Introduction
Breast cancer is the most common malignant primary
tumor in women worldwide, and the incidence is continually on the
increase (1). Triple negative breast
cancer (TNBC), which accounts for 10-20% of newly diagnosed cases
of breast cancer (1,2), is defined by the absence of estrogen
receptor (ER)α, progesterone receptor (PR) and human epidermal
growth factor receptor 2 (HER2) expression. Based on the
pathological features, TNBC is an aggressive subtype with a poor
prognosis, due to a high rate of early recurrence and distant
metastasis (3). The poor prognosis
is due to the lack of efficacy of the current systemic therapies,
including endocrine-based and HER2-targeted therapies (4). Conventional chemotherapy is the
standard strategy for the systemic treatment of advanced TNBC;
however, the therapeutic efficacy in TNBC is not satisfactory. It
has been reported that 34% of patients with newly diagnosed TNBC
will undergo recurrence within five years, following adjuvant or
neoadjuvant chemotherapy (5).
Therefore, combination therapies that enhance the sensitivity and
improve the tolerance of chemotherapy are required for the
effective treatment of TNBC.
ERα is a major determinant in classifying the
various subtypes of breast cancer, and is also an indicator of
endocrine therapy. The role of ERα in breast cancer has been
clearly demonstrated (6). By
contrast, ERβ, another estrogen receptor subtype, is not well
characterized. It has been reported that ERβ, which is expressed in
30% of TNBC cases (7), is a key
regulator of signal transduction and tumor suppression in breast
cancer (8). Furthermore, ERβ
displays an antiproliferative role in TNBC (9). Patients with ERβ-positive TNBC
displayed an improved 5-year survival rate compared with patients
with ERβ-negative TNBC (10).
However, research into the role of ERβ during TNBC has primarily
focused on endocrine therapy, and little has been reported
regarding the role and therapeutic value of ERβ in chemotherapy. A
large-scale retrospective study reported that the upregulation of
ERβ1 (the fully functional isoform of ERβ) predicted an improved
prognosis for patients with TNBC. In the study, 508 out of 571
(89.0%) patients with TNBC were successfully treated with adjuvant
chemotherapy (11). However, whether
the status of ERβ in TNBC is associated with the response to
chemotherapy requires further investigation. Therefore, it is
important to identify the role of ERβ in regulating the response to
chemotherapy and its underlying mechanisms in TNBC.
In the present study, the inhibitory effects of
doxorubicin and a combination therapy [doxorubicin and
liquiritigenin (Liq)] on ERβ-positive TNBC MDA-MB-231 and BT549
cell lines were investigated in vitro. The results suggested
that upregulated ERβ expression in TNBC cells was associated with
improved sensitivity to doxorubicin by inhibiting the PI3K/AKT/mTOR
signaling pathway.
Materials and methods
Cells and reagents
TNBC MDA-MB-231 and BT549 cell lines were purchased
from the American Type Culture Collection. Cells were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA). Liq, an ERβ
agonist, was purchased from Chengdu Biopurify Phytochemicals,
Ltd.
Cell viability assay
To analyze the effects of different treatments on
the viability of MDA-MB-231 and BT549 cells, a Cell Counting Kit-8
(CCK-8) assay (Dojindo Molecular Technologies, Inc.) was performed
according to the manufacturer's protocol. Cells (5x103
cells/well) were plated in 96-well plates at 37˚C for 24 h.
Subsequently, various agents, Liq (0-160 µg/ml, Chengdu Biopurify
Phytochemicals, Ltd.) alone, doxorubicin (DOX; 0-4 ng/ml,
Sigma-Aldrich; Merck KGaA) alone or a combination of DOX (0-4
ng/ml) and Liq (40 µg/ml) were added to each well, whilst the
control cells were treated with DMSO (Sigma-Aldrich; Merck KGaA).
Following a 48-h incubation at 37˚C, 10 µl CCK-8 reagent was added
to each well and incubated for a further 2 h at 37˚C. The
absorbance of each well was measured at a wavelength of 450 nm
using a multi-mode microplate reader (ELx800; Bio-Tek China). The
IC50 of DOX and Liq was calculated using SPSS software
(version 17.0; SPSS, Inc.). Assays were performed in
triplicate.
Lentivirus production and cell
infection
shRNA targeting ERβ or negative control (NC)
scramble sequence were sub-cloned into the GV112 vector (Shanghai
GeneChem Co., Ltd.), respectively. The shRNA sequences were
designed by Shanghai GeneChem Co., Ltd. (shERβ,
5'-GCTGAATGCCCACGTGCTT-3'; shNC; 5'-TTCTCCGAACGTGTCACGT-3'). For
the production of lentivirus, the expression vectors (20 µg) were
co-transfected with packaging plasmid pHelper 1.0 vector (15 µg)
and envelope plasmid pHelper 2.0 vector (10 µg; Shanghai Genechem
Co., Ltd.) into 293T cells using TransIT®-LT1 (Mirus
Bio, LLC). The supernatant was collected 72 h after transfection,
concentrated by ultracentrifugation at 60,000 x g for 90 min at 4˚C
and resuspended with OptiMEM (Gibco; Thermo Fisher Scientific,
Inc.). MDA-MB-231 cells (2x104 cells/well) were cultured
in 12-well plates for 24 h at 37˚C before transduction. The shERβ
or shNC lentivirus particles (multiplicity of infection, 10) were
respectively added into the medium. After 24 h at 37˚C, the culture
medium was removed and replaced with complete medium (Gibco; Thermo
Fisher Scientific, Inc.) containing puromycin. The cells were
incubated for 7 days at 37˚C to obtain stable ERβ knockdown
(ERβ-KD)-MDA-MB-231 cells (an ERα-/ERβ- cell model). Subsequently,
the ERβ-KD-MDA-MB-231 cells were divided into three groups for
subsequent experiments. Western blot analysis was used to assess
the efficiency of transduction.
Colony formation assay
Colony formation assays were performed to evaluate
the effect of the different treatments on cell proliferation.
