Introduction
As a colorless and tasteless gas, carbon monoxide
(CO) is difficult to find when it overflows. The concentration of
CO exceeding 35 ppm will endanger human health (1). Acute carbon monoxide poisoning (ACOP)
is a common acute disease in clinical practice. Its clinical
manifestations range from headache, dizziness to coma and even
death with a mortality rate of 1-3%. A considerable number of
patients have long-term neurological and emotional sequelae after
treatment (2). Its pathogenesis is
not completely clear and its physiological mechanism is mainly
hypoxia stress. In ACOP, the main harm is to organs with high
oxygen demand, including heart and brain, and the severity of the
disease may be related to different concentrations of CO exposure
and duration (3). Among them,
myocardial injuries such as angina pectoris and myocardial
infarction (4) are common in ACOP
patients and are related to the increase of long-term mortality
rate (5). At present, clinical
treatment for ACOP includes hyperbaric oxygen therapy on the basis
of symptomatic treatment, which can effectively reduce the
mortality rate of patients (6).
However, due to the lack of efficacy basis, the application of
hyperbaric oxygen to ACOP patients still lacks relevant evidence
(7). In recent years, due to the
development of extracorporeal circulation technology,
extracorporeal membrane oxygenation (ECMO) technology has been
gradually applied to the treatment of ACOP, and Simonsen et
al (8) reported that ECMO
treatment can improve the survival rate of ACOP patients with
cardiogenic shock, but its exact efficacy is currently less
studied.
Physiological autophagy has a protective effect in
myocardial ischemia, and miR-30a can maintain autophagy reaction of
myocardial cells after hypoxia (9),
so miR-30a is bound to have an important connection with cardiac
injury of ACOP. Therefore, this study judged myocardial injury by
detecting the miR-30a expression level before and after ACOP
patients received hyperbaric oxygen therapy and ECMO respectively,
and detected myocardial enzymes: creatine kinase isoenzyme (CK-MB)
and lactate dehydrogenase (LDH). The aim was further confirm the
efficacy and mechanism of the two treatment methods, to judge which
treatment method is more suitable for clinical use, and to provide
more reliable reference opinions for the future treatment of such
diseases.
Patients and methods
Clinical data
Seventy patients with moderate and severe ACOP
admitted to The Affiliated Hospital of Qingdao University (Qingdao,
China) from January 2017 to December 2018 were studied. On the
basis of routine treatment, they were divided into group A (treated
with hyperbaric oxygen) with 35 cases and group B (treated with
ECMO) with 35 cases according to different combined treatment
methods. Another 30 healthy adults who underwent physical
examination in the hospital were selected as the control group
(CG). The subjects were aged 25-60 years, with an average age of
41.53±8.71 years. This study was approved by the Ethics Committee
of The Affiliated Hospital of Qingdao University, and all patients
and their families signed an informed consent.
Inclusion and exclusion criteria
All the patients involved in this experiment had a
history of CO exposure and met the diagnostic criteria of moderate
or severe ACOP. Exclusion criteria were as follows: i) Mild ACOP
patients; ii) patients with a history of drug allergy; iii)
patients with mental disorders; iv) patients with severe cardiac,
cerebral and renal insufficiency before suffering from this
disease; v) those who were restless and could not cooperate with
the treatment; vi) patients with incomplete pathological data; vii)
patients with coagulation dysfunction.
Methods
On the basis of routine treatment, all patients in
group A received hyperbaric oxygen therapy: stable pressurization
for 20 min, stable oxygen inhalation for 60 min, intermittent rest
for 5 min, gradual decompression for 20 min, hyperbaric oxygen
chamber treatment pressure of 2-2.5 ATA, and hyperbaric oxygen
therapy once a day. Patients in group B received ECMO treatment:
The vein-artery (V-A) auxiliary mode was selected, and ECMO was
composed of a temperature-variable water tank, a centrifugal pump,
an oxygenator and heparin-coated cannulas. They all underwent
ipsilateral femoral artery and femoral vein incision,
heparin-coated cannulas of 15-17FR and 19-21FR were inserted,
appropriate flow paths were set, blood supply to distal limbs of
the same side was ensured, and ECMO was managed and removed
according to the guidelines.
