from a prospective registry (RIETE).
Haematologica. 91:1046–1051. 2006.PubMed/NCBI
|
|
80
|
Lim W, Dentali F, Eikelboom JW and
Crowther MA: Meta-Analysis: Low-molecular-weight heparin and
bleeding in patients with severe renal insufficiency. Ann Intern
Med. 144:673–684. 2006.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Silverstein RL, Bauer KA, Cushman M, Esmon
CT, Ershler WB and Tracy RP: Venous thrombosis in the elderly: More
questions than answers. Blood. 110:3097–3101. 2007.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Agnelli G, Buller HR, Cohen A, Curto M,
Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE,
et al: Oral apixaban for the treatment of acute venous
thromboembolism. N Engl J Med. 369:799–808. 2013.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Hokusai-VTE Investigators. Büller HR,
Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob
GE, Schellong SM, Schwocho L, et al: Edoxaban versus warfarin for
the treatment of symptomatic venous thromboembolism. N Engl J Med.
369:1406–1415. 2013.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Liu X, Johnson M, Mardekian J, Phatak H,
Thompson J and Cohen AT: Apixaban reduces hospitalizations in
patients with venous thromboembolism: An analysis of the apixaban
for the initial management of pulmonary embolism and deep-vein
thrombosis as first-line therapy (AMPLIFY) trial. J Am Heart Assoc.
4(12)2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
van der Hulle T, Kooiman J, den Exter PL,
Dekkers OM, Klok FA and Huisman MV: Effectiveness and safety of
novel oral anticoagulants as compared with vitamin K antagonists in
the treatment of acute symptomatic venous thromboembolism: A
systematic review and meta-analysis. J Thromb Haemost. 12:320–328.
2014.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kvasnicka T, Malikova I, Zenahlikova Z,
Kettnerova K, Brzezkova R, Zima T, Ulrych J, Briza J, Netuka I and
Kvasnicka J: Rivaroxaban-metabolism, pharmacologic properties and
drug interactions. Curr Drug Metab. 18:636–642. 2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Limdi NA, Beasley TM, Baird MF, Goldstein
JA, McGwin G, Arnett DK, Acton RT and Allon M: Kidney function
influences warfarin responsiveness and hemorrhagic complications. J
Am Soc Nephrol. 20:912–921. 2009.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Steffel J, Verhamme P, Potpara TS,
Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke
H, Roldan-Schilling V, et al: The 2018 European heart rhythm
association practical guide on the use of non-vitamin K antagonist
oral anticoagulants in patients with atrial fibrillation. Eur Heart
J. 39:1330–1393. 2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Eriksson BI, Borris LC, Friedman RJ, Haas
S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E,
Misselwitz F, et al: Rivaroxaban versus enoxaparin for
thromboprophylaxis after hip arthroplasty. N Engl J Med.
358:2765–2775. 2008.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Eikelboom JW, Connolly SJ, Bosch J,
Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM,
Anand SS, et al: Rivaroxaban with or without aspirin in stable
cardiovascular disease. N Engl J Med. 377:1319–1330.
2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Khorana AA, Berger JS, Wells PS, Seheult
R, Ashton V, Laliberté F, Crivera C, Lejeune D, Schein J, Wildgoose
P, et al: Risk for venous thromboembolism recurrence among
rivaroxaban-treated patients who continued versus discontinued
therapy: Analyses among patients with VTE. Clin Ther. 39:1396–1408.
2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Coleman CI, Bunz TJ and Turpie AGG:
Effectiveness and safety of rivaroxaban versus warfarin for
treatment and prevention of recurrence of venous thromboembolism.
Thromb Haemost. 117:1841–1847. 2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Larsen TB, Skjøth F, Kjaeldgaard JN, Lip
GYH, Nielsen PB and Sogaard M: Effectiveness and safety of
rivaroxaban and warfarin in patients with unprovoked venous
thromboembolism: A propensity-matched nationwide cohort study.
Lancet Haematol. 4:e237–e244. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Ageno W, Mantovani LG, Haas S, Kreutz R,
Monje D, Schneider J, van Eickels M, Gebel M, Zell E and Turpie AG:
Safety and effectiveness of oral rivaroxaban versus standard
anticoagulation for the treatment of symptomatic deep-vein
thrombosis (XALIA): An international, prospective,
non-interventional study. Lancet Haematol. 3:e12–e21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
95
|
van Bellen B, Bamber L, Correa de Carvalho
F, Prins M, Wang M and Lensing AW: Reduction in the length of stay
with rivaroxaban as a single-drug regimen for the treatment of deep
vein thrombosis and pulmonary embolism. Curr Med Res Opin.
30:829–837. 2014.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Lefebvre P, Coleman CI, Bookhart BK, Wang
ST, Mody SH, Tran KN, Zhuo DY, Huynh L and Nutescu EA:
Cost-Effectiveness of rivaroxaban compared with enoxaparin plus a
vitamin K antagonist for the treatment of venous thromboembolism. J
Med Econ. 17:52–64. 2014.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Merli GJ, Hollander JE, Lefebvre P,
Laliberté F, Raut MK, Germain G, Bookhart B and Pollack CV: Costs
of hospital visits among patients with deep vein thrombosis treated
with rivaroxaban and LMWH/warfarin. J Med Econ. 19:84–90.
2016.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Kline JA, Nordenholz KE, Courtney DM,
Kabrhel C, Jones AE, Rondina MT, Diercks DB, Klinger JR and
Hernandez J: Treatment of submassive pulmonary embolism with
tenecteplase or placebo: Cardiopulmonary outcomes at 3 months:
Multicenter double-blind, placebo-controlled randomized trial. J
Thromb Haemost. 12:459–468. 2014.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Meyer G, Vicaut E, Danays T, Agnelli G,
Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B,
Couturaud F, et al: Fibrinolysis for patients with
intermediate-risk pulmonary embolism. N Engl J Med. 370:1402–1411.
2014.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Bajaj NS, Kalra R, Arora P, Ather S,
Guichard JL, Lancaster WJ, Patel N, Raman F, Arora G, Al Solaiman
F, et al: Catheter-directed treatment for acute pulmonary embolism:
Systematic review and single-arm meta-analyses. Int J Cardiol.
225:128–139. 2016.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Mismetti P, Laporte S, Pellerin O, Ennezat
PV, Couturaud F, Elias A, Falvo N, Meneveau N, Quere I, Roy PM, et
al: Effect of a retrievable inferior vena cava filter plus
anticoagulation vs. anticoagulation alone on risk of recurrent
pulmonary embolism: A randomized clinical trial. JAMA.
313:1627–1635. 2015.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Gnoth MJ, Buetehorn U, Muenster U, Schwarz
T and Sandmann S: In vitro and in vivo P-glycoprotein transport
characteristics of rivaroxaban. J Pharmacol Exp Ther. 338:372–380.
2011.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Song ZK, Cao H, Wei H, Wei Q, Tang M, Yang
S, Liu Y and Qin L: Current status of rivaroxaban in elderly
patients with pulmonary embolism (Review). Exp Ther Med.
19:2817–2825. 2020.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Li SJ, Tu YM, Zhou CS, Zhang LH and Liu
ZH: Risk factors of venous thromboembolism in focal segmental
glomerulosclerosis with nephrotic syndrome. Clin Exp Nephrol.
20:212–217. 2016.PubMed/NCBI View Article : Google Scholar
|