Introduction
Intraoperative anaphylactic reactions may range from
mild, erythema-like to anaphylactic shock, with tension crash and
bronchospasm (1). Usually, severe
allergic reactions which results in vertigo or loss of
consciousness is preceded by intense itching, redness and swelling
over some body areas, new skin eruption or hives, runny nose, itchy
eyes and later on, a feeling of light-headedness, as if the world
is turning, then followed by vertigo. Anaphylaxis during anesthesia
is an unpredictable, severe, and rare reaction. The substances
considered to be most responsible for the occurrence of
intraoperative allergic reactions are neuromuscular blocking
agents, antibiotics and latex (2).
Allergic reactions to latex occur intraoperatively as time is
needed to absorb the allergen through the mucosa or peritoneum.
Patients and methods
A retrospective study was conducted in two hospitals
on a total of 905 patients divided into two groups, depending on
the muscle relaxant used in induction: Succinylcholine or
atracurium.
Because the purpose of the study was to follow the
allergic reactions of succinylcholine versus atracurium, all other
medications used in induction were identical, both as an order of
administration, and as a dose in relation to the patient's
weight.
In order to maintain anesthesia, atracurium was
always used as a muscle relaxant.
Immediate allergic reaction consisting of erythema
on the face, neck and/or upper back, post-induced anesthesia with
succinylcholine or atracurium, within the first 10 min after
administration of myorelaxant, was observed, noted and
described.
The doses administered were 1 mg/kg succinylcholine
(Lysthenon = Suxamethonium chloride, 0.1 g/5 ml; Takeda) and
0.4-0.5 mg/kg over 60 sec, atracurium (Tracrium = atracurium
besylate, 50 mg/5 ml; Aspen) according to ‘Morgan and Mikhail's
Clinical Anesthesiology’ (3).
Inclusion criteria: Patients undergoing surgery
under general anesthesia, scheduled surgical operations, signed
informed consent from the patients involved in the study, according
to ethical requirements (4).
Exclusion criteria: patients in whom surgery was
performed in an emergency, patients whose surgery started with
locoregional anesthesia then converted into general anesthesia,
patients whose anesthesia was performed or continued by other
anesthesiologists, patients with a history of allergic reactions to
drugs or food, patients with asthma.
The present study is a retrospective study based on
the side effects to drugs that were already included in the
treatment protocol, according to ‘Morgan and Mikhail’s Clinical
Anesthesiology’, and are commonly used for anesthesia in the
clinic. Signed informed consent was obtained from each patient.
Statistical analysis
The analyzed database stores the information in the
form of nominal/categorical variables (sex, place of origin, if the
patient had erythema, type of anesthetics, age groups or BMI
groups). The relationships between variables were determined by
calculating the values of the correlation coefficients at nominal
level Φ, C and V, as well as the probabilities associated with
them. If the associated probability, P <α=0.05 (the significance
threshold), it results in variables that are correlated (there is a
dependency relationship between them) (5,6). The
values of the coefficients show how strong the correlation is. The
choice of correlation tests depends on the type of data and the
number of possible variants for each of the variables analyzed.
Another method for determining the degree of
association between two categorical variables is the Pearson
Chi-square test.
The software package used for statistical analysis
was IBM SPSS Statistics version 23.
Results
The 905 patients under study were divided into two
groups. In the first group, consisting of 455 subjects, anesthesia
was induced by succinylcholine, and the second group, consisting of
450 subjects, by atracurium (Table
I).
 | Table IDescriptive statistics of the two
groups: Succinylcholine and atracurium. |
Table I
Descriptive statistics of the two
groups: Succinylcholine and atracurium.