Altogether 3 ml of peripheral blood in all subjects
was taken at different time points before and after treatment,
while from those in the CG 2 ml of venous blood was taken during
physical examination. Myocardial enzyme detection: serum was taken
by low-speed centrifugation at 4˚C for 20 min at 1,000 x g, CK-MB
and LDH levels were detected by Dx800 automatic biochemical immune
analyzer (Beckman Coulter, Inc.) and enzyme-linked immunosorbent
assay (ELISA). CK-MB and LDH kits were from Shanghai Yubo
Biotechnology Co., Ltd. miR-30a detection: Real-time fluorescence
quantitative PCR (RT-PCR) method was used to detect the expression
of target miR-30a (kit from Xiamen Huijia Biotechnology Co., Ltd.,
XWCPK3043). Trizol kit (Shenyang Wanlei Biotechnology Co., Ltd.)
was used to extract the total RNA of cells, and specific steps were
strictly carried out in accordance with the instructions. The
extracted RNA was reverse transcribed to obtain cDNA, which was
used as a template for experiments. The reaction conditions were as
follows: pre-denaturation at 95˚C for 30 sec, denaturation at 95˚C
for 5 sec, and denaturation at 60˚C for 20 sec. Melting conditions:
95˚C, 0 sec; 65˚C, 15 sec; and 95˚C, 0 sec. Samples in each group
were repeated 3 times, internal reference was performed using U6,
and data analysis was performed using 2-∆Ct method. The
primer sequence was designed and synthesized by China Thermo Fisher
Scientific, Inc., as shown in Table
I.
 | Table ImiR-30a primer sequences. |
Table I
miR-30a primer sequences.
| Sequence | miR-30a | U6 |
|---|
| Forward |
5'-ACGGGCGCAACTGTAAACATCC-3' |
5'-GACATCAAGAAGGTGGTGAAGC-3' |
| Reverse |
5'-CAGTGCAGGGTCCGAGGTAT-3' |
5'-TGTCATTGAGAGCAATGCCAGC-3' |
Observation index and efficacy
judgment standard
The efficacy evaluation was divided into three grade
standards, namely markedly effective, effective and
ineffective.
Markedly effective: The symptoms and signs of the
patients were obviously improved, their consciousness was clear,
and the electroencephalogram basically returned to normal.
Effective: The symptoms and signs of the patients were improved and
the consciousness recovered basically, but the EEG examination was
still abnormal. Ineffective: The symptoms and signs of the patients
were not improved, even aggravated or presenting serious
complications.
The effective rate and complications of patients in
the two groups two weeks after treatment were compared. The CK-MB
and LDH levels as well as miR-30a expression in blood samples of
subjects in the three groups at different time points before and
after treatment were observed.
Statistical methods
SPSS24.0 statistical software was used to analyze
and process the data, GraphPad 5 software package was used to draw
relevant images, the counting data were expressed in the form of
rate, and Chi-square test was used for comparison between groups.
The measurement data were expressed as mean ± standard deviation.
Independent-samples t-test was used for inter-group comparison,
one-way analysis of variance was used for multi-group comparison,
LSD t-test was used for post hoc pairwise comparison, repeated
measures analysis of variance was used for multi-time point
expression, and Bonferroni test was used for back testing. The
diagnostic value was analyzed by ROC curve. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of clinical data
There was no significant difference in age, sex,
white blood cell count, exposure time, typing, smoking, and family
history of heart disease between the RG, CG and groups A and B
(P>0.05), proving that subjects in the three groups were
comparable (Table II).
 | Table IIComparison of clinical data
[n(%)]. |
Table II
Comparison of clinical data
[n(%)].