MDA-MB-231 and ERβ-KD-MDA-MB-231 cells were selected. Single cell
suspensions were prepared using 0.25% trypsin at 37˚C for 30 sec.
Subsequently, cells at a density of 1x103 cells/ml were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS, 40 µg/ml Liq, 1 ng/ml DOX or 1 ng/ml DOX
and 40 µg/ml Liq. Cells were incubated at 37˚C with 5%
CO2 for 10-14 days until macroscopic clones appeared.
The colonies were fixed with 2% paraformaldehyde at room
temperature for 15 min, and stained with 0.1% Giemsa (AppliChem
GmbH) at room temperature for 30 min. Colonies containing >50
cells were counted using an inverted light microscope
(magnification, x100). The assay was performed in triplicate.
Western blotting
Western blotting was used to evaluate the protein
expression levels of ERβ, AKT and mTOR. MDA-MB-231 and
ERβ-KD-MDA-MB-231 cells were washed twice with PBS and lysed using
a protein gel buffer (60 mM Tris-HCl, 10% SDS and 10% glycerol)
supplemented with 1 mM phenylmethanesulfonylfluoride for 20 min at
4˚C. Cell lysates were centrifuged at 14,000 x g for 10 min at 4˚C
and the supernatants were collected. Protein concentration was
quantified using a Nanodrop nd-1000 spectrophotometer (Thermo
Fisher Scientific, Inc.). Protein (20 µg) was resolved by 12%
SDS-PAGE and transferred to PVDF membranes. The membranes were
blocked with 5% non-fat milk in TBST for 1 h at room temperature.
Subsequently, the membranes were incubated for 24 h at 4˚C with
primary antibodies targeted against: Phosphorylated (p)-mTOR (cat.
no. 2974; 1:1,000; Cell Signaling Technology, Inc.), mTOR (cat. no.
2983; 1:1,000; Cell Signaling Technology, Inc.), ERβ (cat. no.
sc-8974; 1:2,000; Santa Cruz Biotechnology, Inc.), β-actin (cat.
no. 4970; 1:2,000; Cell Signaling Technology, Inc.), phosphorylated
(p)-AKT (cat. no. 4060; 1:2,000; Cell Signaling Technology, Inc.)
and total AKT (cat. no. 4691; 1:2,000; Cell Signaling Technology,
Inc.). Following primary incubation, the membranes were washed
three times with TBST and subsequently incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. AS014;
1:5,000; ABclonal Biotech Co., Ltd.) for 1 h at room temperature.
Protein bands were visualized using an enhanced chemiluminescence
kit (EMD Millipore). Blots were performed in at least triplicate.
The protein expression levels were quantitatively analyzed using
the Image lab 6.0 software (Bio-Rad Laboratories, Inc.) and
normalized against β-actin loading control.
Statistical analysis
Data are presented as the mean ± SD. One-way ANOVA
followed by Tukey's post-hoc test was used to analyze the data.
Comparisons between multiple groups and across multiple factors
were made using two-way ANOVA followed by Bonferroni's post-hoc
test. Statistical analyses were performed using SPSS software
(version 17.0; SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Liq inhibits the proliferation and
promotes the sensitization of TNBC cells to DOX treatment
To investigate the role of Liq in regulating the
therapeutic response to DOX treatment, the MDA-MB-231 and BT549
TNBC cell lines were used. The CCK-8 assay suggested that the
viability of MDA-MB-231 cells decreased in a dose-dependent manner
following treatment with different concentrations of Liq for 48 h
(Fig. 1A). The results also
indicated that Liq concentrations ≥20 µg/ml significantly decreased
the viability of MDA-MB-231 cells compared with the control group
(Fig. 1A). Therefore, 40 µg/ml Liq
(Liq IC50=69.28 µg/ml) was used for subsequent
experiments. Additionally, the effects of DOX and combination
treatment (1 ng/ml DOX and 40 µg/ml Liq) on the viability of
MDA-MB-231 cells were assessed. DOX treatment alone did not
significantly alter the viability of MDA-MB-231 cells compared with
the negative control group. By contrast, the viability of
MDA-MB-231 cells was significantly decreased by the combination
treatment, even with low concentrations of DOX, compared with the
negative control group (DOX IC50 combination treated
group =0.60 ng/ml vs. DOX IC50 DOX treated
group =1.72 ng/ml; Fig. 1B).
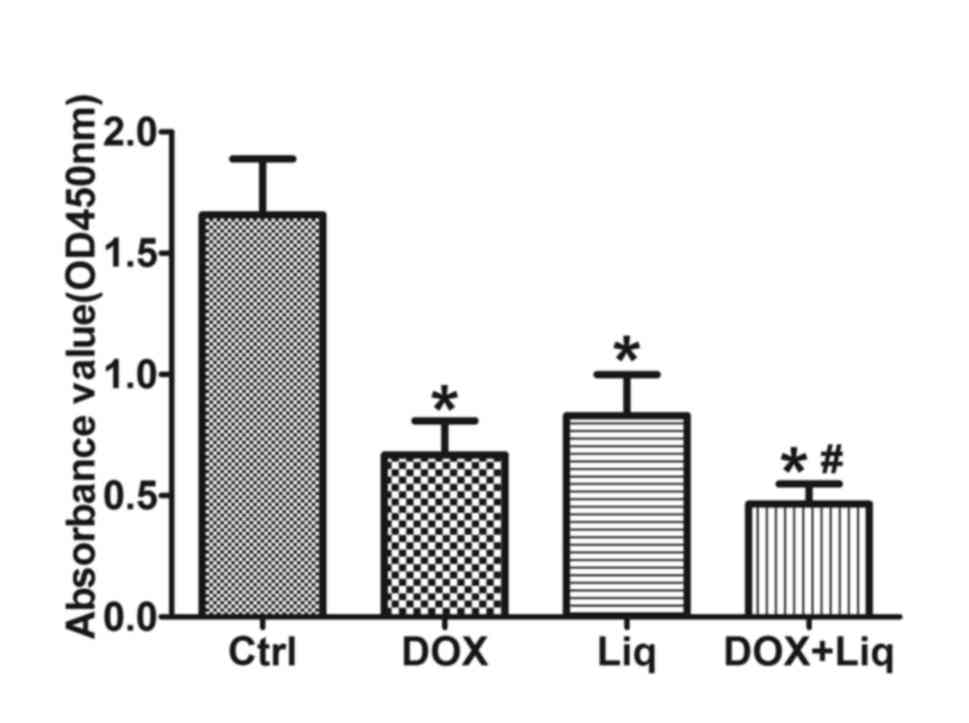
Compared with the control group, DOX (1 ng/ml), Liq (40 µg/ml) and
combination treatment (1 ng/ml DOX and 40 µg/ml Liq) significantly
reduced the viability of MDA-MB-231 cells. Furthermore, combination
treatment (1 ng/ml DOX and 40 µg/ml Liq) significantly decreased
the viability of MDA-MB-231 cells compared with DOX treatment alone
(1 ng/ml) (P<0.05; Fig. 1C).
