Introduction
The first-trimester visualization and analysis of
the fetal brain has become a reality in recent years, thanks to
technological advancements that allow superimposing theoretical
knowledge of early fetal development with sonographic images of
different fetal stages. The road was opened in 2009 by Chaoui et
al (1) in search of early signs
of open spina bifida, and for several years, this was the only
direction for clinical research (2-4).
The detailed description of the posterior fossa allowed the
identification and pursuing the development of different anatomical
structures, from 11 weeks to later in the second trimester. The
initial description of ‘intracranial translucency’ was later
abandoned as it became clear that the structure was the V4.
Standard measurements were performed using the already standard
sagittal section of the fetal head, with the described landmarks
for the nuchal translucency assessment. However, using just the
fetal head mid-sagittal plane, specific intracranial structures,
especially supratentorial, cannot be seen, and their development
cannot be assessed. We need axial, as well as oblique head
sections, for a thorough examination of the fetal brain. Therefore,
the standardization of these sections and measurements is
mandatory.
Patients and methods
From January 2017 to March 2018, all women with a
viable pregnancy presenting for first-trimester screening were
included in the study. Both singleton and multiple pregnancies were
included, provided that a proper examination of all the fetuses was
possible. The main inclusion criteria were fetal crown-rump length
(CRL) between 45-84 mm and patient consent for additional
measurements during the standard fetal examination. Cases were
excluded when fetal anatomical assessment was suboptimal. The
analysis included fetuses with structural defects which were
examined separately. The study was conducted in accordance with the
World Medical Association’s Declaration of Helsinki. The pregnant
women included in the study provided written informed consent and
the study protocol was approved by the Ethics Committee of the
Elias University Hospital (Bucharest, Romania).
After nuchal translucency (NT) measurement, the
probe was rotated by 90˚, and a transverse section of the fetal
head was obtained. The probe needs to be slightly tilted to the
point where the posterior fossa becomes visible. At this level,
three hypoechoic spaces are visible, separated by echogenic lines.
From anterior to posterior, these spaces are the thalamic nuclei
and the cerebral peduncles, fourth ventricle (V4) and cisterna
magna. These structures find their correspondent on the
mid-sagittal section of the fetal head.
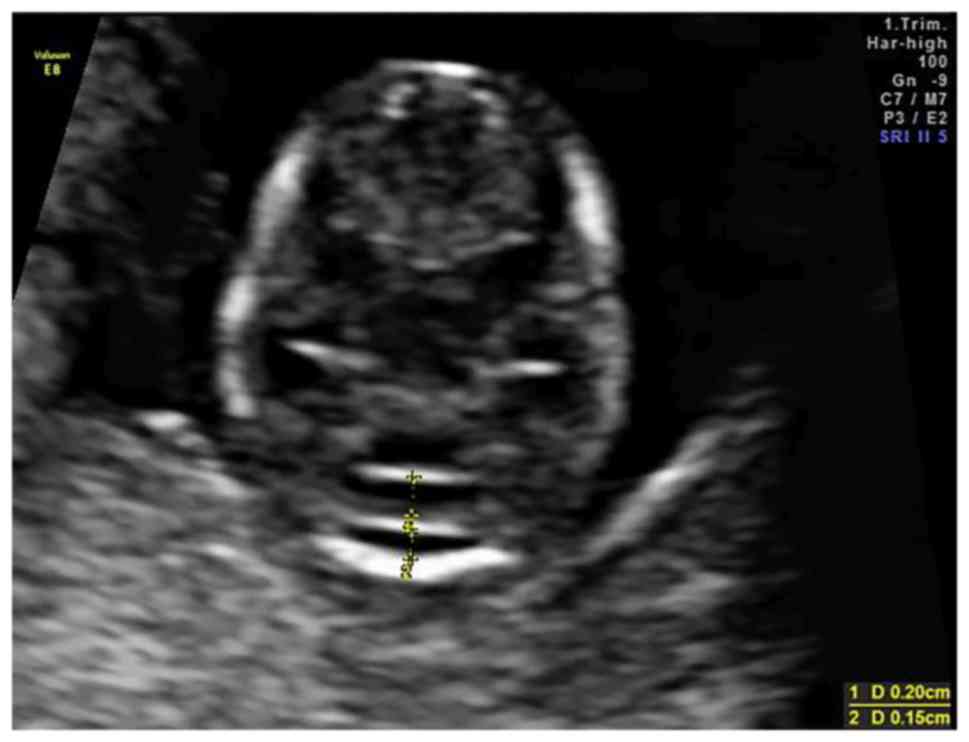
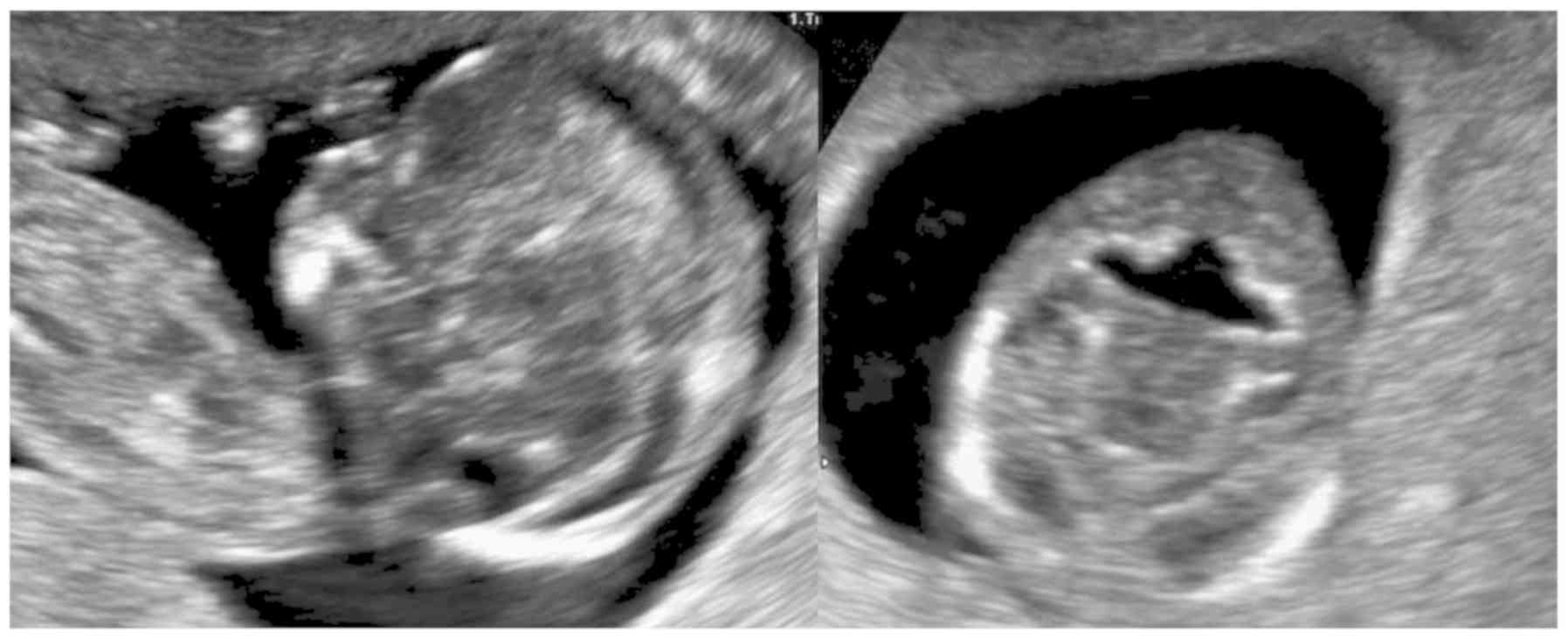
The anteroposterior diameter of the V4 and the
cisterna magna (CM) were measured on an axial view of the posterior
fossa (Fig. 1). All measurements
were performed during the fetal examination, and no post-processing
was required.