| Parameters | Group A (n=35) | Group B (n=35) | Control group
(n=30) | χ2 or F
value | P-value |
|---|
| Age | 39.88±6.41 | 42.25±7.56 | 43.18±7.29 | 1.910 | 0.154 |
| Sex | | | | 2.190 | 0.335 |
|
Male | 14 (40.00) | 19 (54.29) | 17 (56.67) | | |
|
Female | 21 (60.00) | 16 (45.71) | 13 (43.33) | | |
| White blood cell
count (109/l) | 8.53±2.64 | 8.26±2.12 | 7.51±1.96 | 1.715 | 0.185 |
| Exposure time
(h) | 6.43±4.57 | 6.67±5.19 | | 0.205 | 0.838 |
| Typing | | | | 0.245 | 0.621 |
|
Moderate | 23 (65.71) | 21 (60.00) | | | |
|
Severe | 12 (34.29) | 14 (40.00) | | | |
| Family history of
heart disease | | | | 0.688 | 0.709 |
|
Yes | 5 (14.29) | 6 (17.14) | 3 (10.00) | | |
|
No | 30 (85.71) | 29 (82.86) | 27 (90.00) | | |
| Smoking | | | | 1.476 | 0.478 |
|
Yes | 11 (31.43) | 15 (42.86) | 9 (30.00) | | |
|
No | 24 (68.57) | 20 (57.14) | 21 (70.00) | | |
Comparison of the effective rates of
patients in the two groups
The total effective rate of patients in group A was
71.43%, and that of patients in group B was 91.43%. Compared with
patients in the two groups, those in group B were significantly
higher than those in group A (P<0.05) (Table III).
 | Table IIIComparison of therapeutic