| | Succinylcholine | Atracurium |
|---|
| | Frequency | Percent | Frequency | Percent |
|---|
| Variables | (n) | (%) | (n) | (%) |
|---|
| Sex |
|
Female | 376 | 82.6 | 136 | 30.2 |
|
Male | 79 | 17.4 | 314 | 69.8 |
| BMI,
kg/m2 |
|
<18.5 | 71 | 15.6 | 25 | 5.6 |
|
18.5-24.9 | 172 | 37.8 | 215 | 47.8 |
|
25-29.9 | 127 | 27.9 | 139 | 30.9 |
|
30-34.9 | 65 | 14.3 | 46 | 10.2 |
|
35-39.9 | 20 | 4.4 | 25 | 5.6 |
| Age, years |
|
<40 | 65 | 14.3 | 35 | 7.8 |
|
40-60 | 259 | 56.9 | 190 | 42.2 |
|
>60 | 131 | 28.8 | 225 | 50.0 |
| Provenance |
|
Urban | 172 | 37.8 | 225 | 50 |
|
Rural | 283 | 62.2 | 225 | 50 |
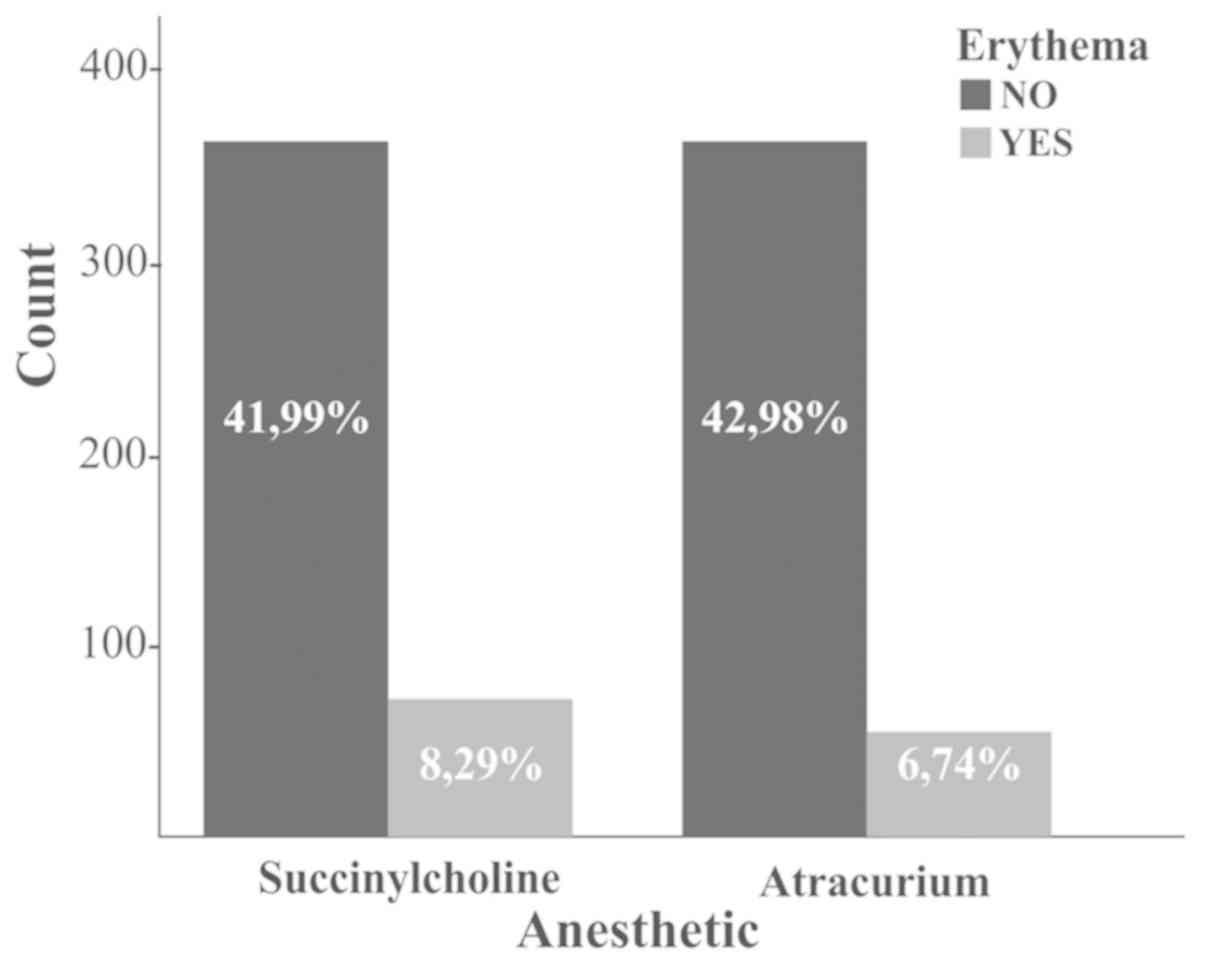
Looking at the entire study group, analyzing the
occurrence of erythema within the first 10 min after injection of
myorelaxant in induction phase of anesthesia, a higher frequency
was found in patients who received succinylcholine (Fig. 1).
The statistical analysis revealed a poor correlation
between the type of subjects and the occurrence of erythema after
the muscle relaxant in the case of succinylcholine (P<0.001
<α=0.05; Φ=-0.204) and in the case of atracurium (P=0.023
<α=0.05; Φ=-0.107), women being the most affected.