A second fetal assessment, was performed routinely
between 19-24 weeks of gestation, measuring the CM width and
transverse cerebellar diameter (TCD) as previously described
(5-7).
All ultrasound examinations were performed on
Voluson E8 Expert or V730 ultrasound machines (GE Healthcare), by a
single operator (MZ). A transabdominal or transvaginal approach was
used, as required for a better evaluation of the fetal anatomy.
Statistical software package SPSS 23.0 (SPSS Inc.)
was used for data analyses. The normality of distribution was
assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests.
Continuous variables were compared using the t-test for independent
means. Correlation between study variables was verified using
Pearson's correlation coefficient. P-value <0.05 was considered
statistically significant.
Results
At the time of the first-trimester scan 132 fetuses
were evaluated. The primary maternal characteristics, ultrasound
measurements, biochemistry results, and the estimated risks of
aneuploidies are detailed in Table
I. Both maternal and fetal population were normally distributed
according to the CRL measurements and maternal age.
 | Table ISummary of the main maternal
characteristics, ultrasound measurements, biochemistry results and
estimated risks for the main aneuploidies. |
Table I
Summary of the main maternal
characteristics, ultrasound measurements, biochemistry results and
estimated risks for the main aneuploidies.
| Variables | Minimum | Mean | Maximum |
|---|
| Maternal age
(years) | 20 | 30.66 | 45 |
| Maternal weight
(kg) | 41.5 | 59.65 | 84.0 |
| CRL (mm) | 47.6 | 63.65 | 81.3 |
| NT (mm) | 1.1 | 1.89 | 5.9 |
| DV PI | 0.8 | 1.1 | 2.1 |
| β-hCG MoM | 0.236 | 1.236 | 5.220 |
| PAPP-A MoM | 0.202 | 1.025 | 2.513 |
| Risk for T21 | 1/30,664 | 1/15-966 | 1/2 |
| Risk for T18 | 1/361,907 | 1/191-525 | 1/136 |
| Risk for T13 | 1/851,951 | 1/426-932 | 1/10 |
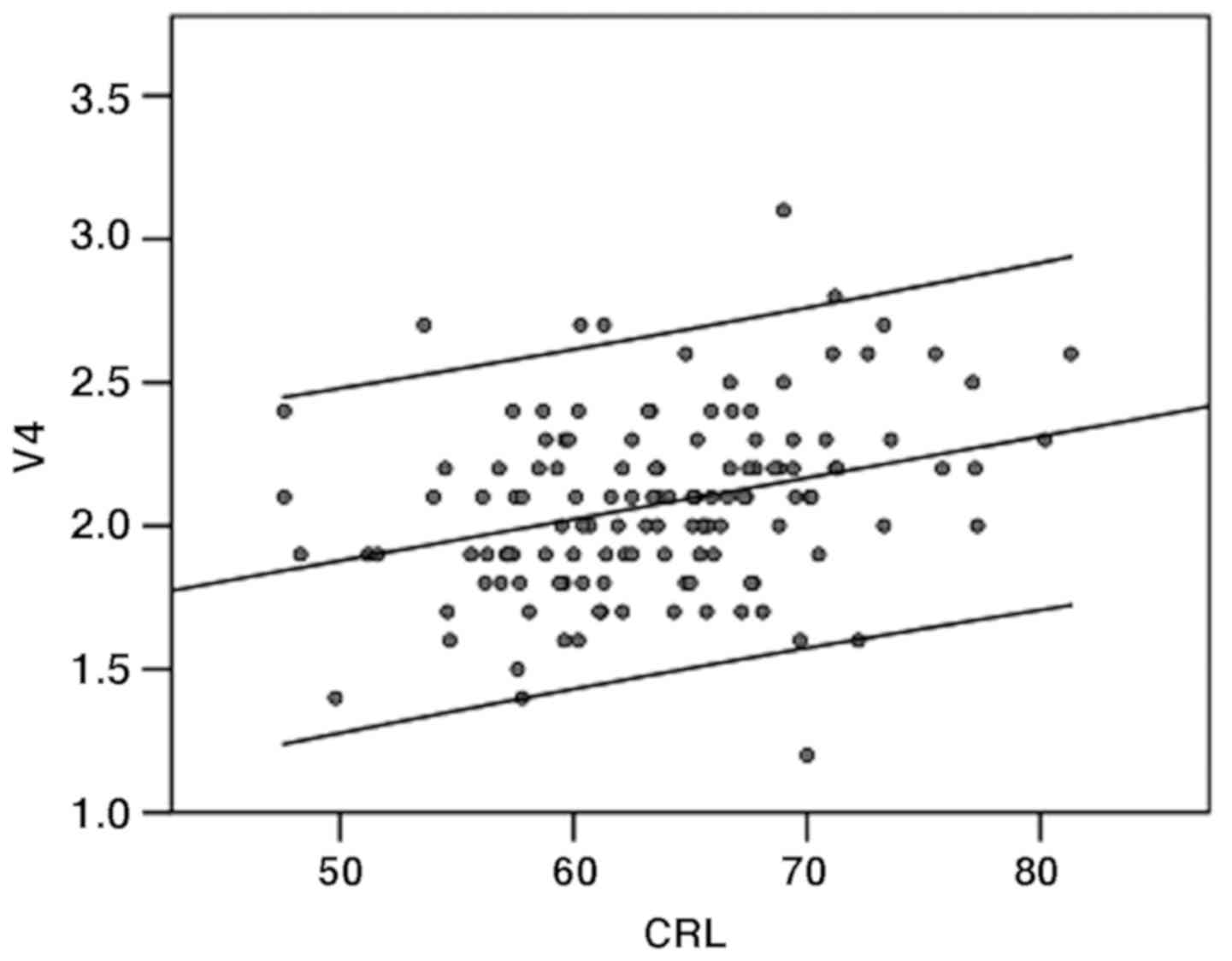
The size of the V4 had a mean of 2.076 mm (range
1.2-3.1 mm) and was significantly correlated with CRL (P<0.01),
according to Pearson r assumption for a normally distributed
population. Its 50th centile varies from 1.8 mm at a CRL of 45-2.4
mm at a CRL of 84 mm (Fig. 2).
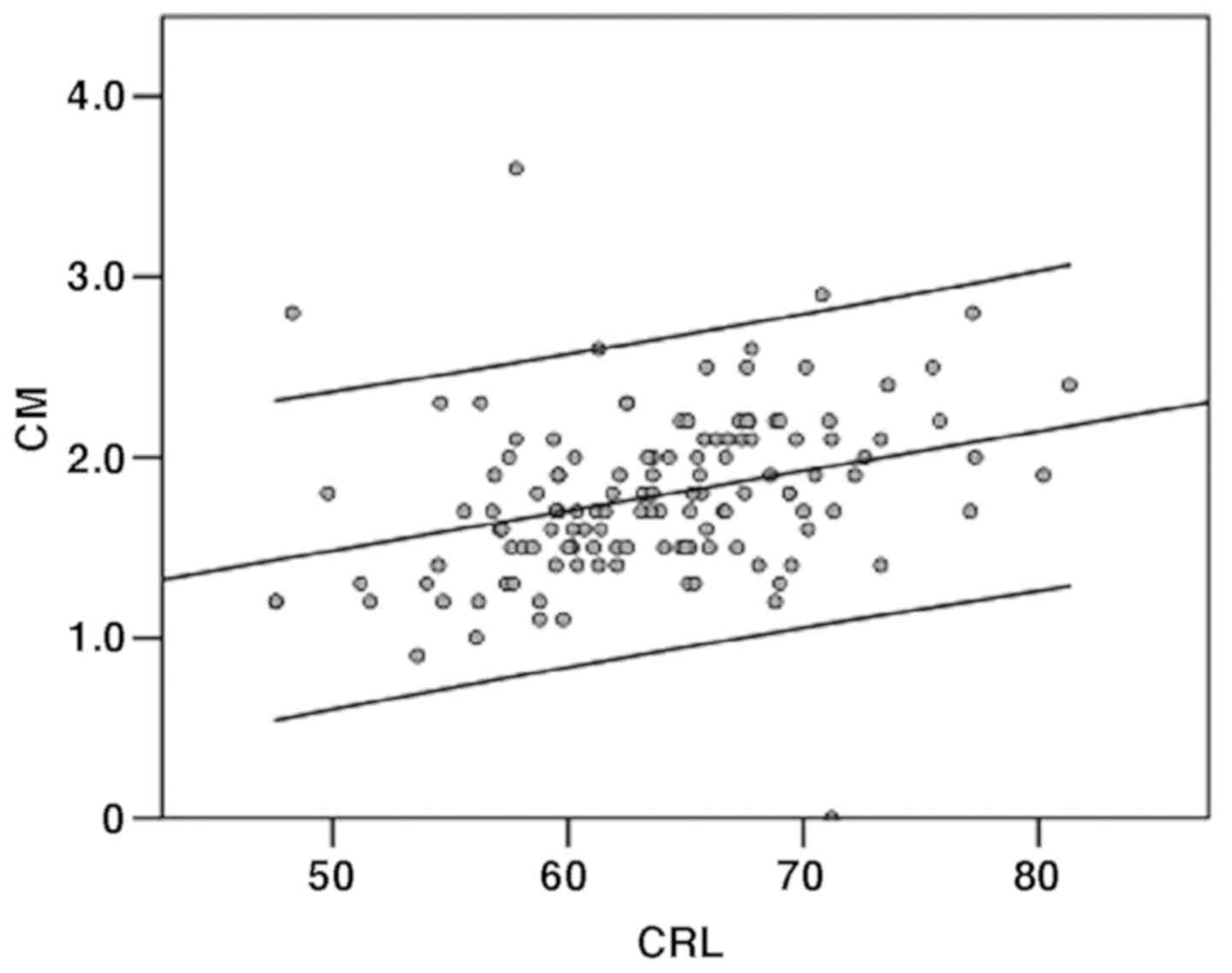
The CM measurements had a mean of 1.784 (range 0-3.6
mm) and CM was significantly correlated with CRL (P<0.01). The
50th centile for CM varies from 1.2 mm at a CRL of 45-2.3 mm and at
a CRL of 84 mm (Fig. 3).
For 81 fetuses, the follow up at 19-24 gestational
weeks was possible. In this group, the Pearson r coefficient
indicated a positive correlation between the first-trimester and
second-trimester measurement of CM, which is not statistically
significant (P=0.465).
Comparing the development of the CM and the V4
between 11 to 14 gestational weeks, it was noted that the size of
the CM is inferior to V4, at the same gestational age. However, its
growth is faster, and it will intersect and surpass the development
of the V4 after the first trimester. There was no correlation
between the size of V4 or CM and maternal characteristics.
It was found that there is a low positive
correlation between the first-trimester V4 measurements and the
second-trimester TCD, and an inverse correlation between the
first-trimester CM and the second-trimester TCD, the results are
not statistically significant.
In the study group, there were a series of cases
with genetic and structural anomalies, in which possible early
deviations in the morphology of the posterior fetal fossa were
analyzed.
There were two cases with confirmed trisomy 21. In
both, the anteroposterior diameter of the V4 was equal to the CM
diameter (2.2 mm and 2.3 mm, respectively). All measurements were
within the normal range for CRL.