efficiency. |
Table III
Comparison of therapeutic
efficiency.
| Efficiency | Group A (n=35) | Group B (n=35) | χ2
value | P-value |
|---|
| Markedly
effective | 14 (40.00) | 18 (51.43) | | |
| Effective | 11 (31.43) | 14 (40.00) | | |
| Ineffective | 10 (28.57) | 3 (8.57) | | |
| Total effective
rate | 71.43% | 91.43% | 4.629 | 0.031 |
Comparison of the incidence rate of
complications between the two groups
The two groups were compared for delayed
encephalopathy, myocardial injury, urinary tract infection, skin
dystrophy and other complications. Analysis revealed that the
incidence rate of complications in group B (14.29%) was
significantly lower than that in group A (37.14%) (P<0.05)
(Table IV).
 | Table IVIncidence rate of complications. |
Table IV
Incidence rate of complications.
| Factors | Group A (n=35) | Group B (n=35) | χ2
value | P-value |
|---|
| Delayed
encephalopathy | 5 (14.29) | 3 (8.57) | | |
| Myocardial
injury | 4 (11.43) | 2 (5.71) | | |
| Urinary tract
infection | 2 (5.71) | 0 (0.00) | | |
| Cutaneous
dystrophy | 2 (5.71) | 0 (0.00) | | |
| Incidence rate of
complications | 37.14% | 14.29% | 4.786 | 0.029 |
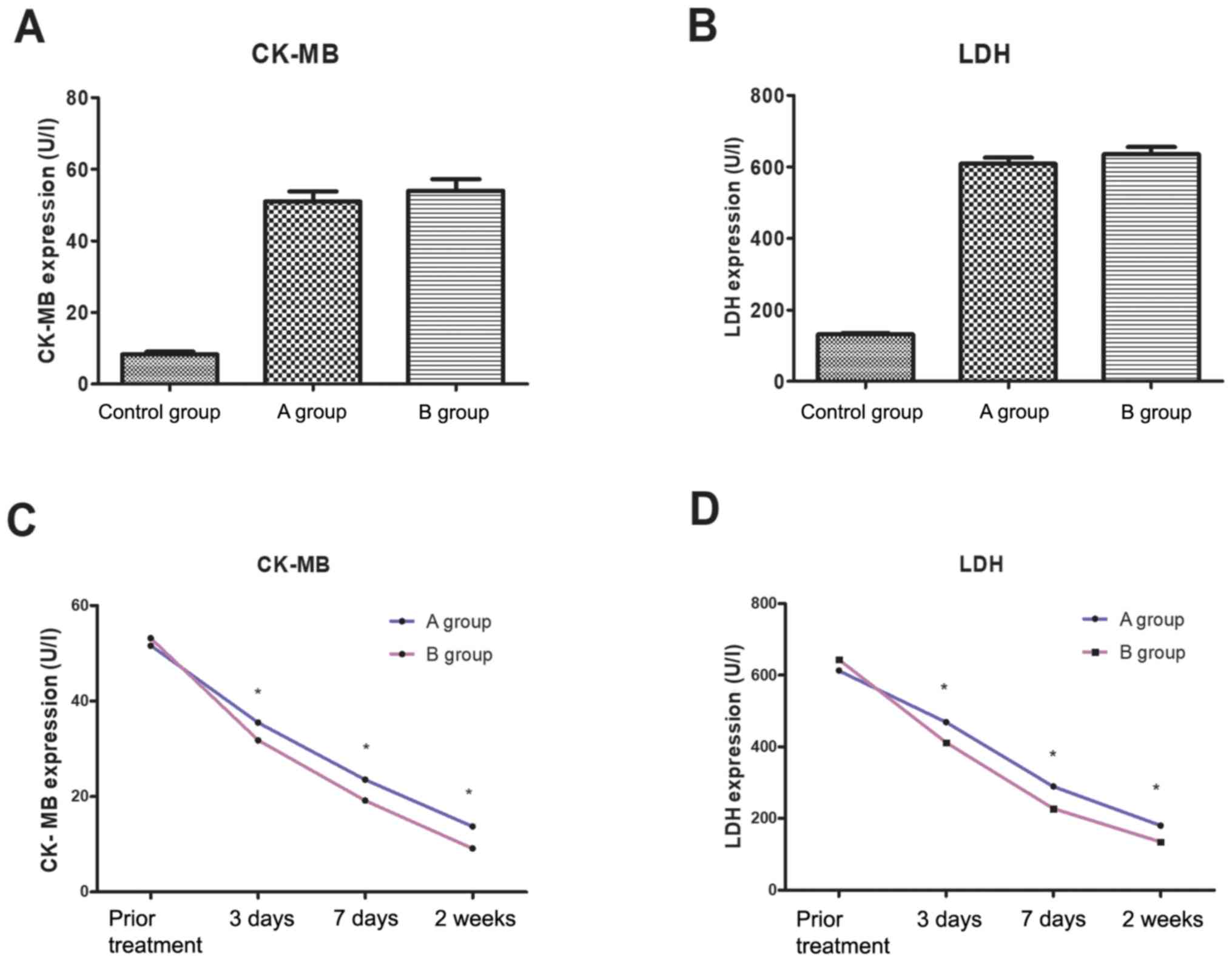
Comparison of CK-MB and LDH levels at