Compared with the control group, the number of cell colonies was
found to be significantly reduced in both DOX-treated and
Liq-treated groups, whilst he number of cell colonies was
significantly decreased in the combination treated group compared
with that in the DOX-treated group (P<0.05; Fig. 1D). Similar results were obtained for
BT549 cells. DOX (1 ng/ml), Liq (40 µg/ml) and combination
treatment (1 ng/ml DOX and 40 µg/ml Liq) significantly reduced the
viability of BT549 cells, compared with that in the control group.
The viability of BT549 cells was significantly decreased in the
combination treatment group, compared with that in the DOX-treated
group (P<0.05; Fig. 2).
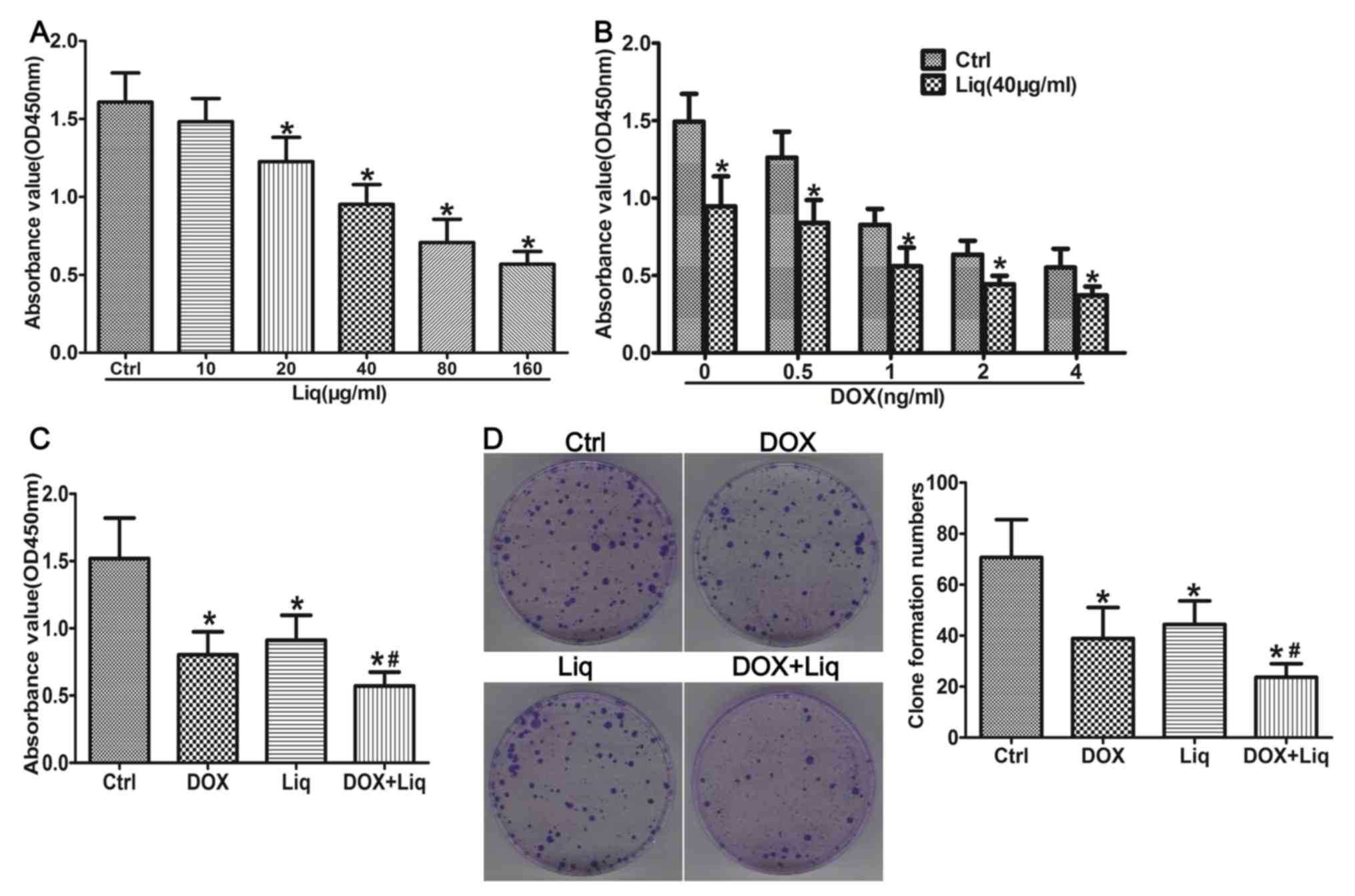 | Figure 1Liq treatment inhibits the viability
and promotes the sensitization of MDA-MB-231 cells to DOX
treatment. (A) MDA-MB-231 cells were treated with (A) Liq (0-160
µg/ml), (B) DOX (0-4 ng/ml) or a combination of DOX (0-4 ng/ml) and
Liq (40 µg/ml), and (C) DOX (1 ng/ml), Liq (40 µg/ml) or a
combination of DOX (1 ng/ml) and Liq (40 µg/ml) for 48 h.