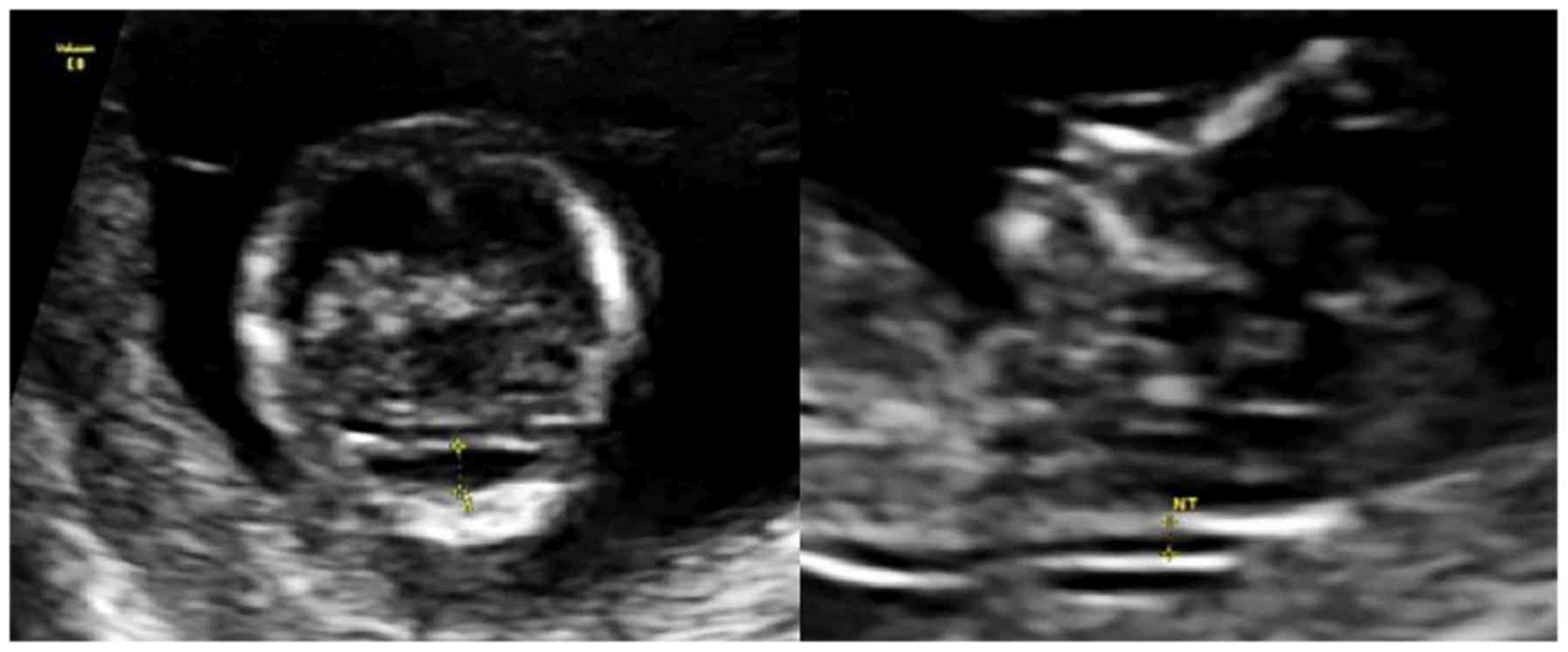
In one case of trisomy 13, the measurement of V4 was
2.8 mm, and the CM was unmeasurable (0 mm). There was no spinal
defect in this case. At a CRL of 70 mm, the size of the V4 is above
the 95th centile. When comparing the images of the posterior fossa
in the mid-sagittal and axial views, the measurements of the two
components are more comfortable to perform, and more evident in the
transversal plane (Fig. 4).
In a fetus with trisomy 18, with a CRL of 50.5 mm,
the measurement of the V4 was 2.5 mm, which is the 95th centile.