different time points before and after treatment
There was no significant difference in CK-MB and LDH
concentrations between group A, group B and control group before
treatment (P>0.05). Three days, seven days and two weeks after
treatment, the levels of CK-MB and LDH in group A were
significantly lower than those in group B (P<0.05) (Fig. 1).
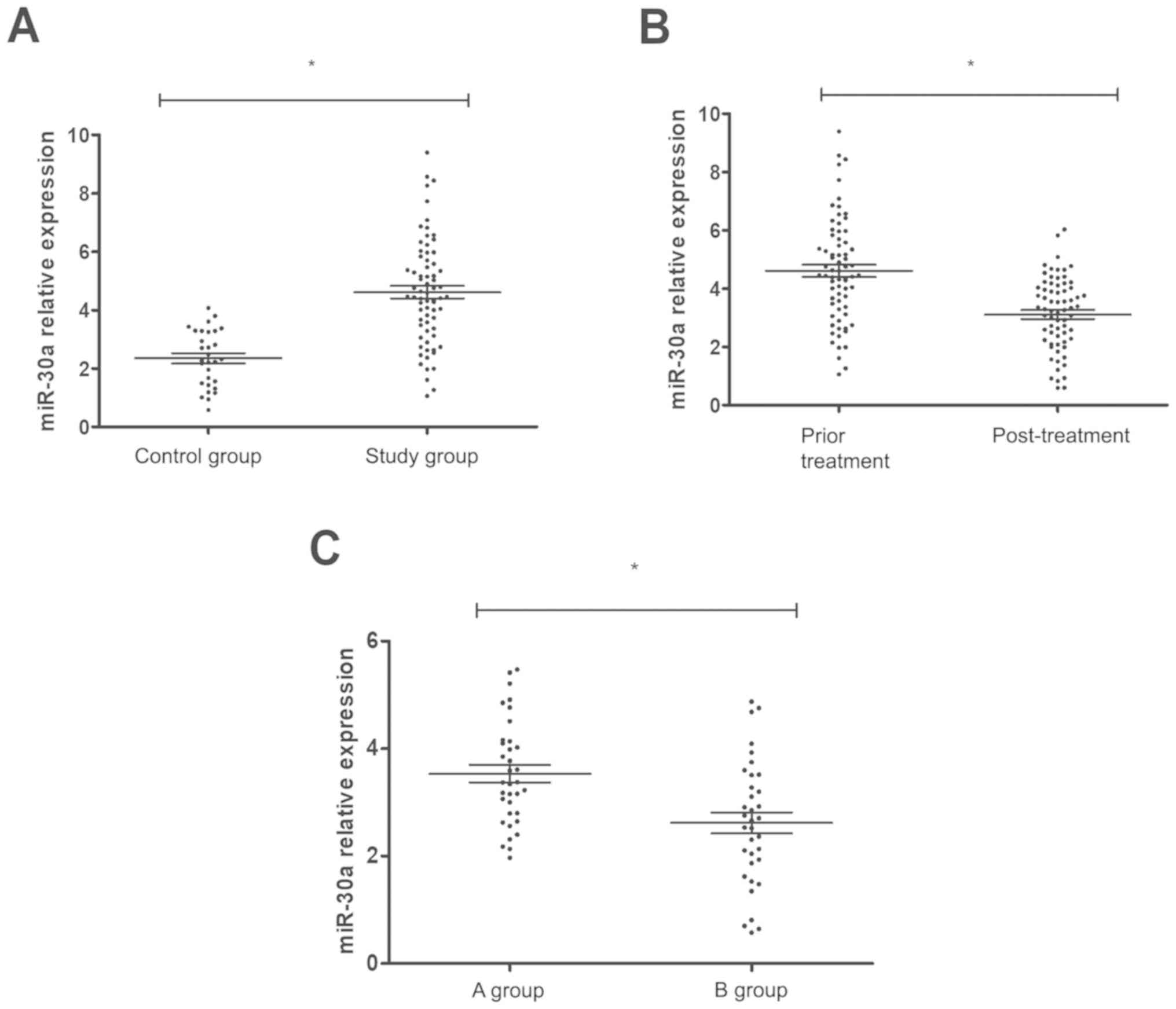
Detection of miR-30a expression
level
Blood samples of 100 subjects were collected before,
and seven days after treatment to detect the expression level of
miR-30a, and the expression level of miR-30a in ACOP patients
before treatment was significantly higher than that in normal
samples (P<0.01), at seven days after treatment it was
significantly lower than that before treatment, and the reduction
level in group B was better than that in group A (P<0.05)
(Fig. 2).
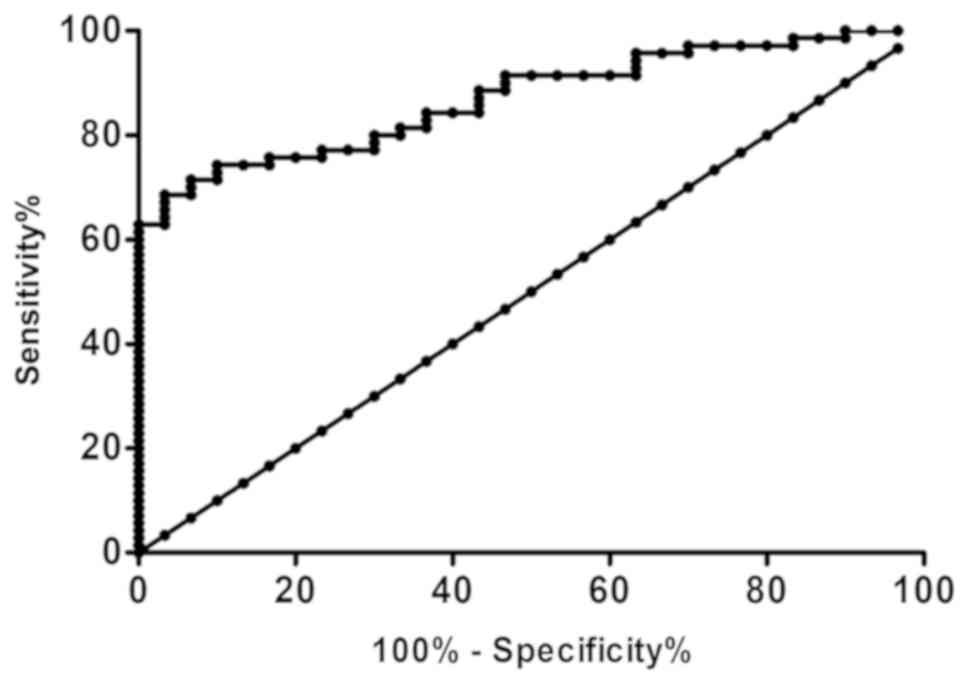
Diagnostic value of miR-30a in
myocardial injury of ACOP
ROC curve analysis showed that the area under the
curve (AUC) was 0.870. When the maximum cut-off value was 3.807,
the sensitivity and specificity of miR-30a in diagnosing myocardial
injury of ACOP were 68.57 and 96.67% (P<0.01) (Fig. 3).