Subsequently, cell viability was determined using the Cell Counting
Kit-8 assay in triplicate. (D) MDA-MB-231 cells were treated with
DOX (1 ng/ml), Liq (40 µg/ml) or a combination of DOX (1 ng/ml) and
Liq (40 µg/ml). Subsequently, cell proliferation was assessed using
a clone formation assay. Magnification, x100. *P<0.05
vs. the negative control group. #P<0.05 vs. the
DOX-treated group. Liq, liquiritigenin; DOX, doxorubicin; OD,
optical density; Ctrl, control. |
Liq enhances the therapeutic efficacy
of DOX by inhibiting the PI3K/AKT/mTOR signaling pathway
To investigate whether Liq enhanced DOX sensitivity
by modulating the PI3K/AKT/mTOR signaling pathway, the protein
expression levels of ERβ, p-AKT, AKT, p-mTOR and mTOR were assessed
by western blotting in DOX-treated, Liq-treated and
combination-treated MDA-MB-231 cells. MDA-MB-231 cells treated with
Liq or the combination treatment displayed increased ERβ expression
levels, and decreased levels of AKT and mTOR phosphorylation,
compared with the control group (Fig.
3A). Subsequently, the ratio of ERβ/β-actin, p-AKT/AKT and
p-mTOR/mTOR was calculated. MDA-MB-231 cells treated with Liq or
the combination treatment displayed significantly increased
expression levels of ERβ, but a significantly decreased ratio of
p-AKT/AKT and p-mTOR/mTOR, compared with the control group
(Fig. 3B). ERβ expression was
significantly increased, and the ratio of p-AKT/AKT and p-mTOR/mTOR
was significantly decreased in the combination treatment group
compared with the DOX-treated group (Fig. 3B).
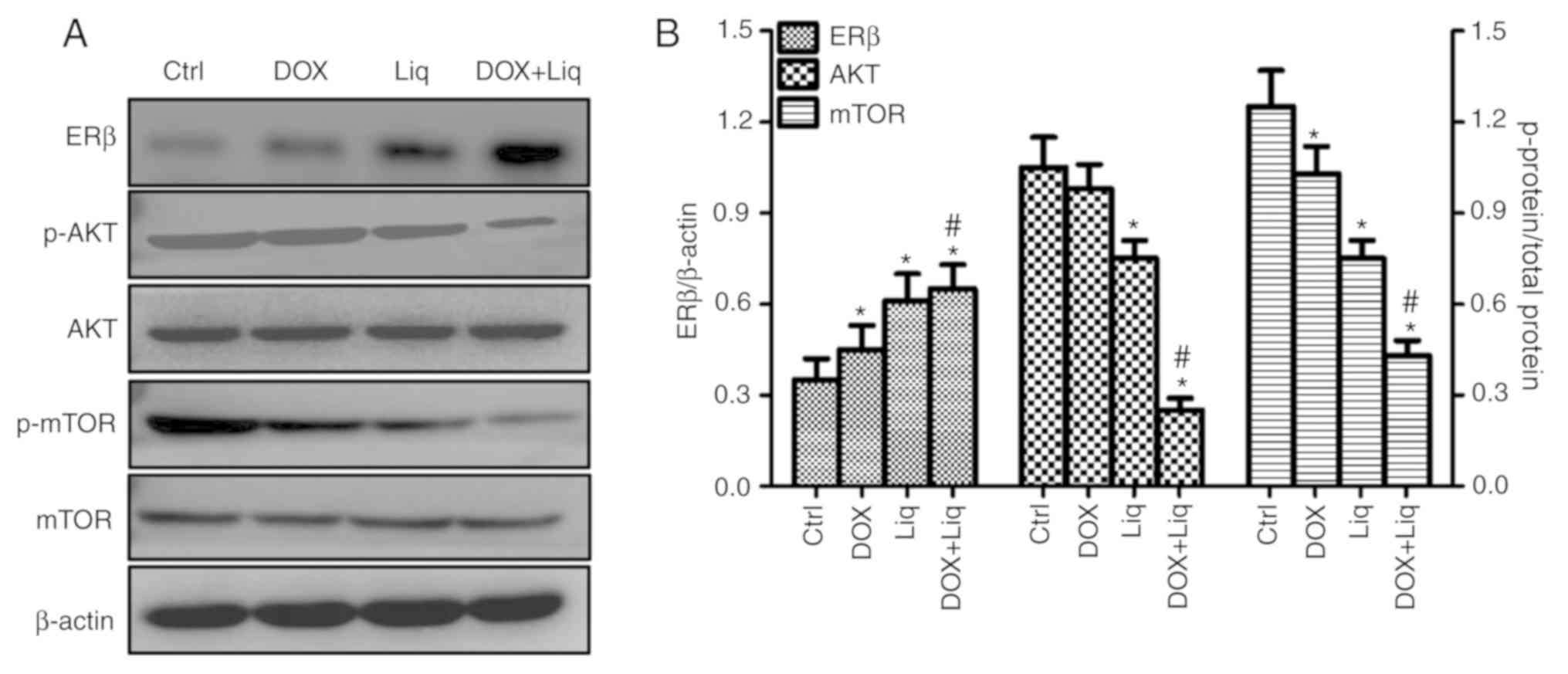 | Figure 3Liq treatment enhances the protein