The CM size was 1 mm, which is just above the 5th centile for
gestational age. The measurements were performed by the
transabdominal approach, but the discrepancy in the size of the two
structures is better seen using the transvaginal probe.
In one case of diandric triploidy, the distortion of
the posterior fossa was so marked that no reliable measurement was
possible. The separation between the V4 and CM was not
identifiable, probably due to a significant delay in vermian
development (Fig. 5). No case of
open spina bifida was found in our series.
Discussion
In literature only one large prospective study was
found, including 692 first-trimester fetuses in which the posterior
fossa was assessed in the axial view of the head (8). The operators measured the
anteroposterior diameter of the V4, CM and TCD on acquired
ultrasound volumes of the fetal head. In their study, the 50th
centile for CM ranges from 1 mm at a CRL of 45-3 mm and at a CRL of
85 mm. The 50th centile of the V4 ranges from 1.7 to 2.4 mm. Even
though the variation range in the study of Egle et al
(8) is narrower than in our study,
we have seen a similar trend in the development of the two
structures, with V4 larger at lower CRL and a steeper increment in
the diameter of cisterna magna as the first trimester comes to an
end. The differences between the present results and these
measurements may be explained by a different technique, as well as
by a larger number of cases.
Papastefanou et al (9) established references for CM and V4
measurements in the mid-sagittal view of the fetal head, at the end
of the first trimester, on 465 fetuses with known outcome.
If we compare the V4 and CM measurements in the
mid-sagittal and axial views, as plotted in the two previous
studies, they are superimposable. The 50th centile for CM on the
sagittal plane ranges from 0.9 to 2.8 mm, and on transverse plane
varies from 1 to 2.8 mm. The 50th centile for the diameter of the
V4 on sagittal view ranges from 1.5 to 2 mm and on the transversal
view ranges from 1.7 to 2.2 mm.
Loureiro et al (10) used volumetric acquisitions in order
to measure the diameter of the V4 on axial views, in a study that
was focused on the assessment of morphologic differences of the
posterior fossa between euploid and aneuploid fetuses. They
compared 62 fetuses with trisomy 21, trisomy 13, trisomy 18, and
triploidy, to 410 euploid fetuses. The fetuses with trisomy 13, 18,
and triploidy, had an increased transverse diameter of the V4,
whereas, in fetuses with trisomy 21, the measurements resembled
euploid fetuses.
A similar conclusion was drawn from other studies
that assessed the posterior fossa at 11-14 gestational weeks, but
the measurements were performed in the mid-sagittal plane. Ferreira
et al (11) measured the
brain stem diameter (BS) and the distance between the brain stem
and occipital bone (BSOB). In trisomy 13, trisomy 18, and
triploidy, the BS diameter and BS to BSOB ratio were significantly
lower than in euploid fetuses. Assessment of the posterior fossa in
a mid-sagittal plane at the time of the nuchal translucency
measurement and on the same section of the fetal head was realized
by Volpe et al (12). They
suggest that the increased amount of fluid, mainly when associated
with loss of the normal anatomy and normal septation in this area,
may be a significant risk factor for future cystic anomalies of the
posterior fossa and should prompt further detailed
investigations.
The increased amount of fluid in the posterior
fossa, starting as early as 11-14 weeks of gestation, is presumed
to be related to altered cerebellar and especially vermian
development, which is more frequently associated with aneuploidies
such as trisomy 18 and 13.
In conclusion, assessment of the anatomical details
and measurements of different structures may be performed with
similar accuracy on both mid-sagittal and axial views of the fetal
head. The results of our study and the anomalies observed and
described in specific clinical cases are in line with the published
data regarding the anatomy of the posterior fetal fossa at 11-14
weeks. From our experience, assessment of the posterior fetal fossa
on the transverse section is feasible even in situations where a
perfect sagittal section cannot be obtained.