Post-induction allergic reaction was found in 19.94% of patients
who received Lysthenon and none was male in this group, and in the
second group of atracurium, in 19.11% of the women and 11.11% of
the men.
Regarding the home environment, a weak correlation
(P<0.001 <α=0.05, Φ=0.224) was observed in the
succinylcholine group, most of the patients experiencing allergic
reaction being urban (22.96% of urban area vs. 5.81% in rural
areas). In the second lot, between these two nominal parameters
there was no statistical correlation (P=0.890 >α=0.05).
In the age groups, each of the two groups was
divided into 3 subgroups: Patients up to 40 years of age, patients
aged 40 to 60, and patients over 60 years of age. Finding an
average correlation between the age and the occurrence of erythema
in the first group (P<0.001 <α=0.05, V=0.414; C=0.382), the
most affected being young patients under the age of 40, 7% of those
who experienced post-injection allergy). No relationship between
these two parameters was observed when using atracurium (P=0.310
>α=0.05.
In both groups there was a weak correlation between
the body mass index and the allergic reactions occurring after
induction, posterior injection of the Lysthenon (P<0.001
<α=0.05, V=0.218, C=0.213) and Tracrium (P=0.002 <0.05,
V=0.197 and C=0.193), with the highest percentage of erythema being
present in overweight patients with BMI between 25-29.9
kg/m2 (40% in the first group and 50.8%, in the
second).
Allergic reactions, in the studied group, were only
of erythematous type, which spontaneously resolved within 15 min of
the occurrence.
Discussion
Lysthenon is a commonly used depolarizing agent. It
is a drug that acts as an agonist at neuromuscular junction
acetylcholine receptors which results in nerve cell depolarization
that leads to sustained cell excitation. Succinylcholine results in
nerve endplate resistance to further activation by acetylcholine,
which paralyses the muscle. Tracrium is a highly selective,
competitive or non-depolarising neuromuscular blocking agent. A
neuromuscular non-depolarizing agent is a form of neuromuscular
blocker that does not depolarize the motor end plate.
Isolated cutaneous reactions appear to be primarily
due to non-IgE mediated anaphylaxis, and severe side effects such
as arterial hypotension, bronchospasm, are due to type IgE mediated
allergy mechanisms (1,7,8). Other
side effects may be induced by first cellular mechanisms
interleukin-modulated because of topical medication or may occur on
common medication and may be locally complicated (9-13).
As an intraanesthetic sensitivity response, the
incidence was reported to be between 1/1,250 and 1/13,000
anesthetic interventions (8-10).
Other studies have reported the incidence of anaphylactic reactions
as being between 1/4,000 to 1/25,000(4) and 1/5,000 of these are caused by
neuro-muscular blockers (14).
The 6th National Audit Project (NAP6) that looked at
perioperative anaphylactic reactions based on 266 cases reported in
the United Kingdom with 3-5 grade anaphylactic reactions over a 1
year period found that 24.4% of these were due to neuromuscular
blocking. Succinylcholine (depolarizing myorelaxant) is the cause
of twice as frequent occurrence of anaphylactic reactions. There
was no difference between non-depolarizing muscle relaxants
(15).
The mechanism by which muscle relaxants cause
anaphylactic reactions is either IgE-dependent, such as
succinylcholine, or by direct activation of mast cells or
basophils, a mechanism encountered in the case of atracurium
(16).
IgE-recognized immunodominant determinant is
represented by the ammonium group at the level of muscle relaxants,
and cross-reactions between muscle relaxants and other
perioperative substances such as neostigmine or morphine may occur
(17,18). IgG-mediated perioperative
anaphylactic reactions are those that most commonly occur after
muscle relaxants, followed by latex and antibiotics (2,19).
Atracurium, being a benzyl-isoquinolinium-type
muscle relaxant, produces a non-IgE-mediated anaphylactoid reaction
(20-29),
releases more histamine than the aminosteroid neuro-muscular
blockers. This could be prevented by slow injection, possibly using
antihistamines before induction (30).
From a clinical point of view, allergic immune and
non-immune recurrences cannot be differentiated.
As a result of clinical phenomena, immediate
reactions of hyper reactivity are classified into several degrees
(31): Grade I: Cutaneous signs,
generalized erythema, angioedema, urticaria; grade II: Not
life-threatening, cutaneous signs, tachycardia, hypotension,
respiratory dysfunction (cough, difficulty in breathing); grade
III: Life threatening, arrhythmias, tachycardia or bradycardia,
bronchospasm, collapse; grade IV: Cardiac/respiratory arrest
(32-34).