expression of ERβ and inhibits the activity of the PI3K/AKT/mTOR
signaling pathway in TNBC cells. MDA-MB-231 cells were treated with
DOX (1 ng/ml), Liq (40 µg/ml) or a combination of DOX (1 ng/ml) and
Liq (40 µg/ml). Subsequently, the protein expression levels of ERβ,
p-AKT, AKT, p-mTOR and mTOR were (A) determined by western blotting
and (B) quantified. ERβ expression levels were increased in
MDA-MB-231 cells treated with Liq (40 µg/ml) or the combined
treatment, whereas the ratio of p-AKT/AKT and p-mTOR/mTOR was
decreased, compared with the control group. *P<0.05
vs. the negative control group. #P<0.05 vs. the
DOX-treated group. Liq, liquiritigenin; ERβ, estrogen receptor β;
DOX, doxorubicin; p, phosphorylated; Ctrl, control. |
ERβ knockdown inhibits the effects of
Liq on proliferation and the therapeutic efficacy of DOX in TNBC
cells
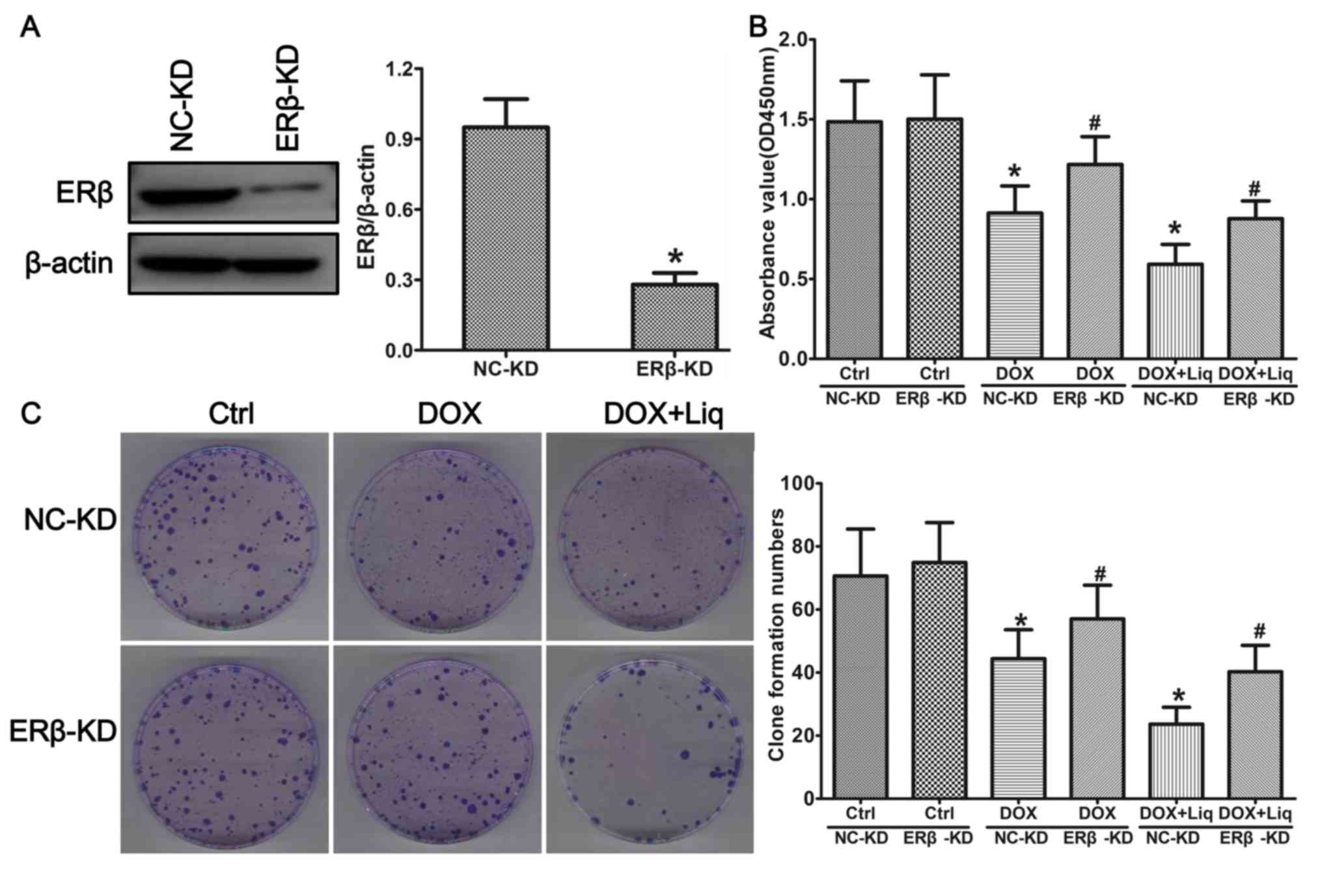
To identify the role of ERβ in regulating DOX
sensitivity in TNBC cells, ERβ knockdown in MDA-MB-231 cells was
performed using lentiviral particles. Subsequently, the viability
and proliferation of MDA-MB-231 (ERα-/ERβ+) and ERβ-KD (ERα-/ERβ-)
cells were assessed. The protein expression levels of ERβ were
significantly decreased in the ERβ-KD group compared with the NC-KD
group (Fig. 4A). ERβ-KD-MDA-MB-231
cells treated with Liq (40 µg/ml) or the combination treatment (1
ng/ml DOX and 40 µg/ml Liq) displayed increased cell viability
compared with the corresponding NC-KD group (Fig. 4B). The number of cell colonies was
also significantly increased in the Liq-treated (40 µg/ml) and
combination-treated (1 ng/ml DOX and 40 µg/ml Liq) ERβ-KD groups
compared with the corresponding NC-KD groups (Fig. 4C).