Correlation analysis between the
miR-30a expression level and clinicopathology of ACOP patients
The expression level of miR-30a was not
significantly correlated with age, sex, family history of heart
disease, or smoking in ACOP patients (P>0.05), but correlated
with co-exposure time and typing (P<0.05) (Table V).
 | Table VCorrelation analysis of the miR-30a
expression level and clinicopathological features of patients in
the RG. |
Table V
Correlation analysis of the miR-30a
expression level and clinicopathological features of patients in
the RG.
| Parameters | n | miR-30a level | t value | P-value |
|---|
| Age (years) |
|
≥41.5 | 41 | 4.23±1.17 | 0.445 | 0.658 |
|
<41.5 | 29 | 4.36±1.25 | | |
| Sex | | | 0.349 | 0.729 |
|
Male | 33 | 4.06±1.12 | | |
|
Female | 37 | 3.97±1.04 | | |
| Exposure time
(h) | | | 2.123 | 0.037 |
|
≥6.5 | 28 | 4.56±1.41 | | |
|
<6.5 | 42 | 3.93±1.07 | | |
| Typing | | | 2.180 | 0.033 |
|
Moderate | 44 | 4.17±1.16 | | |
|
Severe | 26 | 4.82±1.28 | | |
| Family history of
heart disease | | | 0.815 | 0.418 |
|
Yes | 11 | 4.35±1.37 | | |
|
No | 59 | 4.07±0.98 | | |
| Smoking | | | 0.545 | 0.588 |
|
Yes | 26 | 4.26±1.15 | | |
|
No | 44 | 4.11±1.09 | | |
Discussion
CO is a common fatal poison that easily damage the
transportation and utilization of oxygen (5). Mild ACOP symptoms include dizziness,
headache, and limb weakness. Long-term exposure to CO can lead to
central nervous system and cardiovascular system injury and even
death (10,11). ACOP has become one of the most common
types of poisoning causing death in the world (12). As the target organ of ACOP, heart
failure is the main clinical manifestation after poisoning
(13). At present, the determination
of myocardial enzyme is the main clinical indicator of myocardial
cell injury (14). However, due to
abnormal expression of myocardial enzyme in various cardiac
diseases and low specificity, it is of great significance to find
markers for diagnosing ACOP cardiac injury. MircoRNA is a kind of
small molecule non-coding RNA; previous studies have shown that
miR-30a participates in the occurrence and development of
cardiovascular diseases, and plays an important role in myocardial
function, myocardial cell necrosis and apoptosis by regulating its
target genes (15), proving that
miR-30a is abnormally expressed in blood of patients with
myocardial infarction (16). At
present, the treatment of ACOP includes giving high concentration
and high flow of oxygen, ventilation support and detection of
abnormal heart rate (17).
Hyperbaric oxygen is an effective treatment method of ACOP, which
can quickly correct anoxia. Although hyperbaric oxygen treatment is
related to the good consciousness level of CO poisoning patients,
its impact on their mortality or morbidity rate is still unknown,
and is not directly tied to their reduction of mortality rate
(18). However, in ACOP multiple
organ failure of adults and children, there have been cases showing
success of extracorporeal membrane oxygenation (ECMO) treatment
(19). ECMO is an extracorporeal
circulation technology and has been clinically developed as a
necessary means to treat respiratory and circulatory failure
(20). Since there are few reports
comparing the efficacy of the two therapeutic methods on ACOP, this
study compared the efficacy of hyperbaric oxygen therapy and ECMO
on ACOP patients, respectively, and detects the expression of
miR-30a in cardiac injury of ACOP, proving the application value of
the two therapeutic methods, which has important reference value
for clinical ACOP treatment.