In this study, all patients were ranked grade I in severity.
Referring to patient-responsiveness, in a study
published in 2018 that followed the ‘skin test’ response to muscle
relaxants, it was found that 87% of the subjects who tested
positive for this test were female, the most allergenic one being
succinylcholine (35,36). This study is in agreement with the
results of our research, according to which a greater
predisposition of the female to develop allergic reactions
post-administration of muscle relaxants (37-39).
A nationwide analysis in France over the period
2000-2012 and published in 2018 that followed the occurrence of
post-administration anaphylactic reactions of suxamethonium and
rocuronium versus atracurium or cisatracurium found the involvement
of suxamethonium in 64% of the adverse reactions (40).
Finally, we wondered if it would justify skin
testing of all patients preoperatively. The Petipain study
considers that skin tests should be performed more frequently in
order to prevent allergic post-muscle relaxant reactions (40). Sánchez Palacios considered that
testing for all patients was not justified due to the small number
of patients who had allergic reactions (41).
An article published in 2019 draws attention to the
risk of mortality secondary to the administration of atracurium,
independent of previous anesthesia with this neuromuscular drug.
The mechanism can be by producing IgE upon previous contact with
the drug or with quaternary ammonium moiety that can be found in
some foods, as well as secondary to direct activation of cellular
mast cells (42).
In patients whose risk of anaphylactic reactions is
increased and the skin test is inconclusive, complementary tests
such as basophil activation test (BAT) may be used (43).
In conclusion, succinylcholine is more allergenic
than atracurium, the most affected being female patients. The
subjects in the urban area and those under the age of 40 were
statistically more affected only in the succinylcholine group.
Overweight patients were the most susceptible to adverse reactions
in both groups. Regarding the use of pre-operative skin test in all
patients, we leave this topic open considering further studies are
required in order to determine their efficacy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ORC and MNL contributed to the study design,
participated in the entire review process and prepared the
manuscript. DCV, MNM, RGC and NM contributed to the collection of
the relevant literature, as well as the analysis and critical
interpretation of the data. GS, OCC and AG conceived the study and
drafted the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This is a retrospective study based on the side
effects to drugs that were already included in the treatment
protocol, according to ‘Morgan and Mikhail's Clinical
Anesthesiology’, and are commonly used for anesthesia in the
clinic. Signed informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Gupta A, Srivastava U, Saxena A, Mittal A
and Dwivedi Y: Severe anaphylactic reaction to atracurium. Indian J
Pharmacol. 44:144–145. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mertes PM and Laxenaire MC: Allergy and
anaphylaxis in anaesthesia. Minerva Anestesiol. 70:285–291.
2004.PubMed/NCBI
|
|
3
|
Butterworth JF, Mackey DC and Wasnick JD:
Morgan and Mikhail's Clinical Anesthesiology. McGraw Hill, New
York, NY. 2013.
|
|
4
|
Valcea L, Bulgaru-Iliescu D, Burlea SL and
Ciubara A: Patient's rights and communication in the hospital
accreditation process. Rev Cercet Interv Soc. 55:260–270. 2016.
|
|
5
|
Bumbacea RS, Popa LG, Orzan OA, Voiculescu
VM and Giurcaneanu C: Clinical and therapeutic implications of the
association between chronic urticaria and autoimmune thyroiditis.
Acta Endocrinol (Buchar). 10:595–604. 2014. View Article : Google Scholar
|
|
6
|
Mertes PM, Laxenaire MC and Alla F: Groupe
d'Etudes des Réactions Anaphylactoïdes Peranesthésiques.
Anaphylactic and anaphylactoid reactions occurring during
anesthesia in France in 1999-2000. Anesthesiology. 99:536–545.
2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Claudius C, Garvey LH and Viby-Mogensen J:
The undesirable effects of neuromuscular blocking drugs.
Anaesthesia. 64 (Suppl 1):10–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tatu AL and Nwabudike LC: Bullous
reactions associated with COX-2 inhibitors. Am J Ther.