Liq-mediated effects on the
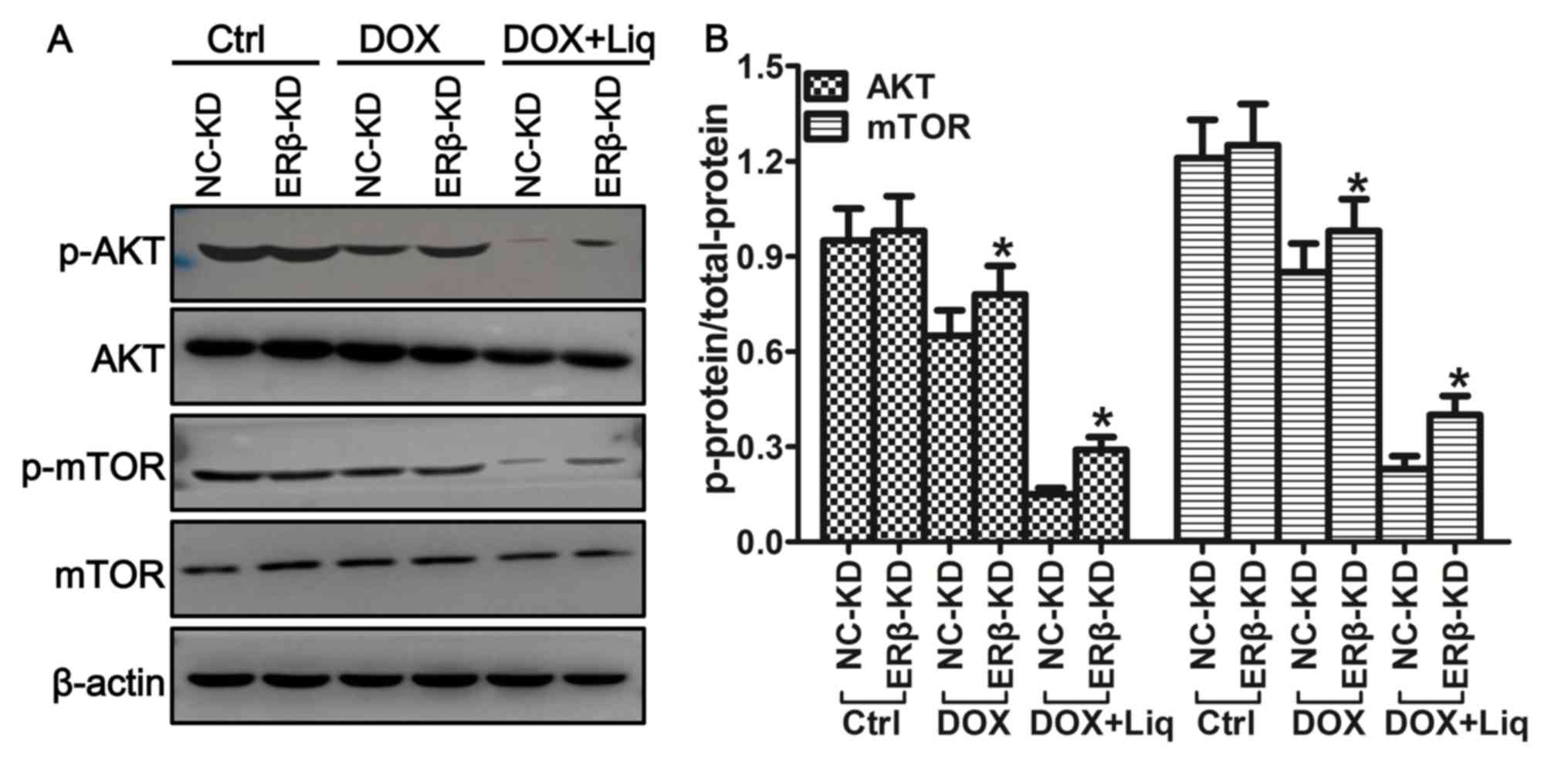
PI3K/AKT/mTOR signaling pathway are ERβ-dependent
Western blotting was used to further investigate the
relationship between the expression of ERβ and the regulation of
the PI3K/AKT/mTOR signaling pathway. MDA-MB-231 cells treated with
Liq (40 µg/ml) or the combination treatment (1 ng/ml DOX and 40
µg/ml Liq) displayed significantly decreased expression levels of
p-AKT and p-mTOR compared with the control group (Fig. 5A). However, ERβ-KD cells treated with
Liq or the combination treatment displayed significantly increased
levels of AKT and mTOR phosphorylation compared with the
corresponding NC-KD groups (P<0.05; Fig. 5B).
Discussion
DOX is one of the most active conventional
chemotherapeutic drugs used for breast cancer in neoadjuvant,
adjuvant and palliative settings (12,13).
DOX, as a cytotoxic agent affiliated with anthracycline, can
inhibit DNA and RNA synthesis, topoisomerase II enzymatic activity,
and block DNA transcription and replication (14-16).
Due to a lack of effective endocrine-based and HER2-targeted
therapies, doxorubicin-containing chemotherapy plays an important
role in patients with TNBC in an adjuvant setting (17). However, the clinical outcome of
conventional chemotherapy is not satisfactory in patients with
TNBC, as resistance to standard anthracycline- and taxane-based
chemotherapy results in treatment failure in some cases (18). In the present study, the ERβ specific
agonist Liq decreased the proliferation of MDA-MB-231 cells and
enhanced the cytotoxic chemotherapeutic effects of DOX. Liq is a
natural compound isolated from the roots of Glcyrrhizae
uralensis (19). Similar to
other ERβ specific agonists, including diarylpropionitrile and
WAY200070, Liq upregulates ERβ expression and displays inhibitory
effects in TNBC cells (20).
ERβ-KD-MDA-MB-231 cells treated with the combination treatment did
not display increased sensitivity to DOX compared with ERβ-positive
cells. The results suggested that the synergistic effect of DOX and
Liq in TNBC was dependent on ERβ. ERβ activation caused by Liq does
not induce cell apoptosis and proliferation of TNBC cells, but does
contribute to cell cycle arrest (21). In 2017, Reese et al reported
that the activation of ERβ resulted in the decreased expression of
a number of cell cycle-related genes, including cyclin B and
cyclin-dependent kinase 1 (CDK1), both in vitro and in
vivo (22). The inhibition of
CDK1 induced G2/M phase cell cycle arrest, which led to
decreased proliferation of MDA-MB-231 cells (22). Collectively, the aforementioned
studies suggest that doxorubicin and ERβ agonists display
synergistic antitumor activity in TNBC, which provides strong
rationale for the combined use of ERβ agonists and conventional
chemotherapeutic agents for the treatment of TNBC.