In this study, 70 patients with confirmed moderate
or severe ACOP were evaluated, the effects of hyperbaric oxygen
therapy and ECMO therapy were compared, and the myocardial enzyme
CK-MB, LDH concentration and miR-30a expression in peripheral blood
in ACOP-induced cardiac injury were detected. The results showed
that ECMO was superior to hyperbaric oxygen in terms of treatment
efficiency and complication rate, and the reason was that it could
remove CO in patients and improve hypoxia state of their tissues
and organs more effectively, which was consistent with the research
results of Choi et al (21).
Furthermore, CK-MB and LDH concentrations in ACOP patients
increased significantly, but decreased significantly three days,
seven days and two weeks after treatment, respectively. However,
the degree of the decrease in the ECMO group was better than that
in hyperbaric oxygen group. RT-PCR was used to detect the miR-30a
expression level in the blood of the study subjects. It was found
that the miR-30a expression level in the blood of ACOP patients was
significantly different from that of the normal control group, and
that of the ACOP patients were significantly higher than normal
people. Seven days after treatment, the expression of miR-30a in
group A and group B decreased significantly compared with that
before, but the decrease in group B was more obvious, which showed
that ECMO was better than hyperbaric oxygen therapy in treating
cardiac injury. ROC curve analysis showed that the cut-off value
was 3.807, miR-30a had a sensitivity of 68.57% and specificity of
96.67% in diagnosing cardiac injury of ACOP, indicating that
miR-30a had certain accuracy. Through further analysis of the
correlation between miR-30a and clinical pathology of ACOP
patients, we found that the miR-30a expression level was not linked
to age, sex, family history of heart disease, or smoking, but
linked to time of exposure to CO and ACOP typing, suggesting that
miR-30a played an important part in the proliferation and apoptosis
of myocardial cells under anoxic conditions, and myocardial cell
damage and recovery could be judged by detecting the miR-30a
expression level in peripheral blood.
Collectively, hyperbaric oxygen therapy and ECMO
therapy have obvious efficacy on ACOP patients, but the latter is
better than the former, and the expression level of miR-30a in
blood of ACOP patients increased significantly, which is positively
correlated with myocardial injury, and it decreased after
treatment. It is believed that miR-30a can provide a reference
index for early diagnosis and prediction of disease progression and
prognosis in cardiac injury of ACOP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SG wrote the manuscript. XH and HXu conceived and
designed the study. JY and DH contributed to observation indexes
analysis. SG and HXin interpreted the data and drafted the
manuscript. XG and RZ performed PCR and ELISA. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Hospital of Qingdao University (Qingdao, China).
Patients who participated in this study had complete clinical data.
Signed informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Xiang W, Xue H, Wang B, Li Y, Zhang J,
Jiang C, Liang F, Pang J and Yu L: Combined application of
dexamethasone and hyperbaric oxygen therapy yields better efficacy
for patients with delayed encephalopathy after acute carbon
monoxide poisoning. Drug Des Devel Ther. 11:513–519.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva
S, Tejero J and Gladwin MT: Carbon monoxide poisoning:
Pathogenesis, management, and future directions of therapy. Am J
Respir Crit Care Med. 195:596–606. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oh S and Choi SC: Acute carbon monoxide
poisoning and delayed neurological sequelae: A potential
neuroprotection bundle therapy. Neural Regen Res. 10:36–38.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee FY, Chen WK, Lin CL and Kao CH: Carbon
monoxide poisoning and subsequent cardiovascular disease risk: A
nationwide population-based cohort study. Medicine (Baltimore).