24(e477-e480)2017. View Article : Google Scholar
|
|
9
|
Tatu AL, Elisei AM, Chioncel V, Miulescu M
and Nwabudike LC: Immunologic adverse reactions of β-blockers and
the skin. Exp Ther Med. 18:955–959. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ciobotaru OR, Voinescu DC, Ciobotaru OC,
Voicu D and Arbune M: Expression of p53 and Ki-67 in distal
oesophageal and gastric cardia adenocarcinomas. Rom Biotechnol
Lett. 20:10800–10808. 2015.
|
|
11
|
Spoerl D, Nigolian H, Czarnetzki C and
Harr T: Reclassifying anaphylaxis to neuromuscular blocking agents
based on the presumed patho-mechanism: IgE-mediated,
pharmacological adverse reaction or ‘innate hypersensitivity’? Int
J Mol Sci. 18(E1223)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghiţă MA, Căruntu C, Rosca AE, Căruntu A,
Moraru L, Constantin C, Neagu M and Boda D: Real-time investigation
of skin blood flow changes induced by topical capsaicin. Acta
Dermatovenerol Croat. 25:223–227. 2017.PubMed/NCBI
|
|
13
|
Branisteanu D, Caruntu C, Negrei C, Ghita
MA, Caruntu A, Badarau AI, Buraga I, Boda D and Albu A: Capsaicin,
a hot topic in skin pharmacology and physiology. Farmacia.
63:487–491. 2015.
|
|
14
|
De Pater GH, Florvaag E, Johansson SG,
Irgens Å, Petersen MN and Guttormsen AB: Six years without
pholcodine; Norwegians are significantly less IgE-sensitized and
clinically more tolerant to neuromuscular blocking agents. Allergy.
72:813–819. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Harper NJ, Cook TM, Garcez T, Farmer L,
Floss K, Marinho S, Torevell H, Warner A, Ferguson K, Hitchman J,
et al: Anaesthesia, surgery, and life-threatening allergic
reactions: Epidemiology and clinical features of perioperative
anaphylaxis in the 6th National Audit Project (NAP6). Br J Anaesth.
121:159–171. 2018. View Article : Google Scholar
|
|
16
|
Galvão VR, Giavina-Bianchi P and Castells
M: Perioperative anaphylaxis. Curr Allergy Asthma Rep.
14(452)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garvey LH: Old, new and hidden causes of
perioperative hypersensitivity. Curr Pharm Des. 22:6814–6824.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ciobotaru OR, Voinescu DC, Barna O, Barna
I and Ciobotaru OC: Influence of the type of anaesthesia used, the
diet and the consumption of sugar and alcohol on the intradermal
skin test to morphine. Biotechnol Biotechnol Equip. 29:935–941.
2015. View Article : Google Scholar
|
|
19
|
Di Leo E, Donne PD, Calogiuri GF, Macchia
L and Nettis E: Focus on the agents most frequently responsible for
perioperative anaphylaxis. Clin Mol Allergy. 16(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Navinés-Ferrer A, Serrano-Candelas E,
Lafuente A, Muñoz-Cano R, Martín M and Gastaminza G:
MRGPRX2-mediated mast cell response to drugs used in perioperative
procedures and anaesthesia. Sci Rep. 8(11628)2018. View Article : Google Scholar
|
|
21
|
Subramanian H, Gupta K and Ali H: Roles of
Mas-related G protein-coupled receptor X2 on mast cell-mediated
host defense, pseudoallergic drug reactions, and chronic
inflammatory diseases. J Allergy Clin Immunol. 138:700–710.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grigore O, Mihailescu AI, Solomon I, Boda
D and Caruntu C: Role of stress in modulation of skin neurogenic
inflammation. Exp Ther Med. 17:997–1003. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014(105950)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ilie MA, Caruntu C, Lixandru D, Tampa M,
Georgescu SR, Constantin MM, Constantin C, Neagu M, Zurac SA and
Boda D: In vivo confocal laser scanning microscopy imaging of skin
inflammation: Clinical applications and research directions. Exp
Ther Med. 17:1004–1011. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ilie MA, Caruntu C, Tampa M, Georgescu SR,
Matei C, Negrei C, Ion RM, Constantin C, Neagu M and Boda D:
Capsaicin: Physicochemical properties, cutaneous reactions and
potential applications in painful and inflammatory conditions. Exp
Ther Med. 18:916–925. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Filip-Ciubotaru F, Manciuc C, Stoleriu G
and Foia L: NADPH Oxidase: Structure and activation mecanisms
(Review). Note I. Rev Med Chir Soc Med Nat Iasi. 120:29–33.