A number of previous studies investigating TNBC have
focused on the therapeutic value of ERβ in endocrine therapy, or
the role of ERβ in tumor invasion and metastasis. For example,
Hinsche and Girgert (21)
co-cultured MG63 osteoblast-like cells with the HCC1806 TNBC cell
line (ERα-/ERβ+), and reported that the ERβ agonists Liq and
ERB-041 increased the expression of ERβ, and inhibited
bone-directed invasion. Thomas et al (23) reported that ERβ1 inhibits EMT and
invasion in TNBC cells in vitro and in vivo. The
present study further suggested that the ERβ agonist Liq increased
the sensitivity of TNBC cells to conventional chemotherapeutic
agents. ERβ agonist-induced chemical sensitization has also been
observed in various types of malignant tumors, including TNBC
(24). Furthermore, Liu et al
suggested that Liq treatment increased the susceptibility of glioma
cells to temozolomide by inhibiting the mTOR signaling pathway
(25).
The PI3K/AKT/mTOR signaling pathway plays a critical
role in regu-lating cell metabolism, growth, survival,
proliferation, migration and differentiation (26). The inappropriate activation or
overactivation of the signaling pathway can result in the
progression of tumors in several malignancies, including TNBC
(27,28). AKT interacts with the DNA-protein
kinase catalytic subunit and induces DNA double-strand break repair
(29). In TNBC, the PI3K/AKT/mTOR
signaling pathway serves as an oncogenic driver (30). PI3K mutations were reported in 73.9%
cfDNA samples and 57.1% tumor samples obtained from patients with
metastatic TNBC (31). In addition,
overexpression of PI3K and overactivation of the PI3K/AKT/mTOR
signaling pathway are associated with chemical drug resistance in
breast cancer cells (32,33). Therefore, some have hypothesized that
combined treatment, including standard chemotherapy and
specifically target components of the PI3K/AKT/mTOR signaling
pathway could be used to effectively treat TNBC (31). However, Park et al (31) previously found that the addition of
the mTOR inhibitor everolimus to the gemcitabine/cisplatin
treatment strategy did not result in a synergistic effect in
patients with metastatic TNBC. In addition, the toxicities of
everolimus, including stomatitis and hematologic toxicities, should
be considered (31,34). The identification of other inhibitors
of the PI3K/AKT/mTOR signaling pathway, which display increased
tolerance and decreased toxicity, is essential for the effective
treatment of TNBC. The present study suggested that increased ERβ
expression levels decreased the level of AKT and mTOR
phosphorylation in TNBC cells. The result was consistent with a
previous study, which reported that ERβ1+/pAKT- status in TNBC
tumor samples predicted the most favorable prognosis. The previous
study also suggested that ERβ activation was associated with
inhibition of the PI3K/AKT/mTOR signaling pathway (11). An explanation for the association
could be that increased ERβ expression results in decreased cell
proliferation, which is primarily controlled by the PI3K/AKT/mTOR
signaling pathway in TNBC cells (35). Furthermore, downregulation of the
signaling pathway results in decreased cell proliferation (35). Alternatively, the ERβ-mediated
inhibition of the PI3K/AKT/mTOR signaling pathway may be associated
with downstream actions that influence the secretion of
amphiregulin and Wnt-10b, which may form part of a cascade that
could potentially regulate the signaling pathway (36). However, the mechanism underlying how
ERβ activation modulates the activity of the PI3K/AKT/mTOR
signaling pathway requires further investigation. Therefore, Liq,
which can specifically target ERβ-positive cells, displays
characteristics of a therapeutic agent with improved tolerance and
reduced toxicity. Furthermore, Liq may display increased
specificity compared with general PI3K/AKT/mTOR signaling pathway
inhibitors, which could result in improved patient outcomes when
used in combination with chemotherapy. To conclude, the in
vitro results of the present study suggested that Liq increased
the sensitivity of TNBC cells to DOX, and indicated that ERβ
agonists in combination with chemotherapy may serve as a novel
therapeutic strategy for TNBC. Additionally, Liq enhanced the
sensitivity of TNBC cells to DOX by inhibiting the PI3K/AKT/mTOR
signaling pathway, in an ERβ-dependent manner.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health and
Family Planning Commission of Hunan Province (grant no. 20180726),
and the Ren Shu Foundation from Hunan Provincial People's Hospital
(grant no. RS201707).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL, PF and AW designed the study. SL, YJ and MF
performed the experiments. MW, CZ, SH and ZH analyzed the data. SL
and AW drafted the manuscript. All authors have read and approved
the final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Papa A, Caruso D, Tomao S, Rossi L,
Zaccarelli E and Tomao F: Triple-negative breast cancer:
Investigating potential molecular therapeutic target. Expert Opin
Ther Targets. 19:55–75. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oakman C, Viale G and Di Leo A: Management
of triple negative breast cancer. Breast. 19:312–321.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Foulkes WD, Smith IE and Reis JS:
Triple-negative breast cancer. New Engl J Med. 363:1938–1948.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Imamov O, Lopatkin NA and Gustafsson JA:
Estrogen receptor beta in prostate cancer. N Engl J Med.
351:2773–2774. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kuiper GG, Enmark E, Pelto-Huikko M,
Nilsson S and Gustafsson JA: Cloning of a novel receptor expressed
in rat prostate and ovary. Proc Natl Acad Sci USA. 93:5925–5930.
1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nilsson S and Gustafsson JA: Estrogen
receptors: Therapies targeted to receptor subtypes. Clin Pharmacol
Ther. 89:44–55. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen JQ and Russo J: ER alpha-negative and
triple negative breast cancer: Molecular features and potential
therapeutic approaches. Biochim Biophys Acta. 1796:162–175.
2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sakamoto G and Honma N: Estrogen receptor
beta status influences clinical outcome of triple negative breast
cancer. Breast Cancer. 16:281–282. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang J, Zhang C, Chen K, Tang H, Tang J,
Song C and Xie X: ERβ1 inversely correlates with PTEN/PI3K/AKT
pathway and predicts a favorable prognosis in triple-negative
breast cancer. Breast Cancer Res Treat. 152:255–269.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
von Minckwitz G, Raab G, Caputo A, Schütte
M, Hilfrich J, Blohmer JU, Gerber B, Costa SD, Merkle E, Eidtmann
H, et al: Doxorubicin with cyclophosphamide followed by docetaxel
every 21 days compared with doxorubicin and docetaxel every 14 days
as preoperative treatment in operable breast cancer: The GEPARDUO
study of the German Breast Group. J Clin Oncol. 23:2676–2685.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gasparini G, Dal Fior S, Panizzoni GA,
Favretto S and Pozza F: Weekly epirubicin versus doxorubicin as
second line therapy in advanced breast cancer. A randomized
clinical trial. Am J Clin Oncol. 14:38–44. 1991.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Momparler RL, Karon M, Siegel SE and Avila
F: Effect of adriamycin on DNA, RNA, and protein synthesis in
cell-free systems and intact cells. Cancer Res. 36:2891–2895.