94(e624)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Clardy PF, Manaker S and Perry H: Carbon
monoxide poisoning. UpToDate 2018. https://www.uptodate.com/contents/carbon-monoxide-poisoning.
|
|
6
|
Huang CC, Ho CH, Chen YC, Lin HJ, Hsu CC,
Wang JJ, Su SB and Guo HR: Hyperbaric oxygen therapy is associated
with lower short- and long-term mortality in patients with carbon
monoxide poisoning. Chest. 152:943–953. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Eichhorn L, Thudium M and Jüttner B: The
diagnosis and treatment of carbon monoxide poisoning. Dtsch Arztebl
Int. 115:863–870. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Simonsen C, Magnusdottir SO, Andreasen JJ,
Rohde MC and Kjærgaard B: ECMO improves survival following
cardiogenic shock due to carbon monoxide poisoning - an
experimental porcine model. Scand J Trauma Resusc Emerg Med.
26(103)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang Y, Li Y, Chen X, Cheng X, Liao Y and
Yu X: Exosomal transfer of miR-30a between cardiomyocytes regulates
autophagy after hypoxia. J Mol Med (Berl). 94:711–724.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin MS, Lin CC, Yang CC, Weng SC, Wang SM,
Chen CY, Huang N and Chou YH: Myocardial injury was associated with
neurological sequelae of acute carbon monoxide poisoning in Taiwan.
J Chin Med Assoc. 81:682–690. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lin CH, Su WH, Chen YC, Feng PH, Shen WC,
Ong JR, Wu MY and Wong CS: Treatment with normobaric or hyperbaric
oxygen and its effect on neuropsychometric dysfunction after carbon
monoxide poisoning: A systematic review and meta-analysis of
randomized controlled trials. Medicine (Baltimore).
97(e12456)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gozubuyuk AA, Dag H, Kaçar A, Karakurt Y
and Arica V: Epidemiology, pathophysiology, clinical evaluation,
and treatment of carbon monoxide poisoning in child, infant, and
fetus. North Clin Istanb. 4:100–107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Garg J, Krishnamoorthy P, Palaniswamy C,
Khera S, Ahmad H, Jain D, Aronow WS and Frishman WH: Cardiovascular
abnormalities in carbon monoxide poisoning. Am J Ther.
25:e339–e348. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maghamiour N and Safaie N: High creatine
kinase (CK)-MB and lactate dehydrogenase in the absence of
myocardial injury or infarction: A case report. J Cardiovasc Thorac
Res. 6:69–70. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang J, Huang C, Luo Y, Liu S and Chen X:
Role of miR-30a in cardiomyocyte autophagy induced by angiotensin
II. J Renin Angiotensin Aldosterone Syst. 16:1–5. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Y, Huang Y, Zhang M, Zhang X, Tang X
and Kang Y: Bioinformatic analysis of the possible regulative
network of miR-30a/e in cardiomyocytes 2 days post myocardial
infarction. Acta Cardiol Sin. 34:175–188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Casillas S, Galindo A, Camarillo-Reyes LA,
Varon J and Surani SR: Effectiveness of hyperbaric oxygenation
versus normobaric oxygenation therapy in carbon monoxide poisoning:
A systematic review. Cureus. 11(e5916)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nakajima M, Aso S, Matsui H, Fushimi K and
Yasunaga H: Hyperbaric oxygen therapy and mortality from carbon
monoxide poisoning: A nationwide observational study. Am J Emerg
Med S0735-6757(19)30087-7, 2019.
|
|
19
|
Rabie AA, Asiri A, Alsherbiny M, Alqassem
W, Rajab M, Mohamed S, I Alenazi WH and Ariplackal L: Management of
carbon monoxide poisoning-induced cardiac failure and multiorgan
dysfunction with combined respiratory and circulatory
extracorporeal membrane oxygenation. Saudi Crit Care J.
3(12)2019.
|
|
20
|
Fan E, Gattinoni L, Combes A, Schmidt M,
Peek G, Brodie D, Muller T, Morelli A, Ranieri VM, Pesenti A, et
al: Venovenous extracorporeal membrane oxygenation for acute
respiratory failure: A clinical review from an international group
of experts. Intensive Care Med. 42:712–724. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Choi JH, Kim SW, Kim YU, Kim SY, Kim KS,
Joo SJ and Lee JS: Application of veno-arterial-venous
extracorporeal membrane oxygenation in differential hypoxia.
Multidiscip Respir Med. 9(55)2014.PubMed/NCBI View Article : Google Scholar
|