2016.PubMed/NCBI
|
|
27
|
Boda D, Negrei C, Nicolescu F and Badalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
28
|
McNeil BD, Pundir P, Meeker S, Han L,
Undem BJ, Kulka M and Dong X: Identification of a
mast-cell-specific receptor crucial for pseudo-allergic drug
reactions. Nature. 519:237–241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Koppert W, Blunk JA, Petersen LJ, Skov P,
Rentsch K and Schmelz M: Different patterns of mast cell activation
by muscle relaxants in human skin. Anesthesiology. 95:659–667.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hariharan U, Shah SB and Bhargava AK:
Local allergic reaction to atracurium besylate: An uncommon and
unique reason. J Anesthesiol Clin Sci. 4:4–6. 2015. View Article : Google Scholar
|
|
31
|
Mertes PM, Lambert M, Guéant-Rodriguez RM,
Aimone-Gastin I, Mouton-Faivre C, Moneret-Vautrin DA, Guéant JL,
Malinovsky JM and Demoly P: Perioperative anaphylaxis. Immunol
Allergy Clin North Am. 29:429–451. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Maranduca MA, Serban IL, Dima N, Badescu
C, Ganceanu-Rusu R, Hurjui LL, Paduraru R, Tanase O, Parlapan A and
Rezus C: Cardiovascular and metabolic comorbidities - therapeutic
difficulties. Case report. Rev Med Chir Soc Med Nat Iasi.
122:304–308. 2018.PubMed/NCBI
|
|
33
|
Grecu C, Grecu A, Serban IL, Hurjui I,
Delianu C, Maranduca MA, Popovici D, Gradinaru I, Mitrea M and
Hurjui LL: Prevalence of nasal carriage of Staphylococcus
aureus with special reference to number of methicillin
resistance and antimicrobial evaluation among apparently people
with good health status. Rev Med Chir Soc Med Nat Iasi.
122:819–825. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maranduca MA, Branisteanu D, Serban DN,
Branisteanu DC, Stoleriu G, Manolache N and Serban IL: Synthesis
and physiological implications of melanic pigments. Oncol Lett.
17:4183–4187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dewachter P, Chollet-Martin S,
Mouton-Faivre C, de Chaisemartin L and Nicaise-Roland P: Comparison
of basophil activation test and skin testing performances in NMBA
allergy. J Allergy Clin Immunol Pract. 6:1681–1689. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ilie MA, Caruntu C, Lupu M, Lixandru D,
Tampa M, Georgescu SR, Bastian A, Constantin C, Neagu M, Zurac SA,
et al: Current and future applications of confocal laser scanning
microscopy imaging in skin oncology. Oncol Lett. 17:4102–4111.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Brănişteanu DE, Pintilie A, Andreş LE,
Dimitriu A, Oanţă A, Stoleriu G and Brănişteanu DC: Ethiopatogenic
hypotheses in lichen planus. Rev Med Chir Soc Med Nat Iasi.
120:760–767. 2016.PubMed/NCBI
|
|
38
|
Schaas BA, Ivan S, Titianu M, Condratovici
CP, Maier A and Schaas CM: Biochemical markers predicting the risk
of gestational diabetes mellitus. Mater Plast. 54:133–136.
2017.
|
|
39
|
Ratiu MP, Purcarea I, Popa F, Purcarea VL,
Purcarea TV, Lupuleasa D and Boda D: Escaping the economic turn
down through performing employees, creative leaders and growth
driver capabilities in the Romanian Pharmaceutical Industry.
Farmacia. 59:119–129. 2011.
|
|
40
|
Petitpain N, Argoullon L, Masmoudi K,
Fedrizzi S, Cottin J, Latarche C, Mertes PM and Gillet P: French
Network of Regional Pharmacovigilance Centres. Neuromuscular
blocking agents induced anaphylaxis: Results and trends of a French
pharmacovigilance survey from 2000 to 2012. Allergy. 73:2224–2233.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sánchez Palacios A, Ortiz Ponce M,
Rodríguez Pérez A, Schamann Medina F and García-Marrero JA:
Modification of mediators of immune reaction after general
anaesthesia. Allergol Immunopathol (Madr). 32:352–360.
2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Schumacher J: Fatal anaphylaxis to
atracurium: A Case Report. A A Pract. 12:145–146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Eberlein B, Wigand S, Lewald H, Kochs E,
Ring J, Biedermann T and Darsow U: Utility of basophil activation
testing to assess perioperative anaphylactic reactions in
real-world practice. Immun Inflamm Dis. 5:416–420. 2017.PubMed/NCBI View
Article : Google Scholar
|