Assessment of the fetal brain in the axial plane
represents an additional step in the first-trimester examination,
but it is utterly necessary for a detailed assessment, especially
in cases with suspected structural or genetic anomalies. We
consider that a skilled practitioner should be able to recognize
the details of normal fetal anatomy in both transverse and sagittal
planes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MEZ designed the study protocol and performed all
measurements within the study. AM, MB and AP were involved in the
recruitment of the patients and provided care to all the patients
included in the study. MEZ and DN performed data analysis. MEZ, AM,
MB, AP and DN wrote the manuscript. MEZ and DN participated in the
review process, prepared the manuscript, and made substantial
intellectual contributions. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the World Medical Association’s Declaration of Helsinki. The
pregnant women included in the study provided written informed
consent and the study protocol was approved by the Ethics Committee
of the Elias University Hospital (Bucharest, Romania).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chaoui R, Benoit B, Mitkowska-Wozniak H,
Heling KS and Nicolaides KH: Assessment of intracranial
translucency (IT) in the detection of spina bifida at the
11-13-week scan. Ultrasound Obstet Gynecol. 34:249–252.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Chaoui R and Nicolaides KH: From nuchal
translucency to intracranial translucency: Towards the early
detection of spina bifida. Ultrasound Obstet Gynecol. 35:133–138.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Chaoui R, Benoit B, Heling KS, Kagan KO,
Pietzsch V, Sarut Lopez A, Tekesin I and Karl K: Prospective
detection of open spina bifida at 11-13 weeks by assessing
intracranial translucency and posterior brain. Ultrasound Obstet
Gynecol. 38:722–726. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lachmann R, Chaoui R, Moratalla J,
Picciarelli G and Nicolaides KH: Posterior brain in fetuses with
open spina bifida at 11 to 13 weeks. Prenat Diagn. 31:103–106.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Brown RN: Reassessment of the normal fetal
cisterna magna during gestation and an alternative approach to the
definition of cisterna magna dilatation. Fetal Diagn Ther.
34:44–49. 2013. View Article : Google Scholar
|
|
6
|
Verburg BO, Steegers EA, De Ridder M,
Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW and Witteman JC:
New charts for ultrasound dating of pregnancy and assessment of
fetal growth: Longitudinal data from a population-based cohort
study. Ultrasound Obstet Gynecol. 31:388–396. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Nemescu D, Bratie A, Mihaila A, Navolan D
and Tanase A: First trimester combined screening for fetal
aneuploidies enhanced with additional ultrasound markers: An 8-year
prospective study. Ginekol Pol. 89:205–210. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Egle D, Strobl I, Weiskopf-Schwendinger V,
Grubinger E, Kraxner F, Mutz-Dehbalaie IS, Strasak A and Scheier M:
Appearance of the fetal posterior fossa at 11 + 3 to 13 + 6
gestational weeks on transabdominal ultrasound examination.
Ultrasound Obstet Gynecol. 38:620–624. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Papastefanou I, Souka AP, Pilalis A,
Panagopoulos P and Kassanos D: Fetal intracranial translucency and
cisterna magna at 11 to 14 weeks: Reference ranges and correlation
with chromosomal abnormalities. Prenat Diagn. 31:1189–1192.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Loureiro T, Ferreira AF, Ushakov F,
Montenegro N and Nicolaides KH: Dilated fourth ventricle in fetuses
with trisomy 18, trisomy 13 and triploidy at 11-13 weeks'
gestation. Fetal Diagn Ther. 32:186–189. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ferreira AF, Syngelaki A, Smolin A, Vayna
AM and Nicolaides KH: Posterior brain in fetuses with trisomy 18,
trisomy 13 and triploidy at 11 to 13 weeks' gestation. Prenat
Diagn. 32:854–858. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Volpe P, Contro E, Fanelli T, Muto B, Pilu
G and Gentile M: Appearance of fetal posterior fossa at 11-14 weeks
in fetuses with Dandy-Walker malformation or chromosomal anomalies.
Ultrasound Obstet Gynecol. 47:720–725. 2016.PubMed/NCBI View Article : Google Scholar
|