1976.PubMed/NCBI
|
|
15
|
Fornari FA, Randolph JK, Yalowich JC,
Ritke MK and Gewirtz DA: Interference by doxorubicin with DNA
unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 45:649–656.
1994.PubMed/NCBI
|
|
16
|
Tewey KM, Rowe TC, Yang L, Halligan BD and
Liu LF: Adriamycin-induced DNA damage mediated by mammalian DNA
topoisomerase II. Science. 226:466–468. 1984.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mamounas EP, Bryant J, Lembersky B,
Fehrenbacher L, Sedlacek SM, Fisher B, Wickerham DL, Yothers G,
Soran A and Wolmark N: Paclitaxel after doxorubicin plus
cyclophosphamide as adjuvant chemotherapy for node-positive breast
cancer: Results from NSABP B-28. J Clin Oncol. 23:3686–3696.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fan Y, Li M, Ma K, Hu Y, Jing J, Shi Y, Li
E and Dong D: Dual-target MDM2/MDMX inhibitor increases the
sensitization of doxorubicin and inhibits migration and invasion
abilities of triple-negative breast cancer cells through activation
of TAB1/TAK1/p38 MAPK pathway. Cancer Biol Ther. 5:617–632.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shanle EK, Hawse JR and Xu W: Generation
of stable reporter breast cancer cell lines for the identification
of ER subtype selective ligands. Biochem Pharmacol. 82:1940–1949.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reese JM, Suman VJ, Subramaniam M, Wu X,
Negron V, Gingery A, Pitel KS, Shah SS, Cunliffe HE, McCullough AE,
et al: ERβ1: Characterization, prognosis, and evaluation of
treatment strategies in ERα-positive and -negative breast cancer.
BMC Cancer. 14(749)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hinsche O, Girgert R, Emons G and Gründker
C: Estrogen receptor β selective agonists reduce invasiveness of
triple-negative breast cancer cells. Int J Oncol. 46:878–884.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Reese JM, Bruinsma ES, Monroe DG, Negron
V, Suman VJ, Ingle JN, Goetz MP and Hawse JR: ERβ inhibits cyclin
dependent kinases 1 and 7 in triple negative breast cancer.
Oncotarget. 8:96506–96521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thomas C, Rajapaksa G, Nikolos F, Hao R,
Katchy A, McCollum CW, Bondesson M, Quinlan P, Thompson A,
Krishnamurthy S, et al: ERbeta1 represses basal-like breast cancer
epithelial to mesenchymal transition by destabilizing EGFR. Breast
Cancer Res. 14(R148)2012.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Shanle EK, Zhao Z, Hawse J, Wisinski K,
Keles S, Yuan M and Xu W: Research resource: Global identification
of estrogen receptor β target genes in triple negative breast
cancer cells. Mol Endocrinol. 27:1762–1775. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu X, Wang L, Chen J, Ling Q, Wang H, Li
S, Li L, Yang S, Xia M and Jing L: Estrogen receptor β agonist
enhances temozolomide sensitivity of glioma cells by inhibiting
PI3K/AKT/mTOR pathway. Mol Med Rep. 11:1516–1522. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khan KH, Yap TA, Yan L and Cunningham D:
Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J
Cancer. 32:253–265. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bitting RL and Armstrong AJ: Targeting the
PI3K/Akt/mTOR pathway in castration-resistant prostate cancer.
Endocr Relat Cancer. 20:R83–R99. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Martelli AM, Evangelisti C, Chiarini F and
McCubrey JA: The phosphate-dylinositol 3-kinase/Akt/mTOR signaling
network as a therapeutic target in acute myelogenous leukemia
patients. Oncotarget. 1:89–103. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Toulany M, Lee KJ, Fattah KR, Lin YF,
Fehrenbacher B, Schaller M, Chen BP, Chen DJ and Rodemann HP: Akt
promotes post-irra-diation survival of human tumor cells through
initiation, progression, and termination of DNA-PKcs-dependent DNA
double-strand break repair. Mol Cancer Res. 10:945–957.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Costa RLB, Han HS and Gradishar WJ:
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast
cancer: A review. Breast Cancer Res Treat. 169:397–406.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Park IH, Kong SY, Kwon Y, Kim MK, Sim SH,
Joo J and Lee KS: Phase I/II clinical trial of everolimus combined
with gemcitabine/cisplatin for metastatic triple-negative breast
cancer. J Cancer. 9:1145–1151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chock KL, Allison JM, Shimizu Y and
ElShamy WM: BRCA1-IRIS overexpression promotes cisplatin resistance
in ovarian cancer cells. Cancer Res. 70:8782–8791. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
O'Brien NA, Browne BC, Chow L, Wang Y,
Ginther C, Arboleda J, Duffy MJ, Crown J, O'Donovan N and Slamon
DJ: Activated phosphoinositide 3-kinase/AKT signaling confers
resistance to trastuzumab but not lapatinib. Mol Cancer Ther.
9:1489–1502. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu J and Tian D: Hematologic toxicities
associated with mTOR inhibitors temsirolimus and everolimus in
cancer patients: A systematic review and meta-analysis. Curr Med
Res Opin. 30:67–74. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Hamilton N, Márquez-Garbán D, Mah V,
Fernando G, Elshimali Y, Garbán H, Elashoff D, Vadgama J, Goodglick
L and Pietras R: Biologic roles of estrogen receptor-β and
insulin-like growth factor-2 in triple-negative breast cancer.
Biomed Res Int. 2015(925703)2015.PubMed/NCBI View Article : Google Scholar
|